Abstract
Aneuploidy is a hallmark of human cancers that originates from abnormal mitoses. Many aneuploid cancer cells also have greater-than-diploid DNA content, suggesting that polyploidy is a common precursor to aneuploidy during tumor progression. Polyploid cells can originate from cell fusion, endoreplication, and cytokinesis failure. Recently we found that cell cannibalism by entosis, a form of cell engulfment involving live cells, also leads to polyploidy, as internalized cells disrupt cytokinesis of their engulfing cell hosts. By this mechanism, cannibalistic cell behavior could promote tumor progression by leading to aneuploidy. Here we discuss cell cannibalism in cancer and other mechanisms that result in the formation of polyploid cancer cells.
Cell cannibalism in human tumors – evidence for entosis
Cell cannibalism has been observed as a phenomenon in mammalian systems for over 100 years(1). Unlike phagocytosis, which involves the engulfment of dead or dying cells, or pathogenic organisms, cannibalism involves the ingestion of live cells, resulting in the unusual appearance of whole cells contained within large vacuoles, or so-called “cell-in-cell” structures. Such cells usually die after they are engulfed, but they can also divide inside of their vacuoles, and some even manage to escape altogether, re-emerging as indistinguishable from their un-engulfed counterparts(1). Whereas the various physiological roles of phagocytosis and the consequences of its dysfunction are clear, by contrast the roles of live cell engulfments under normal or pathological conditions are largely unknown(1). In fact the first physiological function for a program of live cell engulfment in mammals was only recently revealed, by the discovery that hepatocytes ingest live autoreactive T cells in order to block the development of autoimmune disease in mice(2).
Various terms including cannibalism, emperipolesis, entosis, as well as phagocytosis, have been used to describe live cell engulfments that can occur heterotypically (between cells of different types) or homotypically (between cells of the same type)(1). Most heterotypic live cell engulfments involve leukocytes ingested into a variety of host cells (epithelial cells fibroblasts, neural cells, and others). Homotypic live cell engulfment is most often reported between tumor cells, in breast, lung, gall bladder, and gastric carcinoma, as well as metastatic melanoma, malignant mesothelioma, and other cancers(1, 3).
Just as the physiological roles for most forms of cell cannibalism are unknown, so too are the mechanisms that underlie engulfment unclear, as it is the mere appearance of whole – and apparently viable - engulfed cells that is scored as cannibalism, but the actual mechanisms of cell uptake have rarely been investigated. In fact many authors first reporting live cell engulfments speculated that internalized cells were not “eaten” at all, but were instead invaders, not unlike some classes of pathogenic organisms(1). For these authors it was simply the viability of internalized cells that suggested their active involvement(1). It is conceivable that cannibalism of leukocytes, for example, is a related activity to endothelial transmigration, as leukocytes can enter actively into individual endothelial cells in order to exit the blood stream(1). Accordingly, the internalization of live T cells into hepatocytes is argued to occur by an invasion-like mechanism, referred to as suicidal emperipolesis (SE)(2). Conversely, cells could be cannibalized as passive targets when engulfment pathways are overactive, as in SRGP-1 mutant C. elegans where activation of the Rac1 homolog CED-10 leads to the engulfment and killing of viable cells(4). Similarly the ingestion of live leukocytes by metastatic melanoma cells is thought to occur by an active engulfment-like activity of the tumor cells, which is dependent on the cytoskeletal linker protein ezrin(5).
Recently a mode of homotypic cannibalism was reported where epithelial cells exert autonomous control over their uptake, called entosis(6). Cannibalism by entosis involves the formation of adherens junctions between epithelial cells, mediated by E-cadherin, followed by engulfment in a Rho-GTPase and Rho-kinase-dependent manner. Rho activity and Rho-kinase are not required within engulfing cells but within internalizing cells, suggesting that uptake is controlled autonomously by an invasion-like mechanism (Figure 1A)(6). Importantly, cannibalistic cell structures in human breast tumors show clear evidence of a cell-cell adhesion-based mechanism of engulfment, with β-catenin, a component of adherens junctions, at the interface between internalizing cells and their engulfing partners, providing morphological evidence for entosis as a mechanism underlying the appearance of cell cannibalism in vivo(6). The entosis mechanism can also account for the appearance of cell-in-cell-in-cell arrangements of successive live engulfments that are observed in human tumors(6, 7). A mode of heterotypic cannibalism between natural killer (NK) cells and tumor cells was recently reported to involve E-cadherin-mediated cell junctions, as well as ezrin(8). Like entosis, this form of cell cannibalism is suspected to involve at least some degree of active control by the NK cells over their own uptake, suggesting that entosis-like mechanisms involving cell-cell adhesion proteins may not be restricted to homotypic engulfments(8).
