Abstract
Tumor-associated macrophages infiltrate tumors and facilitate tumor growth. Here, we analyzed M1 and M2 marker expression in the course of co-culture-driven macrophage differentiation and investigated the influence of interferons (IFNs) on this differentiation. To generate monocyte-derived macrophages (MDMs) 1×106 monocytes of healthy volunteers were cultivated either with 25×103 adherent A549/mL or in medium containing 50% A549 conditioned medium (CM) for 72 h in the presence or absence of IFN-α, β or γ, respectively. Supernatants were tested for CCL18 (M2 marker) and CXCL10 (M1 marker) by enzyme-linked immunosorbent assay. CCL18 and CXCL10 release by MDM is increased by the presence of A549 cells, but also when cultured in A549 CM. On stimulation with IFN-γ, we observe an increased release of the M1 marker CXCL10 and a decreased release of CCL18. Type I IFNs also increases CXCL10 release. Thus, A549 releases a soluble factor which enhances CCL18 production and M2 polarization, indicating that a localized specific cytokine milieu, as found in the environment of a tumor or in fibrotic lung tissue, favors alternative activation of macrophages. In the presence of IFN-γ, M2 differentiation is attenuated as shown by the decrease of the M2 chemokine CCL18 and by the increase of the M1 chemokine CXCL10. However, CXCL10 levels were also increased by the co-culture, which indicates a simultaneous classical activation (M1) or the formation of a M1/M2 hybrid.
Introduction
Activated macrophages turn into specialized effector cells that perform distinct immunological functions. According to their inflammatory response pattern, they have been characterized as either classically activated macrophage (M1) or alternatively activated macrophages (AAMs; M2) (Mantovani and others 2002; Gordon 2003).
M1 macrophages develop in response to interferon (IFN)-γ, along with a co-stimulatory signal, similar to exposure to lipopolysaccharide. They may be identified by numerous physiological changes found during classical activation, for example, the up-regulation of MHC class II and CD86, the production of NO and O2−, and the expression of pro-inflammatory cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-1, IL-6, CXCL9, CXCL10, CXCL11, CCL3, and CCL2 (Mosser 2003).
On the other hand, M2 macrophages participate in regulating immune responses, promoting angiogenesis and tissue repair. The term M2 macrophage suggests one clearly defined population of cells; however, it becomes more and more clear that M2 macrophages are a very heterogenous group of immune cells. Therefore, they will be referred to as AAMs. AAM play an important role in pathological processes such as fibrosis (Prasse and others 2006), systemic sclerosis (Prasse and others 2006; Pechkovsky and others 2010), and neoplasia (Mantovani and others 2002). Therefore, it is important to understand under what circumstances macrophages get activated in a certain way. It has been shown that IL-4, IL-13, IL-10, as well as collagen and glucocorticoids induce alternative activation of macrophages in vitro (Albert and others 1992; Mantovani and others 2002; Gordon 2003; Prasse and others 2006; Pechkovsky and others 2010). CCL17, CCL18, and CCL22 have been identified as AAM marker cytokines (Martinez and others 2006). Of note, CCL18 enhances collagen production of fibroblasts and, in turn, alveolar macrophages are activated by collagen to differentiate into AAM, which increases their CCL18 release and results in a vicious circle in which fibroblasts respond to CCL18 stimulation by the production of even more collagen (Prasse and others 2006).
Patients suffering from fibrotic lung diseases such as idiopathic pulmonary fibrosis (IPF) and others show high levels of CCL18 in serum, lung tissue, and bronchoalveolar lavage (BAL) fluid (Prasse and others 2007). The main sources of CCL18 in these disorders are alternatively activated alveolar macrophages (Schutyser and others 2005; Prasse and others 2006). Fibrosing pulmonary disorders are treated with steroids and immunosuppressants, however, without convincing success. IFN-γ is discussed as a potential antifibrotic agent in IPF therapy, as IFN-γ inhibits fibroblast proliferation and collagen synthesis in human and animal studies (Gurujeyalakshmi and Giri 1995; Coker and Laurent 1998). Our point of interest is the question whether IFN-γ also has the capability to inhibit M2 polarization and to analyze its potential to be used to down-regulate AAM in a variety of diseases with predominant alternative macrophage activation.
Neoplastic infiltration induces the differentiation of tumor-associated macrophages (TAMs) disclosing a phenotype of alternative activation. Thus, TAM are also referred to as AAM (Mantovani and others 2002). CCL2, released by tumor cells or macrophages, induces transforming growth factor-β (Roca and others 2009), which also promotes the production of fibrotic tissue and inhibits antitumor immune response (Mantovani and others 2002). Therefore, using the human bronchoalveolar carcinoma-derived cell line A549, we developed a model of tumor-cell-induced alternative activation of macrophages well knowing that the mechanisms explaining the induction of AAM by tumor cells are not completely understood.
IFNs might be promising candidates to modulate macrophage activation. IFN-α and β are referred to as type I IFNs, and IFN-γ as type II IFN. Therapeutically, type I IFNs are used to treat hepatitis B and C, as well as melanoma to prolong relapse-free survival (Biron 2001; Agarwala and Case 2010; Halama and others 2010; Kaehler and others 2010).
IFN-γ is an important cytokine in the antineoplastic response of the immune system. It has direct antitumor effects, as it acts antiangiogenic, inhibits proliferation, sensitizes tumor cells to apoptosis, up-regulates MHC class I and II expression, and stimulates antitumor immune activity (Miller and others 2009). It has been successfully used in cases of ovarian cancer (Pujade-Lauraine and others 1996), bladder carcinoma (Giannopoulos and others 2003), and lately in malignant gliomas (Kane and Yang 2010). Therefore, we evaluated its capability to modulate the polarization of macrophages.
