Abstract
Transient A-type K+ channels (IA) in neurons have been implicated in the delay of the spike onset and the decrease in the firing frequency. Here we have characterized biophysically and pharmacologically an IA current in lamprey locomotor network neurons that is activated by suprathreshold depolarization and is specifically blocked by catechol at 100 μM. The biophysical properties of this current are similar to the mammalian Kv3.4 channel. The role of the IA current both in single neuron firing and in locomotor pattern generation was analyzed. The IA current facilitates Na+ channel recovery from inactivation and thus sustains repetitive firing. The role of the IA current in motor pattern generation was examined by applying catechol during fictive locomotion induced by N-methyl-d-aspartate. Blockade of this current increased the locomotor burst frequency and decreased the firing of motoneurons. Although an alternating motor pattern could still be generated, the cycle duration was less regular, with ventral roots bursts failing on some cycles. Our results thus provide insights into the contribution of a high-voltage-activated IA current to the regulation of firing properties and motor coordination in the lamprey spinal cord.
Coordinated motor patterns are generated by neural circuits, the activity of which depends on the intrinsic properties of single neurons and their synaptic interactions. Although a few neural circuits underlying rhythmic motor patterns have been identified and characterized in some detail (1–4), our understanding of their function remains incomplete. A fundamental understanding of how neural circuits generate and control behavior requires knowledge about the role of different subclasses of ion channels in the function of network neurons and thereby in the regulation of information processing, synaptic interactions, and the operation of the network as a whole. The importance of individual classes of ion channel in regulating the activity of neural networks has been studied in a few vertebrate (5–7) and invertebrate (8–10) preparations.
The present study identifies a high-voltage-activated A-type K+ current in the lamprey spinal cord and investigates its importance in controlling the firing properties of single neurons and thereby in the generation of coordinated locomotor patterns. Transient A-type K+ channels can act as a control mechanism for neuronal excitability (11, 12). Different A-type K+ channels have been cloned and characterized biophysically in single-cell expression systems (13). They can be subdivided into low- and high-voltage-activated channels, depending on their voltage activation range. Low-voltage-activated currents generally activate at potentials below the spike threshold, whereas high-voltage-activated currents are only activated by membrane potential depolarizations above the spike threshold. Low-voltage-activated currents delay the onset of action potentials, decrease the firing frequency of neurons (11, 12, 14), influence dendritic integration and propagation of information (15, 16), and gate the propagation of action potentials in branched axons (17). However, because of the lack of specific blockers, the role of the high-voltage-activated channels in regulating the excitability of neurons and the pattern of activity in a neural network has proved more difficult to investigate.
The in vitro preparation of the lamprey spinal cord provides a model system in which a detailed analysis of how ion channels contribute to the generation of motor behavior can be undertaken. In this preparation, the neural network underlying swimming activity has been partially characterized. Here we have used a selective pharmacological agent, catechol, to characterize a high-voltage-activated A-type current in lamprey spinal cord neurons and thus examine its role in single neuron firing and in rhythmic motor pattern generation. We show that this current facilitates Na+ channel recovery from inactivation and thus plays a critical role in the sustained repetitive firing of neurons. Furthermore, a blockade of this current significantly alters the motor pattern in the intact spinal cord, suggesting an intrinsic role for the current in the production of locomotory behavior.
Materials and Methods
Cell Dissociation.
The spinal cord of larval and young adult lampreys (Petromyzon marinus) was dissociated in Leibovitz's L-15 culture medium (Sigma) supplemented with penicillin-streptomycin (2 μl/ml; Sigma); the osmolarity was adjusted to 270 mOsm (18). Before dissociation, motoneurons (MNs) were retrogradely labeled by applying fluorescein-coupled dextran amine to the remaining muscle tissue along the entire length of the preparation. All dorsal roots were cut to allow the transport of the dye only through the ventral roots to label MNs. Crossed caudally projecting interneurons (CCINs) were labeled by injecting fluorescein-coupled dextran amine on one side of the spinal cord and dissociating the contralateral side of the spinal cord rostral to the injection site. After 24 h of incubation to allow transport of the dye, the spinal cord was treated with collagenase (1 mg/ml, 30 min; Sigma) and then protease (2 mg/ml, 45 min; Sigma). The tissue was subsequently washed with the culture medium and triturated through a sterilized pipette. The dissociated cells were distributed in Petri dishes and incubated at 10–12°C for 1–4 days.
Electrophysiology.
