Abstract
Enhanced granulopoietic activity is crucial for host defense against bacterial pneumonia. Alcohol impairs this response. The underlying mechanisms remain obscure. Granulocyte colony-stimulating factor (G-CSF) produced by infected lung tissue plays a key role in stimulating bone marrow granulopoiesis. This study investigated the effects of alcohol on G-CSF signaling in the regulation of marrow myeloid progenitor cell proliferation in mice with Streptococcus pneumoniae pneumonia. Chronic alcohol consumption plus acute alcohol intoxication suppressed the increase in blood granulocyte counts following intrapulmonary challenge with S. pneumoniae. This suppression was associated with a significant decrease in bone marrow granulopoietic progenitor cell proliferation. Alcohol treatment significantly enhanced STAT3 phosphorylation in bone marrow cells of animals challenged with S. pneumoniae. In vitro experiments showed that G-CSF-induced activation of STAT3-p27Kip1 pathway in murine myeloid progenitor cell line 32D-G-CSFR cells was markedly enhanced by alcohol exposure. Alcohol dose-dependently inhibited G-CSF-stimulated 32D-G-CSFR cell proliferation. This impairment of myeloid progenitor cell proliferation was not attenuated by inhibition of alcohol metabolism through either the alcohol dehydrogenase pathway or the CYP450 system. These data suggest that alcohol enhances G-CSF-associated STAT3-p27Kip1 signaling, which impairs granulopoietic progenitor cell proliferation by inducing cell cycling arrest and facilitating their terminal differentiation during the granulopoietic response to pulmonary infection.
Keywords: rodent, bacterial, cell activation, hematopoiesis, signal transduction
Introduction
Representing the largest population of white blood cells in the circulation, polymorph nuclear leukocytes (PMNs or granulocytes) constitute the first line of phagocytic defense. These cells patrol in the circulatory system and quickly migrate to infected tissue sites to eliminate pathogenic microbes by phagocytosis and intracellular killing. Due to the relatively short life span of PMNs, the bone marrow continuously produces these phagocytes from hematopoietic precursors in order to maintain homeostatic levels of circulating PMNs (1). During bacterial infection, bone marrow production of PMNs is significantly enhanced in order to reinforce host defense against invading pathogens. Impairment of granulopoiesis and the granulopoietic response by inherited or acquired abnormalities results in ineffective control of infection with increased morbidity and mortality (2).
Alcohol abuse predisposes the host to severe bacterial infections, particularly pneumonia (3, 4). A prominent feature of alcohol-abusing patients with severe pulmonary infection is the frequent occurrence of granulocytopenia, which predicts poor outcome (3, 5–7). Analysis of bone marrow from these individuals shows a reduction in the number of mature granulocytes with vacuolization of myeloid progenitor cells (8–10). Incubation of bone marrow cells with alcohol at concentrations commonly observed in intoxicated patients has been reported to suppress granulocyte colony formation (8, 11). Currently, the underlying mechanisms for these effects remain unclear.
Granulocyte colony-stimulating factor (G-CSF) is a lineage-specific growth factor that stimulates granulopoiesis in the bone marrow. During bacterial infection, cells of infected tissues produce large quantities of inflammatory mediators, including G-CSF (12–15). Studies on clinical patients and experimental animals have repeatedly shown that the level of G-CSF in the systemic circulation is significantly increased during bacterial infection (16–19). This elevated G-CSF concentration stimulates marrow granulocyte lineage development and enhances PMN mobilization from the bone marrow into the circulation (12, 14, 15, 20). Previous investigations have shown that acute alcohol intoxication causes a temporary inhibition of the G-CSF response to bacterial infection (21). However, the inhibition of the G-CSF response is brief, which suggests that the prolonged impairment of the granulopoietic response during bacterial infection in alcohol abusers may involve other factors yet to be defined (21).
Engagement of G-CSF with its receptor triggers the p44/42-cyclin D pathway, which mediates myeloid progenitor cell proliferation under both normal and emergency conditions (22). G-CSF also activates signal transducer and activator of transcription 3 (STAT3)-cyclin-dependent kinase (CDK) inhibitor p27Kip1 pathway, which serves as a negative signal causing cell cycle arrest at the G1 checkpoint (23–25). These two pathways are complementary in their roles of promoting progenitor cell pool expansion and terminal granulocyte differentiation. In this study, we determined the impact of alcohol on cell signaling regulation of myeloid progenitor cell development. Our observations indicate that alcohol treatment causes a profound enhancement of STAT3-p27Kip1 negative signaling in myeloid precursor cells, which is associated with impairment of the granulopoietic response in mice with S. pneumoniae lung infection.
Materials and methods
Animals
Male BALB/c mice (7 wk old; Charles River Laboratories)with a body weight of 22.7 ± 0.1 g were housed in a specific pathogen-freefacility with a 12-h light/dark cycle. Mice were maintained on the Lieber-DeCarli low fat liquid alcohol diet (LED supplies 36% of calories as ethanol; Dyets#710261, Bethlehem, PA) for 5 days and standard laboratory diet plus 20% alcohol in drinking water for 2 days per week for a total of 5 weeks (26). Control animals were fed the is caloric Lieber-DeCarli low fat liquid control diet (LCD; Dyets #710028) and standard laboratory diet with drinking water utilizing the same schedule. This alcohol feeding protocol is well-tolerated by mice (26). The median alcohol concentration in the blood (from random morning samples) is 8.5 mM (39 mg/dl) (26). Acute alcohol intoxication (i.p. injection with 20% alcohol in saline at a dose of 5g alcohol/kg) was induced after mice had been on chronic alcohol diets for 5 weeks. The blood alcohol levels were 119.7± 1.3, 106.3 ± 1.5, 87.7 ± 3.6, and 48.4± 3.5 mM, respectively, at 45 min, 90 min, 3 h, and 6h post alcohol injection in naïve mice as reported by our group previously (27). Control mice were i.p. injected with an equal volume of saline.
Thirty minutes after i.p. ethanol injection, S. pneumoniae (Type 3, 6303, American Type Culture Collection, Manassas, VA) (1 × 105 CFU in 50 μL of pyrogen-free isotonic saline) or saline was administered intratracheally (i.t.) to mice under isoflurane anesthesia. The animals were sacrificed at scheduled time points after the i.t. challenge as indicated in each figure legend. In a subgroup of mice, bromodeoxyuridine (BrdU, 1 mg in 100 μl of PBS/mouse, BD Biosciences, San Jose, CA) was administered i.v. at the same time of the i.t. challenge. Upon sacrifice, a heparinized blood sample was obtained by cardiac puncture. White blood cells were quantified under a light microscope with a hemocytometer and differential white blood cell counts were performed on Wright-Giemsa smears. Plasma was separated and stored at −80°C. Peripheral blood mononuclear cells (PBMCs) were isolated using Lympholyte-Mammal density separation medium (Cedarlane Laboratories, Burlington, NC) with the procedure provided by the manufacturer. Bronchoalveolar lavage (BAL) was performed to obtain BAL cells as described previously (28). BAL cells were enumerated and differential cell counts were performed. Femurs and tibias were collected. Bone marrow cells were collected as described previously (27). The above described experiments were performed in adherence to the National Institutes of Health guidelines on the use of experimental animals. All experimental protocols were approved by the Louisiana State University Health Sciences Center Animal Care and Use Committee.
Culture of bacteria
For each experiment, frozen stock cultures of S. pneumoniae were added to 100 ml of Todd Hewitt broth and incubated for 14 h in a CO2 incubator. Bacteria were collected and washed twice with PBS. A suspension of bacteria in PBS at a concentration of 1× 108 CFUs/ml was prepared based on its OD at 600 nm. Actual numbers of viable bacteria were verified by standard plate counts of the bacterial suspensions on Columbia agar with sheep blood plates (BBL#221165, BD Biosciences, San Jose, CA) following 24 h culture at 37°C in a 5% CO2 incubator.
Bacterial load was determined in the lungs of control and alcohol-treated animals. Lung samples were collected, weighed, and homogenized with 9 volumes of PBS (mg/μL) using sterilized glass homogenizers equipped with a NSI-12 Fractional Horsepower Motor (Bodine Electric Co., Chicago, IL). Serial dilutions (1:10) of tissue homogenates were plated on Columbia agar with sheep blood plates and cultured for 24 h at 37°C in a 5% CO2 incubator. Bacterial colonies were enumerated after culture.