Given the fact that AAMs are at least in part responsible for the progression of tumor growth (Biswas and others 2006; Schoppmann and others 2006) and part of a pathological vicious circle found in fibrotic diseases (Prasse and others 2006), it is of great importance to understand the processes that lead to alternative activation of macrophages and to find measures to down-regulate or interrupt this activation. Our results demonstrate that co-culture with A549 cells as well as A549 conditioned medium (CM) evokes an AAM phenotype in macrophages, which is attenuated by IFN-γ.
Methods
A549 culture and generation of CM
A549 cells were maintained in culture medium (Dulbecco's Modified Eagle's Medium [DMEM] containing 10% fetal calf serum [FCS] and 1% penicillin/streptomycin). To obtain A549 CM, A549 cells were suspended in culture medium, sowed at a density of 3×105/mL in 5 mL culture medium (DMEM containing 10% FCS and 1% penicillin/streptomycin), and cultured for 24 h at 37°C. After medium change, cells were cultured for an additional 24 h with fresh medium and thereafter, cell free supernatants were cautiously harvested and stored in aliquots at −80°C.
Peripheral blood monocyte isolation and co-culture
Twenty-four hours before experiments, 25×103 A549 cells were cultured in 24-well plates in 1 mL of medium for 24 h to allow adherence. Blood was collected from healthy human volunteers, and peripheral blood mononuclear cells (PBMCs) were isolated by density centrifugation (Pancoll human; PAN Biotech). Monocytes were isolated from PBMC using antihuman CD14 beads applying the MACS system (Miltenyi Biotec) according to the manufacturer's protocol. These preparations consisted of 97% monocytes by immunochemistry with anti-CD14 antibodies and flow cytometry analysis (data not shown). Cells were 97% viable by Trypan blue exclusion. Monocytes were resuspended and adjusted to 1×106 cells/mL in DMEM with 10% FCS and 1% penicillin/streptomycin. For co-culture, the medium was removed from the precultured A549, and 1 mL of the monocytes suspension was added. For control purposes, monocytes and A549 were also separately cultured. All cultures were cultivated for 72 h. During this culture period, monocytes differentiate to monocyte-derived macrophages (MDMs). Stimulations were performed with 10 or 100 U/mL IFN-α, β or γ, respectively, at the beginning of the cultivation period. Furthermore, 106 monocytes/mL were cultivated in medium containing 50% A549 CM (see below). After 72 h, cell-free supernatants were collected and stored at −80°C until cytokine measurements were taken.
Enzyme-linked immunosorbent assay for CCL18 and CXCL10
Supernatants of monocyte cultures, A549, and co-cultures were tested for CCL18 and CXCL10 by enzyme-linked immunosorbent assay (ELISA). Cytokine concentrations in supernatants were quantified by ELISA (DuoSet ELISA Development System Kits; R&D Systems) using the protocols suggested by the supplier. The detection limit for both ELISAs was 7 pg/mL.
RNA isolation, reverse transcription, and real-time polymerase chain reaction
Total RNA was extracted from 5×105 to 1×106 cells using TRIzol Reagent according to the manufacturer's protocol (Invitrogen). Total RNA was reverse transcribed with StrataScript RT (Stratagene) using oligo (dT)12–18 primer according to the manufacturer's protocol. Primers for human glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and TNF were designed with Primer3 software (Whitehead Institute for Biomedical Research; http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi), Amplify1.2 software (University of Wisconsin; http://engels.genetics.wisc.edu/amplify) using GenBank database (National Center for Biotechnology Information; www.ncbi.nlm.nih.gov). Accession code numbers and primer sequences for the targets are listed in Table 2. All primers are intron spanning and synthesized by biomers.net (biomers.net). The real-time polymerase chain reaction (PCR) was performed with iQ SYBR Green SuperMix, iCycler thermocycler, and iCycler iQ 3.0 software (Bio-Rad Laboratories) according to the manufacturer's protocol. To control for specificity of the amplification products, a melting curve analysis was performed. No amplification of unspecific products was observed in all reactions. The data were analyzed to calculate a cycle threshold value (Ct) for each sample independently in duplicate for TNF and GAPDH. The relative level of TNF mRNA was calculated by the following formula: 2(Ct GAPDH-Ct CYTOKINE)×10,000 for each cDNA sample and given as a factor (rE) without a unit.
Table 2.
Primers Used for Polymerase Chain Reaction
| Target | Accession code | Forward | Reverse |
|---|---|---|---|
| TNF | NM_000594 | CCCAGGGACCTCTCTCTAATC | GCTGGTTATCTCTCAGCTCCA |
| GAPDH | NM_002046 | CACCAGGGCTGCTTTTAACT | GATCTCGCTCCTGGAAGATG |
TNF, tumor necrosis factor; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Statistical analysis
Data are depicted as box plots. Horizontal lines represent median and 25th and 75th percentiles; small lines characterize 10th and 90th percentiles. Statistical analysis was performed using StatView 5.0 software (SAS Institute). Comparisons between the cytokine production of the different cell-cultures, monocytes, A549, and co-cultures, thus, nonlinked variables, were performed by Mann–Whitney U test for nonparametric analysis. Linked variables such as cytokine production of identical cell-cultures that underwent different stimulations were compared by Wilcoxon Signed Rank Test. Spearman rank correlation coefficient was used to study correlations between the amount of IFN added and cytokine concentrations in the supernatant. Probability values were considered significant if they were <0.05.