Whole-cell recordings were made of identified MNs and CCINs and unidentified neurons with an Axopatch 200A patch-clamp amplifier (Axon Instruments, Foster City, CA). The unidentified neurons included in this study were either monopolar or multipolar and had a small diameter corresponding mainly to MNs and interneurons (18). The mechanosensory dorsal cells could easily be identified by their round and large cell bodies (18). Because these neurons are not part of the locomotor network they were not included in this study. The recordings had a series resistance ranging from 4 to 10 MΩ that was compensated for electronically by 75–85%. Linear leak and residual capacity currents were subtracted on-line with the use of a P/4 subtraction protocol. Neurons were clamped at a holding potential of −80 mV or −120 mV, and currents were evoked by 30- to 100-ms depolarizing voltage steps applied at 10-s intervals. To isolate K+ currents, Na+ and Ca2+ channels were blocked. The cells were perfused through a gravity-driven six-barrel microperfusion system with the nozzle positioned close to the recorded cell. The control solution contained 124 mM NaCl, 2 mM KCl, 1.2 mM MgCl2, 5 mM CaCl2, 10 mM glucose, and 10 mM Hepes, with the pH adjusted to 7.6. For whole-cell recordings, the pipettes were filled with a solution containing 102 mM KCH3SO3, 1.2 mM MgCl2, 1 mM CaCl2, 10 mM EGTA, 10 mM glucose, and 10 mM Hepes, with the pH adjusted to 7.6 with KOH. The liquid junction potential ranged between 3 mV and 5 mV and was not corrected for. Membrane currents and voltages were controlled and recorded with a personal computer and pclamp software (Axon Instruments). Current and voltage signals were sampled at 10 kHz or 100 kHz, and the analysis was performed with the use of pclamp8 or origin (Microcal Software, Northampton, MA).
Extracellular measurements of ventral root activity were performed on spinal cord/notochord preparations of adult Lampetra fluviatilis. Intracellular recordings were made from the isolated spinal cord preparation (5). The preparation was mounted in a cooled (8–12°C) Sylgard-lined chamber, which was continuously perfused with a solution of the following composition: 138 mM NaCl, 2.1 mM KCl, 1.8 mM CaCl2, 1.2 mM MgCl2, 4 mM glucose, 2 mM Hepes, and 0.5 mM l-glutamine. The solution was bubbled with O2 and the pH was adjusted to 7.4. Fictive locomotion was induced by a bath application of N-methyl-d-aspartate (100 μM). Intracellular recordings were made of gray matter neurons with thin-walled microelectrodes filled with K-acetate (4 M). Current injections were made in discontinuous current-clamp mode with an Axoclamp 2B amplifier.
Drugs.
Cathecol, tetrodotoxin, tetraethylammonium, and 4-aminopyridine (4-AP) were purchased from Sigma. Dendrotoxin-I and α-dendrotoxin) were obtained from the Peptides Institute (Barnet, U.K.), and N-methyl-d-aspartate was from Tocris Cookson (Bristol, U.K.).
Analysis.
To obtain the K+ current activation and inactivation curves, values of the chord conductance (G) were calculated from the respective peak currents assuming ohmic behavior. The K+ equilibrium potential was determined by using the Nernst equation. Spike peak corresponds to the maximum potential reached by the action potential. Spike width was measured at half the spike amplitude. Time to peak was measured as the time between the onset of the stimulus and the peak of the action potential or current. The fast afterhyperpolarization (fAHP) was measured as the voltage minimum directly following the action potential. Experiments with fictive locomotion were analyzed with the use of the datapac analysis program (Run Technologies, Laguna Hill, CA). The cycle duration was calculated as the time interval between midpoints of two successive bursts in a single ventral root and averaged over 600–1,000 cycles. The burst proportion was defined as the ratio of burst duration and cycle duration. Unless otherwise stated the results are expressed as means ± SD. Means were compared with the use of Student's t test or one-way ANOVA (graphpad).
Results
Pharmacological Characterization of the IA Current.
