Abstract
Co-stimulatory and inhibitory receptors are critical regulators of adaptive immune cell function. These pathways regulate the initiation and termination of effective immune responses to infections while limiting autoimmunity and/or immunopathology. This review focuses on recent advances in our understanding of inhibitory receptor pathways and their roles in different diseases and/or infections, emphasizing potential clinical applications and important unanswered mechanistic questions. While significant progress has been made in defining the influence of inhibitory receptors at the cellular level, relatively little is known about the underlying molecular pathways. We will discuss our current understanding of the molecular mechanisms for key inhibitory receptor pathways, highlight major gaps in knowledge and explore current and future clinical applications.
Introduction
A major function, and perhaps a driver for evolutionary development of inhibitory receptors in the immune system, is regulating autoreactivity. Not surprisingly, therefore, inhibitory receptor pathways in T and B cells, including CTLA-4, PD-1, Lag-3 and others, have been implicated in autoimmunity in mice. Importantly, polymorphisms in inhibitory receptor genes are associated with susceptibility to several human autoimmune diseases, including diabetes, multiple sclerosis, and rheumatoid arthritis (1). This regulatory system has also been co-opted, and perhaps diversified, to help temper overzealous immune responses. Many studies have shown that inhibitory receptors are critical negative regulators of the immune response to allografts (2), tumors (3), infections (4), and perhaps even allergens (5).
In some settings, efficient negative regulation by inhibitory receptors may help restrain detrimental immune responses (6, 7). However, inhibitory receptors can also hinder the effective immune responses needed to clear pathogens and tumors (4). Several studies have demonstrated the benefit of both positive and negative manipulation of inhibitory receptor pathways (1–4). In fact, antibodies targeting inhibitory receptor pathways are currently in clinical trials and several have already been FDA approved in settings of autoimmunity and cancer (1,2). With the growing clinical significance of these approaches, better mechanistic insight into these pathways may provide safer and more robust therapeutic opportunities.
Acute Infections
Inhibitory receptors and their ligands play crucial roles in shaping the immune response to pathogenic microbes. The opposing functions of inhibitory and activating pathways provide the immune system with a mechanism to “fine-tune” innate and adaptive immune responses, ensuring pathogen control without excessive immune-mediated damage. The cascade of events involved in T and B cell responses during acute infection provides multiple points where inhibitory receptors could have an impact: i) opposing positive costimulation during priming, ii) curbing effector functions to limit immunopathology or iii) slowing the response at later stages of infection. In addition, there are clearly ways that inhibitory receptors could influence T and B cell responses during acute infections that are cell extrinsic, such as a role for many inhibitory receptor pathways on natural killer (NK) cells, dendritic cells (DCs), macrophages, and regulatory T cells (Tregs) (8). While we still understand relatively little about how and where inhibitory receptors act during acute infections, there are clear examples of the importance of these pathways.
Modulating the PD-1 pathway during acute infection can, in some cases, increase the effectiveness of anti-pathogen immune responses. For example, knocking out or blocking the PD-1 pathway in mice increases immune responses and survival following infection with Histoplasma capsulatum, rabies virus, or respiratory syncitial virus (9–11). In addition, Lag-3 has been shown to negatively regulate the development of CD8+ T cell memory following Sendai virus infection (12). These studies suggest that inhibitory receptors may hinder effective immune responses during some acute infections and that blockade of negative regulatory pathways might improve immunity to pathogens. In contrast, disrupting inhibitory receptor pathways can also be harmful. For example, while PD-1 deficient mice can more efficiently clear adenovirus from the liver than wildtype mice, they also develop more severe hepatocellular injury, likely due to an overaggressive adaptive immune response (13). Other studies have shown that the absence or blockade of PD-L1 reduces early CD8+ T cell responses to influenza virus or Listeria monocytogenes (Lm) in mice. In these examples, inhibitory receptors on innate immune cells, such as DCs and macrophages, play important roles in T cell activation and survival (14–17). Thus, a beneficial aspect of inhibitory receptor pathways during acute infections might be tempering of T cell responses to prevent immunopathology and perhaps sustaining highly activated effector T cells by preventing activation-induced death.
