Abstract
Despite the widespread distribution of Q fever, the prevalence in humans is not accurately known, because many infected people seroconvert without symptoms or with a mild febrile disease. The aim of this study was to determine the seroprevalence of Q fever in different regions of Croatia. During a 2-year period (2008–2010), serum samples from 552 febrile patients with prolonged cough aged 1–88 were tested for the presence of Coxiella burnetii antibodies by using indirect immunofluorescent assay. Sera from 27.5% patients showed IgG antibodies. Serological evidence of C. burnetii infection was found in patients from all parts of Croatia. Seroprevalence rates significantly differed among regions from 21.5% to 41.2% (p=0.001). Men were more often seropositive (31.6%) than women (22.2%; p=0.016). According to age, a progressive increase in the IgG seropositivity rates was observed as ranging from 6.7% in children less than 10 years of age to 39.2% in patients aged 40–49 (p=0.001). Above the age of 50, the IgG seroprevalence remained stable. Patients from rural areas were more often seropositive than patients from urban areas (40.8% vs. 19%), p<0.001). Acute Q fever was confirmed in 5.8% of patients. Cases occurred throughout the year. A majority of cases were reported during summer months.
Key Words: C. burnetii, Croatia, Geographical distribution, Seroprevalence, Q fever
Introduction
Q fever is a worldwide zoonosis caused by obligate intracellular bacterium Coxiella burnetii (Tissot-Dupont and Raoult 2008). Many animal species are reservoirs of C. burnetii in nature. Sheep, goats, and cattle are the main reservoirs and the most important source of infection for humans (Angelakis and Raoult 2010). Infected animals are generally asymptomatic, but shed bacteria in their urine, feces, milk, and in very high concentrations of amniotic fluid during birth or abortion (Arricau-Bouvery and Rodolakis 2005). Transmission to humans primarily occurs through inhalation of contaminated aerosol and, to a lesser extent, ingestion of contaminated dairy products. In humans, Q fever is usually an asymptomatic or nonspecific, flu-like disease with spontaneous recovery. Atypical pneumonia or hepatitis may be observed in more severe cases (Lukšić et al. 2006). In a small proportion of patients, the disease may become chronic, thereby leading to severe, often fatal endocarditis (Tissot-Dupont and Raoult 2008).
Human infections with C. burnetii have been reported in many European countries (Pascual-Velasco et al. 1998, Cisak et al. 2003, Cardenosa et al. 2006, Dorko et al. 2008, McCaughey et al. 2008, Hamzic et al. 2008, Monno et al. 2009), including Croatia. In Croatia, Q fever was first described in 1950 (Mihaljevic 1950). Since then, sporadic cases as well as minor or major outbreaks have been continuously reported (Galinovic-Weisglass et al. 1983, Janic and Golubic 1986, Spiranec et al. 1986, Milotić et al. 2001, Vilibic-Cavlek et al. 2004, Punda-Polic et al. 2007). However, data on the seroprevalence of Q fever are lacking. The aim of the current study was to determine the prevalence and distribution of Q fever among febrile patients residing in urban and rural parts of Croatia.
Materials and Methods
Study population
During a 2-year period (from August 2008 to July 2010), a total of 552 serum samples from febrile patients with prolonged cough (2 weeks or more) aged 1–88 residing in urban, sub-urban, and rural mainland and coastal Croatian regions was collected and tested for C. burnetii antibodies. According to the presence or absence of antibodies, different subgroups of the study population were defined: seronegative (neither IgM nor IgG antibodies), acute Q fever (positive IgM and IgG antibodies), and past infection (negative IgM and positive IgG antibodies).
The study was approved by the Ethics Committee of the Croatian National Institute of Public Health.