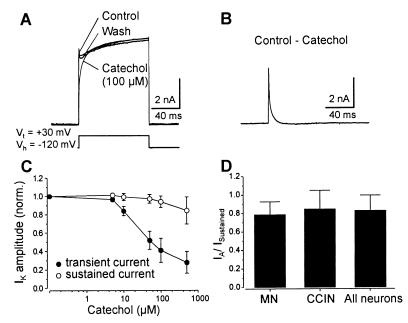
Whole-cell patch-clamp recordings were made of dissociated lamprey spinal cord neurons. The membrane potential of the neurons was held at −120 mV, and depolarizing voltage steps to +30 mV were applied to activate voltage-gated K+ channels, whereas Na+ and Ca2+ channels were blocked by tetrodotoxin (150 nM) and cadmium (100 μM), respectively. All neurons studied (n = 65), including identified MNs (n = 11) and CCINs (n = 5), displayed an outward K+ current that consisted of a transient and a sustained component (Fig. 1A). Catechol (100 μM) reversibly blocked the transient current (Fig. 1A). Fig. 1B shows the fast transient current that was blocked by catechol (100 μM; catechol response subtracted from control). This current had an amplitude of 7.6 nA ± 2.5 nA (n = 65), reached the peak amplitude within 1.0 ms ± 0.2 ms, and inactivated rapidly with a time constant τ = 2.0 ms ± 0.9 ms (Fig. 1B). The effect of catechol on the transient current was dose-dependent (Fig. 1C). Catechol (10–100 μM) specifically blocked the transient current by 59% ± 13% (n = 12) but had no effect on the sustained current (Fig. 1C). Catechol (500 μM) further reduced the amplitude of the transient current by 72% ± 11% but also decreased the sustained current by 15% ± 14% (n = 8; Fig. 1C). The block of the transient current by catechol was not voltage-dependent, as there was no significant difference in the amount of inhibition that occurred at the different test potentials (not shown; n = 15). The fraction of the transient to sustained K+ current was compared between the different types of neurons studied (Fig. 1D). In MNs this fraction was 0.78 ± 0.14 (n = 11) and was not significantly different from that of CCINs (0.83 ± 0.17; n = 5) or that of all neurons examined (0.84 ± 0.20; n = 65).
Figure 1.
Pharmacology of the IA current. (A) Recordings from a MN in which catechol (100 μM) reversibly blocked the transient IA current without affecting the sustained current. (B) The subtracted current blocked by catechol. (C) Dose–response curves showing the effect of catechol on the IA and sustained currents. (D) Ratio of the transient to sustained current in MNs, CCINs, and all of the neurons studied.
The transient current was also blocked by 4-AP (0.5–2 mM; n = 21), which always in addition affected the sustained current (not shown). The effect of 4-AP was poorly reversible after washout. Neither the transient nor the sustained current was affected by α-dendrotoxin or dendrotoxin-I (500 nM; n = 5; not shown). These results show that lamprey spinal cord neurons possess a fast activating and inactivating K+ current corresponding to an IA current that is specifically blocked by catechol at 100 μM.
Biophysical Characterization of the IA Current.
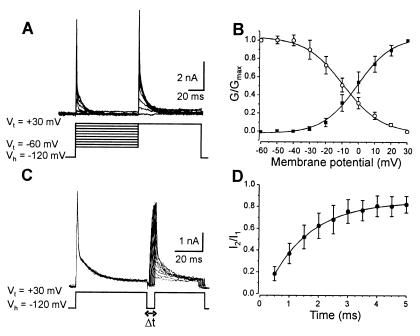
Taking advantage of its sensitivity to catechol, we isolated the IA current and determined the activation, inactivation, and recovery kinetics (Fig. 2). Depolarizing steps between −60 and +30 mV with +10-mV increments were applied from a holding potential of −120 mV in control and in catechol (100 μM). Subtraction of the current evoked in catechol from that evoked in control isolated a rapidly inactivating IA current (Fig. 2A). The activation curve was fitted with a single Boltzmann function with a half-activation potential of −1.0 mV ± 1.0 mV and a slope factor of 10.6 mV ± 0.9 mV (Fig. 2B). The steady-state inactivation was studied by applying conditioning steps (80 ms) to membrane potentials between −60 and +30 mV before a test step to +30 mV in control and in catechol (100 μM). The steady-state inactivation curve was obtained by plotting the mean normalized conductance as a function of conditioning potential (n = 15; Fig. 2B). The data could be fitted with a single Boltzmann function with a half-inactivation potential of −9.3 ± 1.04 mV and a slope factor of −11.7 ± 1.05 mV (Fig. 2B).
Figure 2.
Activation, inactivation, and recovery from inactivation of IA current. (A) A double test voltage step was applied in the control and in catechol (100 μM), the first to Vt between −60 and +30 mV and the second to +30 mV. IA current was isolated by subtracting the current evoked in catechol from that evoked in control. (B) The activation curve (■) was fitted with the best-fitting Boltzmann equation: G/Gmax = (1 + e(V − V1/2)/k)−1. The steady-state inactivation curve (○) was determined by plotting the mean normalized conductance as a function of the membrane potential. (C) A two-voltage-step protocol to +30 mV applied at increasing intervals was used to determine the recovery from inactivation of the IA current. The traces shown correspond to the current blocked by catechol. (D) The recovery from the inactivation curve corresponds to the mean normalized IA current amplitude as a function of the interpulse duration.
