Abstract
AIM: To investigate whether the small bowel transit time (SBTT) influences the diagnostic yield of capsule endoscopy (CE).
METHODS: Six hundred and ninety-one consecutive CE procedures collected in a database were analyzed. SBTT and CE findings were recorded. A running mean for the SBTT was calculated and correlated to the diagnostic yield with a Spearman’s correlation test. Subgroup analyses were performed for the various indications for the procedure.
RESULTS: There was a positive correlation between the diagnostic yield and SBTT (Spearman’s rho 0.58, P < 0.01). Positive correlations between diagnostic yield and SBTT were found for the indication obscure gastrointestinal bleeding (r = 0.54, P < 0.01), for polyposis and carcinoid combined (r = 0.56, P < 0.01) and for the other indications (r = 0.90, P <0.01), but not for suspected Crohn’s disease (r = -0.40).
CONCLUSION: The diagnostic yield in small bowel capsule endoscopy is positively correlated with the small bowel transit time. This is true for all indications except for suspected Crohn’s disease.
Keywords: Capsule endoscopy, Small bowel transit time, Diagnostic yield
INTRODUCTION
Capsule endoscopy (CE) is a very sensitive diagnostic technique to detect small bowel pathology. It has a higher diagnostic yield than conventional diagnostic methods, i.e., push enteroscopy, small-bowel follow-through, conventional CT and angiography[1]. The reported diagnostic yield of CE varies between 38% and 83%[2-11]. In 15%-20% of all CE’s the capsule does not reach the cecum within recording time. Risk factors for incomplete CE procedures include previous small-bowel surgery, hospitalization, moderate or poor bowel cleansing, and a gastric transit time longer than 45 min[12].
For a good and complete evaluation of the small bowel, the capsule should reach the cecum within recording time, which is eight to eleven hours depending on the manufacturer. Therefore, some investigators use a prokinetic agent to speed up the gastric and small bowel transit. However, the short bowel transit time (SBTT) may influence the diagnostic yield of CE. With colonoscopy, the detection rate of neoplastic lesions is higher when the time to withdraw the colonoscope is longer[13-15]. It is conceivable that a similar principle also applies for small bowel CE. We therefore hypothesize that the diagnostic yield of CE depends on the small bowel transit time. To study this, we analyzed the influence of small bowel transit time on the diagnostic yield of CE in 691 consecutive procedures performed in our department.
MATERIALS AND METHODS
Data from all consecutive CE studies performed at the University Medical Center Groningen, the Netherlands, between September 2003 and January 2009 were collected. Data that were collected included patient demographics, indications for the procedure, procedural data, including gastric transit time (GTT) and SBTT, and findings of the procedure. The GTT was defined as the time, in minutes, from the first image of the stomach until the first image of the duodenum. The SBTT was defined as the passage time, in minutes, from the first image of the duodenum until the first image of the cecum. If the capsule did not reach the cecum within recording time, the SBTT was recorded as the time during which small bowel images were captured. CE was considered complete when the cecum was reached within recording time.
CE procedure
During the study period, all patients received bowel preparation. Patients were given standardized instructions before the procedure, and informed consent was obtained. The patients were asked to stop iron supplements seven days before CE and to use a low-fiber diet three days before CE. The patients started a fasting period at midnight before the procedure. Bowel preparation consisted of the ingestion of four liters of a polyethylene glycol solution (Colofort®), 3 L the evening before the procedure and 1 L in the morning. The capsule (Pillcam; Given Imaging Ltd, Yoqneam, Israel) was swallowed in the morning. The patients were allowed to drink fluids after three hours and to consume a light meal after five hours. Before capsule ingestion, 10 mL of antifoam and a prokinetic agent was given, 10 mg of domperidone (before July 1st 2008, n = 641) or 250 mg of erythromycin (after July 1st 2008, n = 50). All CE recordings were reviewed by two gastroenterologists, experienced with CE (RKW and JJK). Controversial findings were discussed, and consensus was reached upon the final diagnosis. The most relevant findings obtained from CE were documented and categorized according to standard terminology[16] as angiectasia(s); ulcer(s); bleeding of unknown origin; erosion (s); polyp(s)/tumor(s); incidental abnormality of esophagus, stomach, or colon; no abnormality; or unable to make a diagnosis.