Colony forming unit (CFU) assay
CFU assay of bone marrow cells was performed by culturing the cells in Methocult GF M3534 medium (Stem Cell Technologies, Vancouver, BC, Canada). One milliliter of Methocult GF M3534 medium containing 20,000 total bone marrow cells were plated in a 35-mm Nunclon dish (Nunc, Rochester, NY). Each sample was cultured in triplicate for 7 days at 37°C in an atmosphere of 5% CO2. Colonies containing 50 or more cells were then enumerated.
Development of 32D-G-CSFR cell line
The 32Dcl3 cell line (ATCC# CRL-11346, Manassas, VA) is an IL-3-dependent murine myeloid progenitor cell line. Switching 32Dcl3 cell line to G-CSF dependence was achieved by electro oration of cells with a pCR®3.1 vector (Invitrogen, Carlsbad, CA) encoding wild type murine G-CSF receptor gene, followed by G418 selection. Expression of G-CSF receptor mRNA and protein was confirmed by real-time RT-PCR and western blot analysis. These selected 32D-G-CSFR cells were then cultured with 1 ng/ml of murine G-CSF for maintenance.
Flow cytometric analysis
Nucleated bone marrow cells suspended in RPMI1640 (Invitrogen Co, Carlsbad, CA) containing 2% FCS (2 × 106 cells in 100 μl of medium) were incubated with 10 μg/ml fluorochrome-conjugated anti-mouse Gr1 (Ly6G, RB6-8C5, BD Biosciences, San Jose, CA), or isotype control antibody in the dark for 15 min at 4°C. For measuring BrdU incorporation, the cells were further processed using a BD BrdU Flow Kit (BD Biosciences, San Jose, CA). At the end of the staining procedure, cells were suspended in 0.5 ml of PBS containing 1% Para formaldehyde. Analysis of cell phenotype and BrdU incorporation was performed on a FACSAria or a LSR-II flow cytometer with FACS Diva software (BD Biosciences, San Jose, CA). For each sample, 300,000 cells were acquired for analysis.
Additionally, 32D-G-CSFR cells were cultured in RPMI-1640 containing 10% FCS, 10 ng/ml G-CSF and various concentrations of alcohol. On day 6 of the culture, BrdU (1 mM) was added to cells. The cells were harvested 90 min later to determine incorporation of BrdU into cells by flow cytometry using a BD BrdU kit and manufacturers protocols. BrdU incorporation was enumerated on a FACS Caliber flow cytometer (BD Biosciences, San Jose, CA). Viability was assessed by trypan blue exclusion for all in vitro and ex vivo studies (Supplemental Table 1).
Analysis of cell proliferation with CFSE staining
32D-G-CSFR cells were incubated in HBSS containing 10% FCS and 5 μM carboxyfluorescein diacetate, succinimidyl ester (CFSE, Molecular Probes/Invitrogen, Eugene, OR) for 5 min. After washing twice with HBSS, the stained cells were suspended in RPMI-1640 containing 10% FCS and recombinant murine G-CSF (10 ng/ml). The cells were cultured with various concentrations of alcohol. In a subset of cell cultures, 4-methylpyrazole (4-MP, which blocks alcohol dehydrogenase (ADH); Sigma Aldrich, St. Louis, MO) or metyrapone (which blocks cytochrome P450 (CYP450) enzymes; Sigma, St. Louis, MO) was added to the culture media. The cells were cultured with G-CSF (10 ng/ml) in the absence and presence of 50 mM alcohol. Analysis of cell proliferation based on the reduction of cell fluorescence was performed on a FACES Caliber flow cytometer using Cell Quest software (BD Biosciences, San Jose, CA).
Sample preparation for Western blot analysis
Nucleated bone marrow cells isolated from naïve mice were plated in a 12-well plate with 5 × 106 cells per well. Cells were incubated in RPMI-1640 containing 10% FCS with different concentrations of alcohol (0, 50, and 100 mM) thirty minutes prior to the addition of G-CSF (10 ng/ml). The cells were harvested 10 min after addition of G-CSF. In a separate set of experiments, 32D-G-CSFR cells were cultured in RPMI-1640 containing 10% FCS with various concentrations of alcohol (0, 50, and 100 mM) for 4 h. G-CSF (10 ng/ml) was then added to the cultures. Following 30 min of stimulation, cells were harvested. Freshly isolated bone marrow cells, cultured bone marrow cells, and cultured 32D-G-CSFR cells were lysed with lysis buffer to prepare cell lysates for Western blot analysis (29).
Western blot analysis of phospho-JAKs and phospho-STAT3
Western blot procedures were performed as previously reported with minor modifications (29). After gel transfer onto a PVDF membrane, the membrane was blocked with 5% milk in TSB-T buffer and hybridized sequentially with primary antibody against phospho-STAT3 (Tyr705), phospho-JAK1 (Tyr1022/1023), phospho-JAK2 (Tyr1007/1008), and phospho-TYK2 (Tyr1054/1055, Cell Signaling Technology, Danvers, MA) and horseradish peroxidase-conjugated goat anti-rabbit IgG (Cell Signaling Technology, Danvers, MA). The membrane was stripped and then reprobed with rabbit anti-β-actin antibody (Cell Signaling Technology, Danvers, MA) and horseradish peroxidase-conjugated goat anti-rabbit IgG to determine β-actin content in each lane of the gel. Semi-quantification was performed using a Kodak Gel Logic 2,200 Imaging System. Data are presented as the normalized mean intensity ratio (MI ratio) of either the phospho-STAT3 or phospho-JAK2 protein band versus the corresponding β-actin band.
Preparation of p27Kip1 RNA standard and real-time RT-PCR determination of p27Kip1 mRNA
Total RNA was isolated from 107 32D-cl3 cells and p27Kip1 cRNA standards were produced as previously reported (30). Forward and reverse cloning primers for p27Kip1 were 5′-ATGTCAAACGTGAGAGTGTCTAACGG-3′ and 5′-TTACGTCTGGCGTCGAAGGC-3′, respectively. For each subsequent real-time quantitative RT-PCR assay, standard curves of p27Kip1 and 18S ribosomal RNA (Taqman Applied Bios stems) (ranging from 102 to 109 copies of p27Kip1 RNA per reaction and 10−5 to 101 ng of 18S ribosomal RNA per μl of reaction volume) were generated by serial dilution of stock standard RNA aliquots. Real-time RT-PCR determination of p27Kip1 mRNA expression by 32D-G-CSFR cells was performed as described previously (29). The amplification primers and probes used for determination of p27Kip1 expression were: forward primer, 5′-GACTCGTCAGACAATCAGGCT; reverse primer, 5′-CCCTTTTGTTTTGCGAAGAAGAATC; and probe, 5′-AGGTCGCTTCCTCATCCCTGGACACTG. This set of primers and probe was designed using Primer Express software (Applied Bios stems, Carlsbad, CA). The primers and probe for detection of 18S ribosomal RNA were purchased from Applied Bios stems (Carlsbad, CA). The p27Kip1 mRNA quantity in each sample was determined by comparing its cycle threshold number with those of the p27Kip1 RNA standard curve and then normalized to the content of 18S rRNA in each sample. The results are expressed as copies mRNA/ng rRNA.
Statistical analysis
Data are presented as mean ± SEM. The sample size is indicated in the legend of each figure. Statistical analyses of data were conducted using unpaired Student’s t test(for comparison between two groups) and one-way ANOVA, followed by Student-Newman-Keuls test or Proc Mixed (SAS Institute; 2004)two-way ANOVA (for comparisons among multiple groups). Differences were considered statistically significant at p < 0.05.