Results
Monocyte-to-macrophage maturation in vitro
We chose the chemokine CCL18 as a marker for alternative activation. Compared with freshly isolated alveolar macrophages, nonstimulated monocytes release negligible amounts of CCL18 (Pechkovsky and others 2010). It is generally accepted that monocytes cultured for several days differentiate into MDMs. Although there are various cell culture periods for MDM maturation found in the literature, we chose a short 72 h period, because after this period, MDM releases significantly higher amounts of CCL18 compared with earlier time points but minimizes cell loss.
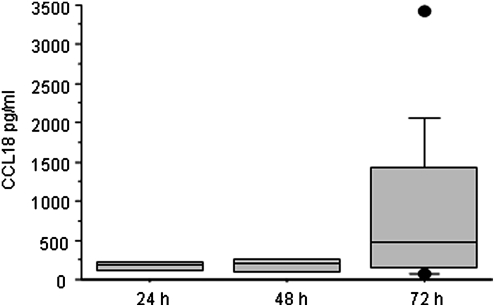
The CCL18 amount released rises along with the maturation of monocytes to macrophages. After 24 and 48 h, nonstimulated monocytes released only very small amounts of CCL18; however, that changed significantly after 72 h of culture (Fig. 1).
FIG. 1.
CCL18 release of monocytes in culture after 24, 48, and 72 h. A strong increase of CCL18 release is observed after 72 h of culture time only (n=4). Data are depicted as Box Plots. The horizontal line within the box represents the median value, the box itself indicates the 25th and 75th percentile, and dots indicate data below 10th or above 90th percentile.
Co-culture of monocytes with A549 induces CCL18 and CXCL10 release
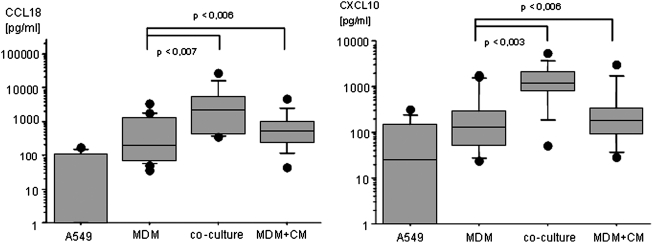
In order to study cytokine expression during macrophage polarization, we established a co-culture model using freshly isolated monocytes co-cultured with A549 cells. Co-culture of monocytes with A549 but without any cytokine stimulation increased CCL18 release significantly. However, the same effect is achieved when A549 cells are substituted by their cell culture supernatant in a 1:1 ratio with fresh medium (Fig. 2). Thus, cell-cell contact is not essential for the observed effect. In the co-cultures and the MDM cultures with A549 conditioned media, CXCL10 levels were also increased to a similar extent as CCL18 (Fig. 2). Nonstimulated A549 release CCL18 and CXCL10 close to the detection limit of the assay. TNF mRNA was only marginally expressed in the MDM cultured alone. Co-culture of MDM with A549 cells induced a down-regulation of TNF mRNA close to the detection limit (data not shown). Nonstimulated MDM show strong individual fluctuations in the amount of CCL18 and CXCL10 released, as well as in the expression of TNF mRNA.
FIG. 2.
Co-culture effect on cytokine release. Cytokine release of nonstimulated A549 cells, Monocyte derived macrophages (MDMs), co-culture of A549 and MDM, and MDM cultured with A549 CM for 72 h. CCL18 and CXCL10 release of A549 cells is low. MDM produce variable amounts of cytokines. The nonstimulated co-culture shows a significant increase of cytokine release, CCL18 augments, and CXCL10. The effect of the CM is similar to the co-culture, elevating cytokine release of MDM (n=8). Data are depicted as Box Plots. The horizontal line within the box represents the median value, the box itself indicates the 25th and 75th percentile, and dots indicate data below 10th or above 90th percentile. Y-axis shows cytokine release in pg/mL in logarithmic scale. X-axis refers to the type of cell culture setting.
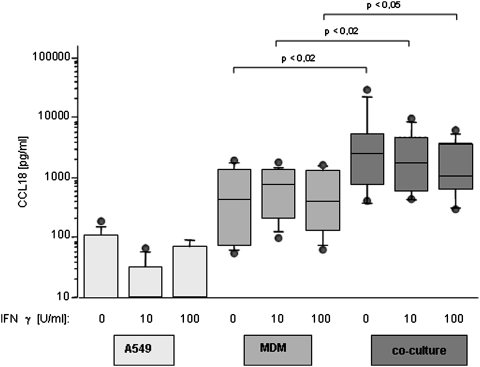
IFN-γ attenuates A549-induced CCL18 release
IFN-γ is one of the most important activators of classical macrophage activation (Martinez and others 2008), but its influence on alternative activation is not fully understood. A549 produce CCL18 close to the detection limit of the assay, which is not altered by stimulation with IFN-γ (Fig. 3). As just described, CCL18 release of MDM single culture shows significant inter-individual fluctuations; however, the spontaneous CCL18 release did not change on stimulation with IFN-γ. In contrast, the elevated CCL18 release of MDM in the co-culture is decreased by IFN-γ (Fig. 3), although these changes did not reach statistical significance. In all cases, MDM in A549 co-cultures release significantly higher amounts compared with a single culture (Fig. 3). Type I IFNs induce no changes in CCL18 expression, neither in monocyte single culture (Table 1), nor in the co-culture of monocytes with A549 cells (data not shown).
FIG. 3.
