Abstract
The number of West Nile virus (WNV)–infected mosquitoes aboard aircraft from the United States that arrive in the United Kingdom each summer was determined using a quantitative risk assessment. In the worst-case scenario, when WNV levels in mosquitoes are high (at epidemic levels) the probability of at least one WNV-infected mosquito being introduced into the United Kingdom was predicted to be 0.99. During these periods, a mean of 5.2 infected mosquitoes were estimated to be aboard flights from the United States to the United Kingdom during May to October, with 90% certainty that the exact value lies between one and ten mosquitoes. Heathrow airport was predicted to receive the majority of the infected mosquitoes (72.1%). Spatial analysis revealed the region surrounding Heathrow satisfies the criteria for potential WNV exposure as both WNV-competent mosquitoes and susceptible wild bird species are present. This region is, therefore, recommended for targeted, risk-based surveillance of WNV-infected mosquitoes in addition to an increased awareness of the risks to horses, birds and humans.
Key Words: Arbovirus(es), GIS, Mosquito(es), Risk analysis, West Nile virus
Introduction
West Nile virus (WNV) is a member of the Flaviviridae family: it is a single-stranded RNA virus encoding 10 proteins (Hayes et al. 2005). There are two principal lineages: lineage 1 is detected throughout the world, whereas lineage 2 is detected in sub-Saharan Africa (Hayes et al. 2005) and more recently in Hungary (Bakonyi et al. 2006). Recent data suggest that WNV should be classified into five genetic lineages, with Indian viruses constituting the distinct genetic lineage 5 (Bondre et al. 2007). Other Flaviviruses include St. Louis Encephalitis, Japanese Encephalitis, Dengue, Yellow Fever, Usutu, and Tick-borne Encephalitis. All are arboviruses and are transmitted by bites from infected arthropods.
WNV circulates between infected mosquitoes (mainly members of the Culex genus) and birds (principally corvids, raptors and passerines). The disease causes encephalitis in horses (Ostlund et al. 2000) and humans (Mostashari et al. 2001). Mammals are incidental dead-end hosts (Ligon 2004) and are not considered to play a significant role in the epidemiology of the disease.
The arrival of WNV in America in 1999 was heralded by an outbreak of human encephalitis cases in Queens, New York City (NYC); a cluster of dead exotic birds from the Bronx Zoo, NYC; and a die-off of crows (Corrus brachyrhynchos and C. ossifragus) in the region (Lanciotti et al. 1999). The virus has since become endemic across the United States and spread into neighboring countries (Planitzer et al. 2009). Early cases in humans were misdiagnosed as St. Louis Encephalitis (McCarthy 2001).
Another flavivirus, Usutu virus, was identified in Vienna, Austria, in August 2001 (Weissenböck et al. 2002). Similar to WNV in the United States, the incursion was confirmed after mass bird mortalities of passerines and fatalities in captive birds at the city's zoo (Weissenböck et al. 2002). The first case of human neuro-invasive Usutu virus infection has been diagnosed recently in Italy: the isolate was similar to that found in Austria (Pecorari et al. 2009). WNV has also been identified in Vienna: the virus was isolated from three birds (two sparrow hawks, Accipiter nisus, and a Kea, Nestor notabilis) in the northern part of Austria (Anonymous 2008, 2009). More recently, WNV has been identified in humans in Greece (Papa et al. 2010). The emergence of such viruses may be the result of a number of factors, such as increasing movement of people, animals, plants, and goods world wide, which favors spread of viruses and their vectors (Weissenböck et al. 2010). Similar concerns have been reported regarding the spread of malarial vectors: air and ship travel have assisted in the spread of malaria by transporting infected mosquitoes around the globe (Gratz et al. 2000, Tatem et al. 2006), aided by climatic similarity and increasing traffic volume on long-haul international routes (Tatem and Hay 2007, Randolph and Rogers 2010), and have resulted in malaria cases in the United Kingdom (Whitfield et al. 1984). It is possible the same transport routes resulted in the introduction of WNV in NYC, as this city is a major international port and early WNV incursions were identified close to La Guardia International Airport.
Risk assessments for Barbados (Douglas et al. 2007), Galapagos (Kilpatrick et al. 2006), and Hawaii (Kilpatrick et al. 2004) have indicated that mosquitoes aboard aircraft pose the greatest threat for WNV introduction to an island, so it could be hypothesized that there is a risk of WNV introduction into the United Kingdom by infected vectors aboard international aircraft.
This study addressed the questions: (1) what is the probability of importing WNV-infected mosquitoes into the United Kingdom by aircraft from the United States per vector season (taken to be May to October)? (2) How many WNV-infected mosquitoes enter the United Kingdom on flights from the United States each vector season? The imported WNV-infected mosquitoes are distributed among the UK airports. In addition, these airports are mapped with the location and density of recorded WNV-competent mosquitoes and survey counts of susceptible wild bird species. This shows where WNV exposure would be possible and the regions that would be at a greater risk of exposure. This risk assessment focuses on WNV from the United States, which was perceived to pose a substantial risk by introducing a WNV-infected mosquito into the United Kingdom. This pathway could act as a model for the potential spread of WNV-infected mosquitoes between countries.
Materials and Methods
Model framework
The questions posed consider disease introduction and so constitute a release assessment using World Organization for Animal Health risk assessment methodology (Murray 2004). Airports in the United States were divided geographically into three regions (Table 1), allowing differences in the prevalence of WNV in mosquitoes to be incorporated. Only the season May to October (a period of 184 days) was considered: this represents the period of highest risk (Medlock et al. 2007), and the period when mosquito populations will be present in both the United Kingdom and United States.
Table 1.