Results
Alterations of bone marrow granulocyte production
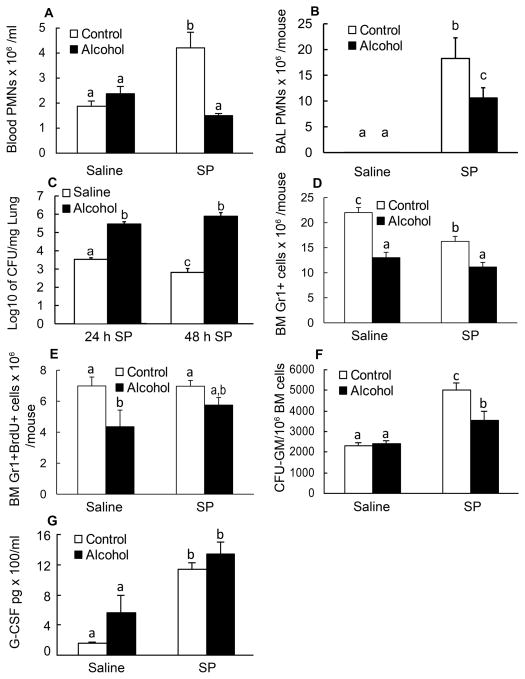
To examine the effects of alcohol treatment on the circulating levels of granulocytes, blood was harvested 24 h after i.t. challenge of saline or S. pneumoniae. Alcohol had no effect on blood PMNs from mice receiving saline; however, alcohol suppressed the increase in the number of circulating PMNs in response to pneumococcal infection (Fig. 1A). Alcohol treatment also impaired PMN recruitment into the alveolar space following pulmonary infection with S. pneumoniae (Fig. 1B). This impaired recruitment of PMNs was associated with a significantly higher bacterial burden in the lung at both 24 and 48 h post S. pneumoniae inoculation (Fig. 1C). Bacterial clearance was evident in the lungs from mice without alcohol treatment at 48 h. To assess the role of the bone marrow in this PMN response, we analyzed total nucleated bone marrow cells for Gr1 expression at 24 h post the challenge. Gr1 is a surface marker of the Ly6 family that is found on cells of the granulocyte lineage. Alcohol treatment diminished the bone marrow pool of Gr1+ cells in both saline- and pneumococcus-inoculated animals (Fig. 1D). To determine if this decreased bone marrow granulocyte pool was due to inhibited cell proliferation, we analyzed BrdU incorporation into the Gr1+ cells in the bone marrow (Fig. 1E). Alcohol treatment decreased the number of Gr1+BrdU+ cells in saline-challenged mice, suggesting an impaired proliferation capacity of the myeloid progenitors. We next performed CFU-GM assays on bone marrow cells from mice sacrificed 48 h post challenge with pneumococcus to examine granulopoietic potential. Pneumococcal infection enhanced the CFU-GM activity of the bone marrow progenitors, and alcohol inhibited this enhancement (Fig. 1F). Since G-CSF is a major cytokine stimulating granulocyte lineage development, we tested whether the effects of alcohol on progenitor proliferation was due to inhibited G-CSF secretion by the infected tissues. The circulating level of G-CSF 10 h post-inoculation of S. pneumoniae was significantly increased as compared to the control values (Fig. 1G). Alcohol treatment did not affect pneumococcal infection-induced increase in plasma G-CSF concentration at this time point. This observation would suggest that the granulopoietic progenitor cell response to G-CSF may play an important role in alcohol-elicited adverse effects on granulocyte production in the bone marrow following pulmonary infection with S. pneumoniae.
Figure 1. Granulopoietic response to pneumonia.
A: changes in blood PMN counts 24 h post i.t. challenge. B: BAL PMN counts 24 h post i.t. challenge. C: bacterial load in the lung 24 and 48 h post i.t. inoculation of S. pneumoniae. D: changes in the number of bone marrow Gr1+ cells 24 h post i.t. challenge. E: changes in the number of bone marrow Gr1+BrdU+ cells 24 h post i.t. challenge. F: changes in CFU-GM activity in bone marrow cells 48 h post i.t. challenge. G: plasma G-CSF concentration 10 h after i.t. challenge. Saline: intratracheal challenge with saline; SP: intratracheal challenge with S. pneumoniae. N = 4–8. Bars with different letters in each panel are statistically different (p<0.05).
Alcohol enhances STAT3 phosphorylation
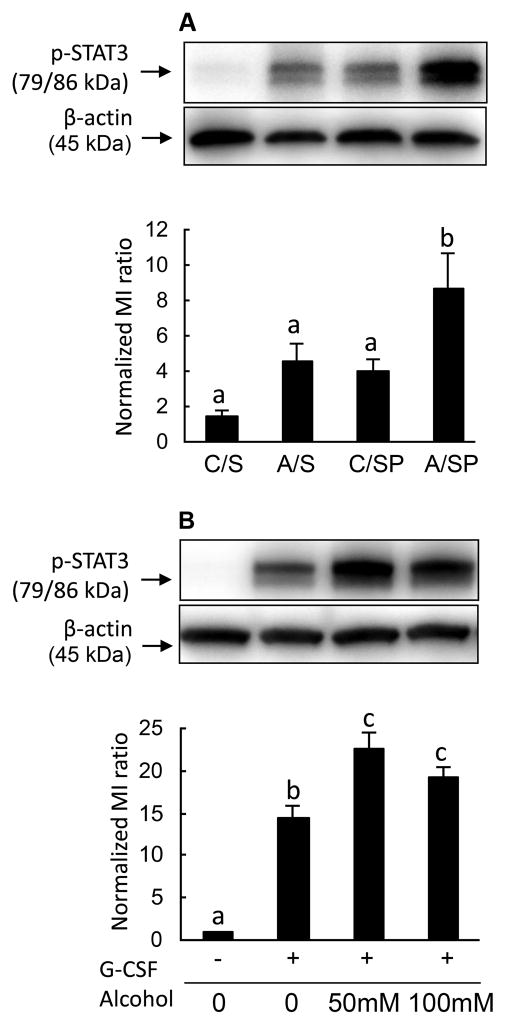
In an effort to elucidate the mechanism by which alcohol impairs neutrophil number and function during infection, we assessed the activation of STAT3 in nucleated bone marrow cells. Under control conditions, mice displayed a low level of Tyr705 phosphorylation on STAT3. Alcohol treatment enhanced STAT3 activation in bone marrow cells 10 h post pulmonary infection with S. pnumoniae (Fig. 2A). This pattern of alcohol-enhanced STAT3 activation was evident as early as 6 hours post the S. pnumoniae challenge (data not shown). STAT3 phosphorylation is a key effector molecule in the signal transduction cascades for many cytokine receptors including the G-CSF receptor (G-CSFR). To assess the role of G-CSFR in alcohol-enhanced p-STAT3 levels, nucleated bone marrow cells isolated from naïve mice were cultured in vitro with G-CSF and various concentrations of alcohol. As shown in Fig. 2B, G-CSF increased STAT3 phosphorylation in cultured bone marrow cells. Exposure to 50 and 100 mM alcohol enhanced G-CSF-induced STAT3 phosphorylation in these cells. These ex vivo results mirror those seen from pneumococcus-challenged animals, indicating G-CSF signaling through STAT3 is enhanced by alcohol treatment.
Figure 2. STAT3 phosphorylation in bone marrow cells.
A: In vivo effects of alcohol treatment on STAT3 activation (Tyr705 phosphorylation) in bone marrow cells 10 h post intratracheal challenge with S. pneumoniae. Western blot images are representative of 3–4 experiments. Each lane was loaded with 20 μg protein. C/S: control mice with i.t. saline; A/S: alcohol-treated mice with i.t. saline; C/SP: control mice with i.t. S. pneumoniae; A/SP: alcohol-treated mice with i.t. S. pneumoniae. B: In vitro effects of alcohol on STAT3 activation in bone marrow cells 10 min following G-CSF (10 ng/mL) stimulation. The western blot images are representatives of 3 experiments. Each lane was loaded with 20 μg protein. Bars with different letters in each panel are statistically different (p<0.05).
