Abstract
Clostridium sordellii and Clostridium perfringens are infrequent human pathogens; however, the case-fatality rates for the infections are very high, particularly in obstetric C. sordellii infections (>90%). Deaths from Clostridium sordellii and Clostridium perfringens toxic shock (CTS) are sudden, and diagnosis is often challenging. Formalin-fixed, paraffin-embedded (FFPE) tissues usually are the only specimens available for sudden fatal cases, and immunohistochemistry (IHC) for Clostridia is generally performed but it cannot identify species. A clear need exists for a rapid, species-specific diagnostic assay for FFPE tissues. We developed a duplex PCR-based microsphere assay for simultaneous detection of C. sordellii and C. perfringens and evaluated DNA extracted from 42 Clostridium isolates and FFPE tissues of 28 patients with toxic shock/endometritis (20 CTS, 8 non-CTS, as confirmed by PCR and sequencing). The microsphere assay correctly identified C. sordellii and C. perfringens in all known isolates and in all CTS patients (10 C. sordellii, 8 C. perfringens, 2 both) and showed 100% concordance with PCR and sequencing results. The microsphere assay is a rapid, specific, and cost-effective method for the diagnosis of CTS and offers the advantage of simultaneous testing for C. sordellii and C. perfringens in FFPE tissues using a limited amount of DNA.
1. Introduction
Clostridium species are ubiquitous Gram-positive, anaerobic, spore-forming bacteria that are generally found in soil and in the intestinal tract of humans and other animals. Clostridium sordellii has been reported to cause a variety of diseases including peritonitis, endocarditis, pneumonia, arthritis, cellulitis, and myonecrosis [1–4]. Fulminant toxic shock syndrome and sepsis among previously healthy persons have been described most often in cases associated with gynecologic infections and neonatal omphalitis [3, 5]. Clostridium perfringens is also responsible for a number of clinical conditions in humans ranging from acute food poisoning and enteritis to gas gangrene, enterotoxemia, and endometritis [6–8]. In the last couple of years, several studies reported pregnancy-associated fatal toxic shock syndrome cases due to C. sordellii and C. perfringens infections [8–10]. Among all C. sordellii infections reported in the literature, the overall case fatality ratio is 70%, while, for obstetric infections, it is more than 90% [8]. Pregnancy-associated C. perfringens fulminant septicemia also carries a very high mortality rate [8]. Deaths from C. sordellii and C. perfringens toxic shock (CTS) are sudden, and most patients die from hypotension and multiorgan failure within hours to a few days after the initial presentation.
A presumptive clinical diagnosis of CTS can be challenging and often confounded by nonspecific symptoms, an absence of fever, and the low prevalence of these infections [10]. A confirmatory diagnosis can only be performed by conventional microbiological isolation and characterization methods including bacterial culture, biochemical analysis, or enzyme-linked immunosorbent (ELISA) assay. However, the fastidious anaerobic growth, variable staining characteristics, and complex biochemical profiles of Clostridium species make them difficult to isolate [11, 12]. In addition, for the fatal cases, involving sudden death, unavailability of appropriate specimens in adequate amount poses further challenge. Formalin-fixed, paraffin-embedded (FFPE), archival autopsy or biopsy tissues are often the only specimens available for the fatal cases, and they are unsuitable for microbial methods. For FFPE tissues, generally tissue-based diagnostic methods such as histopathology, special stains, and immunohistochemistry (IHC) are used; however, these methods cannot identify specific Clostridium species.
In the last couple of years, we reported detection of C. sordellii and C. perfringens in FFPE tissues of several fatal obstetrical cases using the conventional, agent-specific toxin genes PCR assays and sequencing [8–10]. These assays can identify species; however, the conventional PCRs and sequencing can be time-consuming, laborious, and expensive. In addition, to perform multiple conventional PCR assays, an adequate amount of patient specimen is required, which is often limiting in cases of sudden death. All the above-mentioned factors highlighted the need to develop a rapid, sensitive, and specific multiplex diagnostic assay such as multiplex PCR. However, traditional multiplex PCR/real time PCR can be challenging because amplification conditions for multiple targets are often incompatible and the combination of several primers, probes, and reagents in a single reaction generally results in loss of sensitivity and specificity for each of the individual target species. In addition, primer dimer formation and spectral overlap of fluorescent labels when using TaqMan or Molecular Beacons may compromise the ability to interpret quantitative data [13]. Luminex xMAP (multianalyte profiling) technology (Luminex Corp., Austin, TX) addresses these issues via application of microsphere-based suspension arrays and allows for multiplexing. Multiplexed microsphere-based detection of bacteria using the Luminex platform has been described previously [14, 15].
The objective of this study was to develop a rapid duplex PCR-based microsphere assay that can simultaneously detect C. sordellii and C. perfringens in different clinical specimens, including FFPE tissues, and to evaluate its application as a potential diagnostic tool for pregnancy-associated toxic shock syndrome cases in comparison with other tissue-based diagnostic methods, including IHC and conventional PCR.
