Abstract
Background
In pediatric cardiac surgery, infection is a leading cause of morbidity and mortality. We created a model to predict risk of major infection in this population.
Methods
Using the Society of Thoracic Surgeons Congenital Heart Surgery Database, we created a multivariable model in which the primary outcome was major infection (septicemia, mediastinitis, or endocarditis). Candidate independent variables included demographic characteristics, comorbid conditions, preoperative factors, and cardiac surgical procedures. We created a reduced model by backward selection and then created an integer scoring system using a scaling factor with scores corresponding to percent risk of infection.
Results
Of 30,078 children from 48 centers, 2.8% had major infection (2.6% septicemia, 0.3% mediastinitis, and 0.09% endocarditis). Mortality and postoperative length of stay were greater in those with major infection (mortality: 22.2% vs. 3.0%; length of stay > 21 days: 69.9% vs. 10.7%). Young age, high complexity, previous cardiothoracic operation, preoperative length of stay >1 day, preoperative ventilator support, and presence of a genetic abnormality were associated with major infection after backward selection (p<0.001). Estimated infection risk ranged from <0.1% to 13.3%; the model discrimination was good (c-index 0.79).
Conclusions
We created a simple bedside tool to identify children at high risk for major infection after cardiac surgery. These patients may be targeted for interventions to reduce the risk of infection and for inclusion in future clinical trials.
Keywords: Congenital heart disease, Infection, Outcomes
Infections in children are frequent (13-31%) after cardiac surgery [1-7]. Many are surgical site infections (incidence 2.3-8%) [4, 5, 7-9], however, many are more serious, such as septicemia (incidence 6.3-15%) [3, 7, 10], mediastinitis (0.2-3.3%) [5, 8, 9, 11-13], and endocarditis (incidence 0.2%) [14-16]. Infections result in significant morbidity, (e.g. antibiotic usage, re-operation, prolonged hospital and intensive care unit (ICU) stays, and longer periods of mechanical ventilation and inotropic support), and contribute to an increase in mortality [2, 3, 5, 17].
Several studies have evaluated risk factors for post-operative infection such as: longer pre-operative and ICU stay, longer length of admission, open chest after surgery, cyanotic heart disease, younger age, and higher complexity score [1, 3, 7]. These studies, however, have been performed at single centers and are limited by small sample sizes. There have been few attempts to use risk factors to create a risk stratification system for post-operative infection; those that have attempted to create such a system have failed to adequately risk stratify patients in the setting of pediatric cardiac surgery [18-20].
The Society of Thoracic Surgeons (STS) Congenital Heart Surgery Database is the largest congenital heart surgery registry in North America. We utilized this dataset to identify risk factors for major infection in children after cardiac surgery, and to create and validate a bedside scoring system that can be used to estimate a patient’s risk of major infection.
Patients and Methods
Data Source
The STS Congenital Heart Surgery Database was founded in 2002 to support quality improvement in heart surgery. Data elements include demographic information, cardiac and non-cardiac anomalies, comorbid conditions, type of operation, and outcomes including in-hospital mortality, major complications, and length of stay. We obtained approval from the Duke Institutional Review Board for waiver of consent because of the de-identified nature of the data.
Patient Population
We evaluated patients ≤age 18 years at operation from 1/1/2002 to 12/31/2006. We excluded hospitals (n = 10) with >10% missing data on preoperative risk factors, non-cardiac abnormalities, or postoperative complications. We excluded all solely thoracic surgical operations by excluding those that did not meet the criteria for “cardiopulmonary bypass” or “no cardiopulmonary bypass cardiovascular” (n = 7191) and those that consisted of procedures not related to congenital heart disease surgery (n = 6044). In addition, we excluded 803 operations for which both the Risk Adjustment for Congenital Heart Surgery (RACHS-1) category and the Aristotle Basic Complexity score were undefined. We also excluded those that underwent a heart or lung transplant (n = 505), a catheter related procedure (n = 6), and ligation of a patent ductus arteriosus in infants weighing <2500 grams (n = 2954). We excluded patients if they had preoperative endocarditis (n = 154) or septicemia (n = 309), if there were missing data on age (n = 3), weight (n = 83), sex (n = 8), or if the recorded weight was implausible (n = 244; defined as 7 standard deviations below or 5 standard deviations above the patient’s predicted weight according to growth charts from the Centers for Disease Control (CDC) and Prevention [21]); or if there were no data on postoperative complications regarding infection (n = 147). Finally, we included the first operation per hospital admission and excluded subsequent operations (n = 1073) within the same admission. The final population consisted of 30,078 patients from 48 institutions.