Statistical analysis
The SBTT was not normally distributed (tested with a Kolmogorov-Smirnov test) in the study population. To demonstrate the correlation between average diagnostic yield and average SBTT, the average yield was calculated of 50 consecutive transit times and plotted. Diagnostic yield was expressed as 0 for absence of abnormalities and 1 for presence of abnormalities. In this way, a running mean for the SBTT was calculated for 50 consecutive patients and correlated to the diagnostic yield with a Spearman’s correlation test. A rho’s correlation coefficient was calculated. Comparison of SBTT between groups was performed using a Mann-Whitney U test.
Subgroup analyses were performed for the various indications for the procedure. P-values below 0.05 were considered significant. SPSS 14.0 for Windows software (SPSS Inc., Chicago, IL, United States) and Microsoft Office Excel 2003 were used for statistical analyses.
RESULTS
Six hundred and ninety-one consecutive CE procedures were analyzed. The mean age of the patients was 54 years (range 9-93, SD 18 years). 55% of the patients were male. Indications for CE were obscure gastrointestinal bleeding (OGIB) (67%), suspected Crohn’s disease (22%), polyposis (4%), carcinoid (3%) and other (4%). CE findings were as follows in the investigated patients: angiectasia(s) in 121 cases (18%), ulcer(s) in 42 (6%), erosion(s) in 83 (12%), bleeding of unknown origin in 30 (4%), polyp(s)/tumor(s) in 56 (8%), abnormality of esophagus, stomach, or colon in 15 (2%), stenosis in 2 (0.3 %), unable to make a diagnosis in 6 (1%) and no abnormalities in 336 cases (48%). Overall, the diagnostic yield was 51%.
The cecum was reached in 82% of all procedures. The overall median small bowel transit time was 246 min (25 and 75 percentiles: 190 and 342). In CE cases with positive findings, the median SBTT was 254 min (25 and 75 percentiles: 200 and 361), in negative CE procedures, the median SBTT was 239 (25 and 75 percentiles 178 and 320), this difference was significant (P = 0.012). There was a positive correlation between the diagnostic yield and SBTT (Figure 1) indicating that the longer the SBTT, the higher the diagnostic yield (Spearman’s rho 0.58, P < 0.01).
Figure 1.
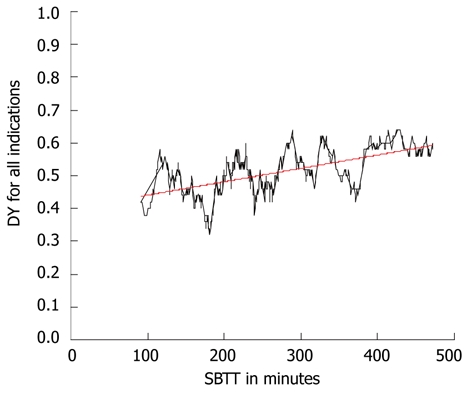
Correlation between the small bowel transit time in minutes and the diagnostic yield for all patients. Spearman’s rho coefficient 0.58 ( P < 0.01) shown by the black line. The trend of this correlation is shown by the red line. DY: Diagnostic yield; SBTT: Small bowel transit time.
Next, patients were excluded in whom the cecum was not reached (n = 125) within recording time, leaving 566 procedures with complete visualization of the small intestine. The overall median SBTT was 233 min (25 and 75 percentiles: 178 and 295). In cases with positive findings, the median SBTT was 236 (25 and 75 percentiles: 186 and 300), in negative CE procedures the median SBTT was 229 min (25 and 75 percentiles: 121 and 281), this difference was not significant (P = 0.078). A positive correlation was again observed between the diagnostic yield and SBTT (Spearman’s rho 0.40, P < 0.01, Figure 2).
Figure 2.
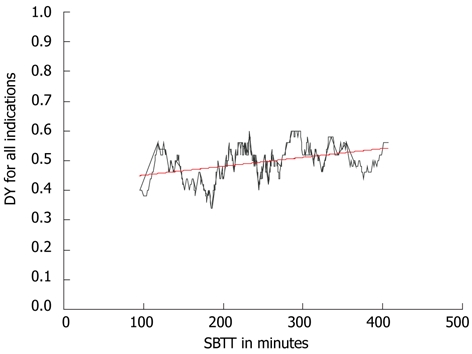
Correlation between the small bowel transit time in minutes and the diagnostic yield in patient with complete capsule endoscopy. Spearman’s rho coefficient 0.40 (P < 0.01) shown by the black line. The trend of this correlation is shown by the red line. DY: Diagnostic yield; SBTT: Small bowel transit time.
