Abstract
AIM: To analyzes the decision whether patients with chronic hepatitis C virus (HCV) infection are treated or not.
METHODS: This prospective cohort study included 7658 untreated patients and 6341 patients receiving pegylated interferon α 2a/ribavirin, involving 434 physicians/institutions throughout Germany (377 in private practice and 57 in hospital settings). A structured questionnaire had to be answered prior to the treatment decision, which included demographic data, information about the personal life situation of the patients, anamnesis and symptomatology of hepatitis C, virological data, laboratory data and data on concomitant diseases. A second part of the study analyzes patients treated with pegylated interferon α2a. All questionnaires included reasons against treatment mentioned by the physician.
RESULTS: Overall treatment uptake was 45%. By multivariate analysis, genotype 1/4/5/6, HCV-RNA ≤ 520 000 IU/mL, normal alanine aminotransferase (ALT), platelets ≤ 142 500/μL, age > 56 years, female gender, infection length > 12.5 years, concomitant diseases, human immunodeficiency virus co-infection, liver biopsy not performed, care in private practice, asymptomatic disease, and unemployment were factors associated with reduced treatment rate. Treatment and sustained viral response rates in migrants (1/3 of cohort) were higher than in German natives although 1/3 of migrants had language problems. Treatment rate and liver biopsy were higher in clinical settings when compared to private practice and were low when ALT and HCV-RNA were low.
CONCLUSION: Some reasons against treatment were medically based whereas others were related to fears, socio-economical problems, and information deficits both on the side of physicians and patients.
Keywords: Hepatitis C virus; Interferon, Ribavirin; Liver cirrhosis; Migrants; Treatment barrier
INTRODUCTION
Approximately 170 million humans worldwide are estimated to have a chronic hepatitis C virus (HCV) infection including 400 000 in Germany[1,2]. More than 20 % of these patients will progress to cirrhosis, hepatocellular carcinoma, liver transplantation or death[3,4]. Therefore, all patients are candidates for antiviral therapy[5]. Its benefits need to be determined based on the individual’s disease stage and on the likelihood of adherence and success[5,6]. Probably only 20 % of HCV-infected subjects know of their infection[3]. This diagnostic deficit is caused by various factors; e.g., physicians do not follow guidelines to screen for HCV infection when alanine aminotransferase (ALT) is elevated[7,8]. In addition only 11%-41% of known infections are treated[9-12]. Only some reasons for this therapeutic deficit have been identified including co-morbidity, drug abuse and psychosocial factors[9,12-15]. Considering that therapy cures the disease in 50% of patients, treatment rate should be increased. The present study evaluates which factors influence the treatment decision in daily German practice.
MATERIALS AND METHODS
The study which is ongoing was started in March 2003; the present data analyzes the treatment decision in patients included between March 2003 and May 2008. Throughout Germany 434 physicians (377 in private practice and 57 in hospital settings) contributed a mean number of 35 patients with chronic hepatitis C. The study included only one academic center. Basic data of the cohort have been published[16] and are only briefly mentioned here. The study was approved by health authorities and ethical committees. Due to its observational character it did not affect individual medical decisions. A structured questionnaire had to be answered prior to the treatment decision; a second part of the study analyzes patients treated with pegylated interferon α2a (Pegasys®, Roche Pharma AG) and ribavirin. This part is not fully analyzed here; only those aspects are analyzed which are relevant to the treatment decision. All questionnaires included reasons against treatment mentioned by the physician. After July 2004 questionnaires also asked why patients denied therapy (n = 7658). Language skills were assessed after January 2006. Fibrosis was staged according to Desmet and Scheuer from F0 to F4[17]. Among the total 15 137 patients 7658 subjects did not receive any treatment (“untreated patients”) while 6341 received pegylated interferon α2a and ribavirin (“treated patients”) and 1138 alternative treatments. Details on alternative therapies (92.5% silymarin, 2.8% ursodesoxycholic acid, 4.9% other interferons) are not given because their characteristics were similar to the group receiving pegylated interferon α2a/ribavirin. Thus, in the following text the total cohort consists of 13 999 patients separated by the treatment decision into “treated patients” (n = 6341) and “untreated patients” (n = 7658). Specific procedures were not mandatory for inclusion except for documentation of chronic hepatitis C. There were no exclusion criteria except for patients below age 18 years and those with Child B/C cirrhosis. Thus, the study represents a real life scenario of a rather unselected cohort including a significant fraction of all patients diagnosed with hepatitis C in Germany.
Statistical analysis
For continuous variables, receiver operating characteristic analyses estimated the best cut-off point for treatment decision; these cut-off points were 56 years for age, 520 000 IU/mL for basal HCV-RNA, ≥ one concomitant disease, ≥ 12.5 years for infection length, and 142 500/μL for platelets. Categorical variables were used for continuous variables using these cut-off points. Association of various factors with treatment decision and sustained virological response (SVR = negative HCV-RNA 24 wk after end of therapy) were analyzed in an univariate fashion using Fisher’s exact test. Only those variables which were significant in the univariate analysis were included in the multivariate analysis.
RESULTS
Effects of various factors on treatment rate by univariate analysis
Basic characteristics of treated vs untreated patients are shown in Table 1. Many characteristics were similar for genotypes 1 (n = 8625), 4 (n = 440), 5 (n = 22) and 6 (n = 27) and for genotypes 2 (n = 1000) and 3 (n = 3885) (data not shown); thus, further analyses were done in two subgroups, i.e., genotypes 1/4/5/6 vs 2/3. Table 2 summarizes treatment and SVR rates in the total cohort (45.3% and 49.6%, respectively) and in treated vs untreated patients.
Table 1.