Clinical Endpoint
The primary endpoint of the analysis was “major infection,” defined as septicemia, mediastinitis, or endocarditis before hospital discharge, or after discharge if it was attributed to the operation. Mediastinitis was defined as “postoperative infection involving the sternum and/or mediastinum” including ≥1 of the following: 1) wound opened with drainage of fluid and/or excision of tissue, 2) positive culture, or 3) treatment with antibiotics or antifungals. Septicemia was defined as “postoperative bloodstream infection requiring positive blood cultures” and excluded line contaminants. Endocarditis was defined as “postoperative intracardiac infection with echocardiographic and/or blood culture confirmation.” However, in equivocal cases, clinical data such as splenic infarcts, Janeway lesions, and thromboemboli were sufficient to make the diagnosis. It should be noted, however, that these definitions were adopted in 2006, after much of the data had been collected.
Statistical Analysis/Model Development
We selected candidate variables based on risk factors for infection after cardiac surgery previously identified [1, 3-5, 7-9, 17, 22-25] as well as potential risk factors based on clinical suspicion of the authors (including pediatric cardiologists, cardiovascular surgeons, and an infectious disease specialist). We included only risk factors identified prior to the operation. Candidate risk factors were: procedure type (grouped into categories by Aristotle Basic Complexity (ABC) [26] and Risk Adjustment for Congenital Heart Surgery, version 1 (RACHS-1) [27] scores), age, weight-for-age-and-sex percentile [21], previous cardiac surgery, preoperative length of stay >1 day, preoperative mechanical ventilation or tracheostomy, preoperative acidosis/circulatory support/shock, identified genetic abnormality, and year of surgery. Weight (as opposed to weight-for-age-and-sex) was not included as a candidate variable because of its correlation with age.
After the selection of candidate variables, we performed a univariable analysis to determine the incidence of major infection in relationship to the candidate variables. We then developed a logistic regression model including all of the candidate explanatory variables (“full model”). Age was modeled continuously using restricted cubic splines with knots at 90 days, 1 year, and 3 years. Weight-for-age-and-sex was modeled as three categories: <5th %ile, 5-50th %ile, and >50th %ile. Parameters of the logistic model were estimated using generalized estimating equations methodology with an exchangeable working correlation structure in order to account for clustering of subjects within hospitals.
We adjusted for procedure by grouping procedure types into strata and entering them in the model as a set of category indicator variables. Initially, we allowed any procedure with ≥20 occurrences to be its own stratum. Remaining procedures were categorized by cross-classifying the procedure’s ABC score with the procedure’s RACHS-1 score. This approach resulted in 37 strata. For simplicity, we also developed a model using only 3 strata (“low complexity”=ABC < 3 and RACHS-1 <3; “high complexity” if ABC >= 4 or RACHS-1 >=5; “medium complexity”= all others). Results were similar; therefore, we used the model using only these three strata for the remainder of the analysis. Of note, where there were multiple procedures per operation, they were assigned to the stratum of the most complex procedure.
Reduced model/bedside tool
We created a reduced model by applying backward selection to the set of candidate variables, using a significance criterion of 0.05 for eliminating variables. To facilitate bedside scoring, we replaced the spline terms for age with a set of age categories (0-30 days, 31-90 days, 90 days – 1 year, 1-3years, 3-5 years, 5+ years.) We forced year of surgery into the model to ensure that the weighting of patient-level risk factors would not be confounded by changes in risk factor prevalence and infection risk over time. To create the bedside risk tool we multiplied each regression coefficient by 5 and rounded to the nearest integer. Although year of surgery was included in the model, it was omitted from the bedside risk tool. The risk score for each patient was defined by summing the points across risk factors. Finally, we determined the relationship between patient-specific risk scores and infection risk using logistic regression. In this model, infection was the outcome variable and risk score (modeled as a cubic polynomial function) was the explanatory variable.
We validated the models internally by calculating measures of calibration and discrimination. We assessed calibration graphically by comparing observed vs. predicted values across levels of predicted risk and used the Hosmer-Lemeshow test to assess whether the observed differences were statistically significant. We assessed discrimination by calculating the C-statistic. Because the models were developed and validated in the same sample, we used the method of bootstrap resampling to adjust the C statistic to obtain an approximately unbiased assessment of future model performance.
Results
Demographic characteristics and infection
We analyzed a total of 30,078 cases in the STS Congenital Cardiac Surgery Database. From this cohort, 857 patients (2.8%) had major infection (2.6% septicemia, 0.3% mediastinitis, 0.09% endocarditis). Thirty-two patients had >1 type of infection. Of patients with major infection, the mean age was 6.5 months (vs. 2.4 years for the entire cohort). Fifty-five percent of patients were male in both the entire cohort and in those with major infection. Both mortality and length of stay were greater in the group of patients that developed major infection (mortality: 22.2% [CI 19.4-25.1] vs. 3.0% [CI 2.8-3.2]; length of stay >21 days: 69.9% [CI 66.7-73.0] vs. 10.7% [CI 10.3-11.0]). The percentage of patients with infection decreased over time from 3.7% in 2002 to 2.2% in 2006.