Allocation of U.S. Airports into Western, Central, and Eastern Regions (Region i)
| Western (w) | Central (c) | Eastern (e) |
|---|---|---|
| Los Angeles | Dallas/Fort Worth | Atlanta |
| Las Vegas | Denver | Baltimore |
| Phoenix | Houston | Bangor |
| San Diego | Minneapolis–St. Paul | Boston |
| San Francisco | New Orleans | Charlotte |
| Seattle (Tacoma) | Chicago (O'Hare) | |
| Cincinnati | ||
| Cleveland | ||
| Detroit | ||
| Fort Lauderdale | ||
| Louisville | ||
| Miami International | ||
| New York (JFK) | ||
| New York (Newark) | ||
| Orlando | ||
| Philadelphia International | ||
| Raleigh | ||
| Sanford | ||
| Tampa | ||
| Washington (Dulles) |
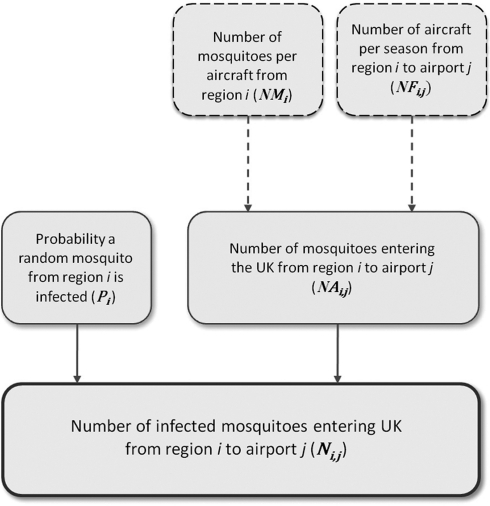
The pathway and parameters required for determining the number of WNV-infected mosquitoes that arrive in the United Kingdom from each region is shown (Fig. 1). In the worst-case scenario, when WNV prevalence in mosquitoes is at an epidemic level in the United States, the total number of infected mosquitoes arriving in the United Kingdom from the United States per season (N) is
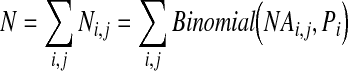 |
(1) |
FIG. 1.
Model framework used to determine the number of West Nile virus (WNV)–infected mosquitoes introduced to the United Kingdom on aircraft from the United States. All values correspond to the vector season (May to October). Region i refers to the three regions of the United States (western, central, and eastern; see Table 1) and airport j refers to the UK destination airport (Table 3).
where i is the U.S. region; j is the UK airport. The parameter NAi,j is the number of mosquitoes entering the United Kingdom from region i to airport j and is calculated by NAi,j=NMi x NFi,j where NMi is the number of mosquitoes per aircraft from region i; and NFi,j is the number of flights from region i to airport j. The parameter Pi is the probability a random mosquito in region i is infected. Ni,j is the simulated number of infected mosquitoes entering the United Kingdom from region i to airport j. All values correspond to the vector period of May to October only. Input parameter values are given in Table 2.
Table 2.
Input Parameters Used to Estimate the Number of West Nile Virus–Infected Mosquitoes from U.S. Region i to UK Airport j From May to October (Ni,j)
| Parameter description | Notation | Value | Reference |
|---|---|---|---|
| Prevalence of WNV in mosquitoes in region ia | Pi, i=w, c, e | Pw=0.00205 | Anonymous (2005) |
| Pc=0.00443 | Bolling et al. (2007) | ||
| Pe=0.00136 | Bernard et al. (2001) and Mans et al. (2004) | ||
| Conversion factor for epidemic to nonepidemic periods | X | 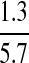 |
Bell et al. (2006) |
| Number of mosquitoes per aircraft | NMi, i=w, c, e | 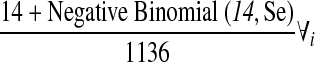 |
Haseyama et al. (2007) |
where Seb = Uniform (minimum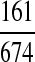 , maximum , maximum ) ) |
Lindsay et al. (1989) | ||
| Number of flights from region i to airport j per season | NFi,j | Table 3 | Anonymous (2009) |
Due to the large sample sizes (552358, 2030, and 327773 for western, central, and eastern United States, respectively) no uncertainty was included in the estimation of Pi, i=w, c, e.
Se is the sensitivity of the flashlight search method.
WNV, West Nile virus.
When WNV in mosquitoes is at an endemic level, the equation becomes
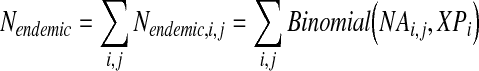 |
(2) |
where conversion factor X is taken from published data of the change in infection levels between epidemic and endemic periods (Table 2).
The probability of at least one WNV-infected mosquito arriving in the United Kingdom from the United States per season (PN>0) was
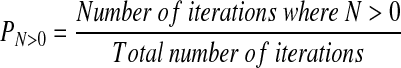 |
(3) |
It was assumed that transatlantic flights would not be sprayed with insecticide.
The model was developed in Office Excel™ (Microsoft) using @Risk™ (Palisade) to incorporate uncertain parameters stochastically: 50,000 iterations were run to ensure model convergence.
Probability a random mosquito is infected with WNV in region i (Pi)
Differences in WNV competence between North American mosquito species have been investigated (Sardelis et al. 2001, Turell et al. 2005) but, due to a lack of data, it was not possible to stratify to this level. It was, therefore, assumed that all American mosquito species were present in equal numbers and were equally likely to be tested and infected.
For each region in the United States, the level of WNV infection in mosquitoes was researched in published literature using PubMed, CAB Direct, and Web of Knowledge search engines. Articles that listed results from all mosquitoes trapped and tested with PCR were included. The studies used were assumed to be equally valid.
For reasons of practicality, studies that aim to estimate the level of WNV infection in mosquitoes sample from pools of mosquitoes, where typically a minimum infection rate (MIR) is estimated. The MIR is defined as the number of infected mosquitoes per 1000 tested. Therefore, dividing the MIR by 1000 provides an estimate of prevalence. However, this can lead to an underestimation of the true prevalence as it is implicitly assumed that there is only one infected mosquito within a positive pool (Gu et al. 2004). Statistical methods have been developed to enable a more accurate measure of prevalence to be calculated (e.g., maximum likelihood estimation), but these are not applied here as the prevalence is deemed to be very low. Under such circumstances there is little difference between the MIR and maximum likelihood estimates (Walter et al. 1980).
Therefore, we define Pi (i=w, c, e) as NP+/TM, where NP+ is defined as the number of positive pools and TM as the total number of mosquitoes tested. Each set of WNV prevalence studies was kept separate to represent the different U.S. regions, except for the east region where the results from 2 studies by Bernard et al. (2001) and Mans et al. (2004) were added together. Due to the large sample sizes from the four studies used to estimate Pi, i=w, c, e no uncertainty was included in the parameter estimate (Table 2).