The recovery from inactivation was studied according to a double-pulse protocol. The IA current was inactivated by the first pulse, and a second pulse was applied at increasing intervals to determine the time necessary for the current to recover from inactivation (Fig. 2C). The recovery from inactivation was determined by plotting the mean normalized current amplitude as a function of the interpulse duration and was approximated by a single exponential with a time constant τ = 1.4 ms ± 0.07 ms (mean ± SEM; n = 15; Fig. 2D). The IA current displays a fast recovery from inactivation, and it is available for reactivation within 4 ms after the end of the pulse.
The IA Current Is Important for Repetitive Firing in Neurons.
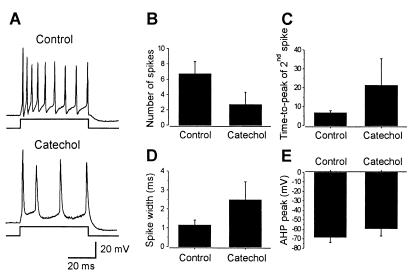
The specificity of catechol in blocking the IA current enabled us to analyze the importance of these channels in neuronal firing properties. Whole-cell recordings were made under current clamp conditions to measure changes in the firing; spike onset, amplitude, and duration; and the amplitude of the fAHP. In these experiments Ca2+ channels (and, indirectly, Ca2+-dependent K+ channels) were blocked by cadmium (100 μM). In the control an application of depolarizing current pulses activated multiple action potentials, and catechol (100 μM) reversibly reduced the number of action potentials elicited by the same stimulus to one (n = 11 of 15) or two (n = 4 of 15; Fig. 3A) spikes. To determine whether catechol affects the repetitive firing of neurons at different current strengths, the relationship between the current injected and the number of spikes evoked was examined in the control and in catechol (Fig. 3B). In catechol (100 μM), the neurons fired only one or two spikes at each current strength (Fig. 3B; n = 7). These changes were accompanied by a significant increase in the peak amplitude of the first spike from +66 ± 3.6 mV in the control to + 77 ± 11.2 mV in catechol (P < 0.05; n = 8; Fig. 3C, black bars) and a decrease in the peak of the second spike, when evoked, from +61 ± 4.2 mV to +33 ± 18.6 mV (P < 0.05; n = 4; Fig. 3C, white bars). When a second spike occurred in catechol, its time to peak was always delayed, from 4 ± 1.6 ms to 7 ± 1.1 ms (75% increase; P < 0.05; n = 4; Fig. 3D, white bars), whereas neither the threshold (Fig. 3A) nor the time to peak of the first spike was affected (Fig. 3D, black bars). A blockade of the IA current also induced changes in the spike width and in the amplitude of the fAHP. In the control, the first spike width was 0.54 ± 0.11 ms, and it was increased to 1.12 ± 0.25 ms by catechol (105% increase; P < 0.001; n = 8; Fig. 3E). The fAHP following the first spike reached a membrane potential of −75 ± 8.7 mV in the control, and in catechol it reached −50 ± 4.3 mV (P < 0.001; n = 8; Fig. 3F). All of the measured parameters recovered to control values after washout of catechol. In addition, catechol had no effect on the membrane potential or input resistance of the recorded neurons. The IA current thus plays an important role in repetitive firing and spike repolarization.
Figure 3.
Role of IA current in repetitive firing in neurons in culture. (A) Application of a depolarizing current to isolated neurons in culture elicited repetitive firing of action potentials. Catechol blocked the repetitive firing. The membrane potential of the neuron was held at −80 mV. (B) Relationship between the current injected and the number of spikes evoked in the control and catechol (100 μM). (C) Catechol reversibly increased the amplitude of the first action potential and decreased that of the second action potential when it occurred. (D) The time to peak of the first action potentials was unaffected by catechol, whereas it was reversibly delayed for the second action potential. (E) The width of action potentials was reversibly increased by catechol. (F) The amplitude of the fAHP was reversibly reduced by catechol.
To determine whether similar changes in firing properties occur in neurons in situ, intracellular recordings with sharp electrodes were carried out in the intact spinal cord in vitro. In these experiments Ca2+ channels were not blocked. Repetitive firing was elicited by a depolarizing current injection in control (Fig. 4A). Application of catechol (100 μM) reduced the average number of spikes per stimulus (60 ms), calculated from all of the neurons tested (n = 6), from 6.7 ± 1.63 in the control to 2.7 ± 1.63 in catechol (P < 0.001; Fig. 4B). The time to peak of the second spike was increased from 6.7 ± 1.2 ms to 21.3 ± 14.1 ms (P < 0.02; n = 6; Fig. 4C), and the width of the first spike was increased from 1.1 ± 0.27 ms to 2.5 ± 0.95 ms (P < 0.01; n = 6; Fig. 4D). The peak fAHP following the first spike was −68 ± 5.8 mV in the control, and it was reduced to −59 ± 8.0 mV in catechol (P < 0.02; n = 6; Fig. 4E). Catechol thus had similar effects on spike width, the fAHP, and time to peak of the second spike in neurons in situ as compared with neurons in culture.