A number of variables were associated with major infection in univariable anaylsis (Table 1), including ABC score, RACHS-1 score, age, weight, weight-for-age-and-sex, preoperative ventilatory support or tracheostomy, longer preoperative stay, preoperative acidosis, preoperative shock, the presence of certain known genetic abnormalities (e.g. 22q11 deletion, DiGeorge syndrome, asplenia), and year of surgery. The presence of a genetic abnormality was also associated with major infection in univariable analysis; however, there were some specific abnormalities that were not associated with an increased risk of infection, such as Trisomy 21, Marfan syndrome, Alagille syndrome, and Williams-Beuren syndrome.
Table 1.
Clinical characteristics of the patient population and univariable analysis
| Variable | Level | Total N (30078) |
Overall % | Number with infection (857) |
Percentage with infection |
P-value+ |
|---|---|---|---|---|---|---|
| Operative | ||||||
| Aristotle Basic Complexity | N/A* | 325 | 1.1 | 6 | 1.9 | <.0001 |
| Level | 1 | 3512 | 11.7 | 29 | 0.8 | |
| 2 | 13895 | 46.2 | 296 | 2.1 | ||
| 3 | 8163 | 27.1 | 240 | 2.9 | ||
| 4 | 4183 | 13.9 | 286 | 6.8 | ||
| RACHS-1 Category | N/A* | 2252 | 7.5 | 73 | 3.2 | <.0001 |
| 1 | 4090 | 13.6 | 28 | 0.7 | ||
| 2 | 11819 | 39.3 | 201 | 1.7 | ||
| 3 | 8387 | 27.9 | 276 | 3.3 | ||
| 4 | 2246 | 7.5 | 113 | 5.0 | ||
| 5 | 6 | <0.1 | 1 | 16.7 | ||
| 6 | 1278 | 4.3 | 165 | 12.9 | ||
| Demographics | ||||||
| Age | <30 days | 6621 | 22.0 | 426 | 6.4 | <.0001 |
| 1 – 3 months | 2702 | 9.0 | 104 | 3.9 | ||
| 4 – 12 months | 8603 | 28.6 | 205 | 2.4 | ||
| 1 – 9 years | 9095 | 30.2 | 107 | 1.2 | ||
| ≥ 10 years | 3057 | 10.2 | 15 | 0.5 | ||
| Weight (kilograms) | <2.50 | 1005 | 3.3 | 80 | 8.0 | <.0001 |
| 2.50 - 4.99 | 9911 | 33.0 | 508 | 5.1 | ||
| ≥ 5.00 | 19162 | 63.7 | 269 | 1.4 | ||
| Weight for age/sex (percentile) |
>50th %ile | 11633 | 38.7 | 239 | 2.1 | 0.0005 |
| 5th to 50th %ile | 7864 | 26.2 | 261 | 3.3 | ||
| <5th %ile | 10581 | 35.2 | 357 | 3.4 | ||
| Gender | Male | 16537 | 55.0 | 472 | 2.9 | 0.9379 |
| Female | 13541 | 45.0 | 385 | 2.8 | ||
| Race | Missing | 5488 | 18.3 | 143 | 2.6 | 0.1269 |
| Caucasian | 13209 | 43.9 | 368 | 2.8 | ||
| Black | 3105 | 10.3 | 112 | 3.6 | ||
| Hispanic | 5042 | 16.8 | 161 | 3.2 | ||
| Asian | 756 | 2.5 | 19 | 2.5 | ||
| Native American | 153 | 0.5 | 8 | 5.2 | ||
| Other | 2325 | 7.7 | 46 | 2.0 | ||
| Surgery year | 2002 | 3276 | 10.9 | 120 | 3.7 | 0.0310 |
| 2003 | 4523 | 15.0 | 150 | 3.3 | ||
| 2004 | 5685 | 18.9 | 182 | 3.2 | ||
| 2005 | 7825 | 26.0 | 213 | 2.7 | ||
| 2006 | 8769 | 29.2 | 192 | 2.2 | ||
| Operative | ||||||
| Operative type | Bypass | 24971 | 83.0 | 716 | 2.9 | 0.4899 |
| Non-bypass | 5107 | 17.0 | 141 | 2.8 | ||
| Cardiovascular | ||||||
| Procedure Stratum** | Low Complexity | 12917 | 43.0 | 152 | 1.2 | <.0001 |
| Medium Complexity | 12831 | 42.7 | 406 | 3.2 | ||
| High Complexity | 4330 | 14.4 | 299 | 6.9 | ||
| Risk Factors | ||||||
| Preoperative length of stay (days) |