Alcohol inhibits myeloid progenitor cell proliferation
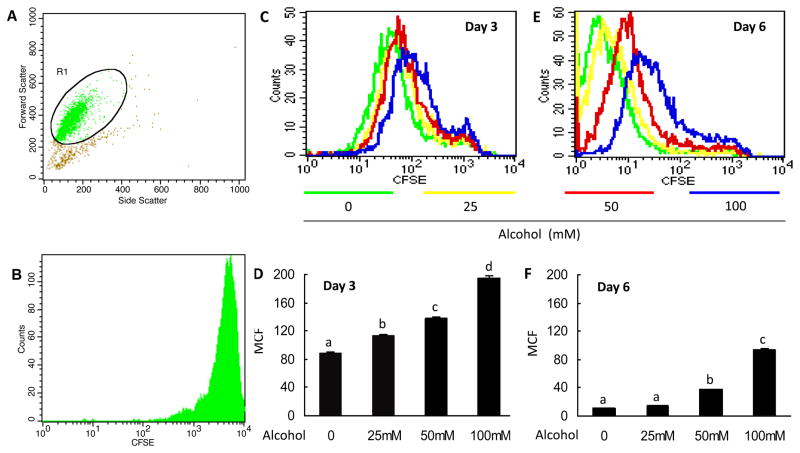
To further test if alcohol inhibits myeloid progenitor cell proliferation, we employed a cell culture model using 32D-G-CSFR cells prepared in our laboratory. The IL-3-dependent parent cell line, 32D-cl3, was derived from mouse bone marrow and maintains an immature, proliferate myeloid phenotype. Cells were labeled with CFSE and grown in the presence of G-CSF and various concentrations of alcohol for 3 and 6 days. Fluorescence of CFSE-labeled cells decreases proportionally with cell divisions. Gating strategy and initial CFSE loading intensity are shown in Fig. 3A and 3B. Alcohol dose-dependently inhibited 32D-G-CSFR proliferation at day 3 of culture (Fig. 3C and 3D). The anti-proliferative effects of the lowest alcohol dose (25 mM) were abrogated by day 6 (Fig. 3E and 3F). However cells treated with 50 and 100 mM alcohol still showed a dose-dependent impairment of cell proliferation by day 6, as reflected by the higher mean channel fluorescence (MCF; Fig. 3E and 3F).
Figure 3. Effects of alcohol on G-CSF-stimulated 32D-G-CSFR cell proliferation determined by CFSE reduction.
A: Gating strategy for CFSE experiments. Potential dead cells and cell debris were not gated for analysis. B: Representative histogram of initial CFSE loading intensity. C and E: Representative histograms for CFSE fluorescence on days 3 and 6, respectively. D and F: Quantified mean channel fluorescence (MCF) for days 3 and 6, respectively. N = 5. Bars with different letters in panels B and D are statistically different (p < 0.05).
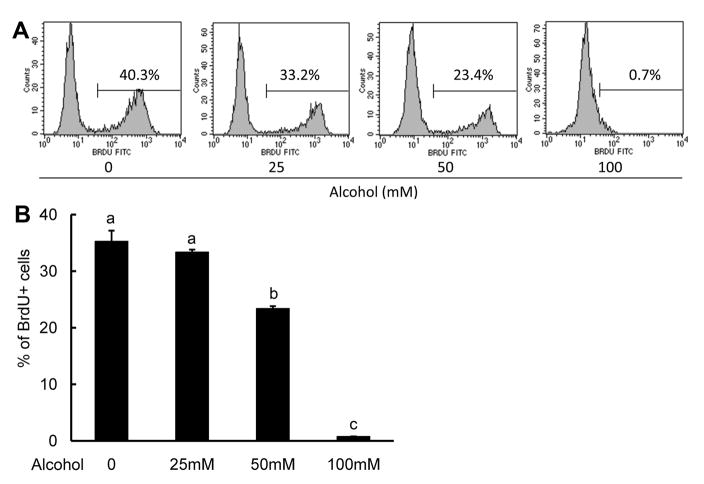
To supplement the CFSE studies, we also treated the 32D-G-CSFR cells with various concentrations of alcohol for 6 days and performed BrdU incorporation assays. BrdU is a thymidine analogue that incorporates into DNA during the S phase of the cell cycle. Consistent with our CFSE findings, alcohol dose-dependently prevented BrdU incorporation into the 32D-G-CSFR cells at 50 and 100 mM (Fig. 4).
Figure 4. Effects of alcohol on BrdU incorporation into 32D-G-CSFR cells.
Cells were cultured with the indicated alcohol concentrations for 6 days in G-CSF (1 ng/mL). BrdU incorporation was assessed 90 min after the addition of BrdU to the cultures. A: Representative histograms are shown. B: MCF was quantified as shown in the bar graph. N = 4. Bars with different letters are statistically different (p < 0.05).
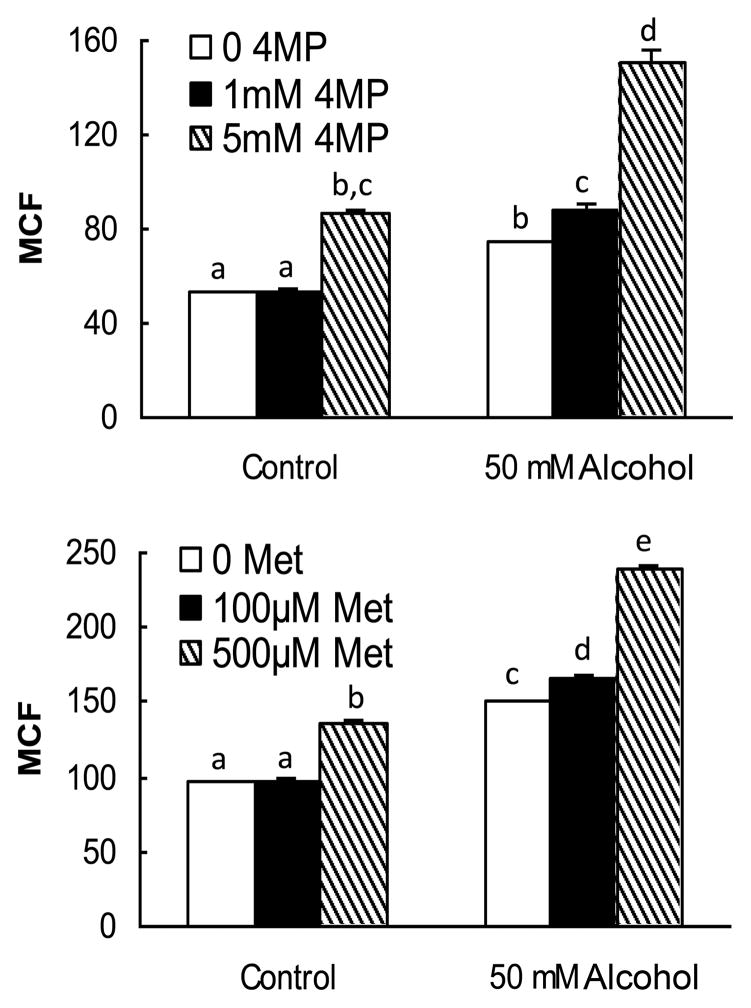
To determine if the effects that we observed were caused by either the direct action of alcohol or alcohol metabolism, we cultured CFSE-labeled 32D-G-CSFR cells in 50 mM alcohol with either 4-MP or metyrapone for 3 days. Inhibition of ADH with 4-MP or CYP450 with metyrapone further enhanced the alcohol-induced inhibition of 32D-G-CSFR cell proliferation in response to G-CSF (Fig. 5). These data indicate that alcohol is acting directly on the cells to inhibit their proliferative response to G-CSF.
Figure 5. Effects of 4-MP and metyrapone (MET) on alcohol-induced inhibition of 32D-GCSFR cell proliferation.
Cells were loaded with CFSE prior to the initiation of culture with indicated alcohol doses, G-CSF (10 ng/mL) and the respective inhibitors. MCF was measured following 3 days of cell culture. N = 5–6. Bars with different letters in each panel are statistically different (p < 0.05).