Figure 4.
Role of IA current in repetitive firing in neurons in the isolated spinal cord. (A) Application of a depolarizing current to neurons recorded in the intact spinal cord elicited repetitive firing of action potentials. Catechol reduced the repetitive firing. The neuron had a resting membrane potential of −75 mV. (B) Catechol reduced the number of action potentials elicited by current injection and increased the time to peak of the second action potential (C) and the width of action potentials (D). (E) The amplitude of the fAHP was reduced by catechol.
The IA Current Maintains Repetitive Firing by Promoting Recovery of Na+ Channel Inactivation.
The experiments reported above show that the IA current is important for sustained repetitive firing. How does the activation of IA current contribute to repetitive firing? The fAHP mediated by the IA current could facilitate the recovery of Na+ channels from inactivation, and when this current is blocked, Na+ channels remain inactivated for a much longer time after the first spike and repetitive firing cannot be sustained. To test for this possibility, experiments were performed on dissociated neurons in culture, where a test depolarizing pulse was preceded by a conditioning pulse with decreasing time intervals (Fig. 5A). In these experiments Ca2+ channels were blocked by cadmium (100 μM). In the control, there was no change in the firing properties of neurons with a decreasing interval between the conditioning and the test pulse (Fig. 5A) or in the spike amplitude (Fig. 5B, open circles), time to peak (Fig. 5C, open circles), or fAHP. Catechol (100 μM) significantly decreased the amplitude of spikes elicited by the test pulse when it was delivered ≤4.5 ms after the conditioning pulse (P < 0.01; one-way ANOVA; n = 9; Fig. 5 A and B, filled circles). In neurons that fired two spikes in catechol, a decrease in the interval between the conditioning and test pulses resulted in a significant delay of the time to peak of the first spike, whereas the second spike was not elicited when the interval was ≤4.5 ms (P < 0.01; one-way ANOVA; n = 9; Fig. 5C, filled circles). There was no significant difference in the membrane potential measured just before the onset of the test pulse in the control and in catechol (Fig. 5D). This lack of a difference in the potential indicates that the changes in spike amplitude and time to peak induced by catechol cannot be accounted for by a depolarization of the membrane potential, when the two pulses occurred close together.
Figure 5.
IA current facilitates Na+ channel recovery from inactivation. (A) Action potentials were elicited in a neuron in culture by a conditioning pulse followed by a test pulse with decreasing time intervals. Catechol decreased the repetitive firing induced by both the conditioning and test pulse. The membrane potential of the neuron was held at −80 mV. (B) In the control, the amplitude of action potentials induced by the test pulse was unaffected by decreasing the interval between the conditioning and test pulse. Catechol significantly decreased the amplitude of action potentials when the test pulse was delivered at ≤4.5 ms after the conditioned pulse. (C) Catechol significantly increased the time to peak when the interval between the conditioning and test pulse was ≤4.5. (D) The membrane potential at the start of the test pulse was not significantly changed by a decrease in the interval between the two pulses in the control and catechol.
The spike broadening produced by blocking KA channels with catechol will result in more Na+ channels being inactivated during the action potential. Moreover, because of a blockade of the fAHP, the rate of recovery of Na+ channel inactivation following the action potential will be slower, resulting in a significant decrease in the amount of recovery during the interspike interval. Thus, fewer Na+ channels will be available to depolarize the neuron in the period leading up to the next spike.
Blocking the IA Current Modulates the Activity of the Locomotor Pattern Generator.
To examine the role of the IA current in network operation, catechol (100 μM) was applied during locomotor activity in the intact spinal cord. Fictive locomotion was induced by N-methyl-d-aspartate (100 μM) (19), and rhythmic activity was recorded simultaneously in ventral roots and motoneurons in the intact spinal cord in vitro (n = 7). Under control conditions, the swimming motor pattern consisted of ventral root burst activity that alternated between the left and right sides with a cycle duration of 0.71 s ± 0.43 s (n = 7), whereas MNs received synaptic locomotor drive input and fired action potentials. After the IA current was blocked by catechol (100 μM), the cycle duration decreased to 0.43 ± 0.15 s (P < 0.0001, n = 7; Fig. 6 A and B), and MNs fired only one or two action potentials per cycle (Fig. 6 A and E). Although an alternating pattern still occurred, the cycle duration was less regular, with missing ventral root bursts (Fig. 6A). The ventral root burst proportion of the locomotor cycle decreased significantly from 0.30 ± 0.09 in the control to 0.22 ± 0.10 in catechol (P < 0.0001, n = 7; Fig. 6C). The alternation phase relationship between left and right ventral roots was unchanged, however (Fig. 6D). In all MNs recorded, the number of action potentials per cycle decreased from 2.5 ± 1.49 in the control to 1.1 ± 0.75 in catechol (P < 0.0001, n = 7; Fig. 6E). Thus, the IA current plays a critical role in coordinated locomotor activity.