CCL18 release under the influence of IFN-γ. Influence of IFN-γ, 10 and 100 U/mL, on CCL18 release of A549 cells, MDM, and the co-culture of these 2 cell types. IFN-γ does not influence CCL18 release of A549 cells, which stays undetectable in supernatants of stimulated as well as nonstimulated A549 cells. MDM production of CCL18 does not change on stimulation with IFN-γ. The co-culture of these 2 cell types shows the most stimulating effect on CCL18 release. This enhancing influence of the co-culture is diminished by IFN-γ (n=6). Data are depicted as Box Plots. The horizontal line within the box represents the median value, the box itself indicates the 25th and 75th percentile, and dots indicate data below 10th or above 90th percentile. Y-axis shows cytokine release in pg/mL in logarithmic scale. X-axis refers to the type of cell culture setting and the quantity of stimulation with IFN-γ. IFN, interferon.
Table 1.
CCL18 and CXL10 Release Under the Influence of Type I Interferons CCL18 and CXCL10 Release in Supernatants of Monocyte-Derived Macrophages, Cultured for 72 h, Nonstimulated as Well as with 10 and 100 U/ml of Interferon-α and β Respectively
| Nonstimulated | IFN-α 10 U/mL | IFN-α 100 U/mL | IFN-β 10 U/mL | IFN-β 100 U/mL | |
|---|---|---|---|---|---|
| CCL18 (pg/mL) | 775±665 | 864±715 | 1,041±1,170 | 1,096±1,116 | 815±511 |
| CXCL10 (pg/mL) | 518±690 | 548±642 | 874±838* | 680±824* | 626±675 |
Stimulation produces only insignificant changes in CCL18 production. CXCL10 release is increased significantly by IFN-α (100 U/mL). IFN-β (10 U/mL) also augment CXCL10 release significantly (*P<0.05) n=8.
IFN, interferon.
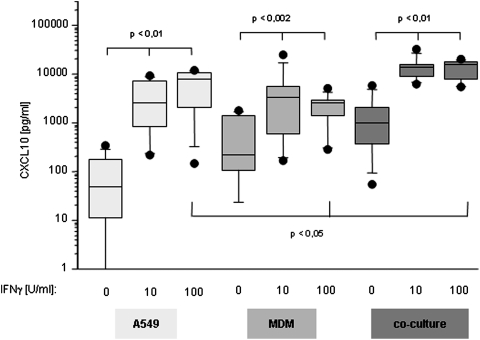
CXCL10 release by MDM
As just indicated, nonstimulated A549 cells release CXCL10 close to the detection limit of the assay; however, stimulation with IFN-γ induced a significant and dose-dependent increase in CXCL10 release (Fig. 4). In contrast to A549, MDM released detectable amounts of CXCL10 already without any stimulation. In the presence of 10 and 100 U/mL IFN-γ, CXCL10 concentrations measured in the supernatant rose in a dose-dependent manner (Fig. 4), which is in line with the fact that IFN-γ is a potent inducer of classical activation of macrophages. The amount of CXCL10 found in supernatants of the co-culture of monocytes with A549 cells was significantly higher than in both single cultures and exceeded the sum of the single cultures. Again, stimulation with IFN-γ induced a highly significant increase in CXCL10 production in the co-culture of monocytes and A549, outperforming any other stimulation.
FIG. 4.
CXCL10 release under the influence of IFN-γ. Influence of IFN-γ (10 and 100 U/mL respectively) on CXCL10 release of A549 cells and MDM and the co-culture of these 2. CXCL10 release by A549 cells, MDM, and the co-culture is increased by stimulation with IFN-γ in a dose-dependant manner (n=6). Data are depicted as Box Plots. The horizontal line within the box represents the median value, the box itself indicates the 25th and 75th percentile, and dots indicate data below 10th or above 90th percentile. Y-axis shows cytokine release in pg/mL in logarithmic scale. X-axis refers to the type of cell culture setting and the quantity of stimulation with IFN-γ.
TNF mRNA expression by MDM
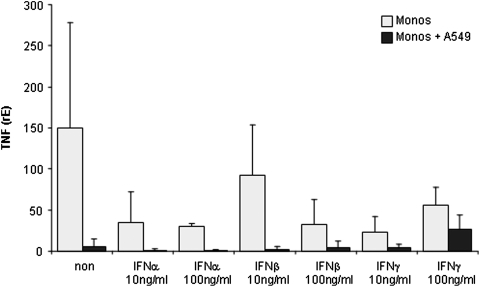
MDM in a single culture expressed very low levels of TNF mRNA. Addition of type I and type II IFNs decreased TNF mRNA expression markedly with IFN-α as the most potent suppressor. However, due to the marked inter-individual differences, none of these reductions gained statistical significance (Fig. 5).
FIG. 5.
TNF mRNA expression in MDM either cultured alone (grey) or in co-culture with A549 cell (black) stimulated with the indicated concentration of IFN-α, β or γ. The expression is given as relative expression normalized with GAPdH (rE, see Material and Methods section). TNF, tumor necrosis factor.
Co-culture of MDM and A549 cells decreased TNF mRNA expression close to the detection limit of our PCR system. The addition of IFN-α and IFN-β did not change TNF expression. In contrast, the addition of IFN-γ to the A549/MDM co-cultures markedly increased TNF mRNA expression (Fig. 5). Again, due to the marked inter-individual differences, this increase did not reach statistical significance.
Type I IFN induce CXCL10 release
Although the therapeutical use of type I IFN in autoimmune and inflammatory disorders is common, the mechanisms of action are largely unknown. Therefore, we investigated the influence of type I IFN on macrophage polarization by measuring their cytokine release in the presence of type I IFN. Type I IFNs used in the same concentration, as IFN-γ also have the capability to induce CXCL10 in monocytes, however, to a lesser extent than IFN-γ. Stimulation of monocytes with 100 U/mL IFN-α increased CXCL10 release significantly. About 10 U/mL IFN-β also stimulate CXCL10 release significantly (Table 1) without further treatment. In the co-culture of monocytes with A549, stimulation with type I IFN did not change CXCL10 release compared with nonstimulated co-cultures (data not shown). No significant influence of type I IFN on CCL18 expression could be observed; neither in single-cultured MDM (Table 1) nor in co-culture with A549 cells (data not shown).