Demographic data and basic characteristics
| Characteristics | Not treated (n = 7658) | Treated (n = 6341) |
| % of the 13 999 patients | 55.7 | 45.3 |
| Genotypes 1/4/5/6 (%) | 69.8 | 59.4 |
| Genotypes 2/3 (%) | 30.2 | 40.6 |
| Age (yr, median) | 44.0 | 41.0 |
| BMI (kg/m2, median) | 24.2 | 24.3 |
| Gender (male %) | 56.6 | 61.1 |
| Regulär employment (%) | 35.3 | 50.2 |
| Infection length (yr, median) | 11.0 | 10.0 |
| Ultrasound performed (%) | 76.8 | 87.6 |
| Liver biopsy performed (%) | 12.8 | 30.2 |
| Fibrosis score F 0-1 | 72.8 | 58.6 |
| Fibrosis score F 2-4 | 27.2 | 41.4 |
| Active drug or alcohol abuse (%) | 28.3 | 13.8 |
| HIV co-infection (%) | 6.7 | 3.7 |
| Psychiatric disease (%) | 14.8 | 9.2 |
| Severe language problems (%) | 9.6 | 10.0 |
| Initial HCV-RNA (IU/mL, median) | 482 500 | 500 000 |
| ALT (U/L, median) | 61.0 | 78.0 |
| Thrombocytes (/μL, median) | 217 000 | 218 000 |
| At least on concomitant disease (%) | 62.3 | 42.6 |
BMI: Body mass index; HIV: Human immunodeficiency virus; HCV: Hepatitis C virus; ALT: Alanine aminotransferase.
Table 2.
Treatment and sustained virological response rates in various subgroups
|
Fischer’s exact test, two-sides P value |
|||||
| Treatment rate % | SVR % | Number | Treatment rate | SVR | |
| Total | 45.3 | 49.6 | 13 999 | ||
| Genotypes 1/4/5/6 | 41.4 | 42.7 | 9114 | < 0.0001 | < 0.0001 |
| Genotypes 2/3 | 52.7 | 59.8 | 4885 | ||
| Clinical setting | 63.9 | 49.8 | 1298 | < 0.0001 | NS |
| Private practice | 43.4 | 49.6 | 12 701 | ||
| Male | 47.2 | 47.9 | 8214 | < 0.0001 | < 0.01 |
| Female | 42.6 | 52.3 | 5785 | ||
| Age ≤ 56 yr | 49 | 51.3 | 11 497 | < 0.0001 | < 0.0001 |
| Age > 56 yr | 28.2 | 36.7 | 2502 | ||
| BMI ≤ 23 (kg/m2) | 44.3 | 51.8 | 4762 | < 0.01 | < 0.05 |
| BMI > 23 (kg/m2) | 46.9 | 48.6 | 8846 | ||
| No employment | 38.9 | 47.3 | 8113 | < 0.0001 | < 0.001 |
| Regular employment | 54.1 | 52 | 5886 | ||
| Bad German language skills | 47 | 52.5 | 824 | NS | NS |
| Good German language skills | 45.8 | 47.8 | 7565 | ||
| Migrants | 53.3 | 52.6 | 2663 | < 0.0001 | < 0.0001 |
| German natives | 41.7 | 45.4 | 5465 | ||
| Infection length ≤ 12.5 yr | 62.8 | 51.6 | 3639 | < 0.0001 | < 0.01 |
| Infection length > 12.5 yr | 37.2 | 48 | 3165 | ||
| Ultrasound not performed | 30.7 | 47.5 | 2568 | < 0.0001 | NS |
| Ultrasound performed | 48.6 | 50 | 11 431 | ||
| Liver biopsy not performed | 39.9 | 50.1 | 11 100 | < 0.0001 | NS |
| Liver biopsy performed | 66.1 | 48.5 | 2899 | ||
| Fibrosis scores F0-1 | 60.9 | 52.4 | 1766 | < 0.0001 | < 0.01 |
| Fibrosis scores F2-4 | 74.6 | 44.1 | 1017 | ||
| Clinical symptoms absent | 42.2 | 47.8 | 4430 | < 0.0001 | NS |
| Clinical symptoms present | 46.7 | 50.4 | 9569 | ||
| No concomitant disease | 55.7 | 51.8 | 6527 | < 0.0001 | < 0.0001 |
| At least one concomitant disease | 36.2 | 46.8 | 7472 | ||
| Psychiatric disease absent | 46.9 | 49.8 | 12 281 | < 0.0001 | NS |
| Psychiatric disease present | 34.1 | 48.4 | 864 | ||
| Active drug or alcohol abuse absent | 49.9 | 49.7 | 10 960 | < 0.0001 | NS |
| Active drug or alcohol abuse present | 28.7 | 49.4 | 3039 | ||
| HIV co-infection absent | 46.1 | 50 | 13 254 | < 0.0001 | < 0.01 |
| HIV co-infection present | 31.4 | 39.3 | 745 | ||
| Good quality-of-life | 43.8 | 49.5 | 11 348 | < 0.0001 | NS |
| Reduced quality-of-life | 51.8 | 50.1 | 2651 | ||
| ALT normal (< 50 U/L for men, < 30 U/L for women) | 34.8 | 50.8 | 3297 | < 0.0001 | NS |
| ALT elevated (U/L) | 49.6 | 49.7 | 10 105 | ||
| Thrombocytes ≥ 142 500 /μL | 48 | 51.6 | 11 284 | < 0.0001 | < 0.0001 |
| Thrombocytes < 142 500 /μL | 38.9 | 36.2 | 1816 | ||
| HCV-RNA ≤ 520 000 IU/mL | 45.4 | 54.8 | 6810 | < 0.0001 | < 0.0001 |
| HCV-RNA > 520 000 IU/mL | 49.7 | 43.3 | 5904 | ||
| No concomitant disease | 55.7 | 51.8 | 6527 | < 0.0001 | < 0.0001 |
| At least one concomitant disease | 36.2 | 46.8 | 7472 | ||
| HIV co-infection absent | 46.1 | 50 | 13 254 | < 0.0001 | < 0.01 |
| HIV co-infection present | 31.4 | 39.3 | 745 | ||
SVR: Sustained virological response; BMI: Body mass index; HIV: Human immunodeficiency virus; ALT: Alanine aminotransferase; HCV: Hepatitis C virus; NS: Not significant.