Figure 6.
Blockade of IA current affected the locomotor pattern. (A) Intracellular recording was made from a MN (trough potential = −70 mV), and alternating ventral root bursts were recorded in ipsilateral (i-vr) and contralateral (c-vr) ventral roots. Catechol increased the frequency of the locomotor rhythm, which became irregular. (B) Catechol decreased the cycle duration and the burst proportion (C). (D) The left–right phase relationship was unchanged in catechol. (E) The number of action potentials fired by MNs during each locomotor cycle was reduced by catechol.
Discussion
This study provides insights into the contribution of a high-voltage-activated IA current to the control of single neuron firing properties and to the operation of the neural circuit underlying rhythmic locomotor activity.
Similarities Between the Lamprey IA Current and Cloned Mammalian K+ Channels.
Our results show that lamprey spinal cord neurons possess a fast activating and inactivating A-type K+ current that activates at a relatively high voltage range and is blocked selectively by catechol at 100 μM. The IA current in lamprey spinal cord neurons activates rapidly, reaching the peak current within 1 ms after the onset of the stimulus. It has a half-activation potential of about −1 mV and inactivates rapidly, with a time constant of 2.0 ms and a half-inactivation potential around −10 mV. This current is blocked specifically by catechol and is sensitive to 4-AP but not to dendrotoxin. 4-AP also affected the sustained current. Catechol has been shown to block low-voltage-activated IA current (20–23) but has no effect on Na+ or Ca2+ currents (data not shown; see ref. 20) or on Ca2+-dependent K+ currents (24).
Among the cloned mammalian A-type channels only those belonging to the Kv3 gene family show a high voltage range for activation (13, 25). This observation suggests that the lamprey native channels might correspond to one of the members of the Kv3 gene family. Four Kv3 channels (3.1, 3.2, 3.3, 3.4) have been identified, two of which (Kv3.1 and Kv3.2) correspond to the delayed-rectifier-type current in expression systems, whereas the other two (Kv3.3 and Kv3.4) express an A-type current. The latter channels show different rates of inactivation, with Kv3.4 being inactivated an order of magnitude faster (τ ≈ 10 ms) than Kv3.3 (τ ≈ 240 ms). Kv3.4 is blocked by 4-AP with an IC50 of 0.5–0.6 mM but is not affected by dendrotoxin (see ref. 13). Because the lamprey native A-type channels display a rapid inactivation and are sensitive to 4-AP but not to dendrotoxin, they are more similar to Kv3.4 rather than to Kv3.3.
The IA Current Supports Sustained Repetitive Firing by Facilitating Na+ Channel Recovery from Inactivation.
The specificity of the block of this current by catechol allowed us to determine its role in controlling the firing properties of neurons and the pattern generation in the locomotor network. Blockade of the IA current by catechol had no effect on the spike onset and threshold; however, it severely affected the sustained firing of neurons. When the IA current was available, neurons fired several action potentials in response to a depolarizing current pulse, but fired only one or two action potentials after a blockade of the IA current. In contrast, low-voltage-activated IA currents have a different role (11, 12, 26). They activate below the threshold for action potentials and are inactivated at the resting membrane potential. When activated, they tend to oppose the depolarizing drive and thus delay the onset of the action potential. During repetitive firing the low-voltage-activating IA currents slow down the frequency of repetitive discharges by decreasing the rate of decay of the AHP following the action potential (14). In lamprey spinal cord neurons, the high-voltage-activated IA current is instead responsible for the action potential repolarization and the fAHP. Blockade of this current broadens the action potentials and decreases the amplitude of the fAHP. Because these channels have a fast activation and recovery from inactivation, they are sufficiently activated by the brief action potential and will thus determine the rate of repolarization. Furthermore, the duration of the fast hyperpolarization is sufficient for the IA current to recover from inactivation and become available during the next action potential. This current thus has specific biophysical properties, including its high-voltage activation and fast recovery from inactivation, that will allow it to support sustained firing in neurons. How can these properties account for the role of the IA current in maintaining sustained firing? The fact that this current activates above the threshold for action potentials may allow it to facilitate Na+ channel recovery from inactivation by rapidly repolarizing the membrane potential and thus shortening the duration of the action potential. After a blockade of the IA current, the action potentials become broader, resulting in a greater Na+ channel inactivation during the action potential. Furthermore, the rate of recovery from inactivation of Na+ channels following the spike is slowed down because of the blockade of the fAHP. These effects would result in a significant decrease in the amount of recovery from inactivation during the interspike interval, and thus fewer Na+ channels would be available to generate the next spike. The results of experiments in which a test current pulse was preceded by a conditioning pulse support this interpretation. This study thus presents experimental evidence suggesting that the high-threshold-activated, Kv3.4-like IA current can maintain sustained repetitive firing by facilitating Na+ channel recovery from inactivation.