Discussion
We chose CXCL10 and TNF as markers for classical activation of macrophages, and the chemokine CCL18 as a marker for alternative activation. The latter was chosen, as it was recently shown that all M2 marker cytokines highly correlate (Pechkovsky and others 2010). Interestingly, the release of both CCL18 as well as CXCL10 by MDM was increased in the presence of A549 cells, as well as by the culture of MDM in A549 CM. In contrast, although TNF mRNA expression was low in MDM, it was further down-regulated in the presence of A549 cells. On stimulation with IFN-γ, we observed an increased release of the M1 marker CXCL10 and TNF and a decreased release of CCL18. Type I IFNs also increased CXCL10 release but did not have any effect on CCL18 and TNF release.
A localized specific cytokine milieu, as found in the environment of a tumor or in fibrotic lung tissue, favors alternative activation of macrophages, which are involved in the perpetuation of pathological processes (Maher and others 2007). The prospect of modulating macrophage activation is of great value, as an unbalanced macrophage population has the potential to harm the host and to trigger and promote the progress of diseases such as fibrosis and tumor formation. These diseases exhibit an elevated number of AAM, which have been found responsible for deposition of extra-cellular matrix (Prasse and others 2006), angiogenesis (Mantovani and others 2002), and the formation of new lymph vessels (Grimshaw and Balkwill 2001; Schoppmann and others 2006).
The alternative activation of MDM described in this work has been observed in the presence of A549 or A549 CM. TAMs have been identified in various tumors as, for example, in human ovarian, breast, and lung cancers, intrahepatic cholangiocarcinoma (Hasita and others 2010), as well as in various murine tumors. In addition, we could demonstrate that NSCLC and SCLC cell lines also induce alternative activation (data not shown).
In order to learn more about the interactions between macrophages and tumor cells, we established a model using a co-culture of monocytes and A549 cells. This model helps in studying macrophage polarization in vitro, but in a context that more likely resembles the in vivo situation. We could show that CCL18 release is strongly increased in the co-culture of monocytes with A549 cells, while TNF is down-regulated, leading to the conclusion that tumor cells foster M2 polarization in MDM. This effect can also be induced by cultivating monocytes in A549 CM leading to a phenotype similar to TAM. Thus, our results support the theory of alternative activation of macrophages by cellular contact or by yet unknown soluble factors. Mosser and Edwards (2008) suggest that tumor-derived agents, such as prostaglandins, hypoxia, or extra-cellular nucleotides, induce a M2 macrophage subset that has regulatory functions, that is, TAM. These M2 macrophages produce high levels of IL-10 (Sica and others 2000; Mantovani and others 2002) and deactivate neighboring macrophages, thus promoting tumor growth. In addition, CCL2 has also been shown to induce M2 polarization (Tsuda and others 2004; Roca and others 2009). Interestingly, CCL2 is a major product released by A549 (Pechkovsky and others 2005) as well as by other tumor cells (Soria and others 2008; Zhang and others 2010).
Surprisingly, in the co-cultures of monocytes and A549 cells, CXCL10 levels were also increased to a similar extent. This increase in CXCL10 release was also observed in cultures of monocytes with A549 CM; thus, it is independent of cell-cell contacts. CXCL10 has been described as a chemokine that is clearly released by M1 activated macrophages (Martinez and others 2006). Interestingly, the presence of macrophages presenting with a mixed pattern has been described in colon cancer (Rigo and others 2010).
These findings may be interpreted in different ways. Seemingly, tumor cells generate a cytokine milieu enabling both classical and alternative activation of macrophages as demonstrated by the concomitant up-regulation of CXCL10 and CCL18. It is also possible that that MDM, matured in the presence of tumor cells (here A549), represent a special subpopulation with a mixed cytokine expression pattern not fitting into the M1/M2 paradigm. This assumption is supported by the findings of Biswas (Biswas and others 2006) in the murine system. They observed that resting and activated TAM produce IFN-inducible cytokines such as CXCL10, CXCL9, CXCL16, and CCL5, but also express IL-10 and CCL2 as expected. However, a clear cytokine profile of TAM in human neoplasia has not yet been defined.
In addition, in the chosen setting, we did not add stimuli triggering NFκB activation, which is an important transcription factor regulating TNF expression. Hence, the expression of TNF is very low in our cultures. In contrast, an important regulatory element for CXCL10 is STAT1 (Saha and others 2010), which is also likely to be a regulatory element for CCL18 (Politz and others 2000). Thus, although CXCL10 is in many cases referred as being an M1-associated chemokine, the CXCL10 regulatory elements make it likely that this mediator is also increased in a M2 stimulatory environment.
There are more and more hints that macrophages keep their plasticity beyond maturation and even polarization is reversible (Porcheray and others 2005). Extra-cellular matrix and cytokines may push maturing monocytes toward a specialized state in between the poles of M1 and M2. The intensity of this drag toward M2 can be attenuated by IFN-γ as observed in the co-culture, where high CCL18 release is decreased by IFN-γ, but not completely inhibited. CCL18 is a constitutively expressed cytokine that is additionally regulated by various transcription factors (Politz and others 2000) and, thus, IFN-γ might not be sufficient for complete inhibition. Moreover, since IFN-γ triggers STAT1, it is likely that at least in part, the remaining CCL18 release is triggered by IFN-γ-induced STAT1 activation. Thus, our results show that only tumor-cell-induced CCL18 production can be constrained by IFN-γ.