Geographical features
Based on the geographic characteristics of the area as well as the economic activities of the population, five different regions were defined: Zagreb macroregion, Slavonia, Lika, Gorski Kotar, and the Adriatic area: Istria and Dalmatia. The Zagreb area is an urban zone, whereas its surrounding areas (Kordun, Banovina, Moslavina, and Prigorje) are formed of hills. The many villages and small towns spread across the hillside. Slavonia is a geographical region in eastern Croatia. It is generally known as a lowland. Mountains higher than 500 m are rare and of an insular character. Most of this area is being used for agricultural activities and livestock breeding. Pigs and cattle are the predominant livestock. An inner mountainous region (Lika and Gorski Kotar) separates the continental mainland from the coast. It consists of mountains as high as 1500 m. Their inhabitants are involved in farming activities, including breeding stock, mainly sheep. The Adriatic area includes the narrow coastal belt and islands. Istria is the largest peninsula located in the north coastal region. About 30% of the Istrian population lives in rural zones where farmers breed mostly sheep and goats. Dalmatia is a region located in the south of Croatia. It consists of an urban zone (towns with their suburbs located in the coast), islands, and the rural mainland (Zagora). Zagora is a mountainous area with valleys surrounding small towns and villages. Its inhabitants are involved in agriculture and stock breeding (sheep and goats).
Serologic testing
Specific IgM and IgG antibodies to C. burnetii were detected by indirect immunofluorescence assay by using commercial slides containing C. burnetii phase II antigen obtained from culture on Vero cells (Coxiella burnetii-Spot IF; Biomerieux, Marcy l'Etoile, France) and fluorescein-isothiocyanate labeled anti-human IgM/IgG immunoglobulins (Fluoline M/G; Biomerieux). For IgM detection, sera were absorbed with anti-IgG (RF absorbent; Biomerieux). IgM titer ≥40 and IgG titer ≥80 were considered positive.
Statistical analysis
Mann–Whitney U and Fisher's exact tests were used to compare differences between groups of ordinal and nominal variables, respectively. Assumption of binomial distribution was used for calculation of confidence intervals. For statistical analysis, STATA/IC version 11.1 software was used. p<0.05 was considered statistically significant.
Results
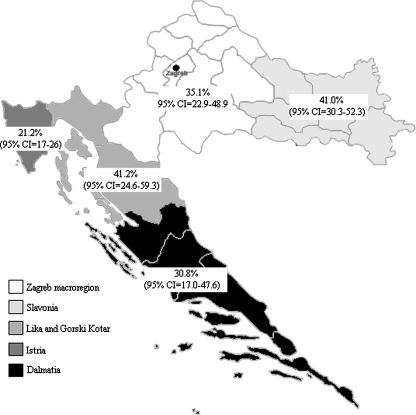
Serum samples from 152 of 552 tested patients or 27.5% (95% CI=23.8–31.5) had IgG antibodies, and 32 or 5.8% (95% CI=4.0–8.1) had IgM antibodies to C. burnetii. Of all seropositive subjects, 120/152 or 78.9% (95% CI=71.6–85.1) showed serologic evidence of past infection, and 32/152 or 21.1% (95% CI=14.9–28.4) showed evidence of acute Q fever. The seroprevalence rates differed (p=0.001) from one region to another (Fig. 1): Zagreb macroregion, 35.1%; Slavonia, 41.0%; Lika and Gorski Kotar, 41.2%; Istria, 21.5%; and Dalmatia, 30.8%.
FIG. 1.
Prevalence of Coxiella burnetii IgG antibodies in Croatia.
The prevalence of C. burnetii IgG antibodies according to the characteristics of the participants is shown in Table 1. There was a significant difference in IgG seroprevalence (p=0.016) between men (31.6%; 95% CI=26.5%–37.1%) and women (22.2%; 95% CI=17.1–28.0).
Table 1.