Subgroup analysis for the different indications was performed for OGIB, suspected Crohn’s disease, polyposis and carcinoid combined and other indications. The indications polyposis and carcinoid were taken together because both groups were too small for separate subgroup analysis. For these indications, positive correlations between diagnostic yield and SBTT were found for OGIB (r = 0.54, P < 0.01), for polyposis plus carcinoid (r = 0.56, P < 0.01) and for the other indications (r = 0.90, P < 0.01). However this was not observed for Crohn’s disease (r = -0.40). These results are depicted in Figure 3.
Figure 3.
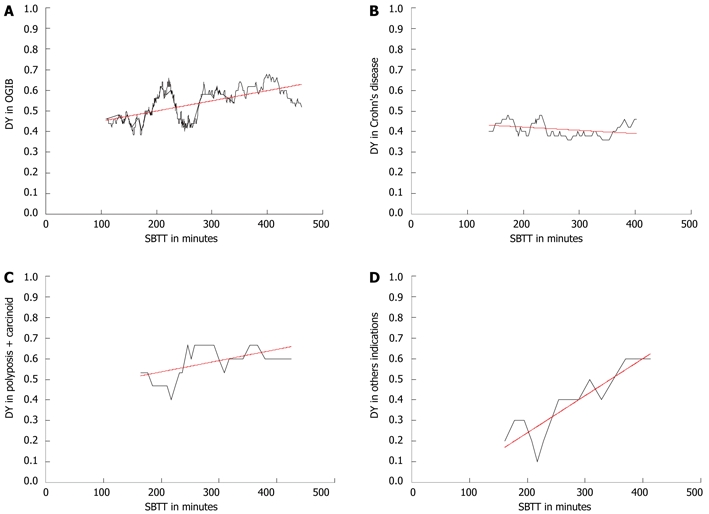
Correlations between small bowel transit time and diagnostic yield. A: Obscure gastrointestinal bleeding (OGIB; r = 0.54, P < 0.01); B: Suspected Crohn’s disease (r = -0.40); C: Polyposis plus carcinoid (r = 0.56, P < 0.01); D: Other indications (r = 0.90, P < 0.01), shown by the black line. The trend of this correlation is shown by the red line. DY: Diagnostic yield; SBTT: Small bowel transit time.
DISCUSSION
In this study, we found a positive correlation between the diagnostic yield of CE and small bowel transit time, irrespective of whether the capsule had reached the cecum within recording time. These findings are in accordance with those from colonoscopy studies, which show higher diagnostic yields for detecting neoplastic lesions when the withdrawal time during colonoscopy is longer[10-13] and from one previous study on the effect of SBTT on the diagnostic yield of CE[17]. Most of these colonoscopy studies divided the withdrawal time into more or less than a chosen number of minutes. In CE there are no known standard SBTT times, so we judged that it would not be correct to randomly divide the SBTT in two or more randomly chosen groups. Therefore we calculated a running mean to determine whether the diagnostic yield correlated with the SBTT. We found a positive correlation between the two, meaning that a longer transit time, implicating more images of the small bowel, resulted in a higher diagnostic yield.
For colonoscopy, the correlation between diagnostic yield and withdrawal time was only investigated in subjects undergoing screening for neoplastic lesions. In this study we looked at all indications for CE. We found a positive correlation between the diagnostic yield and SBTT for the indications OGIB and polyposis/carcinoid and for other indications, but not for the indication suspected Crohn’s disease. The latter is probably due to the multiple and widespread small bowel lesions usually seen in Crohn’s disease. Therefore the endoscopist may be less dependent upon the mucosal inspection time to make the diagnosis. Furthermore, a previous study showed reduced capsule transit times in Crohn’s disease[18].