Missing | 8 | <0.1 | 0 | 0.00 | <.0001 |
| 0 | 18537 | 61.6 | 237 | 1.3 | ||
| 1 | 2389 | 7.9 | 74 | 3.1 | ||
| 2 | 1549 | 5.2 | 64 | 4.1 | ||
| ≥3 | 7595 | 25.3 | 482 | 6.4 | ||
| Previous cardiac operation | Missing | 563 | 1.9 | 37 | 6.6 | 0.0867 |
| No | 21337 | 70.9 | 608 | 2.9 | ||
| Yes | 8178 | 27.2 | 212 | 2.6 | ||
| Preoperative acidosis | No | 29462 | 98.0 | 811 | 2.8 | <.0001 |
| Yes | 616 | 2.1 | 46 | 7.5 | ||
| Preoperative circulatory support |
No | 30022 | 99.8 | 853 | 2.8 | 0.0518 |
| Yes | 56 | 0.2 | 4 | 7.1 | ||
| Preoperative shock | No | 29740 | 98.9 | 836 | 2.8 | 0.0011 |
| Yes | 338 | 1.1 | 21 | 6.2 | ||
| Preoperative tracheostomy | No | 29982 | 99.7 | 845 | 2.8 | 0.0016 |
| Yes | 96 | 0.3 | 12 | 12.5 | ||
| Preoperative ventilatory support |
No | 26922 | 89.5 | 564 | 2.1 | <.0001 |
| Yes | 3156 | 10.5 | 293 | 9.3 | ||
| Any genetic abnormality | No | 21811 | 72.5 | 487 | 2.2 | <.0001 |
| Yes | 8267 | 27.5 | 370 | 4.5 | ||
| Mortality | ||||||
| Discharge mortality | Missing | 36 | 0.1 | 1 | 2.8 | <.0001 |
| No | 28963 | 96.3 | 666 | 2.3 | ||
| Yes | 1079 | 3.6 | 190 | 17.6 |
Not assigned
“low complexity”=ABC < 3 and RACHS-1 <3; “high complexity” if ABC >= 4 or RACHS-1 >=5; “medium complexity”= all others
Multivariable Regression Model
Independent variables associated with increased infection risk in multivariable analysis were age (modeled as a continuous variable), previous cardiac operation, preoperative length of stay >1 day, preoperative ventilator support or tracheostomy, any genetic abnormality, medium or high complexity score, and year of surgery (Table 2). In the reduced model, variables that remained after backward selection were age <90 days, age 90 days-3 years, age 3-5 years, medium complexity score, high complexity score, length of stay >1 day, preoperative ventilator support or tracheostomy, previous cardiothoracic operation, and any genetic abnormality (Table 3). The variables most strongly associated with major infection were age <90 days, age 90 days-3 years, and high complexity score.
Table 2.
Full model
| Variable | OR (95% CI) |
|---|---|
| Age | |
| 7 days (vs. 1 year) | 1.82 (1.43, 2.31) |
| 30 days (vs. 1 year) | 1.74 (1.39, 2.18) |
| 90 days (vs. 1 year) | 1.56 (1.31, 1.87) |
| 3 years (vs. 1 year) | 0.66 (0.59, 0.73) |
| 10 years (vs. 1 year) | 0.39 (0.29, 0.52) |
| Weight (for age and sex) | |
| 5th – 50th %ile | 0.91 (0.79, 1.04) |
| <5th %ile | 1.18 (0.97, 1.43) |
| Previous cardiothoracic operation | 2.14 (1.71, 2.68) |
| Preoperative stay > 1 day | 1.78 (1.54, 2.07) |
| Preoperative ventilatory support | 2.02 (1.66, 2.45) |
| Preoperative acidosis, circulatory support, shock | 0.90 (0.69, 1.16) |
| Genetic abnormality | 1.69 (1.44, 1.98) |
| Surgery year | 0.90 (0.82, 0.98) |
| Medium complexity* | 1.84 (1.51, 2.24) |
| High complexity** | 3.00 (2.24, 4.03) |
Aristotle Basic Complexity Score = 3 or RACHS-1 = 3-4 (and not “high complexity”)
Aristotle Basic Complexity Score > 3 or RACHS-1 > 4
Table 3.