By univariate analysis reduced treatment uptake and reduced SVR were seen in these groups: (1) genotypes 1/4/5/6 vs 2/3; (2) age > 56 years vs ≤ 56 years; (3) platelets ≤ 142 500/μL vs > 142500/μL; (4) disease duration >12.5 years vs ≤ 12.5 years; (5) human immunodeficiency virus (HIV)/HCV co-infection vs HCV mono-infection; (6) presence vs absence of concomitant diseases; (7) German natives vs migrants; and (8) absence vs presence of regular employment.
Treatment uptake was reduced but SVR was higher in the following groups: (1) women vs men; (2) fibrosis F0-1 vs F2-4; and (3) basal HCV-RNA > 520000 IU/mL vs ≤ 520 000 IU/mL.
Treatment uptake was reduced while SVR was similar in the following groups: (1) normal vs elevated ALT; (2) good vs reduced quality of life; (3) treatment in private practice vs clinical setting; (4) presence vs absence of psychiatric disease; (5) presence vs absence of alcohol or drug abuse; and (6) liver biopsy (and ultrasound) not performed vs performed.
History of i.v. drug abuse was the most frequent mode of infection (44.6%) followed by history of blood transfusions (17.0%). By multivariate analysis infection mode did influence neither treatment uptake nor SVR (data not shown). In the total cohort only 20.7 % of patients had a liver biopsy. Biopsy was done more often in genotypes 1/4/5/6 when compared to genotypes 2/3 (23.6% vs 15.3%, P < 0.001) and in patients with elevated ALT (75.4% had elevated ALT) when compared to those with normal ALT (21.6% vs 18.4%, P < 0.05). Biopsy rate was three-times higher in hospital settings when compared to practioners (53.4% vs 17.4%, P < 0.001). Alcohol or drug abuse was a frequent treatment barrier in particular in patients with psychiatric diseases or HIV co-infection and in jobless people (Table 2). Treatment rates were similarly low in drug abusers with or without substitution (data not shown). Patients with alcohol or drug abuse refused therapy less often compared to patients without abuse (50.2% vs 67.9%, P < 0.001). Thus, the decision not to treat was made primarily by the physician. About 1/3 of all patients were migrants among whom 1/3 had severe language problems. Nevertheless, treatment and SVR rates were higher in migrants than in German natives while language problems did not affect treatment and SVR rates. Treatment uptake decreased with an increasing number of socio-economical and psychiatric problems; HIV infection on top of other problems reduced treatment uptake to 7 % (Table 3). SVR was unaffected even by presence of several socio-economical problems but was drastically reduced when there was a HIV co-infection on top of other problems.
Table 3.
Treatment and sustained virological response rates vs socio-economic problems and concomitant diseases
| Characteristics | Treatment rate % | SVR % | n |
| Drug abuse absent and employed without psychiatric disease or HIV co-infection | 58.2 | 52.7 | 4382 |
| Drug abuse absent and employed without psychiatric disease | 58.2 | 52.4 | 4560 |
| Drug abuse absent and employed | 57.1 | 52.6 | 4929 |
| Drug abuse absent | 49.2 | 49.6 | 10 839 |
| Drug abuse present | 32.0 | 49.9 | 3160 |
| Drug abuse present and unemployed | 29.1 | 51.6 | 2203 |
| Drug abuse present and unemployed with psychiatric disease | 25.1 | 50.8 | 470 |
| Drug abuse present and employed with psychiatric disease and HIV co-infection | 7.1 | 0.0 | 56 |
HIV: Human immunodeficiency virus.
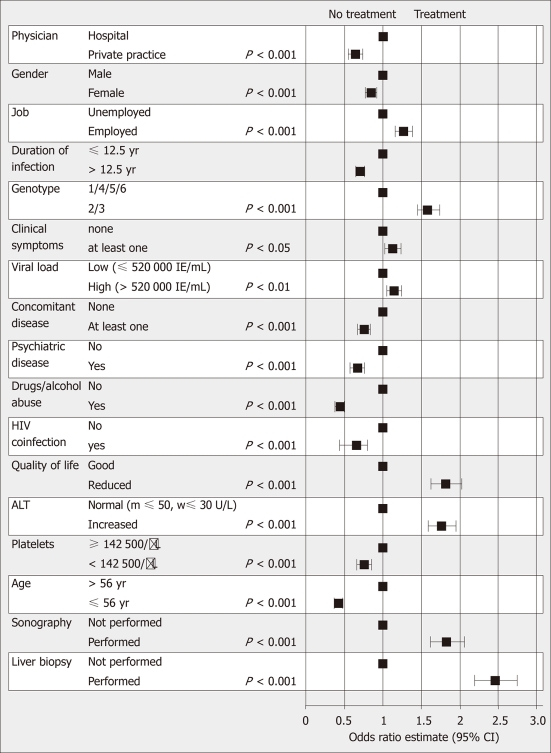
Multivariate regression analysis
Gender, age, genotype, HCV-RNA, ALT, platelets, symptoms, infection length, occupational status, concomitant diseases, HIV co-infection, alcohol and drug abuse, performance of liver biopsy and ultrasound, and quality-of-life significantly affected the treatment decision in the multivariate analysis (Figure 1). In patients with genotypes 1/4/5/6 the same factors as for the total cohort affected the treatment decision except for presence of symptoms; in patients with genotypes 2/3 the same factors as for the total cohort affected the treatment decision except for symptoms, platelets, employment, and performance of liver biopsy (data not shown). SVR was associated with various factors in the univariate analysis (Table 2). By multivariate analysis SVR was associated only with gender, genotype, HCV-RNA, age, platelets, symptoms, employment and HIV co-infection (data not shown).
Figure 1.
Multivariate regression analysis of treatment rates vs various factors. HIV: Human immunodeficiency virus; ALT: Alanine aminotransferase.