Inhibitory receptors clearly play a role during some acute infections, but exactly how different inhibitory receptors regulate these responses remains poorly understood. While PD-1 (18), CTLA-4 (19), Lag-3 (12), CD200:CD200R (20) and some Ly49 family members (21) have been examined on T cells during acute infection, the functions of many other inhibitory receptors have yet to be investigated. Several other important unanswered questions remain. Whether additional inhibitory receptors are acting individually or synergistically to regulate adaptive immune cells during acute infections is not known. There is also evidence for distinct functions of inhibitory receptors on different cell types, emphasizing the importance of studying these negative regulators in multiple adaptive and innate immune cells. Given the role of inhibitory receptors in modulating T cell activation, it is also possible that inhibitory receptors shape the populations of effector and memory cells that develop during acute infections. For example, it will be interesting to determine how inhibitory receptors might affect terminally-differentiated versus memory-precursor subsets of CD8+ T cells and perhaps even subsequent memory formation. In this regard, recent work has demonstrated that functional memory CD8+ T cells responding to an overwhelming secondary infection undergo terminal differentiation and fail to persist long-term (22). The loss of these memory CD8+ T cells was associated with upregulation of the activating/inhibitory receptor 2B4/CD244 and could be reversed in 2B4-deficient memory CD8+ T cells. Because of the complicated nature of 2B4 signaling (23), future work will be needed to shed light on how this inhibitory receptor regulates immune responses to secondary infections. Finally, defining how inhibitory receptors influence the balance between pathogen control and immunopathology should help in the development of safer therapeutic manipulation of these pathways.
Chronic Infections
Inhibitory receptors play a major role during persisting infections. During many chronic infections, antigen-specific T cells are initially activated and gain effector functions, but progressively lose functionality over time. Exhaustion of CD8+ T cells during chronic infection is hierarchical with early defects in proliferation, IL-2 production, and cytotoxicity, followed by the loss of TNF, IFN-γ, and β-chemokine production at late stages (4). Additional alterations also occur in the development of memory properties, such as antigen-independent maintenance and responsiveness to IL-7 and IL-15 (4). T cell exhaustion occurs in many animal models of infection, as well as during human chronic infections (24). Exhausted CD8+ T cells express high levels of inhibitory receptors, while their ligands are upregulated on antigen presenting cells (APCs) (24, 25). Consequently, exhausted CD8+ T cells are more likely to receive inhibitory signals, resulting in decreased effector function.
One of the first inhibitory receptors implicated in T cell exhaustion was PD-1 (26). Unlike functional effector T cells where PD-1 expression is transient, surface expression of PD-1 is upregulated and sustained on exhausted CD8+ T cells. The importance of PD-1 in exhaustion was highlighted by the ability to partially reverse CD8+ T cell dysfunction and lower viral load with in vivo blockade of the PD-1/PD-L pathway during chronic LCMV infection (26). Soon thereafter, several groups showed upregulation of PD-1 expression on exhausted CD8+ T cells during human viral infections, such as HIV, HCV, and HBV, and demonstrated improved function of T cells following in vitro PD-1/L1 blockade or in vivo blockade of the PD-1 pathway in SIV-infected primates (27–30). Increased expression of PD-1 and its ligands also impairs the effector responses against persisting pathogens such as Helicobacter pylori, Mycobacterium tuberculosis (M. tb), Schistomsoma mansoni, Leishmania donovani, and Toxoplasma gondii (31–35). Thus, the PD-1/PD-L pathway is a central negative regulator of immune responses during persisting infections.
Global transcriptional profiling of exhausted CD8+ T cells led to the discovery of other inhibitory receptors that are also upregulated on T cells during chronic infection, including Lag-3, 2B4, CD160, CTLA-4, PIR-B, and GP49b (36). Many of these inhibitory receptors are co-expressed on exhausted CD8+ T cells and the pattern of this co-expression has important functional implications (36). In general, the severity of exhaustion correlates with the number of different receptors expressed, as well as the level of expression of each individual receptor. Patterns of co-expression or cooperative negative regulation by these receptors on CD8+ and CD4+ T cells exist for PD-1, Lag-3, CTLA-4, Tim-3, etc. during infections in mice and humans (36–39). These studies, and others, identified functionally distinct subpopulations of exhausted CD8+ T cells that express unique combinations of inhibitory receptors and that respond differently to inhibitory receptor blockade.
