Abstract
Some of the efforts that have been made to document tooth wear are reviewed here with an emphasis on nonhuman mammals, literature with which dentists may not be very familiar. We project a change in research strategy from the description of wear at various scales of measurement towards investigation of the mechanical mechanisms that actually create the texture of a worn surface. These studies should reveal exactly how tooth tissue is lost and what aspects of the structure of dental tissues affect this. The most important aspects of the interaction between the tooth surface and wear particles would appear to be particle size, particle shape, their mechanical properties with respect to those of tooth tissues, and the influence of saliva.
1. Introduction
The teeth of humans are used several thousand times a day [1], a figure that is probably an order of magnitude lower than in our nearest primate relatives [2]. This newly discovered discrepancy has been attributed to the effects of cooking food [3], which reduces the importance of food breakdown in the mouth [4]. Even so, humans make millions of potentially damaging mechanical contacts in a lifetime, and there are no current solutions to the engineering of any material that could compare to the exceptional damage tolerance of human dental enamel [5, 6].
Damage to teeth via contact with either ingested foods, extraneous particles ingested with them, or opposing teeth takes place at various scales. The largest events can lead to catastrophic fractures of the tooth crown or roots, fragmenting them [7]. The mechanisms that produce at least some types of crown fracture are now beginning to be elucidated and show that preexisting cracks in enamel are often implicated [8, 9]. An exception is seen in millimetre-scale enamel chipping, where it is clear that no preexisting flaws are involved [10]. However, a much more common result than these events is crown wear, which is the loss of volume caused by the accumulated loss of microscopic amounts of hard tissue over an extended time period. Such tooth wear is an enormously important aspect of oral biology, of interest to many researchers on vertebrates. However, this range of research interest has resulted in knowledge being spread across a wide range of journals. Little of this comparative interest is apparent in the dental literature. While, for the most part, wear studies in nonhumans present wear as a mechanical process [11, 12], the accepted view in dentistry seems to be that chemical dissolution is always involved [13–16]. Despite this discrepancy, the final removal of tooth tissue even in modern humans nearly always involves a force, leading to the need for a mechanical analysis, albeit one that needs suitable adjustment for the relevant mechanical properties of the tissue at the instant of removal [17].
The first aim of this paper is to make a brief survey of what has been established in wear studies in various organisms and to indicate how this information has been used. Secondly, our intention is to suggest how future studies at nanoscale could establish the actual mechanisms of dental hard tissue removal and what questions could be asked. Since most work has been reported on enamel rather than dentine, we restrict our focus here to this tissue.
2. Types of Wear Studies
The best method of reviewing the literature seems a classification of method based on scale of observation. Macrowear is the surface texture visible on a tooth either with the naked eye or low-power light microscopy. With the use of oblique lighting, shiny-surfaced facets can be distinguished easily from more matte areas on lightly-worn teeth. About 60 years ago, it was established that these patches of wear establish themselves in fairly fixed locations and that the direction of scratches on the shiny facets, evident even if they cannot be individually resolved, could be used to determine how the jaws moved into and out of occlusion [18]. A numbering system for the wear facets on the molars of early mammals was developed [19], which was later expanded to other lineages including the primates [20, 21]. Little has been done since on the evolution of wear in this sense. However, rather than being outdated, macrowear research shows signs of being reinvigorated in several ways. For example, accurate three-dimensional mapping of these facets has been used to reconstruct the jaw movements in prehistoric humans, coupling this to collision detection software to indicate exactly which parts of opposing teeth would have been in contact at any point in time [22]. The results show how the patterns of stress in dental tissues are influenced by the varying direction of the bite force. In addition, macroscopic patterns of postcanine tooth wear have recently been examined with respect to the biological fitness of individual animals [23–29]. Primates that have heavily worn molar crowns consisting only of dentine surrounded by an enamel ridge appear to have very compromised health.