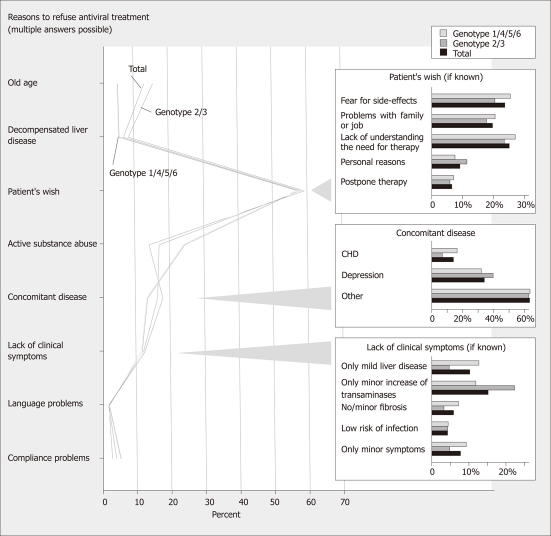
Analysis of specific reasons against treatment
The analysis looked at reasons mentioned by physicians and patients (Figure 2). The patients’ wish was the most common reason against treatment (62.9 %). Among these patients lack of understanding the need of therapy, fear of side-effects, and problems with family and job were frequent reasons. Fear of side-effects was mentioned more often in women than in men (29.9% vs 18.8%, P < 0.001). Alcohol or drug abuse and concomitant diseases (most commonly depression) were also frequent treatment barriers. Among patients who did not see a need for therapy reasons included lack of liver disease, symptoms, fibrosis and bad prognosis as well as normal ALT. In patients with normal ALT minor disease activity was mentioned by the physician as a reason to wait in 24.1% whereas this reason was mentioned in only 6.6% when ALT was elevated (P < 0.001). In contrast, a similar percentage of patients mentioned the lack of disease activity as a treatment barrier irrespective of whether ALT was normal or elevated (27.1% vs 24.4%; NS). In patients with a HCV-RNA ≤ 520 000 IU/mL minor disease activity was mentioned by the physician as a reason to wait in 15.8% whereas this reason was mentioned in only 6.7% when HCV-RNA was > 520 000 IU/mL (P < 0.01). The percentage of patients mentioning lack of disease activity as a treatment barrier was similar when looking at high or low HCV-RNA (data not shown). In patients who had liver biopsy minor disease activity was mentioned by the physician as a treatment barrier in 21.4 % whereas this reason was mentioned in only 10.3 % of patients without a liver biopsy (P < 0.01). Patients mentioned fear of side effects and lack of understanding the need for therapy less often when treated in hospital settings as compared to private practice (18.5% vs 24.1% and 17.4% vs 25.9%, P < 0.01, respectively). In patients with drug/alcohol abuse, this abuse was the main treatment barrier mentioned by physicians (48.1 %). In contrast, patients with abuse refused therapy less often than those without (50.2% vs 67.9%, P < 0.001). In HIV co-infection concomitant diseases and drug/alcohol abuse were more frequent treatment barriers than in mono-infection (25.0% vs 16.6% and 25.2% vs 16.4%, P < 0,01). HIV co-infected patients refused therapy less often than mono-infected patients (59.1% vs 63.2%, P < 0.05). Similarly, in patients with psychiatric diseases, the psychiatric disease was the main treatment barrier (46.2%); among patients with psychiatric disease drug and alcohol abuse was another common barrier (24.5% vs 15.7% in patients without psychiatric disease; P < 0.001). Older age was associated with a reduced treatment rate (49.0% vs 28.2% in patients ≤ 56 years vs patients > 56 years) (Table 2; Figure 1); in patients aged between 65 and 70 years treatment rate was 26.3% (158/600) and thus similar to the rate of 28.2% seen at ages > 56 years.
Figure 2.
Reasons to refuse antiviral treatment.
DISCUSSION
Treatment uptake in the present cohort (45%) is one of the highest reported in the literature. Since the cohort included a significant fraction of all HCV-infected patients in Germany, the high treatment rate is probably not due to selection bias. In the literature treatment uptake tends to decrease with increasing number of subjects studied[9,12-15,18] with the lowest rate of 12% reported for the largest group of subjects studied[15]. There is little preselection in the present cohort; only patients with Child B/C cirrhosis were excluded as well as those under age 18 years. The present study did not include a relevant number of academic centers where most previous studies had been done. The community-based character of the present cohort incorporating 434 physicians and hospitals throughout the country reflects daily life in Germany probably better than looking at academic centres. However, one needs to keep in mind that most of the 434 physicians were not general practioners, but gastroenterologists or at least physicians who treat hepatitis C. In general practioners treatment rates may be lower than the 45% reported here. In the general United States community only 11% of all HCV-infected subjects had been treated[15]. This low treatment uptake suggests that therapeutic deficits are located on level of the general practioner or the health care system itself[7,8]. Recent studies show that knowledge deficits and misperceptions are main treatment barriers[19-21]. A high treatment rate might therefore reflect good knowledge among physicians and patients. In Germany most physicians who treat hepatitis C in private practice are organized in the Association of German Gastroenterologists (“bng”). Via their association gastroenterologists have been involved in the development of national HCV guidelines[6,22]. Many of them are members of the national “hepatitis competence network”. Recent studies have also shown that German patients with hepatitis C are well informed and better than patients with hepatitis B[23-25]. However, some practice aspects did not meet standards in the present cohort including the use of liver biopsy and interpretation of HCV-RNA values. Also, there were misperceptions among patients. Patients’ refusal was a common treatment barrier in the present cohort and in previous studies[9-11]. One of the highest treatment rates (41%) was published by Delwaide et al[9]; in that study only 17% of patients declined therapy. Thus, a high treatment uptake may be associated with low rate of refusal by patients[9]. This association may partly be explained by information deficits. In some subgroups, e.g., in patients with HIV co-infection and those with drug and alcohol abuse, the decision against treatment was often made by the physician whereas patients were rather willing to receive therapy.