Prevalence of Coxiella burnetii IgG Antibodies According to Characteristics of Participants
| Characteristic | Tested (%) | Positive (%) | 95% CI | p-Value |
|---|---|---|---|---|
| Gender | 0.016 | |||
| Male | 313 (56.7) | 99 (31.6) | 26.5–37.1 | |
| Female | 239 (43.3) | 53 (22.2) | 17.1–28.0 | |
| Age group (years) | 0.001 | |||
| <10 | 30 (5.4) | 2 (6.7) | 0.1–22.1 | |
| 10–19 | 60 (10.9) | 10 (16.7) | 8.3–28.5 | |
| 20–29 | 74 (13.4) | 14 (18.9) | 10.7–29.7 | |
| 30–39 | 87 (15.8) | 25 (28.7) | 19.5–39.4 | |
| 40–49 | 79 (14.3) | 31 (39.2) | 28.4–50.9 | |
| 50–59 | 83 (15.0) | 28 (33.7) | 23.7–44.9 | |
| 60–69 | 63 (11.4) | 17 (27.0) | 16.6–39.7 | |
| 70+ | 76 (13.8) | 25 (32.9) | 22.5–44.6 | |
| Place of residence | <0.001 | |||
| Urban | 337 (61.1) | 64 (19.0) | 14.9–23.6 | |
| Rural | 213 (38.6) | 87 (40.8) | 34.2–47.8 | |
A progressive increase in the IgG seropositivity rates according to age was observed (p=0.001) as ranging from 6.7% (95% CI=0.1–22.1) in children less than 10 years of age to 39.2% (95% CI=28.4–50.9) in patients aged 40–49. IgG seroprevalence remained stable above the age of 50.
Patients who reside in rural areas are more often seropositive (p<0.001) than those who reside in urban areas (40.8%; 95% CI=34.2%–47.8% vs. 19%; 95% CI=14.9–23.6).
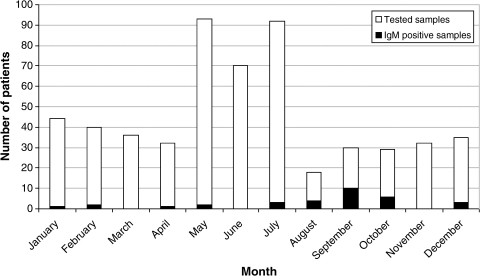
The seasonal distribution of acute Q fever cases (positive IgM antibodies) is presented in Figure 2. Cases occurred throughout the year with the highest incidence (from 20.7% to 33.3%) during summer and autumn months.
FIG. 2.
Seasonal distribution of acute Q fever cases.
Discussion
Q fever continues to be a public health problem in many European countries. The prevalence of Q fever in humans is not accurately known. Since many infected people (50%–60%) seroconvert without symptoms or with a mild febrile disease, Q fever is probably underestimated (Arricau-Bouvery and Rodolakis 2005). Reported seroprevalence rates in European countries vary greatly ranging from 1% to 60% (Hellenbrand et al. 2001, Cardenosa et al. 2006, Coulombier 2010). In certain risk groups, such as farmers and veterinarians, the prevalences are higher, up to 84% (McCaughey et al. 2008, Monno et al. 2009).
There are a few published studies on the prevalence of C. burnetii infections in Croatia. C. burnetii was documented as an etiological agent in 12%, 5.9%, and 3% of patients who presented with atypical pneumonia in 1992 (Mlinaric-Galinovic et al. 1995), 1998–2002, and 2002, respectively (Vilibic-Cavlek et al. 2004, 2006). Although some parts of Croatia are well-known endemic areas for Q fever (Galinovic-Weisglass et al. 1983, Milotić et al. 2001, Luksic et al. 2006, Punda-Polic et al. 2007), there are very few data available on the seroprevalence of C. burnetii antibodies.
In this study, 27.5% of patients showed evidence of exposure to C. burnetii. The IgG seropositivity rates significantly varied (p=0.001) among regions from 21.2% to 41.2%. These differences can be attributed to the variations in the proportion of population involved in farming activities.