Alcohol selectively enhances the JAK2-STAT3-p27Kip1 pathway
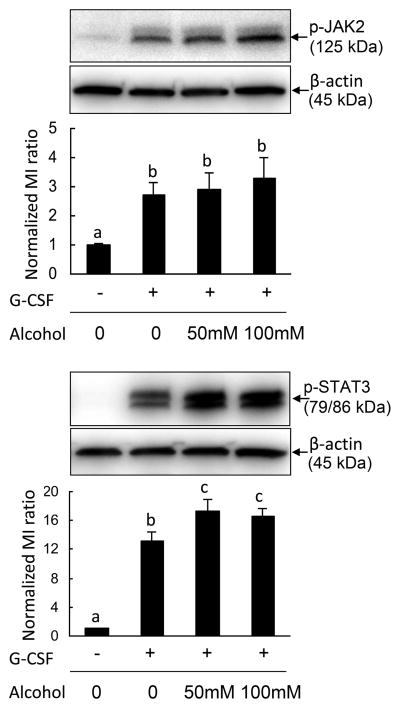
G-CSF signaling through the STAT3-cyclin dependent kinase inhibitor p27Kip1 pathway causes cell cycling arrest (23). Janus kinases (JAK1, JAK2, and TYK2) associated with the G-CSFR are activated after receptor ligation. To further test how alcohol affects this pathway, 32D-G-CSFR cells pre-cultured with various concentrations of alcohol were stimulated with G-CSF, and as expected, G-CSF treatment led to enhancement of JAK2 phosphorylation (Fig. 6A). However, alcohol treatment had no effect on phospho-JAK2 levels (Fig. 6A). We did not detect JAK1 and TYK2 in this culture system (data not shown). STAT3 is immediately downstream of JAK2 in the G-CSFR signal transduction cascade. Consistent with our in vivo and primary cell culture results, phospho-STAT3 levels were elevated by G-CSF treatment. Alcohol exposure enhanced G-CSF-induced STAT3 phosphorylation.
Figure 6. Effects of alcohol on phospho-JAK2 (Tyr1007/1008) and phospho-STAT3 (Tyr 705) levels in 32D-G-CSFR cells.
32D-G-CSFR cells were cultured in alcohol 4 h prior to the addition of G-CSF (10 ng/ml). Cells were harvested 30 min post G-CSF exposure. Each lane was loaded with 20 μg of protein. The image is a representative of 4–5 experiments. Bars with different letters in each panel are statistically significant (p < 0.05).
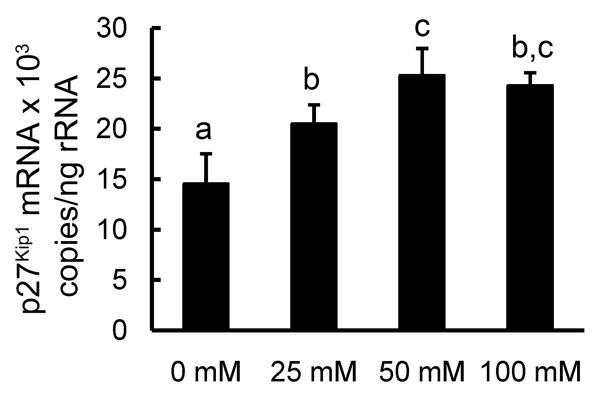
Activated STAT3 directly increases cyclin dependent kinase (CDK) inhibitor p27Kip1 mRNA transcription which is largely responsible for G1 cell cycle arrest and terminal differentiation in hematopoietic progenitor (23, 31, 32). To determine if the alcohol-induced increase in phospho-STAT3 was associated with changes in p27 Kip1 expression, we harvested 32D-G-CSFR cells after 24 h of culture in various concentrations of alcohol in the presence of 10 ng/ml of G-CSF. Indeed, exposure to 25 and 50 mM alcohol caused a dose-dependent increase in p27 Kip1 mRNA expression (Fig. 7). However, no further enhancement of p27 Kip1 expression was observed when alcohol concentration exceeded 50 mM treatment.
Figure 7. Effects of alcohol on p27Kip1 mRNA expression by 32D-G-CSFR cells.
Cells were cultured for 24 h in the indicated alcohol doses with G-CSF (10 ng/mL). N = 5. Bars with different letters are statistically different (p < 0.05).
Discussion
During bacterial infection, hematopoieticactivity in the bone marrow is shifted toward granulocyte production, which is critical for enhancing host defense against invading pathogens. G-CSF is the predominant cytokine stimulating granulopoiesis. G-CSF binding to its receptor can activate multiple signal transduction cascades. The activation of p44/42-cyclin D pathway through ligand engagement of G-CSF receptor mediates myeloid progenitor cell proliferation (33, 34). In contrast, activation of the STAT3-CDK inhibitor p27Kip1 pathway following G-CSF receptor stimulation can cause cell cycle arrest and terminal differentiation (23, 32). These opposite signaling events differentially function to expand the granulopoietic progenitor cell pool (p44/42 pathway) and to promote terminal differentiation of PMNs (STAT3 pathway)(32).
The function of STAT3 signaling in the regulation of cell proliferation has drawn wide attention in recent years. The impact of STAT3 activation is largely context dependent. Multiple tyrosine and serine residues can be phosphorylated in the native STAT3 protein, often eliciting different effects (35, 36). Phosphorylation of tyrosine 705 is required for STAT3 dimerization, nuclear translocation, and transcriptional activation (23). The CDK inhibitor p27Kip1 is a direct target of STAT3-mediated transcription (37). STAT3-induced p27Kip1 expression promotes cell cycle arrest and causes terminal differentiation in myeloid precursor cells (23, 32, 38). Thus alcohol may be causing premature terminal differentiation in the myeloid progenitor cells.
Alcohol has been reported to suppress mitogenesis in a variety of tissues and cell lines (39–44). However, knowledge regarding mechanisms underlying the effects of alcohol on myeloid progenitor cell proliferation remains limited. A few in vitro studies have shown that alcohol inhibits CFU-GM formation in cultured bone marrow cells (45). Recently, our group showed that alcohol intoxication impairs proliferation of bone marrow lineage-c-kit+Sca-1+ cells, which include the short- and long-term repopulating hematopoietic stem cells in mice (27, 46). These studies demonstrated that alcohol impairs hematopoietic precursor cell responses to inflammatory stimulants including cytokines and microbe-derived cell wall components. In light of these observations, we extended our investigations to determine if alcohol impairs granulocyte production via disrupting cell signaling at the myeloid progenitor cell stage during bacterial pneumonia. The results of our current study showed that alcohol treatment suppressed the increase in the number of circulating granulocytes following S. pneumoniae infection in the lung, which was associated with impairment of PMN recruitment into the alveolar space and clearance of bacteria from the lung. Alcohol-treated mice exhibited a reduced storage pool of PMNs in the bone marrow in the absence and presence of pulmonary infection with S. pneumoniae. The inability to increase the number of circulating granulocytes during infection in alcohol-treated animals can partially be explained by attenuated progenitor cell amplification. Chronic alcohol consumption plus acute alcohol intoxication significantly inhibited proliferation of Gr1+ bone marrow cells in uninfected mice. Further, alcohol treatment attenuated S. pneumoniae-stimulated increases in CFU-GM activity in the bone marrow. Since the CFU-GM assay primarily tests progenitor cell amplification potential, these results suggest that in vivo alcohol exposure limits the number of myeloid progenitor cell divisions before terminal differentiation. Because alcohol did not significantly affect plasma G-CSF concentration in our model, we predicted that the inhibited granulopoietic response was most likely occurring at the level of the bone marrow myeloid progenitor cell.
Western blot analysis in our current study showed that chronic alcohol consumption plus acute alcohol intoxication markedly enhanced STAT3 activation in nucleated bone marrow cells following pulmonary infection with S. pneumoniae. Furthermore, in vitro exposure to alcohol markedly enhanced G-CSF-induced STAT3 phosphorylation in cultured bone marrow cells. Previous investigations have shown that alcohol exposure may exert contrasting effects on STAT3 signaling in different cell types (47–49). Our results showed that alcohol dose-dependently inhibited G-CSF-induced proliferation of these myeloid progenitor cells. These data strongly support our in vivo observations in alcohol-treated animals and further confirm that alcohol impairs granulopoietic precursor cell proliferation in response to G-CSF stimulation.