2. Materials and Methods
2.1. Samples
DNA was extracted from various Clostridium isolates (n = 42) and FFPE tissues of patients with toxic shock/necrotizing endometritis (n = 28) and evaluated by the duplex PCR-based microsphere assay for C. sordellii and C. perfringens. DNA samples of 42 known Clostridium isolates were obtained from the Laboratory Branch, Division of Healthcare Quality Promotion, Centers for Disease Control and Prevention (CDC). All 28 cases were submitted to the Infectious Diseases Pathology Branch (IDPB), CDC, from 2004 to 2009 for diagnostic consultation. Clinical and demographic information on the cases were collected when available from medical records. For the confirmation, identification of all the isolates and diagnosis of all the cases were also performed by conventional diagnostic methods. Clinical information as well as conventional diagnostic assay (PCR, sequencing and IHC) results of all C. sordellii and all except 2 C. perfringens cases were previously published as case reports by our group, and, in this study, these cases were used for validation of the microsphere assay [8–10, 16].
2.2. Conventional Diagnostic Methods
DNA was extracted from FFPE tissues of all the cases using the QIAamp DNA mini kit (QIAGEN) following the tissue extraction protocol as previously described [18]. The DNA samples were evaluated by C. sordellii and C. perfringens specific conventional PCR assays targeting the lethal toxin and alpha toxin genes and sequencing, as we described before [8]. To monitor the quality of extraction and to verify the presence of amplifiable DNA, samples were also tested for the amplification of the house-keeping gene human beta-globin [19]. FFPE tissues of all the cases were analyzed by routine hematoxylin-eosin (H&E) and special stains to evaluate histopathological changes and IHC for Clostridia species was performed to localize Clostridia antigens in deparaffinized, rehydrated 3 μm sections of tissue samples, as we described previously [20]. For the diagnosis of C. sordellii and C. perfringens PCR and IHC negative cases, PCR and/or IHC assays for other suspect bacterial agents were also performed [20–25].
2.3. PCR-Based Microsphere Assay
2.3.1. PCR Primers, Oligonucleotide Capture Probes, and Probe Coupling to Microspheres
The primers, probes sequences, gene targets, and amplification product sizes are summarized in Table 1. The primers and probes were synthesized in the Biotechnology Core Facility, CDC, Atlanta, GA. The reverse primers were biotinylated. The probe sequences were labeled at the 5′end with an amino-modified 6-carbon linker and covalently conjugated to individual carboxylated microspheres (Luminex Corp., Austin, TX) using a carbodiimide coupling procedure [26]. A Beckman Coulter Z2 (Hialeah, FL) was used to count conjugated microsphere stocks to determine concentration. Conjugated microspheres were stored in the dark at 4°C in TE buffer. For each probe, a corresponding 5′ biotinylated, complementary oligonucleotide was designed for testing the specificity and effectiveness of microsphere-probe conjugation.
Table 1.
Oligonucleotide primers and probes used in the duplex microsphere assay.
| Primer | Sequence (5′-3′) | Gene target | Product size (bp) |
|---|---|---|---|
| Clostridium sordellii primers and probes | |||
| CSP09-F primer | TGG GAT GAT TGG GAT TAT TCA G | Phospholipase C of C. sordellii (Csp) | 176 bp |
| CSP09-BR primer | TCA GTT CCT GCA TAT TCA TTG T | Phospholipase C of C. sordellii (Csp) | |
| CSP09 probe | AGA AGC GAT AAA AAA TTC TCA A | Phospholipase C of C. sordellii (Csp) | |
| Clostridium perfringens primers [17] and probes | |||
| PL3-F primer | AAG TTA CCT TTG CTG CAT AAT CCC | Phospholipase C of C. perfringens (plc) | 283 bp |
| PL7-BR primer | ATA GAT ACT CCA TAT CAT CCT GCT | Phospholipase C of C. perfringens (plc) | |
| CP1 probe | TTT AGC AAA ACC TCT TG | Phospholipase C of C. perfringens (plc) |
2.3.2. PCR Amplification
DNA samples of all the cases and isolates were evaluated by the microsphere assay. Initially, phospholipase C genes of C. sordellii and C. perfringens were amplified using a High Fidelity PCR Kit (Roche), 0.3 μM of each primer (final concentration), and 5 μL of template DNA sample in a 50 μL final reaction volume, following the manufacturer's instructions. Amplification was carried out on a GeneAmp 9700 thermocycler (Applied Biosystems), using the following cycle conditions: 94°C/2 minutes; 35 cycles of 94°C/20 seconds, 60°C/30 seconds, and 72°C/30 seconds, followed by a final extension of 72°C/5 minutes.
2.3.3. Hybridization of PCR Amplicons to Microsphere Conjugated Probes and Detection
For hybridization, nonpurified biotinylated PCR products were hybridized to the probe-microsphere complexes under optimized reaction conditions in a 96 conical well PCR plate (Costar no. 6509). Each sample was run in triplicate with six reaction blanks per plate. The probe-conjugated microspheres were pulse-vortexed and sonicated for at least 40 seconds, and a working microsphere mixture was prepared by adding calculated volumes of both sets of conjugated microspheres (C. sordellii and C. perfringens) to 1.5X TMAC buffer (4.5 M tetramethyl ammonium chloride, 75 mM Tris-HCl, pH 8.0, 6 mM EDTA, and 0.15% sarkosyl) to achieve a concentration of 1500 microspheres per set per well.