Reduced and Integer Models
| Variable | OR (95%CI) | Points | p-value |
|---|---|---|---|
| Age < 90 days | 6.3 (4.1-9.8) | 9 | <0.0001 |
| Age 90 days-3 years | 4.1 (2.7-6.1) | 7 | <0.0001 |
| Age 3-5 years | 1.9 (1.1-3.4) | 3 | 0.027 |
| Medium complexity* | 1.8 (1.5-2.2) | 3 | <0.0001 |
| High complexity** | 3.0 (2.4-3.7) | 6 | <0.0001 |
| Preoperative length of stay > 1 day | 1.8 (1.5-2.2) | 3 | <0.0001 |
| Preoperative ventilator support | 2.1 (1.8-2.5) | 4 | <0.0001 |
| Previous cardiothoracic operation | 2.1 (1.7-2.5) | 4 | <0.0001 |
| Genetic abnormality | 1.9 (1.7-2.2) | 3 | <0.0001 |
Aristotle Basic Complexity Score = 3 or RACHS-1 = 3-4 (and not “high complexity”)
Aristotle Basic Complexity Score > 3 or RACHS-1 > 4
Risk Scoring System
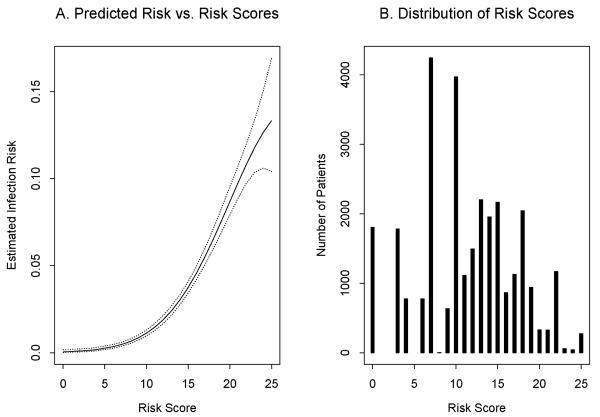
The bedside tool model had good predictive ability (bootstrap-adjusted c-index 0.781). Table 4 was generated to be used clinically to estimate risk of major infection given a specific point score. Estimated risk of infection exhibited a non-linear relationship with risk score and ranged from 0-13.3% (Figure 1A). Distribution of risk scores is shown in Figure 1B. The most common procedures and the observed vs. predicted infection rates are listed in Table 5.
Table 4.
Bedside Tool Risk Look-up Table
| Risk Score | Probability of infection (%) | 95% CI |
|---|---|---|
| 0 | 0.0 | 0.0-0.2 |
| 3 | 0.1 | 0.1-0.2 |
| 4 | 0.2 | 0.1-0.3 |
| 6 | 0.3 | 0.3-0.5 |
| 7 | 0.5 | 0.4-0.6 |
| 8 | 0.6 | 0.5-0.8 |
| 9 | 0.9 | 0.7-1.0 |
| 10 | 1.1 | 1.0-1.3 |
| 11 | 1.5 | 1.3-1.7 |
| 12 | 1.9 | 1.7-2.1 |
| 13 | 2.4 | 2.2-2.7 |
| 14 | 3.0 | 2.7-3.3 |
| 15 | 3.8 | 3.4-4.1 |
| 16 | 4.6 | 4.2-5.0 |
| 17 | 5.5 | 5.1-6.0 |
| 18 | 6.5 | 6.0-7.1 |
| 19 | 7.6 | 6.9-8.3 |
| 20 | 8.7 | 7.9-9.5 |
| 21 | 9.8 | 8.9-10.8 |
| 22 | 10.8 | 9.8-12.0 |
| 23 | 11.8 | 10.4-13.4 |
| 24 | 12.7 | 10.6-15.1 |
| 25 | 13.3 | 10.4-16.9 |
Figure 1.
Panel A: Bedside tool model of predicted risk in relation to risk score. Solid line represents model estimate. Dotted line represents 95% confidence interval. X axis denotes risk score and Y axis denotes estimated infection risk. Panel B: Distribution of study population by risk score category. X axis denotes risk score and Y axis represents total number of patients.
Table 5.