Infection rates vary between epidemic and endemic periods and this was investigated using the conversion factor X as shown in equation (2). An epidemic was defined as a period of intense WNV activity shortly after the first WNV incursion into a state: this was usually the first full year after disease incursion.
Number of mosquitoes per aircraft (NMi)
A study undertaken at Narita Airport in Japan, identified 14 mosquitoes (SM) on 1136 aircraft (FM) from North and South America, 8 (57%) of which were Culex species (Haseyama et al. 2007). The authors searched each aircraft's cabin and hold using a flashlight. Flights were not randomly selected, but it was assumed that this did not affect the results. It was also assumed that all mosquitoes were alive at collection. Although other studies have searched for mosquitoes on planes (Le Maitre and Chadee 1983, Russell et al. 1984, Takahashi et al. 1984, Hutchinson et al. 2005), the data from Haseyama et al. (2007) were selected as it only considered flights arriving from the Americas, searched for mosquitoes in both the cargo and passenger areas and is also the most recent.
The sensitivity of the flashlight search (Se) was derived from a study that compared this method to knockdown with an insecticide spray (Lindsay et al. 1989): this assumed that the insecticide method was a gold-standard test, regardless of the insecticide used. Uncertainty was incorporated by estimating the number of mosquitoes missed using a negative Binomial distribution (Table 2).
Given the limited data, it was assumed the number of mosquitoes per aircraft was independent of region and airport and, therefore, NMe=NMc=NMw.
Due to the uncertainty associated with the data used to estimate the number of mosquitoes present on an aircraft, both the sensitivity of the flashlight search (Se) and SM were included in a sensitivity analysis, where the effect of alternative values of these parameters are investigated.
Number of flights from region i to airport j per season (NFi,j)
Parameter NFi,j was estimated from 2008 data provided by the United Kingdom's Civil Aviation Authority (Anonymous 2009) using the number of passengers on each route and the estimated average number of passengers per aircraft (Table 3), assuming a constant ratio of cargo to passenger flights.
Table 3.
Number of Flights Arriving at UK Airport j from U.S. Region i (NFi,j)
| |
Region i |
|
||
|---|---|---|---|---|
| Airport j | Western (w) | Central (c) | Eastern (e) | All regions |
| Heathrow | 11905 | 4276 | 35048 | 51229 |
| Gatwick | 1702 | 1193 | 8708 | 11603 |
| Manchester | 375 | 8 | 6461 | 6844 |
| Glasgow | 6 | 1 | 1076 | 1083 |
| Edinburgh | 0 | 0 | 883 | 883 |
| Birmingham | 0 | 2 | 488 | 490 |
| Belfast International | 7 | 0 | 517 | 524 |
| Bristol | 0 | 0 | 436 | 436 |
| Stanstead | 0 | 0 | 286 | 286 |
| Luton | 0 | 0 | 138 | 138 |
| Newcastle | 0 | 0 | 125 | 125 |
| Cardiff Wales | 0 | 0 | 86 | 86 |
| East Midlands Int. | 0 | 2 | 53 | 55 |
| Leeds Bradford | 0 | 0 | 7 | 7 |
| Bournemouth | 0 | 1 | 1 | 2 |
| Humberside | 0 | 0 | 1 | 1 |
| Doncaster Sheffield | 0 | 1 | 0 | 1 |
| Exeter | 0 | 1 | 0 | 1 |
| Total | 13995 | 5485 | 54314 | 73794 |
Number of flights from May to October (a period of 184 days) from the three U.S. regions estimated from published data for 2008 from the Civil Aviation Authority. Estimates for the number of flights assume that the average number of passengers is 124 and a constant cargo to passenger aircraft ratio.
It was assumed that the number of flights from region i was constant throughout the year, thereby allowing the number of flights for the 184-day vector period to be calculated.
Exposure map
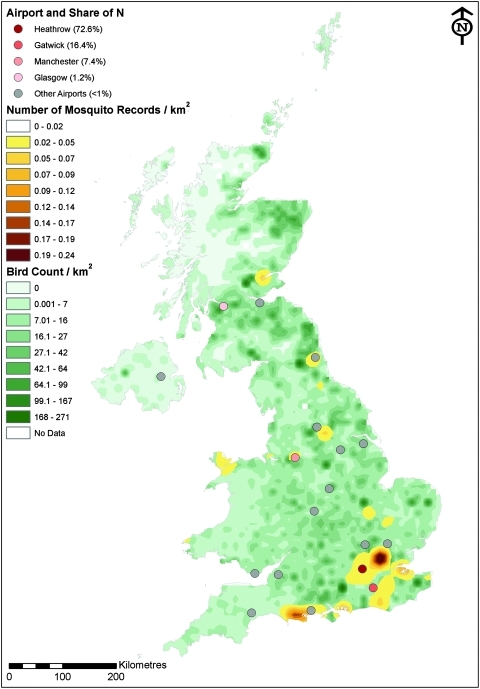
Maps were produced using ESRI ArcGIS™ v9.3 software. The identification and distribution of records of candidate WNV vectors in Britain has been published (Medlock et al. 2005): these data include regional surveys and localized reports of individual mosquitoes and is available to download from the British Mosquito Recording Scheme via the National Biodiversity Network Gateway (http://data.nbn.org.uk/). Kernel smoothing was undertaken using a 25 km kernel to indicate areas with the highest number of recorded mosquitoes.
Laboratory studies have shown that bird species in the orders Passeriformes, Charadriiformes, Strigiformes, and Falconiformes can infect feeding mosquitoes (Komar et al. 2003, Hayes et al. 2005) and are therefore potential WNV hosts. The UK distribution and maximum count per day per 100 km2 for selected members of these orders (listed in the legend of Fig. 6) was supplied by the British Trust for Ornithology (BTO) from the last completed Winter Atlas (Lack 1986). These data do not give an absolute density figure, but rather a relative one, relative to the other species present. Only species with similar summer and winter distributions (P. Lack, BTO, United Kingdom, personal communication) were included in the analysis, as the data were collected in winter but the season of interest is the summer. Data for all species were combined, and these data were plotted using their embedded coordinates. The data were then converted from points into a raster format with a 100 km2 pixel size (i.e., where one 100 km2 count area was represented by one 100 km2 pixel). Subsequently, the values of each pixel were divided by 100 to give a raster representing bird density (wild birds/km2) across the United Kingdom.