In each sample well of the PCR plate, 30 μL of microsphere mix and 20 μL of sample mix, composed of 1–5 μL biotinylated PCR amplification product in TE buffer (pH 8.0), were added. Reaction blanks contained only microsphere mix and TE buffer. The PCR plate was covered, and incubated in a thermocycler at 95°C for 5 minutes, followed by 48°C for 30 minutes. After incubation, the reactions were transferred to a filter plate and washed two times with 1X TMAC buffer using a vacuum manifold. Reporter mixture that contained a fresh 1 : 100 dilution of streptavidin-R-phycoerythrin (SA-PE; Molecular Probes, Eugene, OR) in 1X TMAC buffer was added to each well (75 μL), carefully mixed by hand pipetting and transferred to a PCR plate. The plate was covered, and the samples were heated to 48°C for 15 minutes and analyzed at 48°C on the Bio-Plex 200 system. BioPlex Manager Software v. 5.0 (Bio-Rad Hercules, CA) was used for data acquisition. The hybridization of biotinylated PCR amplicons to the probe sequences on the respective microsphere populations generated the Median Fluorescence Intensity (MFI), which represented the SA-PE fluorescence of 100 biotinylated amplicon-bound microspheres. The reported signal for each probe was obtained by subtracting the mean MFI of the blanks from the MFI values of the samples. The MFI value greater than 4 times the mean MFIs of the negative control isolates was designated as the cut-off value for positive signal detection.
3. Results
Organism specific PCR and sequencing identified C. sordellii in 10 cases, C. perfringens in 8 cases, and mixed infection of C. sordellii and C. perfringens in 2 cases of CTS. In the non-CTS cases, PCR and/or IHC assays detected C. difficile in 2 cases and Streptococcus pyogenes, Streptococcus agalactiae, Staphylococcus aureus, Neisseria meningitides, Neisseria gonorrhoeae, or Bacillus cereus was identified in one case each. Demographic and clinical information, pathologic findings, results of conventional diagnostic assays, including organism specific PCR, sequencing, and IHC assay, and the duplex microsphere assay for all the CTS cases are listed in Table 2.
Table 2.
Demographic, clinicopathological findings, and case-by-case comparison of IHC, PCR, and microsphere assay results.
| Case no. | Age | Association with pregnancy | Outcome | Tissue tested | Pathological findings | Clostridia IHC | C. sordellii PCR | C. perfringens PCR | Microsphere assay |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 18 | Medical abortion | Fatal | Uterus | Necrotizing endomyometritis | Positive | Positive | Negative | Positive for C. sordellii |
| 2 | 22 | Medical abortion | Fatal | Uterus | Necrotizing endomyometritis | Positive | Positive | Negative | Positive for C. sordellii |
| 3 | 34 | Medical abortion | Fatal | Uterus | Necrotizing endomyometritis | Positive | Positive | Negative | Positive for C. sordellii |
| 4 | 21 | Medical abortion | Fatal | Uterus | Necrotizing endomyometritis | Positive | Positive | Negative | Positive for C. sordellii |
| 5 | 26 | Medical abortion | Fatal | Endometrium | Necrotizing endomyometritis | Positive | Positive | Negative | Positive for C. sordellii |
| 6 | 25 | Spontaneous abortion | Non-fatal | Placenta | Severe acute chorioamnionitis | Positive | Positive | Negative | Positive for C. sordellii |
| 7 | 32 | No association | Fatal | Uterus | Necrotizing endometritis | Positive | Positive | Negative | Positive for C. sordellii |
| 8 | 18 | Medical abortion | Fatal | Unknown | Necrotizing endometritis | Positive | Positive | Negative | Positive for C. sordellii |
| 9 | 29 | Medical abortion | Fatal | Uterus | Necrotizing endometritis | Positive | Positive | Negative | Positive for C. sordellii |
| 10 | 21 | Medical abortion | Fatal | Decidual and chorionic tissue | Acute inflammation and necrosis | Positive | Positive | Negative | Positive for C. sordellii |
| 11 | 24 | Medical abortion | Fatal | Uterus | Necrotizing endomyometritis | Positive | Negative | Positive | Positive for C. perfringens |
| 12 | 28 | Medical abortion | Fatal | Uterus | Necrotizing endomyometritis | Positive | Negative | Positive | Positive for C. perfringens |
| 13 | 40 | Postpartum | Fatal | Uterus | Acute endomyometritis | Positive | Negative | Positive | Positive for C. perfringens |
| 14 | 41 | Postpartum | Fatal | Uterus | Necrotizing endometritis | Positive | Negative | Positive | Positive for C. perfringens |
| 15 | 37 | Medical abortion | Fatal | Uterus | Necrotizing endometritis | Negative | Negative | Positive | Positive for C. perfringens |
| 16 | 26 | Postpartum | Fatal | Uterus | Necrotic and hemorrhagic uterus | Negative | Negative | Positive | Positive for C. perfringens |
| 17 | 41 | Spontaneous abortion | Fatal | Uterus | Endomyometritis and sepsis | Positive | Negative | Positive | Positive for C. perfringens |
| 18 | 32 | Postpartum | Fatal | GI | Necrotizing enteritis | Positive | Negative | Positive | Positive for C. perfringens |
| 19 | 16 | Medical abortion | Fatal | Appendix | Acute appendicitis and peritonitis | Positive | Positive | Positive | Positive for both |
| 20 | 40 | No association | Fatal | Cervix | Acute cervicitis and endometritis | Positive | Positive | Positive | Positive for both |
The mean age for the C. sordellii patients was 24 years and 33 years for the C. perfringens patients. All cases, except one C. sordellii case, were fatal. Of 10 C. sordellii cases, 8 were medical abortion cases, 1 was a spontaneous abortion case, and 1 had no pregnancy association but was associated with a gynecological procedure. Of 8 C. perfringens cases, 3 were medical abortion, 4 were postpartum cases, and 1 was a spontaneous abortion case. Of 42 isolates included in the study, 9 each were isolates of C. sordellii and C. perfringens and 24 were various other Clostridium species isolates (4 C. difficile and 5 each C. butyricum, C. septicum, C. sporogenes, C. tetani), as confirmed by conventional diagnostic assays.