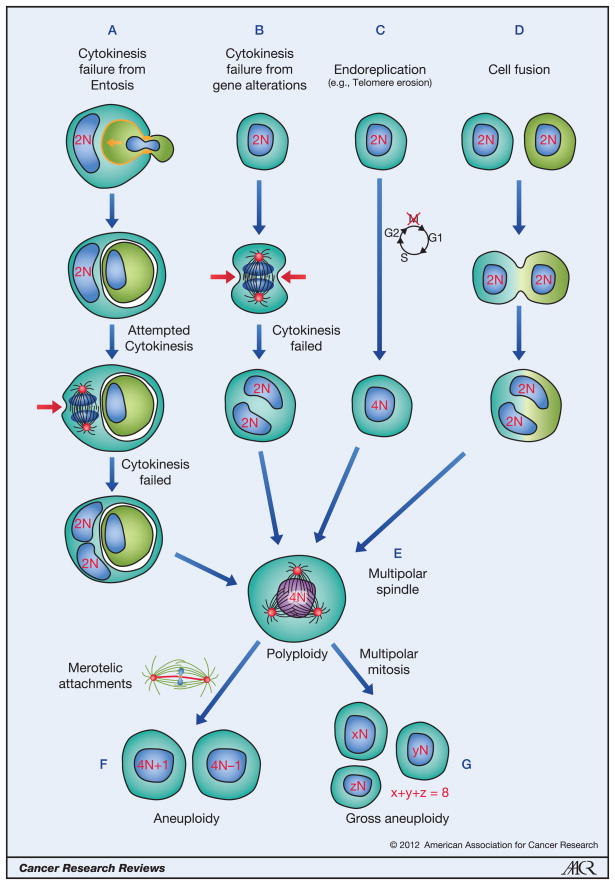
Figure 1. The different mechanisms that lead to polyploidy.
Formation of polyploid cells can result from cytokinesis failure due to entosis (A), cytokinesis failure due to gene dysregulation (B), endoreplication (C) and cell fusion (D). In (A), cannibalism by entosis requires E-cadherin-mediated junctions (orange) between engulfing cells and their targets, and cell uptake is driven by Rho and Rho-kinase activity within internalizing cells that invade into their hosts (orange arrow). Red arrows indicate the cleavage furrow of dividing cells, which is asymmetric in the presence of an internalized cell in (A). Binucleate or tetraploid cells often form multipolar spindles in mitosis due to increased centrosome number (E). Subsequent divisions can be bipolar, but often with merotelic chromosome attachments, resulting in chromosome missegregation (F). Multipolar divisions result in gross aneuploidy, which is often lethal, but can lead to rare aggressive clones (G).
Entosis and ploidy change – a possible mechanism to promote tumor progression
It is becoming clear that homotypic cannibalism in cancer is an indicator of poor prognosis, suggesting that this process may play a role in tumor progression(3). In bladder carcinoma, homotypic cannibalism occurs most frequently in high-grade cancers, where it is a predictor of progression, while generally fewer or no cannibalistic cell structures are found in benign urine samples(3). Similarly, the presence of homotypic cannibalism in breast cancers correlates with high grade(9, 10). High-grade tumors generally exhibit rapid progression, increased recurrence, and decreased overall patient survival compared to low-grade. It is conceivable that cannibalism can promote tumor progression by supplying tumor cells with nutrients, similar to self-digestion by macroautophagy, as demonstrated in culture for heterotypic cannibalism between metastatic melanoma tumor cells and T cells(5). Recently we identified another mechanism whereby cell-in-cell formation may promote tumor progression, by inducing changes in cell ploidy(10).