FIG. 6.
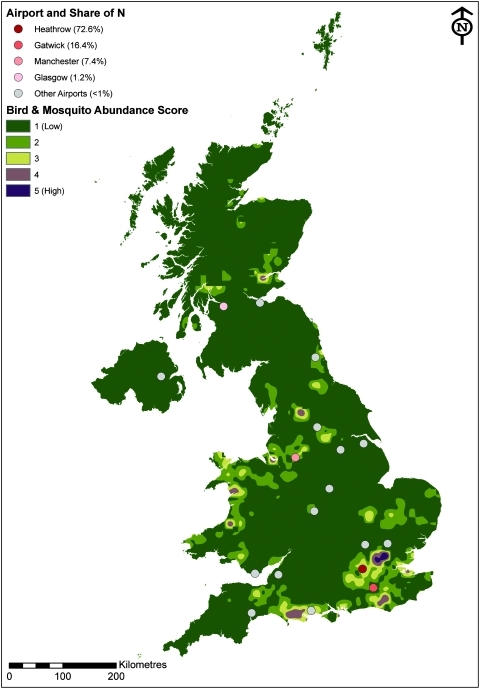
Map of the United Kingdom showing airports with their share of the number of WNV-infected mosquitoes from the United States (N) estimated for the vector season (May to October), with the distribution and relative density of WNV-susceptible wild bird populations and the records of WNV-competent mosquitoes. The lowest mosquito density (0–0.02/km2) has no fill color enabling the bird densities to be seen underneath this layer. The bird density data do not give an absolute density figure, but rather a relative one, relative to the other species of birds present. Bird data presented include the barn owl (Tyto alba); buzzard (Buteo buteo); carrion crow (Corvus corone); hooded crow (Corvus cornix); house sparrow (Passer domesticus); jay (Garrulus garrulus); jackdaw (Corvus monedula); kestrel (Falco tinnunculus); little owl (Athene noctua); magpie (Pica pica); raven (Corvus corax); rook (Corvus frugilegus); sparrowhawk (Accipiter nisus); tawny owl (Strix aluco); and tree sparrow (Passer montanus). Data for Charadriiformes were not available. (Color images available online at www.liebertonline.com/vbz).
A relative abundance score was then calculated as the ratio of the number of recorded WNV-competent mosquitoes per km2 and the wild bird count per km2 (Keeling and Rohani 2007). The abundance raster represents the vector–host ratio and its values range from 0 to 0.043. The abundance raster values were ranked from 1 (low) to 5 (high) using a Natural Breaks (Jenks) classification method (Jenks 1967). The splits generated by the classification were as follows: 0–0.001=Rank 1 (low), 0.001–0.004=Rank 2, 0.004–0.011=Rank 3, 0.011–0.024=Rank 4, 0.024–0.043=Rank 5 (high).
Coordinates for the UK international airports were determined using Get-a-Map™ (Ordnance Survey) and were buffered to a 10 km diameter.
Results
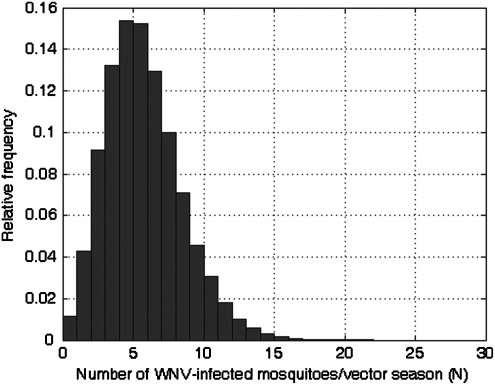
Results for N and Ni, when WNV levels in mosquitoes are at epidemic levels, are shown in Table 4 and Figure 2. When WNV prevalence is at epidemic levels, it is estimated that on average 5.2 (1; 10) WNV-infected mosquitoes arrive in the United Kingdom during the summer period. The probability of at least one infected mosquito arriving during this time (PN>0) is 0.99. Similarly, when WNV is endemic it is estimated that on average 1.2 (0; 3) WNV-infected mosquitoes arrive between May and October, and PN>0=0.68.
Table 4.
The Number of West Nile Virus–Infected Mosquitoes Entering the UK Aboard Aircraft from the United States Estimated for the Summer Season (May–October)
| |
|
Number of WNV-infected mosquitoes (N) |
||
|---|---|---|---|---|
| U.S. region | Parameter | 5th percentile | Mean | 95th percentile |
| All | N | 1 | 5.2 | 10 |
| Western | Nw | 0 | 1.2 | 3 |
| Central | Nc | 0 | 1.0 | 3 |
| Eastern | Ne | 0 | 3.1 | 7 |
50,000 iterations.
FIG. 2.
Relative frequency graph of the number of WNV-infected mosquitoes entering the UK aboard flights from the United States, per season (May to October). The number of iterations was 50,000 and the minimum and maximum values of N were 0 and 26, respectively.
Parameter Ne has the greatest influence on N: this corresponds to the higher number of flights from eastern airports. Ni was lower for flights originating from central and western United States. This again relates to the number of flights from these regions; however, both had slightly higher Pi estimates than Pe (see Tables 2 and 3).
The number of infected mosquitoes arriving in the United Kingdom per season was calculated for each UK airport (parameter Nj). The vast majority of the mosquitoes arrive at Heathrow airport: the model estimated that 72.1% of the mean number of WNV-infected mosquitoes would arrive here. Gatwick airport was predicted to have the second highest share of imported WNV-infected mosquitoes (16.4%); Manchester airport the third highest (7.4%); and Glasgow airport the fourth highest (1.1%). All other UK airports handling flights from the United States were predicted to receive less than 1% of imported WNV-infected mosquitoes. These proportions remain the same for epidemic and endemic periods.