The duplex microsphere assay correctly identified C. sordellii and C. perfringens in all 18 known isolates of C. sordellii and C. perfringens (9 each). All 24 other Clostridia isolates were negative by the assay (Table 3). Figure 1 shows the MFI readings for the C. sordellii and C. perfringens isolates. The MFI readings for non-clostridia isolates (negative controls) ranged from 0 to 58. Thus, the microsphere assay showed 100% analytical specificity for C. sordellii and C. perfringens. The detection limits of the microsphere and conventional PCR assays were determined by preparing serial dilutions of C. sordellii and C. perfringens isolate DNA for testing by the assays. The detection limit for the conventional PCR assays was 2 ng/μL for the starting DNA sample, while the detection limit of microsphere assay was 1.4 ng/μL. Thus, clinical sensitivity of the duplex microsphere assay was slightly higher than the single-plex conventional PCR assays.
Table 3.
Microsphere assay results of Clostridium species isolates.
| Clostridium species | Number of isolates tested | Number of positive isolates | |
|---|---|---|---|
| For C. sordellii | For C. perfringens | ||
| Clostridium sordellii | 9 | 9/9 (100%) | 0/9 (0%) |
| Clostridium perfringens | 9 | 0/9 (0%) | 9/9 (100%) |
| Clostridium difficile | 4 | 0/4 (0%) | 0/4 (0%) |
| Clostridium butyricum | 5 | 0/5 (0%) | 0/5 (0%) |
| Clostridium septicum | 5 | 0/5 (0%) | 0/5 (0%) |
| Clostridium sporogenes | 5 | 0/5 (0%) | 0/5 (0%) |
| Clostridium tetani | 5 | 0/5 (0%) | 0/5 (0%) |
Figure 1.
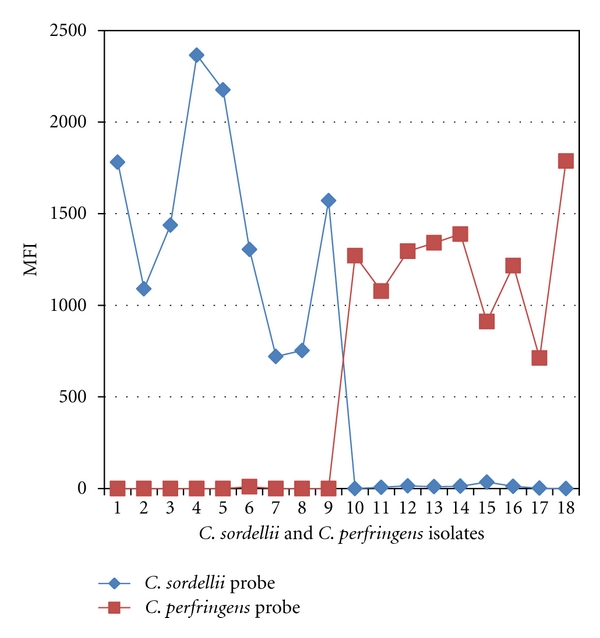
Identification of C. sordellii and C. perfringens isolates by the microsphere assay. Isolates 1–9: C. sordellii isolates. Isolates 10–18: C. perfringens isolates. Note higher MFI values/signals of C. sordellii and C. perfringens probes for the respective isolates only, indicating specificity of the probes.
The microsphere assay also accurately detected C. sordellii and C. perfringens in FFPE tissues of all confirmed CTS cases (10 C. sordellii, 8 C. perfringens) as summarized in Tables 2 and 4. Figure 2 shows the MFI readings of these CTS cases. The assay was also able to identify both C. sordellii and C. perfringens in 2 mixed infection cases. FFPE tissues from 8 patients with non-CTS bacterial infections were negativewith MFI readings ranging from 0 to 23. These results demonstrate that the microsphere assay was 100% concordant with the conventional PCR and sequencing results.
Table 4.