Microwear studies were a later development, utilizing the scanning electron microscope (SEM) to observe selected facets of worn teeth at much higher magnifications [30]. Individual features of the worn surface, such as roughly isodiametric pits and elongated scratches, could be distinguished. Microwear has become an extremely important method of determining the diet of fossil vertebrates, particularly when the morphology of the teeth seemed to be of little help in dietary assessment. A glimpse at microwear analysis across vertebrates demonstrates its achievements in a wide variety of contexts. As an example far from modern humans in evolutionary distance, microwear has shown that some of the earliest jawless vertebrates, called conodonts, used their teeth for breaking down large prey items [31]. In our lineage, much of the evidence for what fossil members of our genus were eating comes from dental microwear [32]. However, microwear does not just inform us about food intake. Some fossil primates almost certainly used their procumbent lower front teeth for grooming activities, just as some living primates do. Hair marks between the incisors are clearly evident in both living and fossil groups [33]. Some fossil giraffes, despite their long necks being associated with browsing on the crowns of trees, probably ate grass [34]. Some fossil horses probably did not, despite the fact that they had tall (hypsodont) molar crowns that would seem redundant when eating tree leaves [35]. The discrepancy between the gross anatomy of the dentitions of these ungulates and the microwear evidence of what these ungulates actually seemed to be eating has led to the need for a reexamination of the long-held view that hypsodonty evolved in response to grazing [36]. Despite these successes, the quantification of microwear patterns has proved difficult because SEM provides a two-dimensional view of the surface that is influenced by the direction at which it is viewed. Considerable recent efforts have been made to make microwear analysis more objective by using white-light confocal microscopy to characterize the three-dimensional texture of the worn surface rather than concentrating on individual features such as pits and scratches [37, 38].
Mesowear has been an even more recent research development involving light microscopy. It is addressed not to the individual features of worn surfaces, but to their overall roughness and to the curvature of the edges of facets [39, 40]. One of its major aims is to establish whether food or teeth were responsible for the major part of the wear. It would appear that browsers, for example, are significantly different from grazers in this respect [40].
3. Causes of Wear
All the above approaches are essentially observational techniques for describing the appearance of worn surfaces. While some experiments have been run to try to determine the causes of particular wear patterns [41–44], the results have been disputed and none have been aimed at demonstrating exactly how tooth material is lost. More broadly, one of the major reasons why research on wear, as a branch of tribology, appears to have been impeded is that the mechanisms that produce it are often unclear. Wear is an accumulation of complex events that can include the combined effects of lubrication, friction, adhesion, and fracture. Practical wear studies often involve a stereotyped kind of apparatus such as a pin on a disk, for which standards are available, but which lack fundamental aims. In vitro studies on dental wear have sometimes been very sophisticated. For example, occluding pairs of teeth have been mounted in a universal testing machine, modified to move as does the jaw with a horizontal, not just a vertical, component of motion [45, 46]. This represents a considerable technical achievement in mimicry but does not help necessarily in defining underlying mechanisms.
One way out of such scenarios is to concentrate on the characteristics of individual events. This requires experimentation at the level of nanotechnology. Nanoindentation equipment has only recently started to be used in the equivalent of wear studies. This has provided evidence that enamel tissue is lost far more easily with a sharp contact than a blunt one [47]; that there seems to be a brittle-ductile transition involved in enamel behaviour controlled by force level [48], and that wetting of the tooth surface improves abrasion resistance [49]. However, all these studies have used diamond tips to abrade the enamel rather than the type of mechanical insult to which tooth surfaces are subjected in life. We thus move now to consider what particles could cause damage to dental tissues and in what way such damage might differ from that produced with diamond.