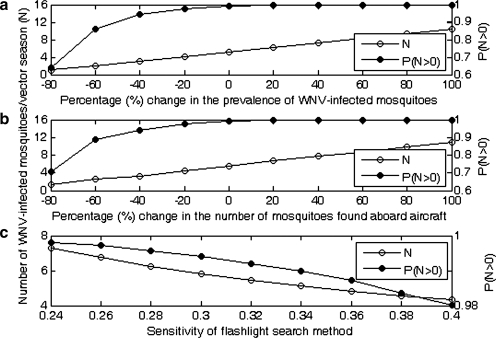
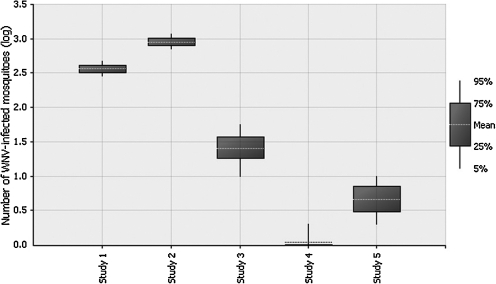
There is considerable uncertainty associated with some of the parameter values within the model, and therefore a sensitivity analysis was carried out to investigate the impact of these parameters. In particular, the probability a randomly selected mosquito is infected (Pi); the number of mosquitoes aboard aircraft (SM) and the sensitivity of the flashlight search method (Se) were selected for further investigation. For each parameter the effect of changing its value on the model outputs N and PN>0 (when WNV is at epidemic levels) was calculated. Figure 3 shows that, as expected, N is linearly related to Pi and SM and nonlinearly related to Se but within the ranges considered only Pi and SM were influential on the risk of release (PN>0) and would therefore benefit from further data generation. Given the uncertainty associated with the values associated with Pi and especially due to the potential under-estimation given the inherent assumptions used to estimate the MIR a scenario analysis was performed. In this analysis the values for Pi were assumed to be of an order of magnitude higher than those used in the baseline model (varying between 1 and 5%), (see Fig. 4). This also assesses the impact of a significant increase in WNV prevalence in mosquitoes in the United States. The scenario analysis shows that, within this range, the prevalence in mosquitoes (Pi) has a very large influence on N. Consequently, if a significant change in WNV prevalence in U.S. mosquitoes is predicted further data generation for Pi would be highly advantageous and the risk of release re-evaluated. The impact of selecting the study by Haseyama et al. (2007) to estimate the number of mosquitoes present on aircraft arriving from the United States was also investigated (Fig. 5). To enable an easier comparison of the five studies Figure 5 has been plotted on a log scale. The analysis shows that if the data from Takahashi et al. (1984) or Russell et al. (1984) were to have been used to estimate the baseline results that N would increase significantly. This demonstrates the importance of this data source within the model. However, the data chosen to parameterize the model (Haseyama et al. 2007) were deemed the most appropriate because this study included only flights out of the Americas, whereas the other studies considered flights out of many different countries and continents.
FIG. 3.
Graph showing the mean number of WNV-infected mosquitoes that enter the United Kingdom from the United States per vector season (N) and the probability that at least one WNV-infected mosquito arrives in the United Kingdom per season (PN>0) with (a) a percentage change in the prevalence of WNV in mosquitoes in all regions (Pi) (where 0% is the baseline (Pw=0.00205; Pc=0.00443; Pe=0.00136)); (b) a percentage change in the baseline number of mosquitoes on aircraft (SM) (where 0% is the baseline (14 mosquitoes)); (c) a change to the sensitivity of the flashlight search method (Se), which in the baseline model is assumed to vary from 0.239 to 0.398. Results shown correspond to WNV-infection in mosquitoes at epidemic levels.
FIG. 4.
Scenario analysis for the prevalence of WNV in mosquitoes in region i (Pi). Graph showing the mean number of WNV-infected mosquitoes that enter the United Kingdom from the United States per vector season (N) and the probability that at least one WNV-infected mosquito arrives in the United Kingdom per season (PN>0). Results shown correspond to WNV-infection in mosquitoes at epidemic levels.
FIG. 5.
Box-plot comparing the log of the mean number of WNV-infected mosquitoes that enter the United Kingdom from the United States per season (Log(N)) if alternative studies are used to estimate the number of mosquitoes present on an aircraft from the United States. Study 1: Takahashi et al. (1984); Study 2: Russell et al. (1984); Study 3: Hutchinson et al. (2005); Study 4: Le Maitre and Chadee (1983); Study 5: Haseyama et al. (2007).
The WNV-susceptible bird distribution is scattered across the country (Fig. 6), although the Scottish borders, and Eastern regions of the country seem to be slightly more represented. The South East has the highest proportion of recorded WNV-competent vectors, including both enzootic and bridge-vector species (Medlock et al. 2007): the highest density of records lies at the north-east London and Essex border (Fig. 7). The proximity of airports to both susceptible mosquito and bird populations is most apparent in Figure 7. Heathrow and Manchester airports lie in regions with moderate bird and mosquito relative abundance scores, whereas Glasgow and Gatwick airports were in low abundance score areas.
FIG. 7.
Map of the United Kingdom (as Fig. 6) with relative mosquito and bird abundance scores: these are the ratio of the records of WNV-competent mosquitoes per km2 and the relative WNV-susceptible bird density. (Color images available online at www.liebertonline.com/vbz).
Discussion
The model presented here indicated that there is a very high risk of at least one WNV-infected mosquito being imported by aircraft from the United States to United Kingdom each summer. Heathrow airport has the greatest risk of an infected mosquito incursion, with much lower risks at all the other UK airports. This is not surprising as Heathrow airport handles 69.4% of all the transatlantic flights modeled. Moreover, spatial analysis indicated the region around Heathrow airport has a moderate vector–host ratio and susceptible vector and bird species could be exposed to imported WNV. In addition, it has been reported that southern England will be a disease hotspot in the future with the potential for the incursion and increased density in insect vectors capable of transmitting exotic viral diseases, the majority of which are zoonotic (Jones et al. 2008). The results presented in this study agree with the conclusion that the south of England is most at risk for incursion of WNV via mosquitoes on transatlantic aircraft. Currently, there is a degree of bias to the presence of mosquitoes in the southeast of England as this region has been specifically surveyed. The vector–host ratio, however, still implies surveillance for WNV should be targeted to this region. Currently, England undertakes targeted surveillance for WNV in mosquitoes as well as surveillance in dead birds (migratory as well as indigenous species). Horse and human surveillance is undertaken by the United Kingdom Department for Environment, Food and Rural Affairs and the Department of Health respectively, by assessing suspect neurological cases.