What does this positive correlation between diagnostic yield and SBTT mean for clinical practice? CE is less valuable when the cecum is not reached within recording time, but on the other hand our study indicates that the diagnostic yield is lower when the SBTT is shorter. So, ideally, the SBTT should be as long as possible, yet the capsule should reach the cecum within recording time. The development of capsule systems with longer battery times may be helpful. One important issue in this matter is whether there is a role for prokinetic agents in CE. In this way, one could influence the small bowel transit time. There is no consensus on this subject[1]. In most of the available studies, there are no data on the influence of prokinetics on the diagnostic yield of CE. Taking our data into account, it may not be wise to use prokinetics that speed up the SBTT. However this must be weighed against the fact that a prolonged GTT is a risk factor for incomplete CE[12]. It may therefore be useful to use an agent which shortens GTT without influencing SBTT.
A well known prokinetic agent is erythromycin. It induces high amplitude gastric propulsive contractions by activating gastric interdigestive migrating motor complexes, thereby accelerating gastric emptying[19-22]. The effects of erythromycin on SBTT are unclear. In the most recent publication on this subject, erythromycin reduced the GTT but had no significant effect on SBTT, total bowel transit time and CE completing rates[22]. Previous studies found similar results[23,24], but in one at the cost of visibility[24]. Others found no effect of erythromycin on either GTT or SBTT[25]. Overall, the data are not very robust. If erythromycin mainly influences GTT, it might be an interesting prokinetic agent to use prior to small bowel CE.
Other prokinetics that have been studied in CE are metoclopramide and mosapride. Both prokinetic agents accelerated GTT and increased capsule completion rates, but had no influence on SBTT[26,27]. In our study, we used domperidone as a prokinetic agent in the majority of patients. Domperidone has shown to be effective in treating diabetic gastroparesis[28], but there are no data on the use this agent in CE.
Another way to use prokinetics may be with the aid of a real-time viewer system. In this way, prokinetics (or water or additional PEG) can be administered when the real-time viewer shows delayed gastric emptying. There are three studies that show a higher diagnostic yield of CE when a real-time viewer is used with on-demand administration of prokinetics, water, PEG or endoscopic-assisted duodenal placement[29-31].
The strength of this study is that this the first study that investigated the relation between diagnostic yield of CE and SBTT in a large study population. This allowed for a subgroup analysis for the different indications of CE. Another strong point of our study in our view is the use of an appropriate statistical method for determining the relation between diagnostic yield and SBTT by using a running mean.
A limitation of this study is that all patients in our population received a prokinetic agent, which might have changed the SBTT and with that also the diagnostic yield. The diagnostic yield might have been higher in this study population when we would not have used a prokinetic agent. Another limitation of this study is that during this study period we switched our prokinetic agent from domperidone to erythromycin. One should realize that this is a retrospective study. Prospective studies are necessary to establish the effect of the use of prokinetics on SBTT and diagnostic yield.
There may be many factors that influence SBTT and thereby diagnostic yield in small bowel CE. In this study we did not analyze such other factors, mainly because the main goal of this study was to determine whether there was a relation between diagnostic yield and SBTT at all. A previous study on the effect of SBTT on diagnostic yield found an independent association between diagnostic yield and SBTT[17]. In that study, no relation was found between diagnostic yield and other potential risk factors such as age, gender, study indication, hospital status, and quality of bowel preparation[17]. Since we also found a positive correlation between diagnostic yield and SBTT it might be very interesting to look further into factors influencing SBTT and thereby diagnostic yield in future studies.
In conclusion, in this study with a large group of patients, we found a positive correlation between the diagnostic yield of small bowel CE and small bowel transit time for all indications except for suspected Crohn’s disease. For clinical practice, these data implicate that it may not be advisable to use prokinetic agents which accelerate small bowel transit although this remains to be proven in future studies.
COMMENTS
Background
Capsule endoscopy (CE) is a very sensitive diagnostic technique to detect small bowel pathology. For a good and complete evaluation of the small bowel, the capsule should reach the cecum within recording time which is eight to eleven hours depending on the manufacturer. Some investigators advocate the use of a prokinetic agent to speed up the gastric and small bowel transit. However, a short bowel transit time (SBTT) may influence the diagnostic yield of CE. With colonoscopy, the detection rate of neoplastic lesions is higher when the time to withdraw the colonoscope is longer. It is conceivable that a similar principle also applies for small bowel CE. Therefore the question is whether the diagnostic yield of CE depends on the small bowel transit time.