IFN-γ has been successfully used in immunologic diseases such as systemic sclerosis (Miller and others 2009) and different kinds of cancer (Giannopoulos and others 2003; Kane and Yang 2010). IFN-γ could be an effective treatment for IPF and neoplasia via multiple pathways. In order to learn more about these complex relations, we are in need of a reliable model that studies macrophage polarization in vitro.
In the context of fibrotic lung diseases, we observe alternative activation of macrophages and high CCL18 levels in lung tissue, BAL, and serum (Prasse and others 2007), which might be attenuated by IFN-γ. Provided that sufficient IFN-γ reaches the lung, the M2 attenuating effect of IFN-γ observed in vitro might also diminish the pathological effects of supernumerous AAMs in vivo.
IFN-γ induces CXCL10 release in monocytes single culture and even more in the co-culture with A549. IFN-γ is the most potent inducer of CXCL10 expression in A549 cells and type II alveolar epithelial cells (Pechkovsky and others 2005) as well as in macrophages and MDM. In fact, the presence of IFN-γ in co-cultures of A549 and MDM increased CXCL10 release the most. CXCL10 concentrations in supernatants of the co-culture stimulated with IFN-γ overtopped the sum of CXCL10 release of the single cultures, arguing for an over-additive effect of these 2 stimuli.
These findings demonstrate that the co-culture induced alternative activation, which is shown by CCL18 release of MDM single culture, can be attenuated by IFN-γ, and shifts to an M1-activation.
Our results demonstrate that type I IFNs also induce CXCL10 in MDM, however, to a lesser extent than IFN-γ. It is known that IFN-α induces CXCL10 in plasmacytoid dendritic cells (Blackwell and Krieg 2003) and both IFN-α and IFN-β induce CXCL10 release in murine bone-marrow-derived macrophages (Narumi and Hamilton 1991; Fleetwood and others 2009). Our results demonstrate that IFN-α and β also induce CXCL10 release in single cultures of human MDM, which is in accordance with the current literature (Bradley and others 1989; Yurkovetsky and others 2007). Interestingly, our data demonstrate that Type I IFNs do not influence CCL18 release.
IFN-α and β trigger STAT1 and STAT2, but they also activate STAT3, for which a regulatory function was suggested by Ho (Ho and Ivashkiv 2006), namely attenuation of STAT1-mediated inflammatory functions. The nature of cellular response to IFN depends on the ratio of STAT1 to STAT3 activation. In the co-culture of monocytes with A549, type I IFNs enhanced neither CXCL10 expression nor CCL18 expression. Thus, the missing effect of type I IFN in the co-culture might be interpreted by higher STAT3 levels, which prevent a strong inflammatory response inducing CXCL10 production. Recently, Hasita suggested that TAM contribute to cancer progression by STAT 3 activation. Possibly, already the presence of tumor cells enhanced STAT3 transcription (Hasita and others 2010). Therefore, no further effect can be obtained by adding type I IFN.
IFN-γ, on the other hand, acts mainly by STAT1 activation, which is probably additionally induced by effects of A549. This might explain the high CXCL10 release of the co-culture stimulated with IFN-γ. To ensure this hypothesis, further experiments are in progress.
Conclusion
This study provides evidence that tumor cells modify the inflammatory mononuclear phagocyte response of the host in direction of a M1/M2-hybrid. IFN-γ attenuates the M2-characteristics brought forward by the tumor cells.
In conclusion, the observed exaggerated release of CCL18 demonstrates an A549-induced M2 polarization. Thus, tumor cells induce a pro-fibrogenic micromilieu leading to increased matrix production and a suppression of antitumor immune reactions. However, a clear-cut differentiation to either M1 or M2 side cannot be observed due to the plasticity of the cells and additional stimuli delivered in the course of the disease might favor a further polarization. A future analysis of the A549-CM will give clues for the cytokine-milieu causing polarization on one hand side and allowing cell plasticity on the other.
Our data might be interpreted in two ways. First, CXCL10 might not be a specific marker to monitor classical pathway activation. The second possibility would be that our model to induce TAM reveals a mixture of classical and alternative activation as monitored by CCL18 and CXCL10 release. Thus, TAMs are probably a specialized subpopulation of macrophages that not only disclose M2 properties, but also express IFN inducible chemokines.
Acknowledgments
The authors wish to thank all their volunteers for blood donations. This work was partly funded by a grant from the German DPLD Network (GOLDnet; German Ministry for Education and Research (BMBF), grant number 01GM0859) and the Deutsche Forschungsgemeinschaft (ZI 495/3-1).
Author Disclosure Statement
No competing financial interests exist.