4. Potential Sources of Wear
At the level of individual events, mechanical wear can be viewed as a type of indentation involving extensive plasticity. A static indentation may produce a pit, while the addition of some translational motion is liable to lead to a scratch [50]. The major material properties of solids that influence the type of damage that they can inflict on each other are their hardness or yield strength (material resistance to plastic deformation), elastic modulus (material resistance to elastic deformation), and fracture toughness (resistance to crack growth). Hardness is more commonly used than the yield stress in studies, but hardness is strictly a measurement while yield stress is a property. For most geometries of contact, the hardness is about three times the yield stress, but this multiple is not fixed [51]. A basic requirement is that any particle that could endanger dental tissues has to be hard enough to indent the other. A rigid particle in this context is one with a hardness more than 2.5 times that of enamel [51]. Even a particle that is just 1.1 times as hard as enamel would be capable of indenting it, but it would also change shape permanently itself. This situation is called mutual indentation and affects calculations of the force involved in producing a mark of any given size [52]. Table 1 indicates the types of particle that have been suggested as wear agents in the vertebrate literature. All lie in the mutual indentation range with respect to enamel. The most obvious cause of wear is the quartz dust found in many natural environments at various particle sizes [53]. However, phytoliths, the amorphous silica particles found particularly in grasses, are also potential wear agents (Table 1). Less likely candidates as indentation agents are seed shells, against which enamel is effectively rigid.
Table 1.
Mechanical properties of some materials that might damage enamel.
5. Mechanisms of Wear
With the exception of quartz dust, there has been considerable dispute about what kind of particles can wear enamel. For example, phytoliths are thought to be important wear agents by some [54], but not by others [55]. Seed eating has been assumed to be the cause of pitting [37], but this is difficult to understand on the basis of Table 1. In addition there is the issue that tooth tissues wear each other. The only way to resolve these issues would be to initiate wear studies at the appropriate experimental level. This is now feasible via nanoindentation equipment and atomic force microscopy. Such nanowear experiments could focus on individual events, which would erase any doubt as to the nature of the interactions involved.
Prior to making such experiments though, it is reasonable to consider what questions could be asked. These questions could contain the kernels of hypotheses to be tested by nanowear experiments. We offer two examples here with potential answers.
(1) Why Are Wear Marks So Small? —
It might be thought that this is because smaller contacts involve lower forces, so that small marks would be predominant. The problem with this answer though is that a tooth surface usually shows almost no large marks at all. This was why it took an SEM to visualize actual wear features. A more likely response concerns the well-known brittle-ductile transition, which is known to alter the behaviour of small particles [5, 58]. There is some evidence already of such a transition in enamel behaviour [48].
(2) Why Do Numbers of Pits and Scratches Often not Match? —
It was noticed from the earliest microwear studies that scratches tend to outnumber pits in most mammals. Jaw movements have been invoked to explain this. For example, in chimpanzees, the third molars, which probably make less of a lateral excursion across each other than more anterior molars, have a higher percentage of pits on their surfaces [59]. However, such considerations do not seem to be sufficient. Rather it would appear that it is likely that scratches form more easily than pits. Some evidence of this comes from considering the mechanics of scratching versus pitting. Figure 1 is redrawn from Sharp et al. [50] to show the differential propensity of materials to be either scratched (sliding indentation) or pitted (static indentation). The axes of these graphs show the important material properties that influence whether a material will behave either elastically, plastically, or fracture. Figure 1(a) simply shows how enamel and dentine match up to other materials. Unsurprisingly, enamel is similar to ceramics like glass, while dentine is akin to some polymers. Figure 1(b) indicates how these materials behave under tension via a subdivision of the map into elastic, plastic, and brittle spaces based on simple equations for tensile behaviour. Again, enamel is brittle, which corresponds with data from tensile tests [1]. However, in Figure 1(c), under the highly compressive stress regime of static indentation, enamel is much less likely to break, showing some plasticity. This is again consistent with data from nanoindentation studies; if the load is small enough, enamel does indeed yield without cracking under static loads. However, when an indenter is slid across a surface to form a scratch, the tensile field behind the indenter is greater and enamel is much more likely to crack (and thus lose tissue) than under a pit. The big difference here is the inclusion of friction in equations for sliding. The frictional coefficient in Figure 1(d) is assumed to be 0.5. Whether such speculations really apply to enamel in “single event,” experiments becomes an important prediction. The time to test such predictions seems to be here.
Figure 1.