Similar to other studies published worldwide (Coulombier 2010), gender distribution in this study indicated a male predominance (31.6% vs. 22.2%, p=0.016), probably due to a greater occupational exposure to C. burnetii in men (Luksic et al. 2006).
Similar to other studies, the present results showed that the prevalence of C. burnetii IgG antibodies tends to increase with age. Higher prevalences at a more advanced age could be explained by longer exposure of older people (Cardenosa et al. 2006).
Risk factors for Q fever include staying in rural areas and contact with livestock (Lukšić et al. 2006). Although patients from rural areas showed a significantly higher IgG seropositivity (40.8%), a high proportion of patients who were seropositive was found in urban areas (19.0%). C. burnetii can survive for months in the environment in the form of spores, which allows it to be transported by wind far away from the original source. This could explain the appearance of Q fever in urban areas, where a majority of patients report no direct contact with animals (Arricau-Bouvery and Rodolakis 2005). Contact with farm animals while travelling to rural areas and contact with pets could be another reason for C. burnetii infection among urban residents (Hellenbrand et al. 2001, Tissot-Dupont and Raoult 2008, Arricau-Bouvery and Rodolakis 2005).
Acute Q fever was documented in 5.8% of febrile patients. In contrast to most previous studies in Croatia, the cases occurred throughout the year. A majority of cases were recorded in summer and autumn. However, recent data from the European Union have shown a seasonal pattern of Q fever with more cases reported during the summer months (Coulombier 2010).
The modern sheep production technology demands well-organized and systematic sheep breeding that implies lambing every 8 months (Pavicic 2010), which may affect the transmission of Q fever throughout the year.
The results of this study suggest that C. burnetii is widespread in several areas of Croatia. About one third of the participants (27.5%) showed evidence of exposure to C. burnetii, and 5.8% had acute Q fever. Active surveillance may be warranted to identify animal sources and control the disease in humans. In endemic regions, the diagnosis of Q fever should be considered in the case of unexplained fever.
Acknowledgments
This research was supported by the Ministry of Science, Education, and Sports of the Republic of Croatia, grant No 005-0053443-3447 (to G.M.G.).
Disclosure Statement
No competing financial interests exist.
References
- Angelakis E. Raoult D. Q fever. Vet Microbiol. 2010;140:297–309. doi: 10.1016/j.vetmic.2009.07.016. [DOI] [PubMed] [Google Scholar]
- Arricau-Bouvery N. Rodolakis N. Is Q fever an emerging or re-emerging zoonosis? Vet Res. 2005;36:327–349. doi: 10.1051/vetres:2005010. [DOI] [PubMed] [Google Scholar]
- Cardenosa N. Sanfeliu I. Font B. Munoz T, et al. Short report: seroprevalence of human infection by Coxiella burnetii in Barcelona (Northeast of Spain) Am J Trop Med Hyg. 2006;75:33–35. [PubMed] [Google Scholar]
- Cisak E. Chmielewska-Badora J. Mackiewicz B. Dutkiewicz J. Prevalence of antibodies to Coxiella burnetii among farming population in Eastern Poland. Ann Agric Environ Med. 2003;10:265–267. [PubMed] [Google Scholar]
- Coulombier D. Query fever: an opportunity to understand the disease better. Euro Surveill. 2010;15:19526. [PubMed] [Google Scholar]