Microsphere, PCR, and IHC assay results of CTS cases.
| Clostridium cases (no. of cases) | Positive for C. sordellii | Positive for C. perfringens | ||||
|---|---|---|---|---|---|---|
| IHC* | PCR** | Microsphere assay | IHC* | PCR** | Microsphere assay | |
| C. sordellii (n = 10) | 10 | 10 | 10 | 10 | 0 | 0 |
| C. perfringens (n = 8) | 6 | 0 | 0 | 6 | 8 | 8 |
| C. sordellii and C. perfringens coinfection (n = 2) | 2 | 2 | 2 | 2 | 2 | 2 |
*IHC assay is specific for Clostridia but cannot speciate further.
**Conventional PCR assay targeting the phospholipase C gene of C. sordellii and C. perfringens.
Figure 2.
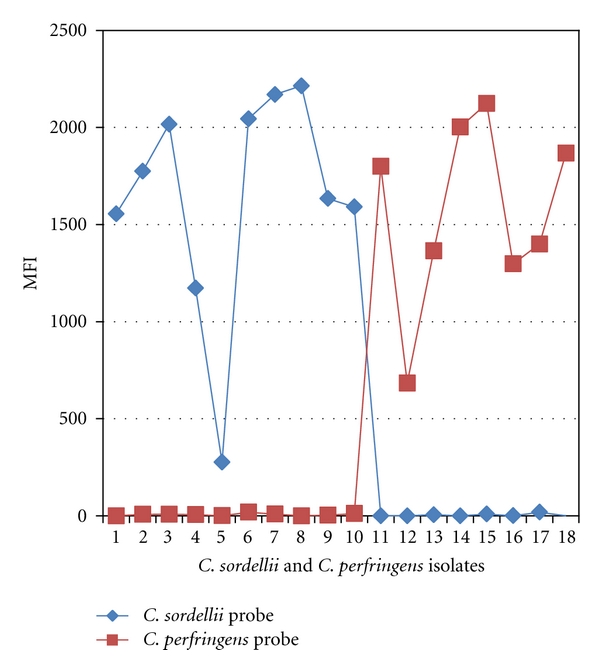
Identification of C. sordellii and C. perfringens in confirmed CTS cases by the microsphere assay. Cases 1–10: C. sordellii cases confirmed by conventional PCR and sequencing. Cases 11–18: C. perfringens cases confirmed by conventional PCR and sequencing. No cross-reactivity was noted.
The Clostridium IHC was positive in all except for 2 C. perfringens cases. Figure 3(a) illustrates the hemorrhage, inflammation, and necrosis of the endometrium and abundant Gram-positive bacilli in a positive C. sordellii case. IHC also detected Clostridial antigens inside the inflammatory cells present in the necrotic endometrial tissues (Figure 3(b)) and in myometrial blood vessels of the positive CTS cases.
Figure 3.
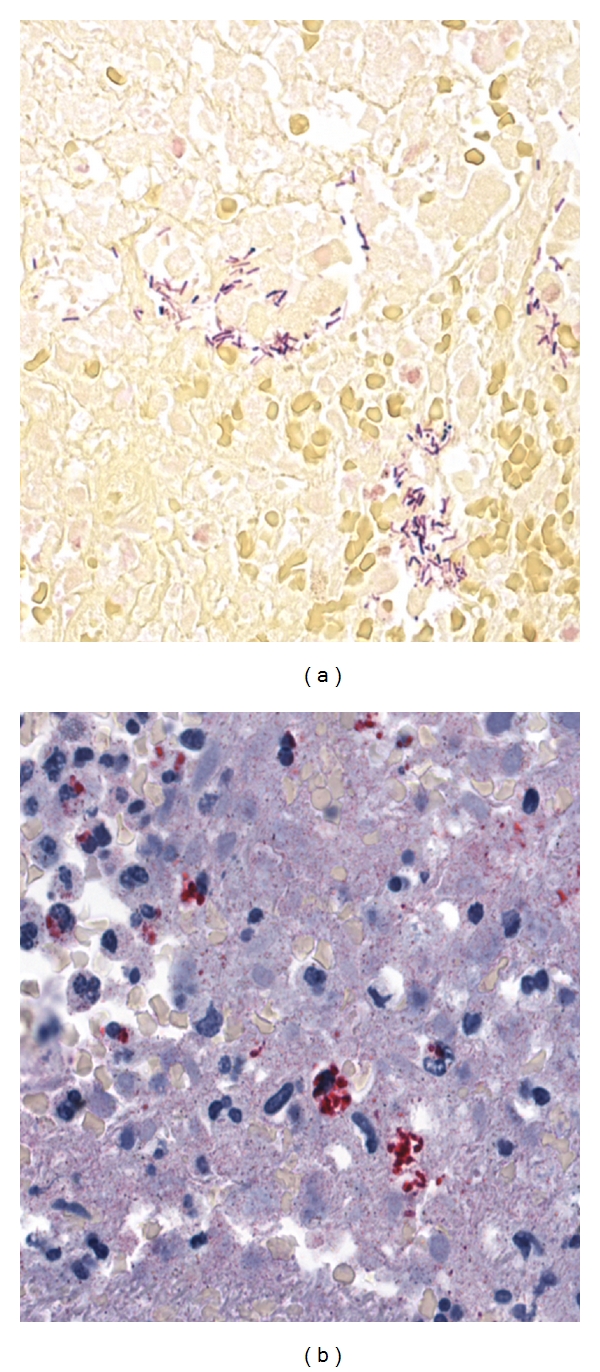
(a) Abundant-gram-positive bacilli in the necrotic endometrial tissues (Gram's stain). (b) Clostridial antigens (red staining) inside the inflammatory cells present in the necrotic endometrial tissues (IHC using polyclonal anti-clostridium species antibody).