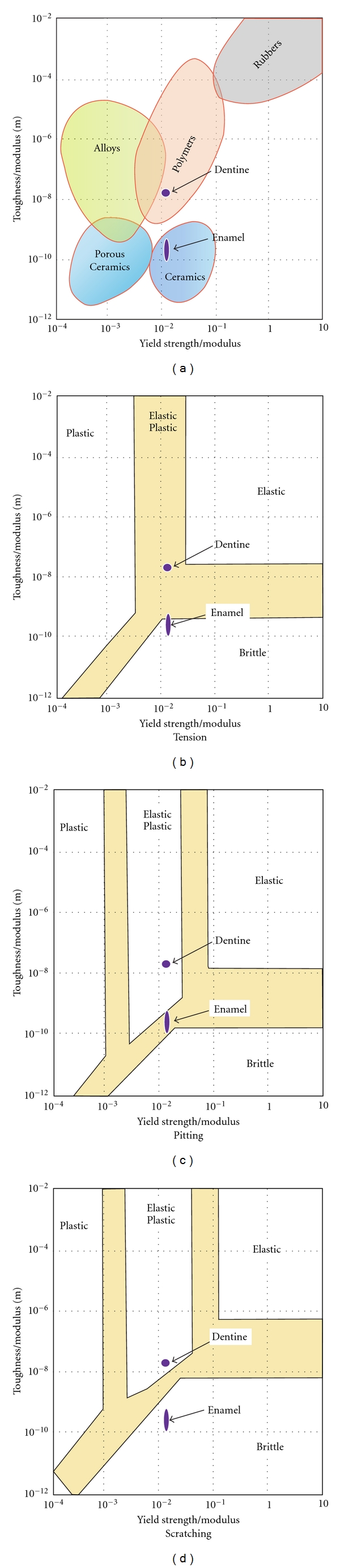
Material property maps (redrawn from [50]). (a) Shows the domains of common types of solids compared to dentine and enamel. Enamel is more like a ceramic in its properties than dentine. Maps (b)–(d) show the effect of different loading regimes on the behaviour of these dental tissues. Under tension (b), enamel is very likely to break in a brittle manner. Under static indentation (c), the highly compressive stresses during pit formation produce a more plastic response, but sliding (d) enhances tension greatly. It can be concluded that enamel is more likely to lose material via a scratch than a pit.
References
- 1.Waters NE. Some mechanical and physical properties of teeth. In: Vincent JFV, Currey JD, editors. The Mechanical Properties of Biological Materials. Cambridge, UK: Cambridge University Press; 1980. pp. 99–135. [PubMed] [Google Scholar]
- 2.Organ C, Nunn CL, Machanda Z, Wrangham RW. Phylogenetic rate shifts in feeding time during the evolution of Homo . Proceedings of the National Academy of Sciences of the United States of America. 2011;108(35):14555–14559. doi: 10.1073/pnas.1107806108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wrangham RW, Jones JH, Laden G, Pilbeam D, Conklin-Brittain N. The raw and the stolen: cooking and the ecology of human origins. Current Anthropology. 1999;40(5):567–594. [PubMed] [Google Scholar]
- 4.Farrell J. The effect of mastication on the digestion of food. British Dental Journal. 1958;100:149–155. [Google Scholar]
- 5.Lucas PW, Constantino P, Wood BA, Lawn BR. Dental enamel as a dietary indicator in mammals. BioEssays. 2008;30(4):374–385. doi: 10.1002/bies.20729. [DOI] [PubMed] [Google Scholar]
- 6.Chai H, Lee JJW, Constantino PJ, Lucas PW, Lawn BR. Remarkable resilience of teeth. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(18):7289–7293. doi: 10.1073/pnas.0902466106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andreasen JO, Andreasen FM, Andersson L. Textbook and Color Atlas of Traumatic Injuries to the Teeth. 4th edition. Oxford, UK: Wiley-Blackwell; 2007. [Google Scholar]
- 8.Chai H, Lee JJW, Kwon JY, Lucas PW, Lawn BR. A simple model for enamel fracture from margin cracks. Acta Biomaterialia. 2009;5(5):1663–1667. doi: 10.1016/j.actbio.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Lee JJ-W, Constantino P, Lucas PW, Lawn BR. Fracture in teeth—a diagnostic for inferring tooth function and diet. Biological Reviews. 2011;86:959–974. doi: 10.1111/j.1469-185X.2011.00181.x. [DOI] [PubMed] [Google Scholar]
- 10.Constantino P, Lee JJ-W, Chai H, et al. Antemortem tooth chipping in early hominins can predict bite forces and diet. Biology Letters. 2010;6:719–722. doi: 10.1098/rsbl.2010.0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teaford MF. A review of dental microwear and diet in modern mammals. Scanning Microscopy. 1988;2(2):1149–1166. [PubMed] [Google Scholar]
- 12.Teaford MF. Dental microwear and dental function. Evolutionary Anthropology. 1994;3(1):17–30. [Google Scholar]
- 13.Smith BGN. Dental erosion, attrition and abrasion. Practitioner. 1975;214(1281):347–355. [PubMed] [Google Scholar]