Previous studies have shown that leukocytes and bone marrow cells metabolize alcohol (50). Alcohol metabolism through both the ADH pathway and the CYP450 system generates reactive oxygen species (51). Reactive oxygen metabolites have been reported to injure various cell functional activities and proliferation (52). Therefore, disruption of G-CSF-stimulated proliferation of myeloid progenitors may also be caused indirectly by alcohol metabolites. To delineate if the observed negative effect of alcohol on myeloid progenitor cell proliferation is directly caused by alcohol or indirectly resulted from alcohol metabolites, myeloid progenitor cells were cultured with 4-MP (ADH inhibitor) or metyrapone (CYP450 inhibitor) to block alcohol metabolism. Our results showed that blockade of alcohol metabolism enhanced the alcohol-induced inhibition of myeloid progenitor proliferation, which may be a result of increased intracellular alcohol concentration. These data indicate that the impairment of myeloid progenitor cell proliferation is directly caused by alcohol.
To identify the level at which alcohol modified G-CSF signal transduction, we examined JAK1, JAK2, and TYK2 activation. Janus kinases are effector molecules immediately proximal to the G-CSF receptor (2, 53–59). JAK1 and TYK2 were not detected in our model system. G-CSF did stimulate JAK2 phosphorylation, but this signal was not altered by alcohol administration. This finding suggested that the target of alcohol was downstream of JAK2. Since activated JAK2 promotes STAT3 phosphorylation, we measured phospho-STAT3 levels after G-CSF addition to the culture system of myeloid progenitor cells in the absence and presence of alcohol. Consistent with our in vivo findings and observations from primary bone marrow cell cultures, alcohol enhanced the level of STAT3 phosphorylation in G-CSF-stimulated myeloid progenitor cells. These data demonstrate that alcohol enhances STAT3 signaling at the level of STAT3 activation. Molecular mechanisms underlying alcohol-enhanced STAT3 phosphorylation remain to be further explored. Since activated STAT3 induces expression of suppressor of cytokine signaling (SOCS) proteins including SOCS1 and SOCS3 and these SOCS proteins in turn inhibit activation of STAT3, one potential mechanism might be that alcohol impairs SOCS expression or function resulting in loss of this negative feedback (60–62). Previous investigations in a rat model have shown that chronic alcohol consumption completely suppresses growth hormone-induced SOCS3 expression in the liver (63).
An important component in the STAT3 signal pathway is that activated STAT3 promotes expression of the CDK inhibitor p27Kip1 which causes G1 cell cycle arrest and promotes terminal neutrophil differentiation (23–25, 32). To determine whether alcohol-induced enhancement of STAT3 activation has a downstream consequence, we analyzed p27Kip1 mRNA expression by myeloid progenitors. The results showed that in the presence of G-CSF stimulation, p27Kip1 transcription in myeloid progenitor cells was dose-dependently increased by alcohol exposure. It has been known that during the process of maturation, hematopoietic progenitors traverse a stage termed transit amplification in which progenitors undergo maximal mitotic expansion (64). Our data support that alcohol enhances STAT3- p27Kip1 negative signaling leading to cell cycle arrest and enhanced terminal differentiation (32). Therefore, the ultimate outcome of chronic alcohol consumption plus acute alcohol administration is restricted transit amplification potential of myeloid progenitor cells during the granulopoietic response to pulmonary bacterial infection.
In summary, the results of our current investigation demonstrate that alcohol enhances STAT3 negative signaling in myeloid progenitor cells in response to pneumococcal infection and G-CSF stimulation. This alcohol-induced STAT3 activation is associated with inhibition of myeloid progenitor cell proliferation. Impaired expansion of the myeloid progenitor cell population restricts the marrow storage pool of granulocytes and consequently limits the increase in the number of circulating granulocytes and recruitment of these phagocytes into the infected alveolar space during the host response to pulmonary infection. These findings highlight an important mechanism underlying alcohol-induced myelosuppression in alcohol abusing hosts.
Supplementary Material
Acknowledgments
We thank Amy B. Weinberg, Joseph S. Soblosky, Rhonda R. Martinez, Jane A. Schexnayder, and Meredith M. Booth for their expert technical assistance. We also thank Connie P. Porretta for expert assistance with flowcytometric analyses and cell sorting.
Footnotes
This work was supported by Public Health Service Grants AA017494, AA019676, AA09803, AA07577, and HL76100.
References
- 1.Pillay J, den Braber I, Vrisekoop N, Kwast LM, de Boer RJ, Borghans JA, Tesselaar K, Koenderman L. In vivo labeling with 2H2O reveals a human neutrophil lifespan of 5.4 days. Blood. 2010;116:625–627. doi: 10.1182/blood-2010-01-259028. [DOI] [PubMed] [Google Scholar]
- 2.Avalos BR. The granulocyte colony-stimulating factor receptor and its role in disorders of granulopoiesis. Leuk Lymphoma. 1998;28:265–273. doi: 10.3109/10428199809092682. [DOI] [PubMed] [Google Scholar]
- 3.MacGregor RR, Louria DB. Alcohol and infection. Curr Clin Top Infect Dis. 1997;17:291–315. [PubMed] [Google Scholar]
- 4.Zhang P, Bagby GJ, Happel KI, Summer WR, Nelson S. Pulmonary host defenses and alcohol. Front Biosci. 2002;7:d1314–d1330. doi: 10.2741/A842. [DOI] [PubMed] [Google Scholar]
- 5.Ruiz M, Ewig S, Torres A, Arancibia F, Marco F, Mensa J, Sanchez M, Martinez JA. Severe community-acquired pneumonia. Risk factors and follow-up epidemiology. Am J Respir Crit Care Med. 1999;160:923–929. doi: 10.1164/ajrccm.160.3.9901107. [DOI] [PubMed] [Google Scholar]
- 6.Fernandez-Sola J, Junque A, Estruch R, Monforte R, Torres A, Urbano-Marquez A. High alcohol intake as a risk and prognostic factor for community-acquired pneumonia. Arch Intern Med. 1995;155:1649–1654. doi: 10.1001/archinte.1995.00430150137014. [DOI] [PubMed] [Google Scholar]
- 7.Saitz R, Ghali WA, Moskowitz MA. The impact of alcohol-related diagnoses on pneumonia outcomes. Arch Intern Med. 1997;157:1446–1452. [PubMed] [Google Scholar]
- 8.Imperia PS, Chikkappa G, Phillips PG. Mechanism of inhibition of granulopoiesis by ethanol. Proc Soc Exp Biol Med. 1984;175:219–225. doi: 10.3181/00379727-175-41792. [DOI] [PubMed] [Google Scholar]
- 9.Nakao S, Harada M, Kondo K, Mizushima N, Matsuda T. Reversible bone marrow hypoplasia induced by alcohol. Am J Hematol. 1991;37:120–123. doi: 10.1002/ajh.2830370210. [DOI] [PubMed] [Google Scholar]
- 10.Yeung KY, Klug PP, Lessin LS. Alcohol-induced vacuolization in bone marrow cells: ultrastructure and mechanism of formation. Blood Cells. 1988;13:487–502. [PubMed] [Google Scholar]