Increased ploidy is common in human breast cancers. Overall, about half of breast tumors contain cells with greater than diploid quantities of DNA, and some are polygenomic, with multiple subclones identified by unique ploidy increases(11). Ploidy increase is most often observed in high-grade tumors compared to low-grade, and is associated with tumor cell proliferation, as well as adverse prognosis in a number of studies (e.g. (12)). We have found that entosis directly promotes polyploidy by disrupting cell division, leading to the formation of binucleate engulfing cells in culture. The engulfing cells, or hosts, of cell-in-cell structures in human breast tumors are also frequently bi- or multinucleated(10). It is the mere presence of cannibalized cells that disrupts division, as they present a barrier to the formation of contractile rings, leading to partial furrows that are often unable to complete cytokinesis (Figure 1A)(10).
The binucleate state has been shown to drive tumor progression when combined with loss of the appropriate checkpoint proteins such as p53(13). Binucleate cells contribute to tumor progression, it is thought, by propagating cell lineages with grossly unstable genomes, which fuels the genetic diversity that drives selection(14). Whereas many of the daughter cells produced by binucleates are nonviable due to the massive chromosome shuffling caused by multipolar divisions, rare clones that have acquired advantages will propagate, potentially forming the clonal or subclonal populations that can be observed in tumors by their unique, and often greater than diploid, DNA content(11). Binucleates formed by division failure also have twice the normal number of centrosomes, which is equally critical to tumor progression as increased DNA content, as both multipolar divisions and abnormal chromosome attachments – merotelic attachments – of single chromatids to two poles, are a direct consequence of having supernumerary centrosomes(15). In tetraploid cells that cluster centrosomes in order to divide with bipolar spindles, merotelic attachments promoted by transient multipolar spindle intermediates lead to the missegregation of single chromosomes, which promotes genetic instability within tetraploid cell lineages without compromising viability to the same extent as multipolar divisions(15).
Other mechanisms that promote ploidy increase in human tumors
The finding that cell cannibalism by entosis can promote polyploidy in cancers by disrupting cell division presents a new and unexpected potential link between cannibalistic cell behavior and tumor progression. As both polyploidy and cell cannibalism have been noted in a variety of human cancers, it is conceivable that this link extends to cancers beyond those of the breast. But besides cell cannibalism, ploidy increase has been shown to occur by other pathways, including additional mechanisms that promote cytokinesis failure, pathways of endoreplication, and also by cell fusion (Figure 1). These various pathways that lead to polyploidy and the evidence that they occur in human cancers are discussed below.
Cytokinesis failure
The failure of cytokinesis remains one of the most commonly observed mechanisms to double cell ploidy, as well as centrosome number, and cytokinesis failure is a likely mechanism underlying many of the ploidy changes that are observed in human tumors. Like the disruption of cytokinesis by cannibalized cells, asbestos fibers lodging within the midbody during cell division were noted long ago to be a physical barrier to completion of cytokinesis, potentially linking this carcinogen mechanistically to tumor formation by induction of a tetraploid intermediate to aneuploidy(16). Although, only some asbestos-associated mesotheliomas are aneuploid, so this mechanism is plausibly one route to tumor formation but clearly not the only tumor-promoting effect of asbestos. Like asbestos fibers or cannibalized cells, chromatin bridges traversing the midbody as well can lead to cytokinesis failure, potentially by acting as a physical barrier, or by a mechanism involving defects in signaling through the Aurora B kinase, which delays abscission and inhibits furrow regression in response to chromatin bridges(17).