High expression of inhibitory receptors has also been documented on B cells during HIV and malaria infections in humans (40, 41). In the context of HIV infection, CD20HiCD27−CD21Lo B cells have a shortened replication history, altered expression of homing receptors, and decreased immunoglobulin diversity. These B cells also express the inhibitory receptors FCRL4, CD22, CD72, and Lair-1 consistent with some functional deficiencies (40). Dysfunction in B cells is currently poorly understood, but inhibitory receptors could influence the exhaustion of HIV- or malaria-specific B cells and subsequent inefficient antibody responses.
The expression and co-expression of many different inhibitory receptors has now been demonstrated on exhausted T and B cells in many chronic infections, revealing complexity and diversity in inhibitory receptor expression (Table 1). While access to distinct ligands is a clear reason for differences in inhibitory receptor expression on various lymphocyte populations, it remains unclear how these patterns of expression are regulated. It is also unclear whether this inhibitory receptor expression diversity is necessary to regulate unique functions of T and B cells or is required for other reasons.
Table 1.
The Role of Inhibitory Receptors on Lymphocytes in Infection
| Infection | Inhibitory Receptor Pathway | Adaptive Immune Cells Regulated | Inhibitory Receptor Blockade or Knock-out | Outcome on Immune response | References | |
|---|---|---|---|---|---|---|
| Chronic | Human Immunodeficiency virus | PD-1, CD160, 2B4, Tim-3, CTLA-4, FCRL4, CD22, CD72, Lair-1 | CD8+ T cells, CD4+ T cells, B cells | PD-1, PD-L1, Tim-3, 2B4 | Improves | 27, 37, 40 |
| Simian Immunodeficiency virus | PD-1 | CD8+ T cells, CD4+ T cells | PD-1 | Improves | 30 | |
| Hepatitis C virus | PD-1, 2B4, CD160, KLRG1, Tim-3, CTLA-4 | CD8+ T cells, CD4+ T cells | Tim-3, PD-L1, PD-1+CTLA-4 | Improves | 29,38 | |
| Hepatitis B virus | PD-1, CTLA-4 | CD8+ T cells | PD-1, CTLA-4 | Improves | 28 | |
| Human T-cell lymphotropic virus | PD-1 | CD8+ T cells | PD-L1 | Improves | 25 | |
| Lymphocytic Choriomeningitis virus | PD-1, Lag-3, CD160, 2B4, Tim-3, CTLA-4, PirB, GP49, BTLA | CD8+ T cells, CD4+ T cells | PD-1, PD-L1, PD-L1+Lag-3, Tim-3 | Improves | 26, 36 | |
| Friend Virus | PD-1, Lag-3, CTLA-4, Tim-3 | CD8+ T cells | Improves | 25 | ||
| Mycobacterium tuberculosis | PD-1 | CD8+ T cells, CD4+ T cells | PD-1 | Improves/Reduces? | 31, 76 | |
| Helicobacter pylori | PD-L1 | CD8+ T cells | PD-L1, CTLA-4 | Improves | 32 | |
| Leishmania donovani/mexicana | PD-L1 | CD8+ T cells | PD-L1 | Improves | 34 | |
| Schistosoma mansoni | PD-L1 | CD4+ T cells, CD8+ T cells | PD-L1 | Improves | 35 | |
| Trypanosoma cruzi | CTLA-4 | CD8+ T cells, CD4+ T cells | CTLA-4 | Improves | 25 | |
| Toxoplasma gondii | PD-1 | CD8+ T cells | PD-L1 | Improves | 33 | |
| Plasmodium falciparum/chabaudi/yoelii | PD-1, Lag-3, FCγRIIb | CD8+ T cells, CD4+ T cells, B cells | PD-L1+ Lag-3 | Improves | 39, 41 | |
| Acute | Rabies virus | PD-L1 | CD8+ T cells | PD-L1 | Improves | 10 |
| Respiratory Syncytial virus | PD-1 | CD8+ T cells | PD-L1 | Improves | 9 | |
| Influenza virus | PD-L1 | CD8+ T cells | PD-L1 | Reduces | 14 | |
| Sendai virus | Lag-3 | CD8+ T cells | Lag-3 | Improves | 12 | |
| Vaccinia virus | PD-1 | CD8+ T cells | PD-1 | Improves | 18 | |
| Adenovirus | PD-1 | CD8+ T cells | PD-1 | Improves | 13 | |
| Herpes Simplex Virus | PD-1, Tim-3 | CD4+ T cells, CD8+ T cells | Tim-3 | Improves | 80 | |
| Histoplasma capsulatum | PD-1 | CD4+ T cells, CD8+ T cells | PD-1 | Improves | 11 | |
| Listeria monocytogenes | PD-L1 | CD4+ T cells, CD8+ T cells | PD-L1 | Improves/Reduces ? | 15–17 |