4. Discussion
This study describes the development of a duplex PCR-based microsphere assay using a suspension array technology, the Luminex xMAP, for rapid, simultaneous detection of C. sordellii and C. perfringens. Although DNA assays developed on the Luminex platform have been used for identification and genotyping of infectious agents such as Mycobacterium, Escherichia coli, Salmonella, Cryptosporidium, Listeria, Candida, and various respiratory bacteria and viruses [14, 15, 26–29] in serum and other clinical specimens, to our knowledge, this represents the first report of the application of this technology for the diagnosis of Clostridium infections, particularly in the FFPE archival tissues. This duplex microsphere assay can have important implications for the pre-mortem and postmortem diagnosis of pregnancy-associated CTS cases.
In recent years, C. sordellii and C. perfringens have been found to be associated with severe or fatal toxic shock syndrome in both postpartum and postabortive women [8–10]. Although pregnancy and gynecologic infections due to C. sordellii and C. perfringens are rare, they progress so rapidly that death often precedes diagnosis. Early confirmatory diagnosis before the development of irreversible toxic shock is critical for implementation of treatment measures and support of epidemiologic investigations. However, presumptive diagnosis of CTS often relies on vague clinical manifestations and is quite challenging. Also, the isolation of Clostridium species is very difficult. Only molecular approaches guarantee identification to the species level, which is important for the selection of treatment as well as for the identification of the source of infection [9]. Generally, several diagnostic assays for different organisms have been performed on the premortem biopsy samples for differential diagnosis, but selecting individual assays in sequential order can delay diagnosis and treatment as well as prolong hospitalization. On the other hand, simultaneous testing by individual assays for each organism can be expensive and requires large volumes of patients' samples, which is usually difficult to obtain, particularly for premortem diagnosis. Even for the fatal cases, autopsies are often not performed and the FFPE archival biopsy tissues are the only specimens available. The microsphere assay addresses these issues and is particularly useful for the rapid, accurate, and simultaneous identification of C. sordellii and C. perfringens in FFPE tissues using only a small volume of starting material. Another added advantage of this assay is the low cost. The total cost of one test using this approach is less than 20 cents excluding costs for lab personnel and equipment. On the other hand, the traditional PCR and automated DNA sequencing methods cost at least $4 per test.
In this study, we compared conventional PCR assays and microsphere assay results for various Clostridia stock strains (isolates) and FFPE tissue specimens from confirmed pregnancy or gynecologic procedure-associated CTS cases to establish analytic sensitivity and specificity of the assay. For the microsphere assays, direct DNA hybridization was used in conjunction with short probes and TMAC buffer to produce a stringent assay. Tetramethyl ammonium chloride (TMAC) binds to the A-T rich regions of the genome and significantly reduces the difference in the melting temperature between the A-T and G-C pairs. The phospholipase C gene was targeted for the primers and probes because of its presence in all types and strains of C. sordellii and C. perfringens. Our results clearly demonstrate the high level of clinical sensitivity and specificity of the microsphere assay for the pathogens examined with 100% concordance with the conventional PCR assay results for all clinical cases and isolates. In addition, the microsphere assay was able to detect mixed infections of C. sordellii and C. perfringens and showed no cross-reactivity with any other organisms tested. The average MFI value for the C. sordellii cases was 1645 and for C. perfringens cases was 1567. No correlation was seen between the MFI values of C. sordellii and C. perfringens cases and the duration of illness, type of infection (medical abortion versus postpartum), IHC results, or any other clinical feature.
We also performed histopathological evaluation and Clostridia IHC on the tissues to define the pathology and to detect Clostridial antigens in the areas of inflammation and pathology. This information is particularly important because Clostridium species are present in the vaginal tract of 4–18% of healthy women [9, 30, 31]. We detected Clostridial antigens by IHC in the areas of pathology, particularly in the necrotic endometrial tissue in all confirmed CTS cases, except for 2 C. perfringens cases. In these IHC negative cases, MFI values of the microsphere assay were quite good (above 1200) and the conventional PCR assays were also positive. The reason for this discrepancy may be the lower sensitivity of the IHC assay in comparison to the PCR and microsphere assay or clearance of antigens by the host immune system.
5. Conclusions
The duplex microsphere assay is a rapid, sensitive, specific, and cost-effective method for the diagnosis of pregnancy-associated CTS cases and offers the advantage of simultaneously testing for C. sordellii and C. perfringens in FFPE tissues using a limited amount of DNA. The assay can be an excellent diagnostic tool for the identification of mixed infections. This technology also allows for further multiplexing of additional pathogens associated with toxic shock syndrome that could be of great value for epidemiologic investigations. In addition, the combination of the microsphere assay and IHC for the analysis of Clostridial DNA and antigens together in the tissues of fatal cases can provide an insight into the disease pathogenesis, improve detection, and have important implications for the diagnosis of CTS. Early recognition and confirmatory diagnosis of CTS, along with an aggressive surgical approach and appropriate antimicrobial therapy, can decrease the mortality associated with this syndrome that occurs primarily among young, otherwise healthy women.