- 14.Johansson A-K. On dental erosion and associated factors. Swedish Dental Journal. Supplement. 2002;(156):1–77. [PubMed] [Google Scholar]
- 15.Johansson A, Johansson A-K, Omar R, Carlsson GE. Rehabilitation of the worn dentition. Journal of Oral Rehabilitation. 2008;35(7):548–566. doi: 10.1111/j.1365-2842.2008.01897.x. [DOI] [PubMed] [Google Scholar]
- 16.Khan F, Young WG. The multifactorial nature of toothwear. In: Khan F, Young WG, editors. Toothwear, the ABC of the Worn Dentition. Chichester, UK: Wiley-Blackwell Press; 2011. pp. 1–14. [Google Scholar]
- 17.Dahl BL, Carlsson GE, Ekfeldt A. Occlusal wear of teeth and restorative materials. A review of classification, etiology, mechanisms of wear, and some aspects of restorative procedures. Acta Odontologica Scandinavica. 1993;51(5):299–311. doi: 10.3109/00016359309040581. [DOI] [PubMed] [Google Scholar]
- 18.Butler PM. The milk-molars of Perissodactyla, with remarks on molar occlusion. Proceedings of the Zoological Society of London. 1952;121:777–817. [Google Scholar]
- 19.Crompton AW. The origin of the tribosphenic molar. In: Kermack DM, Kermack KA, editors. Early Mammals. London, UK: Academic; 1971. pp. 65–97. [Google Scholar]
- 20.Kay RF. The evolution of molar occlusion in the cercopithecidae and early catarrhines. American Journal of Physical Anthropology. 1977;46(2):327–352. doi: 10.1002/ajpa.1330460213. [DOI] [PubMed] [Google Scholar]
- 21.Maier W. Evolution of bilophodont molars of Cercopithecoidea. Function morphological study. Zeitschrift fur Morphologie und Anthropologie. 1977;68(1):26–56. [PubMed] [Google Scholar]
- 22.Benazzi S, Kullmer O, Grosse IR, Weber GW. Using occlusal wear information and finite element analysis to investigate stress distributions in human molars. Journal of Anatomy. 2011;219(3):259–272. doi: 10.1111/j.1469-7580.2011.01396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lanyon JM, Sanson GD. Koala (Phascolarctos cinereus) dentition and nutrition—II. Implications of tooth wear in nutrition. Journal of Zoology. 1986;209:169–181. [Google Scholar]
- 24.McArthur C, Sanson GD. Tooth wear in eastern grey kangaroos (Macropus giganteus) and western grey kangaroos (Macropus fuliginosus), and its potential influence on diet selection, digestion and population parameters. Journal of Zoology. 1988;215:491–504. [Google Scholar]
- 25.Logan M, Sanson GD. The effect of tooth wear on the feeding behaviour of free-ranging koalas (Phascolarctos cinereus, Goldfuss) Journal of Zoology. 2002;256(1):63–69. [Google Scholar]
- 26.DeGusta D, Everett MA, Milton K. Natural selection on molar size in a wild population of howler monkeys (Alouatta palliata) Proceedings of the Royal Society B. 2003;270(1):S15–S17. doi: 10.1098/rsbl.2003.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.King SJ, Arrigo-Nelson SJ, Pochron ST, et al. Dental senescence in a long-lived primate links infant survival to rainfall. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(46):16579–16583. doi: 10.1073/pnas.0508377102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cuozzo FP, Sauther ML. Severe wear and tooth loss in wild ring-tailed lemurs (Lemur catta): a function of feeding ecology, dental structure, and individual life history. Journal of Human Evolution. 