The results show that the risk of importing at least one WNV-infected mosquito per season decreases as the prevalence of WNV decreases in the United States, as was seen when the model was adapted to the endemic situation. As WNV is now endemic in the United States (Planitzer et al. 2009), it is possible that the results calculated for endemic periods more closely approximate the true number of infected mosquitoes being imported. However, less data are available on the prevalence of WNV in mosquitoes during endemic periods as mosquito testing tends to occur during outbreaks.
Data used to estimate the number of mosquitoes per aircraft (SM, FM) were taken from one study from Japan; however, there are many studies that have estimated this and the results vary widely, from 50 mosquitoes on 89863 aircraft (Le Maitre and Chadee 1983) to 686 mosquitoes on 307 aircraft (Russell et al. 1984). There are significant issues concerning the validity of mosquito collection studies: it is possible that the mosquitoes identified could have been present on the airplane for some time (Russell et al. 1984); the season and time of the flights' departures (such as dusk) could affect the number of mosquitoes aboard; and few, if any, of the studies are randomized. The data used in this study corresponded to flights from North and South America: although this dataset will include the United States, it will not represent that country alone. There is, therefore, a high degree of uncertainty over the number of mosquitoes aboard flights from the United States. Moreover, the sensitivity of the flashlight method was estimated by assuming that the insecticide method was a perfect test. However, this is highly unlikely to be the case, which leads to further uncertainty associated with the parameter NMi. Consequently, Se and SM were investigated in a sensitivity analysis, which showed that, within the considered range, the probability of WNV entering the United Kingdom via aircraft is sensitive to SM and less so Se. The parameter SM is the only parameter that could potentially be reduced by a risk mitigating intervention such as insecticide spraying. Insecticide spraying is advocated by the World Health Organization (WHO) for the control of vector-borne diseases such as malaria (Gratz et al. 2000) and could be implemented on flight paths where other mosquito-borne diseases are a concern. However, decreasing the total number of mosquitoes aboard aircraft (Fig. 3) showed that, assuming the estimates for SM and Se are valid, a significant effort would be needed to reduce the risk of WNV-infected mosquitoes from entering the United Kingdom. Indeed, to decrease the probability of at least one infected mosquito arriving in the United Kingdom per vector season to less than 0.9, spraying would need to remove over 50% of the mosquitoes on each plane. As the majority of Culex mosquitoes have been discovered in the cargo hold, particularly when warm-blooded animals are transported (Takahashi 1984), traditional spraying techniques might not be effective.
The identification of mosquitoes was not considered in this model because of a lack of data on the distribution and relative abundance of different species. However, Culex mosquitoes are the main vectors for WNV in the United States (Hayes et al. 2005, Turell et al. 2005) and have high levels of WNV infection (Bernard et al. 2001). Additionally, in many airplane searches the majority of mosquitoes recovered were Culex mosquitoes (Le Maitre and Chadee 1983, Takahashi 1984, Haseyama et al. 2007). If the mosquito species had been taken into account, the risk of WNV-infected mosquitoes arriving into the United Kingdom could be lower (where NMi is overestimated by considering all mosquito species) or higher (where Pi is underestimated by considering all mosquito species) than the risk presented here.
This study has not explicitly modeled the exposure of animals or the consequences of imported WNV-infected mosquitoes in the United Kingdom. A traditional exposure assessment for this pathway would be very complex as there are numerous routes by which indigenous British animals could be exposed to WNV. The exposure assessment would require knowledge of the rate at which mosquitoes leave aircraft and no published data were reported previously on this issue, although it can occur as observed with airport malaria cases (Whitfield et al. 1984, Gratz et al. 2000). Such an exposure assessment would also need to take into account the competence of the native mosquito species present in the United Kingdom. Consequently, a spatial approach has been used here to indicate areas where WNV exposure could occur and so help inform future risk-based surveillance. However, adopting this spatial approach also has disadvantages. For example, it cannot take into account the route by which a bird may be exposed or the degree of exposure. It is also, similar to any model, heavily reliant on the data used to describe bird and mosquito populations within the United Kingdom. It is recognized that the mosquito data incorporate unquantified bias due to the nonrandomized nature of the study used and also that the level of exposure cannot be ascertained at the more local level (e.g., in the immediate vicinity of airports). However, this method, with acknowledgement of the associated caveats, does show where WNV exposure might be possible and the regions that would be at a greater risk of exposure.
The model presented here calculated a high probability of at least one infected mosquito reaching the United Kingdom each year. The question arises, why has the United Kingdom not reported an incursion of WNV in the past? We previously reported that WNV has not been isolated from birds in the United Kingdom (Phipps et al. 2008). In contrast, two serological studies reported the presence of antibodies to WNV in migratory, nonmigratory, and sentinel birds in the United Kingdom (Buckley et al. 2003, 2006) although active virus was not isolated from any of these birds. Possible reasons for the apparent discrepancies between these studies have been discussed by Gould et al. (2006), and include the scenario where UK wildlife are exposed to other Flaviviruses resulting in the generation and detection of cross-reacting antibodies. In the absence of any human WNV cases in the United Kingdom (albeit imported cases) as discussed previously (Phipps et al. 2008), we conclude that although the incursion of WNV-infected mosquitoes may occur sporadically, WNV has not established in UK mosquitoes. We speculate that the formation of an infectious cycle would require an abundance of competent mosquitoes and reservoir hosts linked to optimum climatic conditions. In the United Kingdom, reservoir avian hosts that can act as amplifying hosts for WNV appear to be numerous, although mosquito densities are too low to sustain an infectious cycle (Medlock et al. 2007). We speculate that climate change might be the most important factor for increasing mosquito numbers throughout Europe particularly in southern England during the warmer months and in allowing mosquitoes to survive during the winter period. Understanding the routes by which viruses are introduced into the United Kingdom should enable policy makers to identify the risks of potential incursions, realize the threats to public health, and ultimately enable the development of targeted, risk based surveillance.
Acknowledgments
With thanks to Dr. Peter Lack at the BTO for bird distribution data and Dr. D'Mello and Mr. de Glanville at the Royal Veterinary College (RVC) for advice. This project was funded by the RVC. Dr. Fooks was funded by the EU FP7 Co-ordination Action, ArboZooNet (grant number 211757).