Genotype and viral replication are major factors for estimating the chance for SVR and are therefore considered in the treatment decision. Correspondingly treatment rate and SVR were higher for genotypes 2/3 when compared to genotypes 1/4/5/6. In accordance with most previous studies[5,11,15,22] older age was associated with both reduced treatment uptake and reduced SVR in the present cohort. These results are in contrast to a recent study[18] in which being elderly was not associated with a low SVR. Surprisingly, treatment rate was low in patients with low HCV-RNA. This is a paradox because SVR is low at high replication in the present study and in the literature[26-28]. Thus, there may be misperceptions that high viral load indicates bad prognosis. All evidence shows this is not the case[22,29,30]. Further analyses suggested that physicians (and not patients) carry this misperception.
For many years normal serum aminotransferases were a common treatment barrier because they were thought to indicate good prognosis and reduced efficacy of therapy. In the meantime it has been shown that up to 30% of patients with normal ALT have major fibrosis and that SVR is not associated with ALT as also seen in the present study[22,29-31]. Despite this data, treatment rate was markedly lower in patients with normal ALT when compared to those with elevated ALT. We have reported a similar misperception of ALT for the decision to do HCV antibody tests[8]; many physicians just tested for HCV infection if ALT was markedly increased although most infections were associated with normal or slightly elevated ALT. Thus, ALT values are overestimated both in diagnostic[8] and treatment decisions[9,12].
In contrast to academic trials, only 20% of patients had a liver biopsy in daily German practice. According to guidelines liver biopsy should be considered when the results will influence the treatment decision and in particular when treatment is not initiated[5,22]. However, treatment rate in patients with a liver biopsy was twice that seen in patients without a biopsy; according to guidelines it should be the other way around. Only a single previous study has also shown a positive association between performance of liver biopsy and treatment uptake[32]. It may be speculated that patients who refused liver biopsy may have a general problem to accept medical means. However, further analyses support other explanations. Biopsy rate in hospital settings was more than three-times higher than that in private practice. Although non-invasive means of assessing fibrosis are entering clinical routine, only a minority of community-based physicians use serum markers or sonographic stiffness in daily clinical routine as yet. Thus, physicians in private practice underestimate the value of liver biopsy more often than physicians in hospital settings. The lack of immediate availability of biopsy may explain the low biopsy rate among practioners. Also, treatment uptake was markedly lower for patients treated in private practice when compared to hospital settings. The analysis of specific reasons against treatment may partly explain this difference: patients mentioned fear of side effects and lack of understanding the need for therapy less often when treated in clinical settings when compared to private practice.
The treatment rate of HCV infection was considerably lower in HIV co-infected patients when compared to HCV mono-infection. Although SVR rates were also somewhat lower in co-infected patients, they were still in an acceptable range considering that end-stage liver disease is a common cause of death in HIV/HCV co-infection[33-35]. When compared with the literature the present rates of treatment and SVR (31% and 39%) look favorable. In other studies SVR ranged from 8% to 40% in co-infected patients[36-38]. Nevertheless HIV co-infection was a main treatment barrier also in the present cohort. Among co-infected patients drug and alcohol abuse as well as fear of side-effects were frequent treatment barriers. The present analysis also shows that HIV/HCV co-infected patients refused therapy less often than mono-infected patients; thus the low treatment rate is probably mainly caused by physicians and not by patients. In previous studies only 12%-33% of HIV co-infected patients initiated HCV therapy[36,39-40]; main barriers were non-adherence, patients’ refusal, drug abuse and psychiatric problems. The present results demonstrate that the HIV infection on top of psychiatric and socio-economical problems may not only reduce treatment uptake but almost eliminates chances for SVR.
Recently it has been shown that HCV infection can successfully be treated in patients with drug and alcohol abuse and in those with HIV co-infection provided that there is a good management[35-38,41-43]. This is of great importance because alcohol abuse and co-infections accelerate fibrosis[34,35,44,45]. Although a history of drug abuse did not reduce treatment rate in the present cohort, active alcohol and drug abuse were associated with a markedly reduced treatment uptake as reported previously[10,11,14,15]; SVR was not affected by abuse. In 50% of abusers, physicians specified the abuse as the main treatment barrier. In contrast, patients with alcohol or drug abuse refused therapy less often than did patients without abuse. Thus, the decision not treat was made primarily by the physician. A survey of 320 American Society of Addiction Medicine physicians showed that even among these specialists only a minority were providing HCV treatment or willing to provide treatment[46]. Treatment rates are even lower in the general community and may approach values of less than 1 % in unselected drug addicts[47].
Treatment rate was lower in unemployed patients when compared to those with a job while SRV was similar between these groups. Since jobless people tend to have a low educational state, these results fit to recent United States data showing that psychosocial factors and low education were associated with reduced treatment uptake[12,14,48]. In the present cohort 1/3 of HCV infected patients were migrants among whom 1/3 had severe language problems. Unexpectedly, treatment uptake was not lower but higher in migrants when compared to German natives. These results can not be explained easily. Along this line women had a lower treatment rate when compared to men in this cohort as well as in another previous study[10]. This is also unexpected because men have a lower use of medical services than women both in the United States[49] and in Germany[50]. Thus, good knowledge and care about health issues per se do not necessarily increase treatment uptake for hepatitis C.
ACKNOWLEDGMENTS
The following 20 physicians contributed the largest number of patients in addition to the authors: Thomas Lutz, 60311 Frankfurt; Solange Nzimegne Gölz, 10719 Berlin; Peter Geyer, 36088 Hünfeld; Petra Sandow, 14052 Berlin; Ralph Link, 77654 Offenburg; Henning Schnell-Kretschmer, 35392 Giessen; Oswald Burkhard, 67547 Worms; Stefan Pape, 33098 Paderborn; Bernd Bokemeyer; 32423 Minden; Willi Schiffelholz, 86150 Augsburg; Mathias Freitag, 76133 Karlsruhe; Andreas Zipf, 68161 Mannheim; Peter Buggisch, 20251 Hamburg; Maria Leuschner, 63065 Offenbach; Hanns Löhr, 65185 Wiesbaden; Jörg Cordes, 60596 Frankfurt; Christian Jellinek, 12043 Berlin; Klaus Boeker, 30161 Hannover; Gisela Felten, 44623 Herne; Christine John, 10117, Berlin; Germany (for all contributors).