References
- Agarwala SS. Case S. Everolimus (RAD001) in the treatment of advanced renal cell carcinoma: a review. Oncologist. 2010;15(3):236–245. doi: 10.1634/theoncologist.2009-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert RK. Embree LJ. McFeely JE. Hickstein DD. Expression and function of β 2 integrins on alveolar macrophages from human and nonhuman primates. Am J Respir Cell Mol Biol. 1992;7:182–189. doi: 10.1165/ajrcmb/7.2.182. [DOI] [PubMed] [Google Scholar]
- Biron CA. Interferons alpha and beta as immune regulators—a new look. Immunity. 2001;14(6):661–664. doi: 10.1016/s1074-7613(01)00154-6. [DOI] [PubMed] [Google Scholar]
- Biswas SK. Gangi L. Paul S. Schioppa T. Saccani A. Sironi M. Bottazzi B. Doni A. Vincenzo B. Pasqualini F. Vago L. Nebuloni M. Mantovani A. Sica A. A distinct and unique transcriptional program expressed by tumor-associated macrophages (defective NF-kappaB and enhanced IRF-3/STAT1 activation) Blood. 2006;107(5):2112–2122. doi: 10.1182/blood-2005-01-0428. [DOI] [PubMed] [Google Scholar]
- Blackwell SE. Krieg AM. CpG-A-induced monocyte IFN-gamma-inducible protein-10 production is regulated by plasmacytoid dendritic cell-derived IFN-alpha. J Immunol. 2003;170(8):4061–4068. doi: 10.4049/jimmunol.170.8.4061. [DOI] [PubMed] [Google Scholar]
- Bradley SF. Vibhagool A. Kunkel SL. Kauffman CA. Monokine secretion in aging and protein malnutrition. J Leukoc Biol. 1989;45(6):510–514. doi: 10.1002/jlb.45.6.510. [DOI] [PubMed] [Google Scholar]
- Coker RK. Laurent GJ. Pulmonary fibrosis: cytokines in the balance. Eur Respir J. 1998;11(6):1218–1221. doi: 10.1183/09031936.98.11061218. [DOI] [PubMed] [Google Scholar]
- Fleetwood AJ. Dinh H. Cook AD. Hertzog PJ. Hamilton JA. GM-CSF- and M-CSF-dependent macrophage phenotypes display differential dependence on type I interferon signaling. J Leukoc Biol. 2009;86(2):411–421. doi: 10.1189/jlb.1108702. [DOI] [PubMed] [Google Scholar]
- Giannopoulos A. Constantinides C. Fokaeas E. Stravodimos C. Giannopoulou M. Kyroudi A. Gounaris A. The immunomodulating effect of interferon-gamma intravesical instillations in preventing bladder cancer recurrence. Clin Cancer Res. 2003;9(15):5550–5558. [PubMed] [Google Scholar]
- Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3(1):23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- Grimshaw MJ. Balkwill FR. Inhibition of monocyte and macrophage chemotaxis by hypoxia and inflammation—a potential mechanism. Eur J Immunol. 2001;31(2):480–489. doi: 10.1002/1521-4141(200102)31:2<480::aid-immu480>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Gurujeyalakshmi G. Giri SN. Molecular mechanisms of antifibrotic effect of interferon gamma in bleomycin-mouse model of lung fibrosis: downregulation of TGF-beta and procollagen I and III gene expression. Exp Lung Res. 1995;21(5):791–808. doi: 10.3109/01902149509050842. [DOI] [PubMed] [Google Scholar]
- Halama N. Zoernig I. Jaeger D. Advanced malignant melanoma: immunologic and multimodal therapeutic strategies. J Oncol. 2010;2010:689893. doi: 10.1155/2010/689893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasita H. Komohara Y. Okabe H. Masuda T. Ohnishi K. Lei XF. Beppu T. Baba H. Takeya M. Significance of alternatively activated macrophages in patients with intrahepatic cholangiocarcinoma. Cancer Sci. 2010;101(8):1913–1919. doi: 10.1111/j.1349-7006.2010.01614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho HH. Ivashkiv LB. Role of STAT3 in type I interferon responses. Negative regulation of STAT1-dependent inflammatory gene activation. J Biol Chem. 2006;281(20):14111–14118. doi: 10.1074/jbc.M511797200. [DOI] [PubMed] [Google Scholar]
- Kaehler KC. Sondak VK. Schadendorf D. Hauschild A. Pegylated interferons: prospects for the use in the adjuvant and palliative therapy of metastatic melanoma. Eur J Cancer. 2010;46(1):41–46. doi: 10.1016/j.ejca.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Kane A. Yang I. Interferon-gamma in brain tumor immunotherapy. Neurosurg Clin N Am. 2010;21(1):77–86. doi: 10.1016/j.nec.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Maher TM. Wells AU. Laurent GJ. Idiopathic pulmonary fibrosis: multiple causes and multiple mechanisms? Eur Respir J. 2007;30(5):835–839. doi: 10.1183/09031936.00069307. [DOI] [PubMed] [Google Scholar]
- Mantovani A. Sozzani S. Locati M. Allavena P. Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23(11):549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- Martinez FO. Gordon S. Locati M. Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol. 2006;177(10):7303–7311. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- Martinez FO. Sica A. Mantovani A. Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- Miller CH. Maher SG. Young HA. Clinical use of interferon-gamma. Ann N Y Acad Sci. 2009;1182:69–79. doi: 10.1111/j.1749-6632.2009.05069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser DM. The many faces of macrophage activation. J Leukoc Biol. 2003;73(2):209–212. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- Mosser DM. Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12):958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narumi S. Hamilton TA. Inducible expression of murine IP-10 mRNA varies with the state of macrophage inflammatory activity. J Immunol. 1991;146(9):3038–3044. [PubMed] [Google Scholar]