Unlike the physical barriers to division presented by cannibalized cells or asbestos fibers, which involve no genetic defects underlying the initial generation of tetraploidy, most other mechanisms that disrupt cytokinesis involve dysregulated pathways and checkpoints (Figure 1B). One notable mechanism that has emerged as a potentially common link to aneuploidy and polyploidy in cancers is overactivation of the spindle assembly checkpoint (SAC)(18). The SAC functions to ensure bipolar spindle attachment of sister chromatids prior to anaphase onset. Mad2 is a critical component of the SAC that inhibits the Anaphase Promoting Complex (APC-Cdc20), which targets Securin, a negative regulator of the Cohesin-protease Separase, for destruction by the proteasome(18). Mad2 is transcriptionally upregulated by E2F, which is inhibited the Rb tumor suppressor(18). Overexpression of Mad2, as a consequence of Rb inhibition, induces aneuploidy by allowing chromosome nondisjunctions; and, Mad2-overexpressing cells become polyploid as a result of cytokinesis failure, potentially linked to chromatin bridging(19), or by the Mad2-dependent inhibition of the mitotic kinesin MKlp2 that controls cytokinesis(20), or by slipping out of mitosis(21). As Mad2 overexpression promotes tumor formation and progression in a variety of tissues(19), and Rb is inactivated directly or indirectly in a host of cancers, this mechanism is likely a common inducer of aneuploidy as well as polyploidy in human cancers(18).
Endoreplication
Endoreplication, also known as endoreduplication, is another mechanism to increase cell ploidy, but without cell division (Figure 1C). Pathways of endoreplication generally involve disruptions to the cell division cycle that allow for cells to skip mitosis (referred to as endocycling), abort from mitosis (referred to as endomitosis), or re-initiate DNA replication during S-phase (referred to as re-replication). For both endocycles and endomitoses, the genome is completely replicated in each cycle, giving rise to cells with genomic content of 2N multiples. By contrast, re-replication involves dysfunctional regulation of DNA synthesis leading to multiple origin initiations per S phase, giving rise to cells with indistinct DNA profiles (for a more comprehensive Review see (22)). Pathways associated with endoreplication have been shown to promote cell transformation and tumorigenicity, but the frequency of endoreplication and its role in human cancers is largely unknown.
Recently a new mechanism linking endoreplication to telomere erosion was reported(23). Shortened telomeres result from normal cellular aging, and are thought to link human aging to increased cancer predisposition. Accordingly, shortened telomeres are reported at the early stages of many different human cancers(24). Telomere erosion triggers endoreplication in p53-null cells by induction of a prolonged G2 cell cycle phase, due to inhibition of Cdk1/Cyclin B, which allows for degradation of the replication inhibitor geminin and expression of the replication origin-licensing protein Cdt1. These combined effects allow cells without a p53-dependent G1 arrest, which would normally occur in response to telomere erosion, to re-enter S-phase by skipping mitosis, thereby becoming polyploid. Strikingly, the subsequent restoration of telomere protection allows polyploid cells to enter mitosis; and, due to the accumulation of multiple centrosomes, these cells exhibit multipolar divisions that are known to generate aneuploid progeny(23). As shortened telomeres are a common characteristic of many human cancers at early stages, this mechanism could be a frequent inducer of aneuploidy through a tetraploid intermediate derived from endoreplication(24).
Cell fusion
Perhaps the most direct way double cell ploidy and centrosome number is to simply fuse two cells together (Figure 1D). Conceivably, cell fusion could be linked to cell cannibalism, as the intimate association of viable engulfed cells and their hosts could facilitate membrane fusion, as has been speculated(25). In our laboratory, we have not found cell fusion to occur commonly between cannibalized cells and their hosts. But this outcome may depend on cell type, or potentially on the mechanism of engulfment. Cell fusion occurs normally throughout metazoan development; for example, muscle fibers form by myoblast fusion, trophoblasts can fuse in the placenta, and even fertilization itself is a fusion event(25). And fusion between hematopoietic cells and various targets has been observed during inflammation in adults(25). Whereas the cell products of fusion in normal physiology are terminally differentiated and non-mitotic, hybrid cells formed by fusion between tumor cells with defective checkpoints are predicted to be capable of division. In fact, experimentally-induced cell fusion between cells with disrupted checkpoints has been shown to be sufficient to induce tumor formation, in a manner recapitulating many of the most common aspects of human tumors – ploidy increase, and genotypic and phenotypic diversity(26). The phenotypic traits of parental cells are combined in fusion progeny, including even metastatic organotropism(27), leading to speculation that fusion between normal motile cells and tumor cells could underlie metastasis(28), or between stem cells and tumor cells the acquisition of tumor-initiating capacity(29). Interestingly, the propensity of polyploid cells to metastasize may also relate more directly to their increased size, which favors lodging in the capillary beds of organs like lung and brain(30).