In hippocampal basket interneurons, blockade of Kv3.1, Kv3.2 channels, underlying the high-voltage-activated delayed rectifier K+ current, converted the fast spiking phenotype into a slower spiking pattern (27). Auditory neurons also possess high-voltage-activated delayed rectifier K+ channels with physiological and pharmacological properties similar to those of Kv3.1 (28). Blockade of these channels altered the ability of these neurons to follow stimulus frequencies greater than 200 Hz, but not at lower frequencies (28). In fast spiking neocortical interneurons, blockade of Kv3.1, Kv3.2 currents affects sustained, but not transient, early high-frequency firing (29). In contrast to these studies, our results show that the high-voltage-activated IA current is necessary for the occurrence of the high-frequency firing in the early part of the spike train as well as sustained firing.
Role of the IA Current in Network Function.
The role of two types of distinct K+ currents in neural circuit function has been examined in Xenopus embryos (6). The two currents could be separated by their kinetics and pharmacology: a fast activating current blocked by catechol and a slow current sensitive to dendrotoxin. The slow activating current has been suggested to play a critical role in controlling the excitability of neurons and the generation of locomotor activity. In this preparation a small IA current was present in some neurons, and its role in pattern generation was not examined.
The present study demonstrates that a high-voltage-activated IA current is present in lamprey locomotor network neurons and that its blockade by catechol significantly alters the locomotor network activity. The cycle duration was decreased, as was the ventral root burst proportion and the number of action potentials fired by motoneurons during each locomotor cycle. A decrease in firing of neurons on one side will allow neurons on the contralateral side to become activated earlier as the midcycle inhibition is reduced, resulting in a faster alternation between left and right ventral roots and thereby increasing the locomotor burst frequency. Others factors, brought into action by a blockade of the IA current, could also contribute to the change in the frequency of the locomotor rhythm.
In conclusion, we have characterized a high-voltage-activated IA current with biophysical properties allowing it to regulate the repetitive firing in single neurons and thus to contribute to the generation of a coordinated motor pattern in the lamprey spinal cord.
Acknowledgments
We thank Drs. S. Grillner, P. Kettunen, P. Larsson, D. Parker, and P. Wallén for their comments on the manuscript and Dr. B. Lamotte d'Incamps for valuable discussions. We also thank H. Axegren and M. Bredmyr for skillful technical assistance. This work was supported by the Swedish Medical Research Council (Project 11562), the Swedish Foundation for Strategic Research, the Erikssons Stiftelse, the Jeanssons Stiftelse, the Wiberg Stiftelse, and Karolinska Institutet funds. D.H. received a fellowship from the Deutsche Forschungsgemeinschaft, Germany.