Disclaimer
The findings and conclusions in this paper are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Conflict of Interests
The authors cite no relationships or support, which might be perceived as constituting a conflict of interests.
Acknowledgments
The authors gratefully thank Dr. Brandi Limbago, Duncan MacCannell, and other colleagues in Laboratory Branch, Division of Healthcare Quality Promotion, CDC, Atlanta, for providing DNA specimens of various Clostridium species isolates. The authors would like to greatly acknowledge Dr. Clifford McDonald and Dr. Brandi Limbago, Division of Healthcare Quality Promotion, CDC, Atlanta, for their valuable suggestions throughout the course of this study and all the public health departments, the pathologists, and medical examiners that submitted specimens to the IDPB.
References
- 1.Barnes P, Leedom JM. Infective endocarditis due to Clostridium sordellii. The American Journal of Medicine. 1987;83(3):p. 605. doi: 10.1016/0002-9343(87)90790-x. [DOI] [PubMed] [Google Scholar]
- 2.Soper DE. Clostridial myonecrosis arising from an episiotomy. Obstetrics and Gynecology. 1986;68(3):26S–28S. [PubMed] [Google Scholar]
- 3.McGregor JA, Soper DE, Lovell G, Todd JK. Maternal deaths associated with Clostridium sordellii infection. American Journal of Obstetrics and Gynecology. 1989;161(4):987–995. doi: 10.1016/0002-9378(89)90768-0. [DOI] [PubMed] [Google Scholar]
- 4.Aldape MJ, Bryant AE, Stevens DL. Clostridium sordellii infection: epidemiology, clinical findings, and current perspectives on diagnosis and treatment. Clinical Infectious Diseases. 2006;43(11):1436–1446. doi: 10.1086/508866. [DOI] [PubMed] [Google Scholar]
- 5.Adamkiewicz TV, Goodman D, Burke B, Lyerly DM, Goswitz J, Ferrieri P. Neonatal Clostridium sordellii toxic omphalitis. Pediatric Infectious Disease Journal. 1993;12(3):253–257. doi: 10.1097/00006454-199303000-00020. [DOI] [PubMed] [Google Scholar]
- 6.Sparks SG, Carman RJ, Sarker MR, McClane BA. Genotyping of enterotoxigenic Clostridium perfringens fecal isolates associated with antibiotic-associated diarrhea and food poisoning in North America. Journal of Clinical Microbiology. 2001;39(3):883–888. doi: 10.1128/JCM.39.3.883-888.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsokos M, Schalinski S, Paulsen F, Sperhake JP, Puschel K, Sobottka I. Pathology of fatal traumatic and nontraumatic clostridial gas gangrene: a histopathological, immunohistochemical, and ultrastructural study of six autopsy cases. International Journal of Legal Medicine. 2008;122(1):35–41. doi: 10.1007/s00414-007-0163-9. [DOI] [PubMed] [Google Scholar]
- 8.Cohen AL, Bhatnagar J, Reagan S, et al. Toxic shock associated with Clostridium sordellii and Clostridium perfringens after medical and spontaneous abortion. Obstetrics and Gynecology. 2007;110(5):1027–1033. doi: 10.1097/01.AOG.0000287291.19230.ba. [DOI] [PubMed] [Google Scholar]
- 9.Fischer M, Bhatnagar J, Guarner J, et al. Fatal toxic shock syndrome associated with Clostridium sordellii after medical abortion. The New England Journal of Medicine. 2005;353(22):2352–2360. doi: 10.1056/NEJMoa051620. [DOI] [PubMed] [Google Scholar]
- 10.Ho CS, Bhatnagar J, Cohen AL, et al. Undiagnosed cases of fatal Clostridium-associated toxic shock in Californian women of childbearing age. American Journal of Obstetrics and Gynecology. 2009;201(5):459.e1–459.e7. doi: 10.1016/j.ajog.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura S, Ogura H, Tanaka J, et al. Difference in susceptibility of various cell cultures to cytotoxic culture filtrates of Clostridium sordellii. Microbiology and Immunology. 1984;28(4):493–497. doi: 10.1111/j.1348-0421.1984.tb00700.x. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura S, Yamakawa K, Nishida S. Antibacterial susceptibility of Clostridium sordellii strains. Zentralblatt für Bakteriologie Mikrobiologie und Hygiene. 1986;261(3):345–349. doi: 10.1016/s0176-6724(86)80052-9. [DOI] [PubMed] [Google Scholar]
- 13.Eriksson R, Jobs M, Ekstrand C, et al. Multiplex and quantifiable detection of nucleic acid from pathogenic fungi using padlock probes, generic real time PCR and specific suspension array readout. Journal of Microbiological Methods. 2009;78(2):195–202. doi: 10.1016/j.mimet.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 14.Benson R, Tondella ML, Bhatnagar J, et al. Development and evaluation of a novel multiplex PCR technology for molecular differential detection of bacterial respiratory disease pathogens. Journal of Clinical Microbiology. 2008;46(6):2074–2077. doi: 10.1128/JCM.01858-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dumonceaux TJ, Schellenberg J, Goleski V, et al. Multiplex detection of bacteria associated with normal microbiota and with bacterial vaginosis in vaginal swabs by use of oligonucleotide-coupled fluorescent microspheres. Journal of Clinical Microbiology. 2009;47(12):4067–4077. doi: 10.1128/JCM.00112-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meites E, Zane S, Gould C. Fatal Clostridium sordellii infections after medical abortions. The New England Journal of Medicine. 2010;363(14):1382–1383. doi: 10.1056/NEJMc1001014. [DOI] [PubMed] [Google Scholar]