Research frontiers
This the first study that investigated the relation between diagnostic yield of CE and SBTT in a large study population. This possible relation was also investigated for different indications for CE.
Innovations and breakthroughs
Six hundred and ninety-one consecutive CE procedures were analyzed. This study found a positive correlation between the diagnostic yield of CE and small bowel transit time, irrespective of whether the capsule had reached the cecum within recording time. These findings are in accordance with those from colonoscopy studies. This means that a longer transit time, implicating more images of the small bowel, resulted in a higher diagnostic yield. We found a positive correlation between the diagnostic yield and SBTT for all indications except for suspected Crohn’s disease. The latter is probably due to the multiple and widespread small bowel lesions usually seen in Crohn’s disease.
Applications
What does this positive correlation between diagnostic yield and SBTT mean for clinical practice? CE is less valuable when the cecum is not reached within recording time, but on the other hand our study indicates that the diagnostic yield is lower when the SBTT is shorter. So, ideally, the SBTT is long, but not so long that the capsule does not reach the cecum within recording time. One important issue in this matter is whether there is a role for prokinetic agents in CE to influence small bowel transit time. There is no consensus in the literature on this subject. For clinical practice, these data implicate that it may not be advisable to use prokinetic agents which accelerate small bowel transit, although this remains to be proven in future studies.
Peer review
It is a nice and interesting work. It values an important aspect of the capsule endoscopy not well studied until now: the relation between the diagnostic yield of capsule endoscopy and small bowel transit time.
Footnotes
Supported by A clinical fellow grant (90700281) from the Netherlands Organization for Scientific Research (NWO)
Peer reviewers: Dieter Schilling, MD, PhD, Department of Gastroenterology, Diakonie Hospital Academic Teaching Hospital of the University of Heidelberg, Speyererstrasse 91-93, 68163 Mannheim, Germany; Benito Velayos, PhD, Department of Gastroenterology, Hospital Clínico de Valladolid, Av Ramón y Cajal 3, 47003 Valladolid, Spain
S- Editor Cheng JX L- Editor A E- Editor Zhang DN
References
- 1.Westerhof J, Koornstra JJ, Weersma RK. Capsule endoscopy: a review from the clinician’s perspectives. Minerva Gastroenterol Dietol. 2008;54:189–207. [PubMed] [Google Scholar]
- 2.Matsumoto T, Esaki M, Moriyama T, Nakamura S, Iida M. Comparison of capsule endoscopy and enteroscopy with the double-balloon method in patients with obscure bleeding and polyposis. Endoscopy. 2005;37:827–832. doi: 10.1055/s-2005-870207. [DOI] [PubMed] [Google Scholar]
- 3.Hadithi M, Heine GD, Jacobs MA, van Bodegraven AA, Mulder CJ. A prospective study comparing video capsule endoscopy with double-balloon enteroscopy in patients with obscure gastrointestinal bleeding. Am J Gastroenterol. 2006;101:52–57. doi: 10.1111/j.1572-0241.2005.00346.x. [DOI] [PubMed] [Google Scholar]
- 4.Nakamura M, Niwa Y, Ohmiya N, Miyahara R, Ohashi A, Itoh A, Hirooka Y, Goto H. Preliminary comparison of capsule endoscopy and double-balloon enteroscopy in patients with suspected small-bowel bleeding. Endoscopy. 2006;38:59–66. doi: 10.1055/s-2005-870446. [DOI] [PubMed] [Google Scholar]
- 5.Ohmiya N, Yano T, Yamamoto H, Arakawa D, Nakamura M, Honda W, Itoh A, Hirooka Y, Niwa Y, Maeda O, et al. Diagnosis and treatment of obscure GI bleeding at double balloon endoscopy. Gastrointest Endosc. 2007;66:S72–S77. doi: 10.1016/j.gie.2007.05.041. [DOI] [PubMed] [Google Scholar]