Abbreviations
- CCINs
crossed caudally projecting interneurons
- fAHP
fast afterhyperpolarization
- 4-AP
4-aminopyridine
- MN
motoneuron
References
- 1.Arshavsky Y I, Orlovsky G N, Panchin Y V, Roberts A, Soffe S R. Trends Neurosci. 1993;16:227–232. doi: 10.1016/0166-2236(93)90161-e. [DOI] [PubMed] [Google Scholar]
- 2.Marder E, Calabrese R L. Physiol Rev. 1996;76:687–717. doi: 10.1152/physrev.1996.76.3.687. [DOI] [PubMed] [Google Scholar]
- 3.Grillner S, Ekeberg Ö, El Manira A, Lansner A, Parker D, Tegnér J, Wallén P. Brain Res Rev. 1998;26:184–197. doi: 10.1016/s0165-0173(98)00002-2. [DOI] [PubMed] [Google Scholar]
- 4.Delgado-Lezama R, Hounsgaard J. Prog Brain Res. 1999;123:57–63. doi: 10.1016/s0079-6123(08)62844-7. [DOI] [PubMed] [Google Scholar]
- 5.El Manira A, Tegnér J, Grillner S. J Neurophysiol. 1994;72:1852–1861. doi: 10.1152/jn.1994.72.4.1852. [DOI] [PubMed] [Google Scholar]
- 6.Kuenzi F M, Dale N. J Neurosci. 1998;18:1602–1612. doi: 10.1523/JNEUROSCI.18-04-01602.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thoby-Brisson M, Telgkamp P, Ramirez J M. J Neurosci. 2000;20:2994–3005. doi: 10.1523/JNEUROSCI.20-08-02994.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris-Warrick R M, Coniglio L M, Barazangi N, Guckenheimer J, Gueron S. J Neurosci. 1995;15:342–358. doi: 10.1523/JNEUROSCI.15-01-00342.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baro D J, Levini R M, Kim M T, Willms A R, Lanning C C, Rodriguez H E, Harris-Warrick R M. J Neurosci. 1997;17:6597–6610. doi: 10.1523/JNEUROSCI.17-17-06597.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nadim F, Calabrese R L. J Neurosci. 1997;17:4461–4472. doi: 10.1523/JNEUROSCI.17-11-04461.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rogawski M A. Trends Neurosci. 1985;8:214–219. [Google Scholar]
- 12.Pongs O. FEBS Lett. 1999;452:31–35. doi: 10.1016/s0014-5793(99)00535-9. [DOI] [PubMed] [Google Scholar]
- 13.Coetzee W A, Amarillo Y, Chiu J, Chow A, Lau D, McCormack T, Moreno H, Nadal M S, Ozaita A, Pountney D, et al. Ann NY Acad Sci. 1999;868:233–285. doi: 10.1111/j.1749-6632.1999.tb11293.x. [DOI] [PubMed] [Google Scholar]
- 14.Connor J A, Stevens C F. J Physiol (London) 1971;213:31–53. doi: 10.1113/jphysiol.1971.sp009366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffman D A, Magee J C, Colbert C M, Johnston D. Nature (London) 1997;387:869–875. doi: 10.1038/43119. [DOI] [PubMed] [Google Scholar]
- 16.Schoppa N E, Westbrook G L. Nat Neurosci. 1999;2:1106–1113. doi: 10.1038/16033. [DOI] [PubMed] [Google Scholar]
- 17.Debanne D, Guerineau N C, Gähwiler B H, Thompson S M. Nature (London) 1997;389:286–289. doi: 10.1038/38502. [DOI] [PubMed] [Google Scholar]
- 18.El Manira A, Bussières N. J Neurophysiol. 1997;78:1334–1340. doi: 10.1152/jn.1997.78.3.1334. [DOI] [PubMed] [Google Scholar]
- 19.Grillner S, McClellan A, Sigvardt K, Wallén P, Wilén M. Acta Physiol Scand. 1981;113:549–551. doi: 10.1111/j.1748-1716.1981.tb06937.x. [DOI] [PubMed] [Google Scholar]
- 20.Ito I, Maeno T. J Physiol (London) 1986;373:115–127. doi: 10.1113/jphysiol.1986.sp016038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erdélyi L, Such G. Neurosci Lett. 1988;92:46–51. doi: 10.1016/0304-3940(88)90740-9. [DOI] [PubMed] [Google Scholar]
- 22.Kehl S J. Neurosci Lett. 1991;125:136–138. doi: 10.1016/0304-3940(91)90010-q. [DOI] [PubMed] [Google Scholar]
- 23.Sah P, McLachlan E M. J Neurophysiol. 1992;68:1834–1841. doi: 10.1152/jn.1992.68.5.1834. [DOI] [PubMed] [Google Scholar]
- 24.Inokuchi H, McLachlan E M, Meckler R L. Naunyn-Schmiedeberg's Arch Pharmacol. 1997;355:609–618. doi: 10.1007/pl00004991. [DOI] [PubMed] [Google Scholar]
- 25.Rudy B, Chow A, Lau D, Amarillo Y, Ozaita A, Saganich M, Moreno H, Nadal M S, Hernandez-Pineda R, Hernandez-Cruz A, et al. Ann NY Acad Sci. 1999;868:304–343. doi: 10.1111/j.1749-6632.1999.tb11295.x. [DOI] [PubMed] [Google Scholar]
- 26.Rudy B. Neuroscience. 1988;25:729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- 27.Martina M, Schultz J H, Ehmke H, Monyer H, Jonas P. J Neurosci. 1998;18:8111–8125. doi: 10.1523/JNEUROSCI.18-20-08111.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang L Y, Gan L, Forsythe I D, Kaczmarek L K. J Physiol (London) 1998;509:183–194. doi: 10.1111/j.1469-7793.1998.183bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Erisir A, Lau D, Rudy B, Leonard C S. J Neurophysiol. 1999;82:2476–2489. doi: 10.1152/jn.1999.82.5.2476. [DOI] [PubMed] [Google Scholar]