- 17.Fach P, Popoff MR. Detection of enterotoxigenic Clostridium perfringens in food and fecal samples with a duplex PCR and the slide latex agglutination test. Applied and Environmental Microbiology. 1997;63(11):4232–4236. doi: 10.1128/aem.63.11.4232-4236.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guarner J, Bhatnagar J, Shieh WJ, et al. Histopathologic, immunohistochemical, and polymerase chain reaction assays in the study of cases with fatal sporadic myocarditis. Human Pathology. 2007;38(9):1412–1419. doi: 10.1016/j.humpath.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 19.Tucker RA, Unger ER, Holloway BP, Swan DC. Real-time PCR-based fluorescent assay for quantitation of human papillomavirus types 6, 11, 16, and 18. Molecular Diagnosis. 2001;6(1):39–47. doi: 10.1054/modi.2001.21899. [DOI] [PubMed] [Google Scholar]
- 20.Guarner J, Bartlett J, Reagan S, et al. Immunohistochemical evidence of Clostridium sp, Staphylococcus aureus, and group A Streptococcus in severe soft tissue infections related to injection drug use. Human Pathology. 2006;37(11):1482–1488. doi: 10.1016/j.humpath.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 21.Imrit K, Goldfischer M, Wang J, et al. Identification of bacteria in formalin-fixed, paraffin-embedded heart valve tissue via 16S rRNA gene nucleotide sequencing. Journal of Clinical Microbiology. 2006;44(7):2609–2611. doi: 10.1128/JCM.00572-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guarner J, Sumner J, Paddock CD, et al. Diagnosis of invasive group A streptococcal infections by using immunohistochemical and molecular assays. American Journal of Clinical Pathology. 2006;126(1):148–155. doi: 10.1309/KHGV-R72C-BRM4-FQ58. [DOI] [PubMed] [Google Scholar]
- 23.Thomas LC, Gidding HF, Ginn AN, Olma T, Iredell J. Development of a real-time Staphylococcus aureus and MRSA (SAM-) PCR for routine blood culture. Journal of Microbiological Methods. 2007;68(2):296–302. doi: 10.1016/j.mimet.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Lansac N, Picard FJ, Menard C, et al. Novel genus-specific PCR-based assays for rapid identification of Neisseria species and Neisseria meningitidis. European Journal of Clinical Microbiology and Infectious Diseases. 2000;19(6):443–451. doi: 10.1007/s100960000290. [DOI] [PubMed] [Google Scholar]
- 25.Herrmann B, Nystrom T, Wessel H. Detection of Neisseria gonorrhoeae from air-dried genital samples by single-tube nested PCR. Journal of Clinical Microbiology. 1996;34(10):2548–2551. doi: 10.1128/jcm.34.10.2548-2551.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Das S, Brown TM, Kellar KL, Holloway BP, Morrison CJ. DNA probes for the rapid identification of medically important Candida species using a multianalyte profiling system. FEMS Immunology and Medical Microbiology. 2006;46(2):244–250. doi: 10.1111/j.1574-695X.2005.00031.x. [DOI] [PubMed] [Google Scholar]
- 27.Cowan LS, Diem L, Brake MC, Crawford JT. Transfer of a Mycobacterium tuberculosis genotyping method, Spoligotyping, from a reverse line-blot hybridization, membrane-based assay to the Luminex multianalyte profiling system. Journal of Clinical Microbiology. 2004;42(1):474–477. doi: 10.1128/JCM.42.1.474-477.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dunbar SA, Vander Zee CA, Oliver KG, Karem KL, Jacobson JW. Quantitative, multiplexed detection of bacterial pathogens: DNA and protein applications of the Luminex LabMAP system. Journal of Microbiological Methods. 2003;53(2):245–252. doi: 10.1016/s0167-7012(03)00028-9. [DOI] [PubMed] [Google Scholar]
- 29.Mahony J, Chong S, Merante F, et al. Development of a respiratory virus panel test for detection of twenty human respiratory viruses by use of multiplex PCR and a fluid microbead-based assay. Journal of Clinical Microbiology. 2007;45(9):2965–2970. doi: 10.1128/JCM.02436-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sweet RL, Ledger WJ. Puerperal infectious morbidity: a two year review. American Journal of Obstetrics and Gynecology. 1973;117(8):1093–1100. doi: 10.1016/0002-9378(73)90759-x. [DOI] [PubMed] [Google Scholar]
- 31.Hammill HA. Normal vaginal flora in relation to vaginitis. Obstetrics and Gynecology Clinics of North America. 1989;16(2):329–336. [PubMed] [Google Scholar]