- 6.Kaffes AJ, Siah C, Koo JH. Clinical outcomes after double-balloon enteroscopy in patients with obscure GI bleeding and a positive capsule endoscopy. Gastrointest Endosc. 2007;66:304–309. doi: 10.1016/j.gie.2007.02.044. [DOI] [PubMed] [Google Scholar]
- 7.Fujimori S, Seo T, Gudis K, Tanaka S, Mitsui K, Kobayashi T, Ehara A, Yonezawa M, Tatsuguchi A, Sakamoto C. Diagnosis and treatment of obscure gastrointestinal bleeding using combined capsule endoscopy and double balloon endoscopy: 1-year follow-up study. Endoscopy. 2007;39:1053–1058. doi: 10.1055/s-2007-967014. [DOI] [PubMed] [Google Scholar]
- 8.Kameda N, Higuchi K, Shiba M, Machida H, Okazaki H, Yamagami H, Tanigawa T, Watanabe K, Watanabe T, Tominaga K, et al. A prospective, single-blind trial comparing wireless capsule endoscopy and double-balloon enteroscopy in patients with obscure gastrointestinal bleeding. J Gastroenterol. 2008;43:434–440. doi: 10.1007/s00535-008-2182-9. [DOI] [PubMed] [Google Scholar]
- 9.Kamalaporn P, Cho S, Basset N, Cirocco M, May G, Kortan P, Kandel G, Marcon N. Double-balloon enteroscopy following capsule endoscopy in the management of obscure gastrointestinal bleeding: outcome of a combined approach. Can J Gastroenterol. 2008;22:491–495. doi: 10.1155/2008/942731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arakawa D, Ohmiya N, Nakamura M, Honda W, Shirai O, Itoh A, Hirooka Y, Niwa Y, Maeda O, Ando T, et al. Outcome after enteroscopy for patients with obscure GI bleeding: diagnostic comparison between double-balloon endoscopy and videocapsule endoscopy. Gastrointest Endosc. 2009;69:866–874. doi: 10.1016/j.gie.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 11.Niv Y, Niv G, Wiser K, Demarco DC. Capsule endoscopy - comparison of two strategies of bowel preparation. Aliment Pharmacol Ther. 2005;22:957–962. doi: 10.1111/j.1365-2036.2005.02647.x. [DOI] [PubMed] [Google Scholar]
- 12.Westerhof J, Weersma RK, Koornstra JJ. Risk factors for incomplete small-bowel capsule endoscopy. Gastrointest Endosc. 2009;69:74–80. doi: 10.1016/j.gie.2008.04.034. [DOI] [PubMed] [Google Scholar]
- 13.Benson ME, Reichelderfer M, Said A, Gaumnitz EA, Pfau PR. Variation in colonoscopic technique and adenoma detection rates at an academic gastroenterology unit. Dig Dis Sci. 2010;55:166–171. doi: 10.1007/s10620-008-0703-2. [DOI] [PubMed] [Google Scholar]
- 14.Barclay RL, Vicari JJ, Doughty AS, Johanson JF, Greenlaw RL. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med. 2006;355:2533–2541. doi: 10.1056/NEJMoa055498. [DOI] [PubMed] [Google Scholar]
- 15.Barclay RL, Vicari JJ, Greenlaw RL. Effect of a time-dependent colonoscopic withdrawal protocol on adenoma detection during screening colonoscopy. Clin Gastroenterol Hepatol. 2008;6:1091–1098. doi: 10.1016/j.cgh.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 16.Korman LY, Delvaux M, Gay G, Hagenmuller F, Keuchel M, Friedman S, Weinstein M, Shetzline M, Cave D, de Franchis R. Capsule endoscopy structured terminology (CEST): proposal of a standardized and structured terminology for reporting capsule endoscopy procedures. Endoscopy. 2005;37:951–959. doi: 10.1055/s-2005-870329. [DOI] [PubMed] [Google Scholar]
- 17.Buscaglia JM, Kapoor S, Clarke JO, Bucobo JC, Giday SA, Magno P, Yong E, Mullin GE. Enhanced diagnostic yield with prolonged small bowel transit time during capsule endoscopy. Int J Med Sci. 2008;5:303–308. doi: 10.7150/ijms.5.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fireman Z, Kopelman Y, Friedman S, Ephrath H, Choman E, Debby H, Eliakim R. Age and indication for referral to capsule endoscopy significantly affect small bowel transit times: the given database. Dig Dis Sci. 2007;52:2884–2887. doi: 10.1007/s10620-007-9789-1. [DOI] [PubMed] [Google Scholar]