2006;51(5):490–505. doi: 10.1016/j.jhevol.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Galbany J, Altmann J, Pérez-Pérez A, Alberts SC. Age and individual foraging behavior predict tooth wear in Amboseli baboons. American Journal of Physical Anthropology. 2011;144(1):51–59. doi: 10.1002/ajpa.21368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walker A, Hoeck HN, Perez L. Microwear of mammalian teeth as an indicator of diet. Science. 1978;201(4359):908–910. doi: 10.1126/science.684415. [DOI] [PubMed] [Google Scholar]
- 31.Purnell MA. Microwear on conodont elements and macrophagy in the first vertebrates. Nature. 1995;374(6525):798–800. [Google Scholar]
- 32.Ungar PS, Grine FE, Teaford MF, El Zaatari S. Dental microwear and diets of African early Homo. Journal of Human Evolution. 2006;50(1):78–95. doi: 10.1016/j.jhevol.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 33.Rose KD, Walker A, Jacobs LL. Function of the mandibular tooth comb in living and extinct mammals. Nature. 1981;289(5798):583–585. doi: 10.1038/289583a0. [DOI] [PubMed] [Google Scholar]
- 34.Solounias N, Teaford M, Walker A. Interpreting the diet of extinct ruminants: the case of a non- browsing giraffid. Paleobiology. 1988;14(3):287–300. [Google Scholar]
- 35.MacFadden BJ, Solounias N, Cerling TE. Ancient diets, ecology, and extinction of 5-million-year-old horses from Florida. Science. 1999;283(5403):824–827. doi: 10.1126/science.283.5403.824. [DOI] [PubMed] [Google Scholar]
- 36.Damuth J, Janis CM. On the relationship between hypsodonty and feeding ecology in ungulate mammals, and its utility in palaeoecology. Biological Reviews. 2011;86(3):733–758. doi: 10.1111/j.1469-185X.2011.00176.x. [DOI] [PubMed] [Google Scholar]
- 37.Scott RS, Ungar PS, Bergstrom TS, et al. Dental microwear texture analysis shows within-species diet variability in fossil hominins. Nature. 2005;436(7051):693–695. doi: 10.1038/nature03822. [DOI] [PubMed] [Google Scholar]
- 38.Scott RS, Ungar PS, Bergstrom TS, et al. Dental microwear texture analysis: technical considerations. Journal of Human Evolution. 2006;51(4):339–349. doi: 10.1016/j.jhevol.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 39.Fortelius M, Solounias N. Functional characterization of ungulate molars using the abrasion-attrition wear gradient: a new method for reconstructing paleodiets. American Museum Novitates. 2000;3301:1–36. [Google Scholar]
- 40.Kaiser TM, Brinkmann G. Measuring dental wear equilibriums—the use of industrial surface texture parameters to infer the diets of fossil mammals. Palaeogeography, Palaeoclimatology, Palaeoecology. 2006;239(3-4):221–240. [Google Scholar]
- 41.Covert HH, Kay RF. Dental microwear and diet: implications for determining the feeding behaviors of extinct primates, with a comment on the dietary pattern of Sivapithecus. American Journal of Physical Anthropology. 1981;55(3):331–336. doi: 10.1002/ajpa.1330550307. [DOI] [PubMed] [Google Scholar]
- 42.Gordon KD, Walker AC. Playing ’possum: a microwear experiment. American Journal of Physical Anthropology. 1983;60(1):109–112. doi: 10.1002/ajpa.1330600115. [DOI] [PubMed] [Google Scholar]