Predicted vs. Observed Rates of Major Infection in the 10 Most Common Procedures
| Procedure | Number of procedures |
Infection rate, observed (%) (95% CI) |
Infection rate, predicted (%) |
|---|---|---|---|
| Ventricular septal defect repair |
2527 | 1.3 (0.9 - 1.8) | 1.1 |
| Atrial septal defect repair | 1240 | 0.2 (0.1 - 0.7) | 0.4 |
| Complete atrioventricular septal defect repair |
1218 | 3.6 (2.6 - 4.8) | 3.1 |
| Norwood procedure | 1131 | 13.4 (11.5 - 15.6) | 8.5 |
| Bidirectional Glenn procedure |
1070 | 2.1 (1.3 - 3.1) | 2.3 |
| Coarctation repair, end to end, extended |
1030 | 2.3 (1.5 - 3.5) | 3.8 |
| Modified Blalock-Taussig shunt |
1013 | 5.9 (4.6 - 7.6) | 5.4 |
| Tetralogy of Fallot repair | 943 | 2.2 (1.4 - 3.4) | 2.6 |
| Patent ductus arteriosus closure |
924 | 1.1 (0.5 - 2.0) | 1.7 |
| Right ventricular outflow tract procedure |
897 | 2.0 (1.2 - 3.2) | 1.4 |
Validation of Model
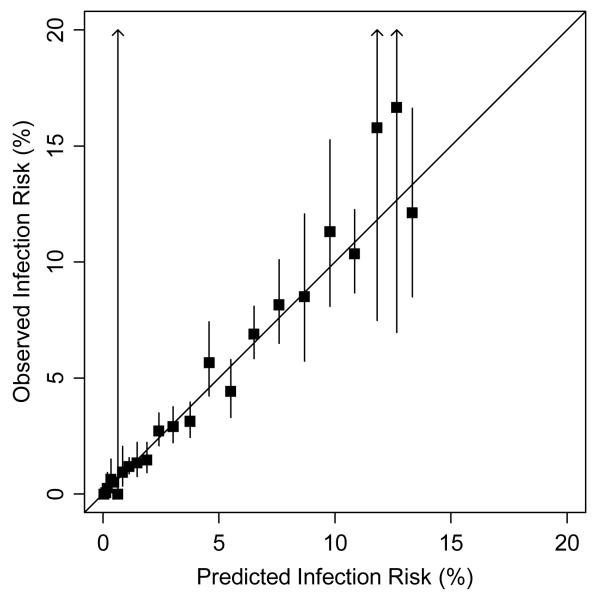
Each model had good predictive ability, with no difference in the predictive ability of the full model (c-index 0.785) vs. the reduced model (c-index 0.786); and only slightly increased predictive ability of both full and reduced models vs. the bedside tool (c-index 0.781). Internal calibration of the risk tool was excellent, with close agreement between predicted and observed infection rates (Figure 2; goodness of fit chi-square = 17.8; p = 0.66). Observed vs. predicted rates of infection for the 10 most common procedures are listed in Table 5.
Figure 2.
Observed vs. predicted risk of infection during internal calibration of bedside model. Squares correspond to observed risk for each unique value of the risk score. Solid lines correspond to 95% confidence intervals. Goodness of fit chi-square = 17.8; p = 0.66. X axis denotes predicted infection risk and Y axis shows observed infection risk.
Comment
Our study confirms that major infection after congenital heart surgery is a complication with major sequelae. Using a large multi-center patient population, we identified risk factors for major infection and created a clinical tool that can be used pre-operatively to estimate a patient’s infection risk. We validated the model internally showing that it has good discrimination.
Previous studies have evaluated risk factors for specific types of post-operative infections. Cardiopulmonary bypass, reintubation, and surgical site infection increase the risk of bloodstream infection [22]. Risk factors for surgical site infection include undergoing >1 cardiothoracic procedure, preoperative infection, surgery on a Monday, higher American Society of Anesthesiologists (ASA) score, higher Pediatric Risk of Mortality (PRISM) score, peri-operative hypothermia, open chest after surgery, need for re-exploration, nasal colonization with Staphylococcus aureus, longer duration of surgery, longer pre-operative stay, and younger age [4, 5, 7-9, 17, 23, 24]. Previously reported risk factors for mediastinitis include having a genetic syndrome, higher ASA score, and longer duration of pacing wires [25]. In contrast to our study, these studies were done at single centers, with relatively small sample sizes, and none evaluated for a clinically significant composite endpoint of “major infection.” Additionally, none of these studies developed a model using pre-operative factors that can be used clinically to predict risk of major infection.
In this study, the factors significantly associated with infection by multivariable analysis were largely similar to risk factors previously identified. The factors that accounted for the greatest increase in risk were young age and high complexity. Weight was associated with infection in univariable analysis, but weight-for-age-and-sex did not remain a risk factor with multivariable analysis. This suggests that weight may be a predictor of major infection as it correlates with age; however, there is no evidence of an association between weight and infection after accounting for age and other risk factors. Likewise, preoperative hemodynamic compromise (preoperative acidosis, circulatory support, or shock) was not a predictor of major infection in either the full or reduced model. Some previous studies have included leaving the chest open post-operatively as a risk factor [4, 7, 24]. Since the purpose of our investigation was to identify pre-operative risk factors, and whether or not the chest will be left open cannot always be determined before surgery, we did not include this candidate variable in our study.
The incidence of infection in our population was less than that previously reported. This may be due to the types of infections included. In this study, only “major infections” were included, defined as sepsis, mediastinitis, and endocarditis, while previous studies have often included other types of infection, such as superficial surgical site infection and pneumonia. The incidence of sepsis in this study was less than that reported in previous studies [3, 7, 10]. The incidence of mediastinitis in this study was similar to some of the previous studies [8, 12, 13], although somewhat lower than in others [5, 9, 11]. Incidence of endocarditis in our population was lower than that previously reported [28].
In this study, the rate of infection decreased over the time period studied. One possible explanation is that with more clinical experience, centers may be more efficient and are decreasing surgical time and the length of ICU and hospital stays, all of which contribute to decreased infection rates. This time period has also correlated with greater emphasis on ICU techniques such as proper hand-washing and protocols regarding central lines. It is possible that this decreasing infection rate is related to underreporting in the sample, though this would be less likely to cause a trend within the data set than it would to cause decreased rates when compared to other studies.