- Pechkovsky DV. Goldmann T. Ludwig C. Prasse A. Vollmer E. Muller-Quernheim J. Zissel G. CCR2 and CXCR3 agonistic chemokines are differently expressed and regulated in human alveolar epithelial cells type II. Respir Res. 2005;6(1):75. doi: 10.1186/1465-9921-6-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechkovsky DV. Prasse A. Kollert F. Engel KM. Dentler J. Luttmann W. Friedrich K. Muller-Quernheim J. Zissel G. Alternatively activated alveolar macrophages in pulmonary fibrosis-mediator production and intracellular signal transduction. Clin Immunol. 2010;137(1):89–101. doi: 10.1016/j.clim.2010.06.017. [DOI] [PubMed] [Google Scholar]
- Politz O. Kodelja V. Guillot P. Orfanos CE. Goerdt S. Pseudoexons and regulatory elements in the genomic sequence of the beta-chemokine, alternative macrophage activation-associated CC-chemokine (AMAC)-1. Cytokine. 2000;12(2):120–126. doi: 10.1006/cyto.1999.0538. [DOI] [PubMed] [Google Scholar]
- Porcheray F. Viaud S. Rimaniol AC. Leone C. Samah B. Dereuddre-Bosquet N. Dormont D. Gras G. Macrophage activation switching: an asset for the resolution of inflammation. Clin Exp Immunol. 2005;142(3):481–489. doi: 10.1111/j.1365-2249.2005.02934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasse A. Pechkovsky DV. Toews GB. Jungraithmayr W. Kollert F. Goldmann T. Vollmer E. Muller-Quernheim J. Zissel G. A vicious circle of alveolar macrophages and fibroblasts perpetuates pulmonary fibrosis via CCL18. Am J Respir Crit Care Med. 2006;173(7):781–792. doi: 10.1164/rccm.200509-1518OC. [DOI] [PubMed] [Google Scholar]
- Prasse A. Pechkovsky DV. Toews GB. Schafer M. Eggeling S. Ludwig C. Germann M. Kollert F. Zissel G. Muller-Quernheim J. CCL18 as an indicator of pulmonary fibrotic activity in idiopathic interstitial pneumonias and systemic sclerosis. Arthritis Rheum. 2007;56(5):1685–1693. doi: 10.1002/art.22559. [DOI] [PubMed] [Google Scholar]
- Pujade-Lauraine E. Guastalla JP. Colombo N. Devillier P. Francois E. Fumoleau P. Monnier A. Nooy M. Mignot L. Bugat R. Marques C. Mousseau M. Netter G. Maloisel F. Larbaoui S. Brandely M. Intraperitoneal recombinant interferon gamma in ovarian cancer patients with residual disease at second-look laparotomy. J Clin Oncol. 1996;14(2):343–350. doi: 10.1200/JCO.1996.14.2.343. [DOI] [PubMed] [Google Scholar]
- Rigo A. Gottardi M. Zamo A. Mauri P. Bonifacio M. Krampera M. Damiani E. Pizzolo G. Vinante F. Macrophages may promote cancer growth via a GM-CSF/HB-EGF paracrine loop that is enhanced by CXCL12. Mol Cancer. 2010;9:273. doi: 10.1186/1476-4598-9-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca H. Varsos ZS. Sud S. Craig MJ. Ying C. Pienta KJ. CCL2 and interleukin-6 promote survival of human CD11b+ peripheral blood mononuclear cells and induce M2-type macrophage polarization. J Biol Chem. 2009;284(49):34342–343454. doi: 10.1074/jbc.M109.042671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha B. Jyothi Prasanna S. Chandrasekar B. Nandi D. Gene modulation and immunoregulatory roles of interferon gamma. Cytokine. 2010;50(1):1–14. doi: 10.1016/j.cyto.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Schoppmann SF. Fenzl A. Nagy K. Unger S. Bayer G. Geleff S. Gnant M. Horvat R. Jakesz R. Birner P. VEGF-C expressing tumor-associated macrophages in lymph node positive breast cancer: impact on lymphangiogenesis and survival. Surgery. 2006;139(6):839–846. doi: 10.1016/j.surg.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Schutyser E. Richmond A. Van Damme J. Involvement of CC chemokine ligand 18 (CCL18) in normal and pathological processes. J Leukoc Biol. 2005;78(1):14–26. doi: 10.1189/jlb.1204712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sica A. Saccani A. Bottazzi B. Polentarutti N. Vecchi A. van Damme J. Mantovani A. Autocrine production of IL-10 mediates defective IL-12 production and NF-kappa B activation in tumor-associated macrophages. J Immunol. 2000;164(2):762–767. doi: 10.4049/jimmunol.164.2.762. [DOI] [PubMed] [Google Scholar]
- Soria G. Yaal-Hahoshen N. Azenshtein E. Shina S. Leider-Trejo L. Ryvo L. Cohen-Hillel E. Shtabsky A. Ehrlich M. Meshel T. Keydar I. Ben-Baruch A. Concomitant expression of the chemokines RANTES and MCP-1 in human breast cancer: a basis for tumor-promoting interactions. Cytokine. 2008;44(1):191–200. doi: 10.1016/j.cyto.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Tsuda Y. Takahashi H. Kobayashi M. Hanafusa T. Herndon DN. Suzuki F. CCL2, a product of mice early after systemic inflammatory response syndrome (SIRS), induces alternatively activated macrophages capable of impairing antibacterial resistance of SIRS mice. J Leukoc Biol. 2004;76(2):368–373. doi: 10.1189/jlb.1203645. [DOI] [PubMed] [Google Scholar]
- Yurkovetsky ZR. Kirkwood JM. Edington HD. Marrangoni AM. Velikokhatnaya L. Winans MT. Gorelik E. Lokshin AE. Multiplex analysis of serum cytokines in melanoma patients treated with interferon-alpha2b. Clin Cancer Res. 2007;13(8):2422–2428. doi: 10.1158/1078-0432.CCR-06-1805. [DOI] [PubMed] [Google Scholar]
- Zhang J. Patel L. Pienta KJ. Targeting chemokine (C-C motif) ligand 2 (CCL2) as an example of translation of cancer molecular biology to the clinic. Prog Mol Biol Transl Sci. 2010;95:31–53. doi: 10.1016/B978-0-12-385071-3.00003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]