Cell fusion may be a more common initiator of ploidy increase in human cancers than is appreciated. Beyond the spontaneous fusion of tumor cells, its induction by fusogenic proteins encoded by viruses, such as herpesvirus, might link common infections to tumor development(14). But the products of cell fusion in human tumors are difficult to distinguish from cytokinesis failure, as each results in the transient appearance of binucleate cells, which ultimately appear mononucleate upon nuclear fusion or cell division. Heterotypic fusion between tumor cells and non-tumor cells can be quantified by examining tumor-specific translocations along with cell lineage markers detected by immunohistochemistry. By this technique, up to 30% of tumor-associated osteoclasts in multiple myeloma were found to be the products of tumor cell and osteoclast fusion, which is postulated to underlie a previously unrecognized mechanism of tumor cell-directed bone destruction(31). But quantification of homotypic fusion between tumor cells relies on identification of premature chromosome condensation (PCC) as a distinguishing feature. Fusion of a mitotic cell with an interphase cell results in premature condensation of the chromatin of the interphase nucleus, which appears with morphological characteristics distinctive from mitotic nuclei, termed PCC. Cells in G1 exhibit PCC with single chromatids, whereas S-phase PCC is described as having a “pulverized” appearance, and G2 nuclei have longer chromatids than mitotic nuclei(32). PCC indicative of cell fusion has been observed in a number of studies and in a range of human cancers from leukemias to various carcinomas(33). Although overall, PCC has been found in a relatively low percentage of cancers where it has been investigated, this technique may under-represent cell fusion as it relies on mitotic cells, which are a very small fraction of the total tumor cell population, to be involved in order for identification. Cell fusion therefore could be a common mediator of ploidy increase in human cancers but the extent of its role remains unclear.
Conclusion
Polyploidy arising from disrupted cytokinesis, defective mitotic entry, or cell fusion, is a demonstrated precursor to aneuploidy that is regarded as a driver of human cancers (Figure 1). Here we have discussed some of the evidence that these various forms of polyploid cell induction occur, or are likely to occur, in human cancers. They are each, essentially, means to a similar end with respect to aneuploidy. But distinguishing features will determine whether, and when, each occurs in various cancers. Some forms are also predicted to have additional effects on tumor progression that could be positively selected, for example cell fusion combines the phenotypic traits of different cells, and cell cannibalism could provide starving cells with nutrients. Telomere erosion is a known early event preceding cancer development, and like mechanical disruptions of cytokinesis, it requires no other pre-existing genetic dysregulations to promote ploidy change, besides p53 loss that is also generally required for the fitness of aneuploid progeny. Cell cannibalism could also occur at early stages of tumor progression, as entosis can occur in culture between cells arising from the first division during attempted colony formation in transformed growth assays. Failure of division of such an engulfing cell could be one mechanism that leads to the formation of polyploid cells in human cancers.
Acknowledgments
We apologize to the authors whose work could not be discussed or cited here due to space limitations. We thank members of the Overholtzer lab for critical reading of the manuscript. Michael Overholtzer is supported by a grant from the National Cancer Institute (RO1CA154649), the Louis V. Gerstner, Jr. Young Investigators Fund, and the Benjamin Friedman Research Fund.