Disclosure Statement
No competing financial interests exist.
References
- Anonymous. London: Civil Aviation Authority; 2009. [Oct 14;2009 ]. UK Airport Statistics: 2008 Annual. [Google Scholar]
- Anonymous. Vector-Borne Diseases in California 2004 Annual Report. Sacramento, CA: Department of Health Services; 2005. [Google Scholar]
- Anonymous. West Nile Virus: Austria. 2008. www.defra.gov.uk/animalh/diseases/monitoring/pdf/wnv-austria.pdf. [May 6;2009 ]. www.defra.gov.uk/animalh/diseases/monitoring/pdf/wnv-austria.pdf
- Anonymous. OIE; 2009. World Animal Health Information Database (WAHID) Interface [database on the Internet] [cited 17 March 2009] [Google Scholar]
- Bakonyi T. Ivanics E. Erdelyi K. Ursu K, et al. Lineage 1 and 2 strains of encephalitic West Nile virus, central Europe. Emerg Infect Dis. 2006;12:618–623. doi: 10.3201/eid1204.051379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell JA. Brewer CM. Mickelson NJ. Garman GW, et al. West Nile virus epizootiology, central Red River Valley, North Dakota and Minnesota, 2002–2005. Emerg Infect Dis. 2006;12:1245–1247. doi: 10.3201/eid1208.060129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard KA. Maffei JG. Jones SA. Kauffman EB, et al. West Nile virus infection in birds and mosquitoes, New York State, 2000. Emerg Infect Dis. 2001;7:679–685. doi: 10.3201/eid0704.010415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolling BG. Moore CG. Anderson SL. Blair CD, et al. Entomological studies along the Colorado front range during a period of intense West Nile virus activity. J Am Mosq Control Assoc. 2007;23:37–46. doi: 10.2987/8756-971X(2007)23[37:ESATCF]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Bondre VP. Jadi RS. Mishra AC. Yergolkar PN, et al. West Nile virus isolates from India: evidence for a distinct genetic lineage. J Gen Virol. 2007;88(Pt 3):875–884. doi: 10.1099/vir.0.82403-0. [DOI] [PubMed] [Google Scholar]
- Buckley A. Dawson A. Gould E. Detection of seroconversion to West Nile virus, Usutu virus and Sindbis virus in UK sentinel chickens. Virol J. 2006;3:71. doi: 10.1186/1743-422X-3-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley A. Dawson A. Moss SR. Hinsley SA, et al. Serological evidence of West Nile virus, Usutu virus and Sindbis virus infection of birds in the UK. J Gen Virol. 2003;84:2807–2817. doi: 10.1099/vir.0.19341-0. [DOI] [PubMed] [Google Scholar]
- Douglas KO. Kilpatrick AM. Levett PN. Lavoie MC. A quantitative risk assessment of West Nile virus introduction into Barbados. West Indian Med J. 2007;56:394–397. [PubMed] [Google Scholar]
- Gould EA. Higgs S. Buckley A. Gritsun TS. Potential arbovirus emergence and implications for the United Kingdom. Emerg Infect Dis. 2006;12:549–555. doi: 10.3201/eid1204.051010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratz NG. Steffen R. Cocksedge W. Why aircraft disinsection? Bull World Health Organ. 2000;78:995–1004. [PMC free article] [PubMed] [Google Scholar]
- Gu W. Lampman R. Novak RJ. Assessment of arbovirus vector infection rates using variable size pooling. Med Vet Entomol. 2004;18:200–204. doi: 10.1111/j.0269-283X.2004.00482.x. [DOI] [PubMed] [Google Scholar]
- Haseyama M. Iizuka S. Omae H. Tsuda Y. Results of mosquito collection from international aircrafts arriving at Narita International Airport, Japan and mosquito surveillance at the airport [in Japanese] Med Entomol Zool. 2007;58:191–197. [Google Scholar]
- Hayes EB. Komar N. Nasci RS. Montgomery SP, et al. Epidemiology and transmission dynamics of West Nile virus disease. Emerg Infect Dis. 2005;11:1167–1173. doi: 10.3201/eid1108.050289a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes EB. Sejvar JJ. Zaki SR. Lanciotti RS, et al. Virology, pathology, and clinical manifestations of West Nile virus disease. Emerg Infect Dis. 2005;11:1174–1179. doi: 10.3201/eid1108.050289b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson RA. Bayoh MN. Lindsay SW. Risk of airport malaria in the UK. Eur Mos Bull. 2005;19:12–13. [Google Scholar]
- Jenks GF. The data model concept in statistical mapping. In: Frenzel K, editor. International Yearbook of Cartography. Vol. 7. International Cartographic Association; 1967. pp. 186–190. [Google Scholar]
- Jones KE. Patel NG. Levy MA. Storeygard A, et al. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeling MJ. Rohani P. Modeling Infectious Diseases in Humans and Animals. Princeton, NJ: Princeton University Press; 2007. [Google Scholar]
- Kilpatrick AM. Daszak P. Goodman SJ. Rogg H, et al. Predicting pathogen introduction: West Nile virus spread to Galapagos. Conserv Biol. 2006;20:1224–1231. doi: 10.1111/j.1523-1739.2006.00423.x. [DOI] [PubMed] [Google Scholar]
- Kilpatrick AM. Gluzberg Y. Burgett J. Daszak P. Quantitative risk assessment of the pathways by which West Nile virus could reach Hawaii. EcoHealth. 2004;1:205–209. [Google Scholar]
- Komar N. Langevin S. Hinten S. Nemeth N, et al. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg Infect Dis. 2003;9:311–322. doi: 10.3201/eid0903.020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lack PC. The Atlas of Wintering Birds in Britain and Ireland. T. & A.D. Academic Press, Inc.; Waltham, MA: [Google Scholar]
- Lanciotti RS. Roehrig JT. Deubel V. Smith J, et al. Origin of the West Nile virus responsible for an outbreak of Encephalitis in the northeastern United States. Science. 1999;286:2333–2337. doi: 10.1126/science.286.5448.2333. [DOI] [PubMed] [Google Scholar]