- Dorko E. Kalinova Z. Pilipcinec E. Seroprevalence of Coxiella burnetii antibodies among students of the Faculty of Medicine in Kosice (Slovakia) Folia Microbiol (Praha) 2008;53:563–568. doi: 10.1007/s12223-008-0090-2. [DOI] [PubMed] [Google Scholar]
- Galinovic-Weisglass M. Borcic B. Aleraj B. Delimar N, et al. An outbreak of Q-fever in Croatia (Yugoslavia) in 1983. G Mal Infett Parassit. 1986;38:569–574. [Google Scholar]
- Hamzic S. Beslagic E. Zvizdic S. Serotesting of human Q fever distribution in Bosnia and Herzegovina. Ann N Y Acad Sci. 2008;1078:13–16. doi: 10.1196/annals.1374.022. [DOI] [PubMed] [Google Scholar]
- Hellenbrand W. Breuer T. Petersen L. Changing epidemiology of Q fever in Germany, 1947–1999. Emerg Infect Dis. 2001;7:789–796. doi: 10.3201/eid0705.010504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janic N. Golubic D. Q fever epidemic in the area of Medimurje 1983. G Mal Infett Parassit. 1986;38:600–601. [Google Scholar]
- Lukšić B. Punda-Polic V. Ivic I. Bradaric I, et al. Clinical and epidemiological features of hospitalized acute Q fever cases from Split-Dalmatia County (Croatia), 1985–2002. Med Sci Monit. 2006;12:126–131. [PubMed] [Google Scholar]
- McCaughey C. McKenna J. McKenna C. Coyle PV, et al. Human seroprevalence to Coxiella burnetii (Q fever) in Northern Ireland. Zoonoses Public Health. 2008;55:189–194. doi: 10.1111/j.1863-2378.2008.01109.x. [DOI] [PubMed] [Google Scholar]
- Mihaljevic P. Q fever (Queenslandska groznica) Lijec Vjesn. 1950;72:246. [Google Scholar]
- Milotić I. Miletić B. Morović M. Clinical, epidemiological and epizootic features of Q fever in the Northern coastal part of Croatia from 1989 to 1998. Acta Med Croat. 2001;55:53–57. [PubMed] [Google Scholar]
- Mlinaric-Galinovic G. Turkovic B. Bace A. Loffler-Badzek A, et al. Etiology of atypical pneumonia in children and adults. Paediatr Croat. 1995;39:247–251. [Google Scholar]
- Monno R. Fumarola L. Trerotoli P. Carone D, et al. Seroprevalence of Q fever, brucellosis and leptospirosis in farmers and agricultural workers in Bari, Southern Italy. Ann Agric Environ Med. 2009;16:205–209. [PubMed] [Google Scholar]
- Pascual-Velasco F. Montes M. Marimon JM. Cilla G. High seroprevalence of Coxiella burnetii infection in Eastern Cantabria (Spain) Int J Epidemiol. 1998;27:142–145. doi: 10.1093/ije/27.1.142. [DOI] [PubMed] [Google Scholar]
- Pavicic Z. Razmnožavanje ovaca (Sheep reproduction) 2010. www.agroklub.com/stocarstvo/razmnozavanje-ovaca/2915/ www.agroklub.com/stocarstvo/razmnozavanje-ovaca/2915/ (In Croatian.)
- Punda-Polic V. Luksic B. Capkun V. Epidemiological features of Mediterranean spotted fever, murine typhus and Q fever in Split-Dalmatia County (Croatia), 1982–2002. Epidemiol Infect. 2007;13:1–8. doi: 10.1017/S0950268807009491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiranec N. Pospis J. Smolic V. Ruza-Bengeri M, et al. Q fever in the Varazdin region. G Mal Infett Parassit. 1986;38:602–604. [Google Scholar]
- Tissot-Dupont H. Raoult D. Q fever. Infect Dis Clin North Am. 2008;22:505–514. doi: 10.1016/j.idc.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Vilibic-Cavlek T. Mlinaric-Galinovic G. Turkovic B. Krizmanic I. Etiology of atypical pneumonias in Croatia in 2002. Results of the Croatian Institute of Public Health. Acta Med Croat. 2004;58:187–192. (In Croatian.) [PubMed] [Google Scholar]
- Vilibic-Cavlek T. Sviben M. Mlinaric-Galinovic G. Atypical pneumonias caused by C. burnetii from 1998–2002: results at Croatian Institute of Public Health. Acta Med. 2006;32:10–17. (In Croatian.) [Google Scholar]