- 11.Tisman G, Herbert V. In vitro myelosuppression and immunosuppression by ethanol. J Clin Invest. 1973;52:1410–1414. doi: 10.1172/JCI107314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Semerad CL, Liu F, Gregory AD, Stumpf K, Link DC. G-CSF is an essential regulator of neutrophil trafficking from the bone marrow to the blood. Immunity. 2002;17:413–423. doi: 10.1016/s1074-7613(02)00424-7. [DOI] [PubMed] [Google Scholar]
- 13.Nelson S, Mason CM, Kolls J, Summer WR. Path physiology of pneumonia. Clin Chest Med. 1995;16:1–12. [PubMed] [Google Scholar]
- 14.Dale DC, Liles WC, Summer WR, Nelson S. Review: granulocyte colony-stimulating factor--role and relationships in infectious diseases. J Infect Dis. 1995;172:1061–1075. doi: 10.1093/infdis/172.4.1061. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Mukaida N, Zhang Y, Ito T, Nakao S, Matsushima K. Enhanced mobilization of hematopoietic progenitor cells by mouse MIP-2 and granulocyte colony-stimulating factor in mice. J Leukoc Biol. 1997;62:503–509. doi: 10.1002/jlb.62.4.503. [DOI] [PubMed] [Google Scholar]
- 16.Shahbazian LM, Quinton LJ, Bagby GJ, Nelson S, Wang G, Zhang P. Escherichia coli pneumonia enhances granulopoiesis and the mobilization of myeloid progenitor cells into the systemic circulation. Crit Care Med. 2004;32:1740–1746. doi: 10.1097/01.ccm.0000132900.84627.90. [DOI] [PubMed] [Google Scholar]
- 17.Kragsbjerg P, Jones I, Vikerfors T, Holmberg H. Diagnostic value of blood cytokine concentrations in acute pneumonia. Thorax. 1995;50:1253–1257. doi: 10.1136/thx.50.12.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pauksen K, Elfman L, Ulfgren AK, Venge P. Serum levels of granulocyte- colony stimulating factor (G-CSF) in bacterial and viral infections, and in atypical pneumonia. Br J Haematol. 1994;88:256–260. doi: 10.1111/j.1365-2141.1994.tb05015.x. [DOI] [PubMed] [Google Scholar]
- 19.Quinton LJ, Nelson S, Boe DM, Zhang P, Zhong Q, Kolls JK, Bagby GJ. The granulocyte colony-stimulating factor response after intrapulmonary and systemic bacterial challenges. J Infect Dis. 2002;185:1476–1482. doi: 10.1086/340504. [DOI] [PubMed] [Google Scholar]
- 20.Friedman AD. Transcriptional regulation of granulocyte and monocyte development. Oncogene. 2002;21:3377–3390. doi: 10.1038/sj.onc.1205324. [DOI] [PubMed] [Google Scholar]
- 21.Bagby GJ, Zhang P, Stoltz DA, Nelson S. Suppression of the granulocyte colony-stimulating factor response to Escherichia coli challenge by alcohol intoxication. Alcohol Clin Exp Res. 1998;22:1740–1745. [PubMed] [Google Scholar]
- 22.Marino VJ, Roguin LP. The granulocyte colony stimulating factor (G-CSF) activates Jak/STAT and MAPK pathways in a trophoblastic cell line. J Cell Biochem. 2008;103:1512–1523. doi: 10.1002/jcb.21542. [DOI] [PubMed] [Google Scholar]
- 23.de Koning JP, Soede-Bobok AA, Ward AC, Schelen AM, Antonissen C, van Leeuwen D, Lowenberg B, Touw IP. STAT3-mediated differentiation and survival and of myeloid cells in response to granulocyte colony-stimulating factor: role for the cyclin-dependent kinase inhibitor p27(Kip1) Oncogene. 2000;19:3290–3298. doi: 10.1038/sj.onc.1203627. [DOI] [PubMed] [Google Scholar]
- 24.Mangan JK, Reddy EP. Activation of the Jak3 pathway and myeloid differentiation. Leuk Lymphoma. 2005;46:21–27. doi: 10.1080/10428190400005320. [DOI] [PubMed] [Google Scholar]
- 25.Rane SG, Mangan JK, Amanullah A, Wong BC, Vora RK, Liebermann DA, Hoffman B, Grana X, Reddy EP. Activation of the Jak3 pathway is associated with granulocytic differentiation of myeloid precursor. cells. Blood. 2002;100:2753–2762. doi: 10.1182/blood.V100.8.2753. [DOI] [PubMed] [Google Scholar]
- 26.Mason CM, Dobard E, Zhang P, Nelson S. Alcohol exacerbates murine pulmonary tuberculosis. Infect Immun. 2004;72:2556–2563. doi: 10.1128/IAI.72.5.2556-2563.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang P, Welsh DA, Siggins RW, 2nd, Bagby GJ, Raasch CE, Happel KI, Nelson S. Acute alcohol intoxication inhibits the lineage- c-kit+ Sca-1+ cell response to Escherichia coli bacteremia. J Immunol. 2009;182:1568–1576. doi: 10.4049/jimmunol.182.3.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walker JE, Jr, Odden AR, Jeyaseelan S, Zhang P, Bagby GJ, Nelson S, Happel KI. Ethanol exposure impairs LPS-induced pulmonary LIX expression: alveolar epithelial cell dysfunction as a consequence of acute intoxication. Alcohol Clin Exp Res. 2009;33:357–365. doi: 10.1111/j.1530-0277.2008.00844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang P, Zhong Q, Bagby GJ, Nelson S. Alcohol intoxication inhibits pulmonary S100A8 and S100A9 expression in rats challenged with intratracheal lipopolysaccharide. Alcohol Clin Exp Res. 2007;31:113–121. doi: 10.1111/j.1530-0277.2006.00269.x. [DOI] [PubMed] [Google Scholar]
- 30.Zhang P, Bagby GJ, Boe DM, Zhong Q, Schwarzenberger P, Kolls JK, Summer WR, Nelson S. Acute alcohol intoxication suppresses the CXC chemokine response during endotoxemia. Alcohol Clin Exp Res. 2002;26:65–73. [PubMed] [Google Scholar]
- 31.Cheng T, Scadden DT. Cell cycle entry of hematopoietic stem and progenitor cells controlled by distinct cyclin-dependent kinase inhibitors. Int J Hematol. 2002;75:460–465. doi: 10.1007/BF02982107. [DOI] [PubMed] [Google Scholar]
- 32.McArthur GA, Foley KP, Fero ML, Walkley CR, Deans AJ, Roberts JM, Eisenman RN. MAD1 and p27(KIP1) cooperate to promote terminal differentiation of granulocytes and to inhibit Myc expression and cyclin E-CDK2 activity. Mol Cell Biol. 2002;22:3014–3023. doi: 10.1128/MCB.22.9.3014-3023.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Torii S, Yamamoto T, Tsuchiya Y, Nishida E. ERK MAP kinase in G cell cycle progression and cancer. Cancer Sci. 2006;97:697–702. doi: 10.1111/j.1349-7006.2006.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miranda MB, Johnson DE. Signal transduction pathways that contribute to myeloid differentiation. Leukemia. 2007;21:1363–1377. doi: 10.1038/sj.leu.2404690. [DOI] [PubMed] [Google Scholar]
- 35.Shi X, Zhang H, Paddon H, Lee G, Cao X, Pelech S. Phosphorylation of STAT3 serine-727 by cyclin-dependent kinase 1 is critical for nocodazole-induced mitotic arrest. Biochemistry. 2006;45:5857–5867. doi: 10.1021/bi052490j. [DOI] [PubMed] [Google Scholar]
- 36.Redell MS, Tsimelzon A, Hilsenbeck SG, Tweardy DJ. Conditional overexpression of Stat3alpha in differentiating myeloid cells results in neutrophil expansion and induces a distinct, antiapoptotic and pro-oncogenic gene expression pattern. J Leukoc Biol. 2007;82:975–985. doi: 10.1189/jlb.1206766. [DOI] [PubMed] [Google Scholar]
- 37.Kortylewski M, Heinrich PC, Mackiewicz A, Schniertshauer U, Klingmuller U, Nakajima K, Hirano T, Horn F, Behrmann I. Interleukin-6 and oncostatin M-induced growth inhibition of human A375 melanoma cells is STAT-dependent and involves upregulation of the cyclin-dependent kinase inhibitor p27/Kip1. Oncogene. 1999;18:3742–3753. doi: 10.1038/sj.onc.1202708. [DOI] [PubMed] [Google Scholar]