COMMENTS
Background
In recent surveys only 20% of hepatitis C virus (HCV)-infected subjects know of their infection and only 20% of the latter are treated. Considering that therapy cures the disease in 50% of patients, treatment rate should be increased.
Research frontiers
Bio-epidemiological research focuses to identify treatment barriers in patients with chronic hepatitis C. As yet only some reasons for the current large therapeutic deficit have been identified including co-morbidity, drug abuse and psychosocial factors. The present study evaluates which factors influence the treatment decision in daily German practice.
Innovations and breakthroughs
Treatment uptake in the present cohort (45%) is one of the highest reported in the literature. A high treatment rate usually reflects good knowledge among physicians and patients. In Germany many physicians who treat hepatitis C are members of the national “hepatitis competence network” which is aimed to implement practice guidelines in the broad medical community. Despite the obvious success of the German hepatitis competence network some practice aspects did not meet standards in the present cohort including the use of liver biopsy and interpretation of HCV-RNA and alanine aminotransferase (ALT) values. Liver biopsy and thus knowledge about fibrosis stage were too low in particular in patients treated in private practice and in those with normal ALT. Also, there were misperceptions among patients as their refusal was a common treatment barrier. Unexpectedly, therapy uptake was higher in migrants despite language problems. Some further reasons against treatment appeared medically based whereas others seemed to be based on fears, socioeconomical problems and information deficits both on the side of physicians and patients.
Applications
The present cohort study includes a significant fraction of all HCV-infected patients in Germany. The community-based character of the present cohort incorporating 434 physicians and hospitals throughout the country reflects daily life in Germany probably better than looking at specialized academic centres.
Terminology
Treatment barrier: Reasons why patients with chronic hepatitis C are not treated with antiviral drugs.
Peer review
This is an important paper with a large HCV patient cohort from Germany including both academic and non-academic centres detailing reasons for treating and not treating HCV.
Footnotes
Supported by Investigator fees from by Roche Pharma AG Germany for contributing data to the study
Peer reviewers: Tamara M Alempijevic, MD, PhD, Assistant Professor, Clinic for Gastroenterology and Hepatology, Clinical Centre of Serbia, 2 Dr Koste Todorovica St., 11000 Belgrade, Serbia; Donald M Jensen, MD, Professor, Director, Center for Liver Diseases, University of Chicago Medical Center, 5841 S. Maryland, MC7120, Chicago, IL 60637, United States
S- Editor Wang JL L- Editor O’Neill M E- Editor Xiong L
References
- 1.Wasley A, Alter MJ. Epidemiology of hepatitis C: geographic differences and temporal trends. Semin Liver Dis. 2000;20:1–16. doi: 10.1055/s-2000-9506. [DOI] [PubMed] [Google Scholar]
- 2.Radun D, Hamouda O. Epidemiologie der Hepatitis B und C in Deutschland. Med Welt. 2004;55:206–210. [Google Scholar]
- 3.McHutchison JG, Bacon BR. Chronic hepatitis C: an age wave of disease burden. Am J Manag Care. 2005;11:S286–S25; quiz S286-S25. [PubMed] [Google Scholar]
- 4.Alter MJ, Kruszon-Moran D, Nainan OV, McQuillan GM, Gao F, Moyer LA, Kaslow RA, Margolis HS. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341:556–562. doi: 10.1056/NEJM199908193410802. [DOI] [PubMed] [Google Scholar]
- 5.Seeff LB, Hoofnagle JH. National Institutes of Health Consensus Development Conference: management of hepatitis C: 2002. Hepatology. 2002;36:S1–S2. doi: 10.1053/jhep.2002.36992. [DOI] [PubMed] [Google Scholar]
- 6.Zeuzem S. [Standard treatment of acute and chronic hepatitis C] Z Gastroenterol. 2004;42:714–719. doi: 10.1055/s-2004-813444. [DOI] [PubMed] [Google Scholar]
- 7.Rossol S, Bartel J. [Chronic hepatitis C virus infection] MMW Fortschr Med. 2006;43:36–37. doi: 10.1007/BF03364806. [DOI] [PubMed] [Google Scholar]
- 8.Niederau C, Zehnter E, Kapagiannidis C, Scaber J, Hüppe D. Werden die Empfehlungen des Robert-Koch-Instituts (RKI) zur Diagnose der Hepatitis C im hausärztlichen Bereich umgesetzt? Eine rospektive Untersuchung von 192 Hausarztpraxen in Deutschland. DGVS. 2006;44:A320. [Google Scholar]
- 9.Delwaide J, El Saouda R, Gérard C, Belaïche J. Hepatitis C infection: eligibility for antiviral therapies. Eur J Gastroenterol Hepatol. 2005;17:1185–1189. doi: 10.1097/00042737-200511000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Morrill JA, Shrestha M, Grant RW. Barriers to the treatment of hepatitis C. Patient, provider, and system factors. J Gen Intern Med. 2005;20:754–758. doi: 10.1111/j.1525-1497.2005.0161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bini EJ, Bräu N, Currie S, Shen H, Anand BS, Hu KQ, Jeffers L, Ho SB, Johnson D, Schmidt WN, et al. Prospective multicenter study of eligibility for antiviral therapy among 4,084 U.S. veterans with chronic hepatitis C virus infection. Am J Gastroenterol. 2005;100:1772–1779. doi: 10.1111/j.1572-0241.2005.41860.x. [DOI] [PubMed] [Google Scholar]