- 43.Kay RF, Covert HH. True grit: a microwear experiment. American Journal of Physical Anthropology. 1983;61(1):33–38. doi: 10.1002/ajpa.1330610104. [DOI] [PubMed] [Google Scholar]
- 44.Teaford MF, Walker A. Dental microwear in adult and still-born guinea pigs (Cavia porcellus) Archives of Oral Biology. 1983;28(11):1077–1081. doi: 10.1016/0003-9969(83)90067-5. [DOI] [PubMed] [Google Scholar]
- 45.Douglas WH, Sakaguchi RL, DeLong R. Frictional effects between natural teeth in an artificial mouth. Dental Materials. 1985;1(3):115–119. doi: 10.1016/S0109-5641(85)80040-3. [DOI] [PubMed] [Google Scholar]
- 46.DeLong R, Douglas WH. An artificial oral environment for testing dental materials. IEEE Transactions on Biomedical Engineering. 1991;38(4):339–345. doi: 10.1109/10.133228. [DOI] [PubMed] [Google Scholar]
- 47.Guidoni GM, Swain MV, Jäger I. Wear behaviour of dental enamel at the nanoscale with a sharp and blunt indenter tip. Wear. 2009;266(1-2):60–68. [Google Scholar]
- 48.Guidoni GM, Swain M, Jäger I. Enamel: from brittle to ductile like tribological response. Journal of Dentistry. 2008;36(10):786–794. doi: 10.1016/j.jdent.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 49.Guidoni GM, Swain M, Jäger I. Nano-scale sliding contact deformation behaviour of enamel under wet and dry conditions. Journal of Materials Science. 2010;21(4):1195–1203. doi: 10.1007/s10856-010-3988-6. [DOI] [PubMed] [Google Scholar]
- 50.Sharp SJ, Ashby MF, Fleck NA. Material response under static and sliding indentation loads. Acta Metallurgica et Materialia. 1993;41(3):685–692. [Google Scholar]
- 51.Atkins AG. Topics in indentation hardness. Metal Science. 1982;16(3):127–137. [Google Scholar]
- 52.Atkins AG, Felbeck DK. Applying mutual indentation hardness phenomena to service failures. Metal Engineering Quarterly. 1974;14(2):55–61. [Google Scholar]
- 53.Ungar PS, Teaford MF, Glander KE, Pastor RF. Dust accumulation in the canopy: a potential cause of dental microwear in primates. American Journal of Physical Anthropology. 1995;97(2):93–99. doi: 10.1002/ajpa.1330970202. [DOI] [PubMed] [Google Scholar]
- 54.Baker G, Jones LHP, Wardrop ID. Cause of wear in sheeps’ teeth. Nature. 1959;184(4698):1583–1584. doi: 10.1038/1841583b0. [DOI] [PubMed] [Google Scholar]
- 55.Sanson GD, Kerr SA, Gross KA. Do silica phytoliths really wear mammalian teeth? Journal of Archaeological Science. 2007;34(4):526–531. [Google Scholar]
- 56.Lucas PW, Gaskins JT, Lowrey TK, et al. Evolutionary optimization of material properties of a tropical seed. Journal of the Royal Society Interface. 2012;9(66):34–42. doi: 10.1098/rsif.2011.0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cuy JL, Mann AB, Livi KJ, Teaford MF, Weihs TP. Nanoindentation mapping of the mechanical properties of human tooth enamel. Archives of Oral Biology. 2002;47(4):281–291. doi: 10.1016/s0003-9969(02)00006-7. [DOI] [PubMed] [Google Scholar]
- 58.Kendall K. Complexities of compression failure. Proceedings of the Royal Society London Series A. 1978;361(1705):245–263. [Google Scholar]
- 59.Gordon KD. A study of microwear on chimpanzee molars: implications for dental microwear analysis. American Journal of Physical Anthropology. 1982;59(2):195–215. doi: 10.1002/ajpa.1330590208. [DOI] [PubMed] [Google Scholar]