References
- 1.Overholtzer M, Brugge JS. The cell biology of cell-in-cell structures. Nat Rev Mol Cell Biol. 2008;9:796–809. doi: 10.1038/nrm2504. [DOI] [PubMed] [Google Scholar]
- 2.Benseler V, Warren A, Vo M, Holz LE, Tay SS, Le Couteur DG, et al. Hepatocyte entry leads to degradation of autoreactive CD8 T cells. Proc Natl Acad Sci U S A. 2011;108:16735–40. doi: 10.1073/pnas.1112251108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma N, Dey P. Cell cannibalism and cancer. Diagn Cytopathol. 2011;39:229–33. doi: 10.1002/dc.21402. [DOI] [PubMed] [Google Scholar]
- 4.Neukomm LJ, Frei AP, Cabello J, Kinchen JM, Zaidel-Bar R, Ma Z, et al. Loss of the RhoGAP SRGP-1 promotes the clearance of dead and injured cells in Caenorhabditis elegans. Nat Cell Biol. 2011;13:79–86. doi: 10.1038/ncb2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lugini L, Matarrese P, Tinari A, Lozupone F, Federici C, Iessi E, et al. Cannibalism of live lymphocytes by human metastatic but not primary melanoma cells. Cancer Res. 2006;66:3629–38. doi: 10.1158/0008-5472.CAN-05-3204. [DOI] [PubMed] [Google Scholar]
- 6.Overholtzer M, Mailleux AA, Mouneimne G, Normand G, Schnitt SJ, King RW, et al. A nonapoptotic cell death process, entosis, that occurs by cell-in-cell invasion. Cell. 2007;131:966–79. doi: 10.1016/j.cell.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 7.Sarode GS, Sarode SC, Karmarkar S. Complex cannibalism: An unusual finding in oral squamous cell carcinoma. Oral Oncol. 2011 doi: 10.1016/j.oraloncology.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 8.Wang S, Guo Z, Xia P, Liu T, Wang J, Li S, et al. Internalization of NK cells into tumor cells requires ezrin and leads to programmed cell-in-cell death. Cell Res. 2009;19:1350–62. doi: 10.1038/cr.2009.114. [DOI] [PubMed] [Google Scholar]
- 9.Abodief WT, Dey P, Al-Hattab O. Cell cannibalism in ductal carcinoma of breast. Cytopathology. 2006;17:304–5. doi: 10.1111/j.1365-2303.2006.00326.x. [DOI] [PubMed] [Google Scholar]
- 10.Krajcovic M, Johnson NB, Sun Q, Normand G, Hoover N, Yao E, et al. A non-genetic route to aneuploidy in human cancers. Nat Cell Biol. 2011;13:324–30. doi: 10.1038/ncb2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Navin N, Krasnitz A, Rodgers L, Cook K, Meth J, Kendall J, et al. Inferring tumor progression from genomic heterogeneity. Genome Res. 2010;20:68–80. doi: 10.1101/gr.099622.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pinto AE, Andre S, Soares J. Short-term significance of DNA ploidy and cell proliferation in breast carcinoma: a multivariate analysis of prognostic markers in a series of 308 patients. J Clin Pathol. 1999;52:604–11. doi: 10.1136/jcp.52.8.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujiwara T, Bandi M, Nitta M, Ivanova EV, Bronson RT, Pellman D. Cytokinesis failure generating tetraploids promotes tumorigenesis in p53-null cells. Nature. 2005;437:1043–7. doi: 10.1038/nature04217. [DOI] [PubMed] [Google Scholar]
- 14.Duelli D, Lazebnik Y. Cell-to-cell fusion as a link between viruses and cancer. Nat Rev Cancer. 2007;7:968–76. doi: 10.1038/nrc2272. [DOI] [PubMed] [Google Scholar]
- 15.Ganem NJ, Godinho SA, Pellman D. A mechanism linking extra centrosomes to chromosomal instability. Nature. 2009;460:278–82. doi: 10.1038/nature08136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jensen CG, Jensen LC, Rieder CL, Cole RW, Ault JG. Long crocidolite asbestos fibers cause polyploidy by sterically blocking cytokinesis. Carcinogenesis. 1996;17:2013–21. doi: 10.1093/carcin/17.9.2013. [DOI] [PubMed] [Google Scholar]