- Le Maitre A. Chadee DD. Arthropods collected from aircraft at Piarco International Airport, Trinidad, West Indes. Mos News. 1983;43:21–23. [Google Scholar]
- Ligon BL. Emerging and re-emerging infectious diseases: review of general contributing factors and of West Nile virus. Semin Pediatr Infect Dis. 2004;15:199–205. doi: 10.1053/j.spid.2004.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay SW. Shenton FC. Snow RW. Greenwood BM. Responses of Anopheles gambiae complex mosquitoes to the use of untreated bednets in The Gambia. Med Vet Entomol. 1989;3:253–262. doi: 10.1111/j.1365-2915.1989.tb00225.x. [DOI] [PubMed] [Google Scholar]
- Mans NZ. Yurgionas SE. Garvin MC. Gary RE, et al. West Nile virus in mosquitoes of Northern Ohio, 2001–2002. Am J Trop Med Hyg. 2004;70:562–565. [PubMed] [Google Scholar]
- McCarthy M. St. Louis encephalitis and West nile virus encephalitis. Curr Treat Options Neurol. 2001;3:433–438. doi: 10.1007/s11940-001-0031-8. [DOI] [PubMed] [Google Scholar]
- Medlock JM. Snow KR. Leach S. Potential transmission of West Nile virus in the British Isles: an ecological review of candidate mosquito bridge vectors. Med Vet Entomol. 2005;19:2–21. doi: 10.1111/j.0269-283X.2005.00547.x. [DOI] [PubMed] [Google Scholar]
- Medlock JM. Snow KR. Leach S. Possible ecology and epidemiology of medically important mosquito-borne arboviruses in Great Britain. Epidemiol Infect. 2007;135:466–482. doi: 10.1017/S0950268806007047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostashari F. Bunning ML. Kitsutani PT. Singer DA, et al. Epidemic West Nile encephalitis, New York, 1999: results of a household-based seroepidemiological survey. Lancet. 2001;358:261–264. doi: 10.1016/S0140-6736(01)05480-0. [DOI] [PubMed] [Google Scholar]
- Murray N. OIE Handbook on Import Risk Analysis for Animals and Animal Products. Third. OIE; Paris, France: 2004. [Google Scholar]
- Ostlund EN. Andresen JE. Andresen M. West Nile encephalitis. Vet Clin North Am Equine Pract. 2000;16:427–441. doi: 10.1016/s0749-0739(17)30087-1. [DOI] [PubMed] [Google Scholar]
- Papa A. Danis K. Baka A. Bakas A, et al. Ongoing outbreak of West Nile virus infections in humans in Greece, July-August 2010. Eur Surveill. 2010:15. doi: 10.2807/ese.15.34.19644-en. [DOI] [PubMed] [Google Scholar]
- Pecorari M. Longo G. Gennari W. Grottola A, et al. First human case of Usutu virus neuroinvasive infection, Italy, August-September 2009. Eur Surveill. 2009:14. [PubMed] [Google Scholar]
- Phipps LP. Duff JP. Holmes JP. Gough RE, et al. Surveillance for West Nile virus in British birds (2001 to 2006) Vet Rec. 2008;162:413–415. doi: 10.1136/vr.162.13.413. [DOI] [PubMed] [Google Scholar]
- Planitzer CB. Modrof J. Yu MY. Kreil TR. West Nile virus infection in plasma of blood and plasma donors, United States. Emerg Infect Dis. 2009;15:1668–1670. doi: 10.3201/eid1510.080711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph SE. Rogers DJ. The arrival, establishment and spread of exotic diseases: patterns and predictions. Nat Rev Mircobiol. 2010;8:361–371. doi: 10.1038/nrmicro2336. [DOI] [PubMed] [Google Scholar]
- Russell RC. Rajapaska N. Whelan PI. Langsford WA. Mosquito and other insect introductions to Australia aboard international aircraft, and the monitoring of disinsection procedures. In: Laird M, editor. Commerce and the Spread of Pests and Disease Vectors. Praeger; Westport, CT: 1984. pp. 109–141. [Google Scholar]
- Sardelis MR. Turell MJ. Dohm DJ. O'Guinn ML. Vector competence of selected North American Culex and Coquillettidia mosquitoes for West Nile virus. Emerg Infect Dis. 2001;7:1018–1022. doi: 10.3201/eid0706.010617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S. Survey on accidental introductions of insects entering Japan via aircraft. In: Laird M, editor. Commerce and the Spread of Pests and Disease Vectors. Praeger; Westport, CT: 1984. pp. 65–79. [Google Scholar]
- Tatem AJ. Hay SI. Rogers DJ. Global traffic and disease vector dispersal. Proc Natl Acad Sci U S A. 2006;103:6242–6247. doi: 10.1073/pnas.0508391103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatem AJ. Hay SI. Climatic similarity and biological exchange in the worldwide airline transportation network. Proc R Soc Lond B. 2007;274:1489–1496. doi: 10.1098/rspb.2007.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turell MJ. Dohm DJ. Sardelis MR. Oguinn ML, et al. An update on the potential of north American mosquitoes (Diptera: Culicidae) to transmit West Nile Virus. J Med Entomol. 2005;42:57–62. doi: 10.1093/jmedent/42.1.57. [DOI] [PubMed] [Google Scholar]
- Walter SD. Hildreth SW. Beaty BJ. Estimation of infection rates in population of organisms using pools of variable size. Am J Epidemiol. 1980;112:124–128. doi: 10.1093/oxfordjournals.aje.a112961. [DOI] [PubMed] [Google Scholar]
- Weissenböck H. Hubálek Z. Bakonyi T. Nowotny N. Zoonotic mosquito-borne flaviviruses: worldwide presence of agents with proven pathogenicity and potential candidates of future emerging diseases. Vet Microbiol. 2010;140:271–80. doi: 10.1016/j.vetmic.2009.08.025. [DOI] [PubMed] [Google Scholar]
- Weissenböck H. Kolodziejek J. Url A. Lussy H, et al. Emergence of Usutu virus, an African mosquito-borne flavivirus of the Japanese encephalitis virus group, central Europe. Emerg Infect Dis. 2002;8:652–656. doi: 10.3201/eid0807.020094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield D. Curtis CF. White GB. Targett GA, et al. Two cases of falciparum malaria acquired in Britain. Br Med J (Clin Res Ed) 1984;289:1607–1609. doi: 10.1136/bmj.289.6458.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]