- 12.Butt AA, Wagener M, Shakil AO, Ahmad J. Reasons for non-treatment of hepatitis C in veterans in care. J Viral Hepat. 2005;12:81–85. doi: 10.1111/j.1365-2893.2005.00547.x. [DOI] [PubMed] [Google Scholar]
- 13.Hare CB, Morris JA, Chu A, Gotz V, Loveland JJ, Hodes D, Klaskala W. Comparison of characteristics of treated and non-treated patients with Hepatitis C infection. Pharmacoepidemiol Drug Saf. 2006;15:71–76. doi: 10.1002/pds.1146. [DOI] [PubMed] [Google Scholar]
- 14.Rowan PJ, Tabasi S, Abdul-Latif M, Kunik ME, El-Serag HB. Psychosocial factors are the most common contraindications for antiviral therapy at initial evaluation in veterans with chronic hepatitis C. J Clin Gastroenterol. 2004;38:530–534. doi: 10.1097/01.mcg.0000123203.36471.70. [DOI] [PubMed] [Google Scholar]
- 15.Butt AA, Justice AC, Skanderson M, Rigsby MO, Good CB, Kwoh CK. Rate and predictors of treatment prescription for hepatitis C. Gut. 2007;56:385–389. doi: 10.1136/gut.2006.099150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hüppe D, Zehnter E, Mauss S, Böker K, Lutz T, Racky S, Schmidt W, Ullrich J, Sbrijer I, Heyne R, et al. [Epidemiology of chronic hepatitis C in Germany--an analysis of 10,326 patients in hepatitis centres and outpatient units] Z Gastroenterol. 2008;46:34–44. doi: 10.1055/s-2007-963691. [DOI] [PubMed] [Google Scholar]
- 17.Desmet VJ, Gerber M, Hoofnagle JH, Manns M, Scheuer PJ. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology. 1994;19:1513–1520. [PubMed] [Google Scholar]
- 18.Tsui JI, Currie S, Shen H, Bini EJ, Brau N, Wright TL. Treatment eligibility and outcomes in elderly patients with chronic hepatitis C: results from the VA HCV-001 Study. Dig Dis Sci. 2008;53:809–814. doi: 10.1007/s10620-007-9926-x. [DOI] [PubMed] [Google Scholar]
- 19.Zickmund SL, Brown KE, Bielefeldt K. A systematic review of provider knowledge of hepatitis C: is it enough for a complex disease? Dig Dis Sci. 2007;52:2550–2556. doi: 10.1007/s10620-007-9753-0. [DOI] [PubMed] [Google Scholar]
- 20.Richmond JA, Dunning TL, Desmond PV. Health professionals’ attitudes toward caring for people with hepatitis C. J Viral Hepat. 2007;14:624–632. doi: 10.1111/j.1365-2893.2007.00849.x. [DOI] [PubMed] [Google Scholar]
- 21.McNally S, Temple-Smith M, Sievert W, Pitts MK. Now, later or never? Challenges associated with hepatitis C treatment. Aust N Z J Public Health. 2006;30:422–427. doi: 10.1111/j.1467-842x.2006.tb00457.x. [DOI] [PubMed] [Google Scholar]
- 22.Fleig WE, Krummener P, Lesske J. [Criteria for the definition of acute and chronic hepatitis C] Z Gastroenterol. 2004;42:707–713. doi: 10.1055/s-2004-813443. [DOI] [PubMed] [Google Scholar]
- 23.Niederau C, Bemba G, Kautz A. [Socioeconomic characteristics, quality of life, and state of knowledge of patients with hepatitis C viral infection in Germany--socioeconomic aspects in hepatitis C] Z Gastroenterol. 2006;44:305–317. doi: 10.1055/s-2006-926510. [DOI] [PubMed] [Google Scholar]
- 24.Niederau C, Fischer C, Kautz A. [Socio-economical aspects, quality of life and state of knowledge in hepatitis B patients. Socio-economical aspects in hepatitis B] Z Gastroenterol. 2007;45:355–368. doi: 10.1055/s-2007-963102. [DOI] [PubMed] [Google Scholar]
- 25.Niederau C, Bemba G, Kautz A. [Changes in socio-economics, quality of life and knowledge of patients with chronic hepatitis C during the Hepatitis Competence Net Project] Z Gastroenterol. 2008;46:22–33. doi: 10.1055/s-2007-963534. [DOI] [PubMed] [Google Scholar]
- 26.Hadziyannis SJ, Sette H, Morgan TR, Balan V, Diago M, Marcellin P, Ramadori G, Bodenheimer H, Bernstein D, Rizzetto M, et al. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004;140:346–355. doi: 10.7326/0003-4819-140-5-200403020-00010. [DOI] [PubMed] [Google Scholar]
- 27.Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 28.Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL, Häussinger D, Diago M, Carosi G, Dhumeaux D, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 29.Niederau C, Lange S, Heintges T, Erhardt A, Buschkamp M, Hürter D, Nawrocki M, Kruska L, Hensel F, Petry W, et al. Prognosis of chronic hepatitis C: results of a large, prospective cohort study. Hepatology. 1998;28:1687–1695. doi: 10.1002/hep.510280632. [DOI] [PubMed] [Google Scholar]
- 30.McHutchison JG. Understanding hepatitis C. Am J Manag Care. 2004;10:S21–S29. [PubMed] [Google Scholar]
- 31.Markowitz JS, Gutterman EM, Hodes D, Klaskala W. Factors associated with the initiation of alpha-interferon treatment in Medicaid patients diagnosed with hepatitis C. J Viral Hepat. 2005;12:176–185. doi: 10.1111/j.1365-2893.2005.00607.x. [DOI] [PubMed] [Google Scholar]