The strengths of this study are that it is based upon a large multi-center cohort, the data were collected prospectively, and that the model created can be easily used in clinical situations. However, there are several limitations. Pneumonia, a common complication, was not included in the scoring system because case definitions are difficult to differentiate from atelectasis, and therefore the model cannot predict this complication. Also, it is possible that complications may be underreported, especially if they occurred after discharge. On-site auditing of roughly 7% of participating centers has not revealed underreporting of major infectious complications, though this process was not implemented until 2007 [29]. In addition, when many of these data were being collected, standard definitions of complications had not yet been adopted, and as such, there may be some variability as to infections reported between or within sites. Pre-operative antibiotic protocols were not recorded in the database, so it impossible to know what influence this may have had on the results. Finally, this study does not address practices to prevent major infection in high risk individuals. There have been some studies evaluating various practices with some success [1, 30].
While the incidence of major infection may be decreasing over time, it remains a source of morbidity and mortality in postoperative pediatric cardiac patients. Further research needs to be done to develop better protocols to decrease infection rates in this setting. This model can be used as a tool for risk-stratification as various interventions are studied. While the model created here has good internal validity, it needs to be validated externally. Additionally, further studies are needed to identify intra-operative and post-operative risk factors for major infection, as some of these may be modifiable.
The model created and validated in this study can have important clinical impact as it provides a preoperative estimate of an individual patient’s risk for major infectious complications. Identification of these high risk patients is useful in pre-operative counseling by helping parents and providers to know what obstacles may lie ahead. In addition, these identified high risk patients may be targeted for future clinical trials and interventions to reduce this complication of cardiac surgery.
Acknowledgments
Disclosures Dr. Benjamin receives support from 1R01HD057956-02, 1R01FD003519-01, 1U10-HD45962-06, 1K24HD058735-01, and HHSN267200700051C and the Thrasher Research Foundation.
References
- 1.Guardia Cami MT, Jordan Garcia I, Urrea Ayala M. Nosocomial infections in pediatric patients following cardiac surgery. An Pediatr (Barc) 2008;69(1):34–8. doi: 10.1157/13124216. [DOI] [PubMed] [Google Scholar]
- 2.Urrea M, et al. Prospective incidence study of nosocomial infections in a pediatric intensive care unit. Pediatr Infect Dis J. 2003;22(6):490–4. doi: 10.1097/01.inf.0000069758.00079.d3. [DOI] [PubMed] [Google Scholar]
- 3.Valera M, et al. Nosocomial infections in pediatric cardiac surgery, Italy. Infect Control Hosp Epidemiol. 2001;22(12):771–5. doi: 10.1086/501861. [DOI] [PubMed] [Google Scholar]
- 4.Pollock EM, et al. Early nosocomial infections in pediatric cardiovascular surgery patients. Crit Care Med. 1990;18(4):378–84. doi: 10.1097/00003246-199004000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Mehta PA, et al. Risk factors for sternal wound and other infections in pediatric cardiac surgery patients. Pediatr Infect Dis J. 2000;19(10):1000–4. doi: 10.1097/00006454-200010000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Mrowczynski W, et al. Infection risk factors in pediatric cardiac surgery. Asian Cardiovasc Thorac Ann. 2002;10(4):329–33. doi: 10.1177/021849230201000411. [DOI] [PubMed] [Google Scholar]
- 7.Levy I, et al. Nosocomial infections after cardiac surgery in infants and children: incidence and risk factors. J Hosp Infect. 2003;53(2):111–6. doi: 10.1053/jhin.2002.1359. [DOI] [PubMed] [Google Scholar]
- 8.Allpress AL, et al. Risk factors for surgical site infections after pediatric cardiovascular surgery. Pediatr Infect Dis J. 2004;23(3):231–4. doi: 10.1097/01.inf.0000114904.21616.ba. [DOI] [PubMed] [Google Scholar]