- 17.Steigemann P, Wurzenberger C, Schmitz MH, Held M, Guizetti J, Maar S, et al. Aurora B-mediated abscission checkpoint protects against tetraploidization. Cell. 2009;136:473–84. doi: 10.1016/j.cell.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 18.Schvartzman JM, Sotillo R, Benezra R. Mitotic chromosomal instability and cancer: mouse modelling of the human disease. Nat Rev Cancer. 2010;10:102–15. doi: 10.1038/nrc2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sotillo R, Hernando E, Diaz-Rodriguez E, Teruya-Feldstein J, Cordon-Cardo C, Lowe SW, et al. Mad2 overexpression promotes aneuploidy and tumorigenesis in mice. Cancer Cell. 2007;11:9–23. doi: 10.1016/j.ccr.2006.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee SH, McCormick F, Saya H. Mad2 inhibits the mitotic kinesin MKlp2. J Cell Biol. 2010;191:1069–77. doi: 10.1083/jcb.201003095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hernando E, Nahle Z, Juan G, Diaz-Rodriguez E, Alaminos M, Hemann M, et al. Rb inactivation promotes genomic instability by uncoupling cell cycle progression from mitotic control. Nature. 2004;430:797–802. doi: 10.1038/nature02820. [DOI] [PubMed] [Google Scholar]
- 22.Lee HO, Davidson JM, Duronio RJ. Endoreplication: polyploidy with purpose. Genes Dev. 2009;23:2461–77. doi: 10.1101/gad.1829209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davoli T, Denchi EL, de Lange T. Persistent telomere damage induces bypass of mitosis and tetraploidy. Cell. 2010;141:81–93. doi: 10.1016/j.cell.2010.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davoli T, de Lange T. The Causes and Consequences of Polyploidy in Normal Development and Cancer. Annu Rev Cell Dev Biol. 2010 doi: 10.1146/annurev-cellbio-092910-154234. [DOI] [PubMed] [Google Scholar]
- 25.Lu X, Kang Y. Cell fusion as a hidden force in tumor progression. Cancer Res. 2009;69:8536–9. doi: 10.1158/0008-5472.CAN-09-2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duelli DM, Padilla-Nash HM, Berman D, Murphy KM, Ried T, Lazebnik Y. A virus causes cancer byinducing massive chromosomal instability through cell fusion. Curr Biol. 2007;17:431–7. doi: 10.1016/j.cub.2007.01.049. [DOI] [PubMed] [Google Scholar]
- 27.Lu X, Kang Y. Efficient acquisition of dual metastasis organotropism to bone and lung through stable spontaneous fusion between MDA-MB-231 variants. Proc Natl AcadSci U S A. 2009;106:9385–90. doi: 10.1073/pnas.0900108106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pawelek JM, Chakraborty AK. The cancer cell--leukocyte fusion theory of metastasis. Adv Cancer Res. 2008;101:397–444. doi: 10.1016/S0065-230X(08)00410-7. [DOI] [PubMed] [Google Scholar]
- 29.Lu X, Kang Y. Cell fusion hypothesis of the cancer stem cell. Adv Exp Med Biol. 2011;714:129–40. doi: 10.1007/978-94-007-0782-5_6. [DOI] [PubMed] [Google Scholar]
- 30.Lu X, Kang Y. Organ-specific enhancement of metastasis by spontaneous ploidy duplication and cell size enlargement. Cell Res. 2010;20:1012–22. doi: 10.1038/cr.2010.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andersen TL, Boissy P, Sondergaard TE, Kupisiewicz K, Plesner T, Rasmussen T, et al. Osteoclast nuclei of myeloma patients show chromosome translocations specific for the myeloma cell clone: a new type of cancer-host partnership? J Pathol. 2007;211:10–7. doi: 10.1002/path.2078. [DOI] [PubMed] [Google Scholar]
- 32.Johnson RT, Rao PN. Mammalian cell fusion: induction of premature chromosome condensation in interphase nuclei. Nature. 1970;226:717–22. doi: 10.1038/226717a0. [DOI] [PubMed] [Google Scholar]
- 33.Kovacs G. Premature chromosome condensation: evidence for in vivo cell fusion in human malignant tumours. Int J Cancer. 1985;36:637–41. doi: 10.1002/ijc.2910360602. [DOI] [PubMed] [Google Scholar]