- 32.Zeuzem S, Diago M, Gane E, Reddy KR, Pockros P, Prati D, Shiffman M, Farci P, Gitlin N, O’Brien CB, et al. Peginterferon alfa-2a (40 kilodaltons) and ribavirin in patients with chronic hepatitis C and normal aminotransferase levels. Gastroenterology. 2004;127:1724–1732. doi: 10.1053/j.gastro.2004.09.050. [DOI] [PubMed] [Google Scholar]
- 33.Greub G, Ledergerber B, Battegay M, Grob P, Perrin L, Furrer H, Burgisser P, Erb P, Boggian K, Piffaretti JC, et al. Clinical progression, survival, and immune recovery during antiretroviral therapy in patients with HIV-1 and hepatitis C virus coinfection: the Swiss HIV Cohort Study. Lancet. 2000;356:1800–1805. doi: 10.1016/s0140-6736(00)03232-3. [DOI] [PubMed] [Google Scholar]
- 34.Sulkowski MS, Moore RD, Mehta SH, Chaisson RE, Thomas DL. Hepatitis C and progression of HIV disease. JAMA. 2002;288:199–206. doi: 10.1001/jama.288.2.199. [DOI] [PubMed] [Google Scholar]
- 35.Qurishi N, Kreuzberg C, Lüchters G, Effenberger W, Kupfer B, Sauerbruch T, Rockstroh JK, Spengler U. Effect of antiretroviral therapy on liver-related mortality in patients with HIV and hepatitis C virus coinfection. Lancet. 2003;362:1708–1713. doi: 10.1016/S0140-6736(03)14844-1. [DOI] [PubMed] [Google Scholar]
- 36.McLaren M, Garber G, Cooper C. Barriers to hepatitis C virus treatment in a Canadian HIV-hepatitis C virus coinfection tertiary care clinic. Can J Gastroenterol. 2008;22:133–137. doi: 10.1155/2008/949582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Torriani FJ, Rodriguez-Torres M, Rockstroh JK, Lissen E, Gonzalez-García J, Lazzarin A, Carosi G, Sasadeusz J, Katlama C, Montaner J, et al. Peginterferon Alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med. 2004;351:438–450. doi: 10.1056/NEJMoa040842. [DOI] [PubMed] [Google Scholar]
- 38.Chung RT, Andersen J, Volberding P, Robbins GK, Liu T, Sherman KE, Peters MG, Koziel MJ, Bhan AK, Alston B, et al. Peginterferon Alfa-2a plus ribavirin versus interferon alfa-2a plus ribavirin for chronic hepatitis C in HIV-coinfected persons. N Engl J Med. 2004;351:451–459. doi: 10.1056/NEJMoa032653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nunes D, Saitz R, Libman H, Cheng DM, Vidaver J, Samet JH. Barriers to treatment of hepatitis C in HIV/HCV-coinfected adults with alcohol problems. Alcohol Clin Exp Res. 2006;30:1520–1526. doi: 10.1111/j.1530-0277.2006.00183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adeyemi OM, Jensen D, Attar B, Ghaoui R, Gallagher M, Wolen D, Cotler SJ. Hepatitis C treatment eligibility in an urban population with and without HIV coinfection. AIDS Patient Care STDS. 2004;18:239–245. doi: 10.1089/108729104323038919. [DOI] [PubMed] [Google Scholar]
- 41.Backmund M, Meyer K, Von Zielonka M, Eichenlaub D. Treatment of hepatitis C infection in injection drug users. Hepatology. 2001;34:188–193. doi: 10.1053/jhep.2001.25882. [DOI] [PubMed] [Google Scholar]
- 42.Schaefer M, Schmidt F, Folwaczny C, Lorenz R, Martin G, Schindlbeck N, Heldwein W, Soyka M, Grunze H, Koenig A, et al. Adherence and mental side effects during hepatitis C treatment with interferon alfa and ribavirin in psychiatric risk groups. Hepatology. 2003;37:443–451. doi: 10.1053/jhep.2003.50031. [DOI] [PubMed] [Google Scholar]
- 43.Mauss S, Berger F, Goelz J, Jacob B, Schmutz G. A prospective controlled study of interferon-based therapy of chronic hepatitis C in patients on methadone maintenance. Hepatology. 2004;40:120–124. doi: 10.1002/hep.20279. [DOI] [PubMed] [Google Scholar]
- 44.Bhattacharya R, Shuhart MC. Hepatitis C and alcohol: interactions, outcomes, and implications. J Clin Gastroenterol. 2003;36:242–252. doi: 10.1097/00004836-200303000-00012. [DOI] [PubMed] [Google Scholar]
- 45.Lieber CS. Hepatitis C and alcohol. J Clin Gastroenterol. 2003;36:100–102. doi: 10.1097/00004836-200302000-00003. [DOI] [PubMed] [Google Scholar]
- 46.Litwin AH, Kunins HV, Berg KM, Federman AD, Heavner KK, Gourevitch MN, Arnsten JH. Hepatitis C management by addiction medicine physicians: results from a national survey. J Subst Abuse Treat. 2007;33:99–105. doi: 10.1016/j.jsat.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grebely J, Raffa JD, Lai C, Krajden M, Kerr T, Fischer B, Tyndall MW. Low uptake of treatment for hepatitis C virus infection in a large community-based study of inner city residents. J Viral Hepat. 2009;16:352–358. doi: 10.1111/j.1365-2893.2009.01080.x. [DOI] [PubMed] [Google Scholar]
- 48.Dollarhide AW, Loh C, Leckband SG, Endow-Eyer R, Robinson S, Meyer JM. Psychiatric comorbidity does not predict interferon treatment completion rates in hepatitis C seropositive veterans. J Clin Gastroenterol. 2007;41:322–328. doi: 10.1097/01.mcg.0000225629.22286.96. [DOI] [PubMed] [Google Scholar]
- 49.Green CA, Pope CR. Gender, psychosocial factors and the use of medical services: a longitudinal analysis. Soc Sci Med. 1999;48:1363–1372. doi: 10.1016/s0277-9536(98)00440-7. [DOI] [PubMed] [Google Scholar]
- 50.Ladwig KH, Marten-Mittag B, Formanek B, Dammann G. Gender differences of symptom reporting and medical health care utilization in the German population. Eur J Epidemiol. 2000;16:511–518. doi: 10.1023/a:1007629920752. [DOI] [PubMed] [Google Scholar]