- 9.Nateghian A, Taylor G, Robinson JL. Risk factors for surgical site infections following open-heart surgery in a Canadian pediatric population. Am J Infect Control. 2004;32(7):397–401. doi: 10.1016/j.ajic.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Shah SS, et al. Bloodstream infections after median sternotomy at a children’s hospital. J Thorac Cardiovasc Surg. 2007;133(2):435–40. doi: 10.1016/j.jtcvs.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 11.Vida VL, et al. Mediastinitis in pediatric cardiac surgery: treatment and cost-effectiveness in a low-income country. Pediatr Cardiol. 2007;28(3):163–6. doi: 10.1007/s00246-006-0008-1. [DOI] [PubMed] [Google Scholar]
- 12.Long CB, et al. Postoperative mediastinitis in children: epidemiology, microbiology and risk factors for Gram-negative pathogens. Pediatr Infect Dis J. 2005;24(4):315–9. doi: 10.1097/01.inf.0000157205.31624.ed. [DOI] [PubMed] [Google Scholar]
- 13.Tortoriello TA, et al. Mediastinitis after pediatric cardiac surgery: a 15-year experience at a single institution. Ann Thorac Surg. 2003;76(5):1655–60. doi: 10.1016/s0003-4975(03)01025-7. [DOI] [PubMed] [Google Scholar]
- 14.Di Filippo S, et al. Current patterns of infective endocarditis in congenital heart disease. Heart. 2006;92(10):1490–5. doi: 10.1136/hrt.2005.085332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fisher MC. Changing Risk Factors for Pediatric Infective Endocarditis. Curr Infect Dis Rep. 2001;3(4):333–336. doi: 10.1007/s11908-001-0070-z. [DOI] [PubMed] [Google Scholar]
- 16.Morris CD, Reller MD, Menashe VD. Thirty-year incidence of infective endocarditis after surgery for congenital heart defect. JAMA. 1998;279(8):599–603. doi: 10.1001/jama.279.8.599. [DOI] [PubMed] [Google Scholar]
- 17.Holzmann-Pazgal G, et al. Case-control study of pediatric cardiothoracic surgical site infections. Infect Control Hosp Epidemiol. 2008;29(1):76–9. doi: 10.1086/524323. [DOI] [PubMed] [Google Scholar]
- 18.Culver DH, et al. Surgical wound infection rates by wound class, operative procedure, and patient risk index. National Nosocomial Infections Surveillance System. Am J Med. 1991;91(3B):152S–157S. doi: 10.1016/0002-9343(91)90361-z. [DOI] [PubMed] [Google Scholar]
- 19.Gaynes RP, et al. Surgical site infection (SSI) rates in the United States, 1992-1998: the National Nosocomial Infections Surveillance System basic SSI risk index. Clin Infect Dis. 2001;33(Suppl 2):S69–77. doi: 10.1086/321860. [DOI] [PubMed] [Google Scholar]
- 20.Kagen J, et al. Risk adjustment for surgical site infection after median sternotomy in children. Infect Control Hosp Epidemiol. 2007;28(4):398–405. doi: 10.1086/513123. [DOI] [PubMed] [Google Scholar]
- 21. http://www.cdc.gov/growthcharts.
- 22.Bakshi KD, et al. Determinants of early outcome after neonatal cardiac surgery in a developing country. J Thorac Cardiovasc Surg. 2007;134(3):765–71. doi: 10.1016/j.jtcvs.2007.04.042. [DOI] [PubMed] [Google Scholar]
- 23.McAnally HB, et al. Hypothermia as a risk factor for pediatric cardiothoracic surgical site infection. Pediatr Infect Dis J. 2001;20(4):459–62. doi: 10.1097/00006454-200104000-00023. [DOI] [PubMed] [Google Scholar]
- 24.Huddleston CB. Mediastinal wound infections following pediatric cardiac surgery. Semin Thorac Cardiovasc Surg. 2004;16(1):108–12. doi: 10.1053/j.semtcvs.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 25.Kagen J, et al. Risk factors for mediastinitis following median sternotomy in children. Pediatr Infect Dis J. 2007;26(7):613–8. doi: 10.1097/INF.0b013e31806166bb. [DOI] [PubMed] [Google Scholar]
- 26.Lacour-Gayet F, et al. The Aristotle score: a complexity-adjusted method to evaluate surgical results. Eur J Cardiothorac Surg. 2004;25(6):911–24. doi: 10.1016/j.ejcts.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 27.Jenkins KJ, et al. Consensus-based method for risk adjustment for surgery for congenital heart disease. J Thorac Cardiovasc Surg. 2002;123(1):110–8. doi: 10.1067/mtc.2002.119064. [DOI] [PubMed] [Google Scholar]
- 28.Morris CD, Reller MD, Menashe VD. Thirty-year incidence of infective endocarditis after surgery for congenital heart defect. JAMA. 1998;279(8):599–603. doi: 10.1001/jama.279.8.599. [DOI] [PubMed] [Google Scholar]
- 29.Clarke DR, et al. Verification of data in congenital cardiac surgery. Cardiol Young. 2008;18(Suppl 2):177–87. doi: 10.1017/S1047951108002862. [DOI] [PubMed] [Google Scholar]
- 30.Kato Y, et al. Effects of controlled perioperative antimicrobial prophylaxis on infectious outcomes in pediatric cardiac surgery. Crit Care Med. 2007;35(7):1763–8. doi: 10.1097/01.CCM.0000269027.50834.FE. [DOI] [PubMed] [Google Scholar]