- 38.Wang L, Arcasoy MO, Watowich SS, Forget BG. Cytokine signals through STAT3 promote expression of granulocyte secondary granule proteins in 32D cells. Exp Hematol. 2005;33:308–317. doi: 10.1016/j.exphem.2004.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Friday KE, Howard GA. Ethanol inhibits human bone cell proliferation and function in vitro. Metabolism. 1991;40:562–565. doi: 10.1016/0026-0495(91)90044-w. [DOI] [PubMed] [Google Scholar]
- 40.Ghiselli G, Chen J, Kaou M, Hallak H, Rubin R. Ethanol inhibits fibroblast growth factor-induced proliferation of aortic smooth muscle cells. Arterioscler Thromb Vasc Biol. 2003;23:1808–1813. doi: 10.1161/01.ATV.0000090140.20291.CE. [DOI] [PubMed] [Google Scholar]
- 41.DeVito WJ, Stone S, Mori K. Low concentrations of ethanol inhibits prolactin-induced mitogenesis and cytokine expression in cultured astrocytes. Endocrinology. 1997;138:922–928. doi: 10.1210/endo.138.3.4964. [DOI] [PubMed] [Google Scholar]
- 42.Gallucci RM, Meadows GG. Ethanol consumption suppresses the IL2-induced proliferation of NK cells. Toxicol Appl Pharmacol. 1996;138:90–97. doi: 10.1006/taap.1996.0102. [DOI] [PubMed] [Google Scholar]
- 43.Prakash O, V, Rodriguez E, Tang ZY, Zhou P, Coleman R, Dhillon G, Shellito JE, Nelson S. Inhibition of hematopoietic progenitor cell proliferation by ethanol in human immunodeficiency virus type 1 tat-expressing transgenic mice. Alcohol Clin Exp Res. 2001;25:450–456. [PubMed] [Google Scholar]
- 44.Ahluwalia BS, Westney LS, Rajguru SU. Alcohol inhibits cell mitosis in G2-M phase in cell cycle in a human lymphocytes in vitro study. Alcohol. 1995;12:589–592. doi: 10.1016/0741-8329(95)02008-x. [DOI] [PubMed] [Google Scholar]
- 45.Meagher RC, Sieber F, Spivak JL. Suppression of hematopoietic-progenitor- cell proliferation by ethanol and acetaldehyde. N Engl J Med. 1982;307:845–849. doi: 10.1056/NEJM198209303071402. [DOI] [PubMed] [Google Scholar]
- 46.Raasch CE, Zhang P, Siggins RW, 2nd, Lamotte LR, Nelson S, Bagby GJ. Acute alcohol intoxication impairs the hematopoietic precursor cell response to pneumococcal pneumonia. Alcohol Clin Exp Res. 2010 doi: 10.1111/j.1530–0277.2010.01291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Norkina O, Dolganiuc A, Shapiro T, Kodys K, Mandrekar P, Szabo G. Acute alcohol activates STAT3, AP-1, and Sp-1 transcription factors via the family of Src kinases to promote IL-10 production in human monocytes. J Leukoc Biol. 2007;82:752–762. doi: 10.1189/jlb.0207099. [DOI] [PubMed] [Google Scholar]
- 48.Norkina O, Dolganiuc A, Catalano D, Kodys K, Mandrekar P, Syed A, Efros M, Szabo G. Acute alcohol intake induces SOCS1 and SOCS3 and inhibits cytokine-induced STAT1 and STAT3 signaling in human monocytes. Alcohol Clin Exp Res. 2008;32:1565–1573. doi: 10.1111/j.1530-0277.2008.00726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jaruga B, Hong F, Kim WH, Sun R, Fan S, Gao B. Chronic alcohol consumption accelerates liver injury in T cell-mediated hepatitis: alcohol disregulation of NF-kappaB and STAT3 signaling pathways. Am J Physiol Gastrointest Liver Physiol. 2004;287:G471–G479. doi: 10.1152/ajpgi.00018.2004. [DOI] [PubMed] [Google Scholar]
- 50.Bond AN, Wickramasinghe SN. Investigations into the production of acetate from ethanol by human blood and bone marrow cells in vitro. Acta Haematol. 1983;69:303–313. doi: 10.1159/000206911. [DOI] [PubMed] [Google Scholar]
- 51.Koop DR. Alcohol metabolism’s damaging effects on the cell: a focus on reactive oxygen generation by the enzyme cytochrome P450 2E1. Alcohol Res Health. 2006;29:274–280. [PMC free article] [PubMed] [Google Scholar]
- 52.Noda T, Iwakiri R, Fujimoto K, Aw TY. Induction of mild intracellular redox imbalance inhibits proliferation of CaCo-2 cells. FASEB J. 2001;15:2131–2139. doi: 10.1096/fj.01-0131com. [DOI] [PubMed] [Google Scholar]
- 53.Nicholson SE, Oates AC, Harpur AG, Ziemiecki A, Wilks AF, Layton JE. Tyrosine kinase JAK1 is associated with the granulocyte-colony-stimulating factor receptor and both become tyrosine-phosphorylated after receptor activation. Proc Natl Acad Sci U S A. 1994;91:2985–2988. doi: 10.1073/pnas.91.8.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tian SS, Tapley P, Sincich C, Stein RB, Rosen J, Lamb P. Multiple signaling pathways induced by granulocyte colony-stimulating factor involving activation of JAKs, STAT5, and/or STAT3 are required for regulation of three distinct classes of immediate early genes. Blood. 1996;88:4435–4444. [PubMed] [Google Scholar]
- 55.de Koning JP, Dong F, Smith L, Schelen AM, Barge RM, van der Plas DC, Hoefsloot LH, Lowenberg B, Touw IP. The membrane-distal cytoplasmic region of human granulocyte colony-stimulating factor receptor is required for STAT3 but not STAT1 homodimer formation. Blood. 1996;87:1335–1342. [PubMed] [Google Scholar]
- 56.Shimoda K, Iwasaki H, Okamura S, Ohno Y, Kubota A, Arima F, Otsuka T, Niho Y. G-CSF induces tyrosine phosphorylation of the JAK2 protein in the human myeloid G-CSF responsive and proliferative cells, but not in mature neutrophils. Biochem Biophys Res Commun. 1994;203:922–928. doi: 10.1006/bbrc.1994.2270. [DOI] [PubMed] [Google Scholar]
- 57.Shimoda K, Feng J, Murakami H, Nagata S, Watling D, Rogers NC, Stark GR, Kerr IM, Ihle JN. Jak1 plays an essential role for receptor phosphorylation and Stat activation in response to granulocyte colony-stimulating factor. Blood. 1997;90:597–604. [PubMed] [Google Scholar]
- 58.Ward AC, Hermans MH, Smith L, van Aesch YM, Schelen AM, Antonissen C, Touw IP. Tyrosine-dependent and -independent mechanisms of STAT3 activation by the human granulocyte colony-stimulating factor (G-CSF) receptor are differentially utilized depending on G-CSF concentration. Blood. 1999;93:113–124. [PubMed] [Google Scholar]
- 59.Imada K, Leonard WJ. The Jak-STAT pathway. Mol Immunol. 2000;37:1–11. doi: 10.1016/s0161-5890(00)00018-3. [DOI] [PubMed] [Google Scholar]
- 60.van de Geijn GJ, Gits J, Touw IP. Distinct activities of suppressor of cytokine signaling (SOCS) proteins and involvement of the SOCS box in controlling G-CSF signaling. J Leukoc Biol. 2004;76:237–244. doi: 10.1189/jlb.0104041. [DOI] [PubMed] [Google Scholar]
- 61.Croker BA, Metcalf D, Robb L, Wei W, Mifsud S, DiRago L, Cluse LA, Sutherland KD, Hartley L, Williams E, Zhang JG, Hilton DJ, Nicola NA, Alexander WS, Roberts AW. SOCS3 is a critical physiological negative regulator of G-CSF signaling and emergency granulopoiesis. Immunity. 2004;20:153–165. doi: 10.1016/s1074-7613(04)00022-6. [DOI] [PubMed] [Google Scholar]
- 62.Kimura A, Kinjyo I, Matsumura Y, Mori H, Mashima R, Harada M, Chien KR, Yasukawa H, Yoshimura A. SOCS3 is a physiological negative regulator for granulopoiesis and granulocyte colony-stimulating factor receptor signaling. J Biol Chem. 2004;279:6905–6910. doi: 10.1074/jbc.C300496200. [DOI] [PubMed] [Google Scholar]
- 63.Lang CH, Liu X, Nystrom G, Wu D, Cooney RN, Frost RA. Acute effects of growth hormone in alcohol-fed rats. Alcohol Alcohol. 2000;35:148–158. doi: 10.1093/alcalc/35.2.148. [DOI] [PubMed] [Google Scholar]
- 64.Attar EC, Scadden DT. Regulation of hematopoietic stem cell growth. Leukemia. 2004;18:1760–1768. doi: 10.1038/sj.leu.2403515. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.