- 19.Prather CM, Camilleri M, Thomforde GM, Forstrom LA, Zinsmeister AR. Gastric axial forces in experimentally delayed and accelerated gastric emptying. Am J Physiol. 1993;264:G928–G934. doi: 10.1152/ajpgi.1993.264.5.G928. [DOI] [PubMed] [Google Scholar]
- 20.Keshavarzian A, Isaac RM. Erythromycin accelerates gastric emptying of indigestible solids and transpyloric migration of the tip of an enteral feeding tube in fasting and fed states. Am J Gastroenterol. 1993;88:193–197. [PubMed] [Google Scholar]
- 21.Bruley des Varannes S, Parys V, Ropert A, Chayvialle JA, Rozé C, Galmiche JP. Erythromycin enhances fasting and postprandial proximal gastric tone in humans. Gastroenterology. 1995;109:32–39. doi: 10.1016/0016-5085(95)90266-x. [DOI] [PubMed] [Google Scholar]
- 22.Niv E, Bonger I, Barkay O, Halpern Z, Mahajna E, Depsames R, Kopelman Y, Fireman Z. Effect of erythromycin on image quality and transit time of capsule endoscopy: a two-center study. World J Gastroenterol. 2008;14:2561–2565. doi: 10.3748/wjg.14.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leung WK, Chan FK, Fung SS, Wong MY, Sung JJ. Effect of oral erythromycin on gastric and small bowel transit time of capsule endoscopy. World J Gastroenterol. 2005;11:4865–4868. doi: 10.3748/wjg.v11.i31.4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fireman Z, Paz D, Kopelman Y. Capsule endoscopy: improving transit time and image view. World J Gastroenterol. 2005;11:5863–5866. doi: 10.3748/wjg.v11.i37.5863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caddy GR, Moran L, Chong AK, Miller AM, Taylor AC, Desmond PV. The effect of erythromycin on video capsule endoscopy intestinal-transit time. Gastrointest Endosc. 2006;63:262–266. doi: 10.1016/j.gie.2005.07.043. [DOI] [PubMed] [Google Scholar]
- 26.Wei W, Ge ZZ, Lu H, Gao YJ, Hu YB, Xiao SD. Effect of mosapride on gastrointestinal transit time and diagnostic yield of capsule endoscopy. J Gastroenterol Hepatol. 2007;22:1605–1608. doi: 10.1111/j.1440-1746.2007.05064.x. [DOI] [PubMed] [Google Scholar]
- 27.Selby W. Complete small-bowel transit in patients undergoing capsule endoscopy: determining factors and improvement with metoclopramide. Gastrointest Endosc. 2005;61:80–85. doi: 10.1016/s0016-5107(04)02462-9. [DOI] [PubMed] [Google Scholar]
- 28.Sugumar A, Singh A, Pasricha PJ. A systematic review of the efficacy of domperidone for the treatment of diabetic gastroparesis. Clin Gastroenterol Hepatol. 2008;6:726–733. doi: 10.1016/j.cgh.2008.02.065. [DOI] [PubMed] [Google Scholar]
- 29.Ogata H, Kumai K, Imaeda H, Aiura K, Hisamatsu T, Okamoto S, Iwao Y, Sugino Y, Kitajima M, Hibi T. Clinical impact of a newly developed capsule endoscope: usefulness of a real-time image viewer for gastric transit abnormality. J Gastroenterol. 2008;43:186–192. doi: 10.1007/s00535-007-2140-y. [DOI] [PubMed] [Google Scholar]
- 30.Hosono K, Endo H, Sakai E, Sekino Y, Uchiyama T, Watanabe S, Iida H, Sakamoto Y, Koide T, Takahashi H, et al. Optimal approach for small bowel capsule endoscopy using polyethylene glycol and metoclopramide with the assistance of a real-time viewer. Digestion. 2011;84:119–125. doi: 10.1159/000323225. [DOI] [PubMed] [Google Scholar]
- 31.Shiotani A, Honda K, Kawakami M, Nishi R, Murao T, Ishii M, Matsumoto H, Kusunoki H, Hata J, Haruma K. Use of an external real-time image viewer coupled with prespecified actions enhanced the complete examinations for capsule endoscopy. J Gastroenterol Hepatol. 2011;26:1270–1274. doi: 10.1111/j.1440-1746.2011.06734.x. [DOI] [PubMed] [Google Scholar]