One factor that is associated with the diversity of inhibitory receptor expression is the type and severity of infection. Indeed, a positive correlation between severity of infection and inhibitory receptor expression has been demonstrated for chronic LCMV, HIV, and HCV infections (27, 29, 36). Since antigen receptor signaling is a key factor for expression of many inhibitory receptors, pathogen burden likely has a major influence in these settings. Other factors including inflammation (42), gamma chain cytokines (43), CD4+ T cell help (44) and availability of ligands for inhibitory or costimulatory pathways might also influence inhibitory receptor expression patterns. For example, lack of CD4+ T cell help during priming leads to higher expression of PD-1 on virus-specific CD8+ T cells upon re-exposure to antigen (45), suggesting that epigenetic changes in the Pdcd1 regulatory regions might retain information about environmental encounters for a T cell during infection. Indeed, methylation of the Pdcd1 gene appears to be a major mechanism of regulating PD-1 expression and this methylation status can be influenced by the type of infection (46). Consequently, it is likely that the design of therapies to target inhibitory receptor pathways will have to be tailored to the type and severity of the infection. Therefore, a major goal in the field is to better understand how these, and other factors, influence exhaustion and the regulation of inhibitory receptors.
Inhibitory receptors play critical immunoregulatory roles in many settings of disease. Similar to chronic infection, CD8+ T cell responses to tumors also become dysfunctional and express high levels of inhibitory receptors, including CTLA-4, PD-1, and Lag-3 (3). In humans, high expression of PD-L1 by tumor cells correlates with poor prognosis in pancreatic cancer, renal cancer, ovarian cancer, and several others (3). In mouse tumor models, knocking out or blocking PD-1 increases anti-tumor immune responses and improves survival, suggesting that targeting PD-1 will also be beneficial during human cancer (47). However, because of the complexity of inhibitory receptor expression and interactions, many questions remain about how to most effectively target these pathways therapeutically. For example, the field lacks a precise understanding of the downstream signaling cascades and potential gene targets for inhibitory receptors in B and T cells. Defining these intracellular pathways should help clarify why inhibitory receptors are expressed on certain cell types and how inhibitory receptor pathways intersect with other regulatory pathways in various disease settings.
Inhibitory Receptor Mechanisms of Action
The first opportunity for inhibitory receptors to negatively regulate immune cell function is at the cell surface, where competition for co-stimulatory ligands can prevent proper activation signals. For example, one proposed mechanism for how CTLA-4 exerts inhibitory effects is by competing with CD28 for their shared ligands, B7.1 (CD80) and B7.2 (CD86), thus preventing proper co-stimulatory signaling. CTLA-4 binds with tenfold higher affinity to the B7 ligands and this inhibitory receptor has been shown to form lattice-like networks that physically prevent CD28 from interacting with CD80 and CD86 (48, 49). Similarly, PD-L1 also binds CD80 in addition to PD-1 and sequesters CD80 away from CD28 (49). By preventing initial activation of adaptive immune cells, inhibitory receptors that utilize this mechanism can have a profound effect on the generation of immune responses.
The most well-described inhibitory receptor mechanism of action is the local and transient intracellular attenuation of positive signals from activating receptors, including antigen-receptors and co-stimulatory receptors. Many inhibitory receptors attenuate TCR or BCR signaling events by targeting these activating receptor complexes directly or their downstream signaling molecules (50). As a consequence, inhibitory receptors cause a broad, quantitative reduction in activation-induced signal transduction and downstream gene expression. In addition, inhibitory receptors also interfere with co-stimulatory signaling pathways, resulting in qualitative effects on cell survival, proliferation, and metabolism.
To mediate this negative regulation, many inhibitory receptors exploit sequence motifs in their cytoplasmic tails to recruit effector molecules (50). Perhaps the most widely used of these is the immunoreceptor-based inhibitory motif (ITIM). Inhibitory receptor ligation results in ITIM tyrosine phosphorylation and recruitment of cytosolic phosphatases containing Src homology-2 (SH2) domains, including SHP-1, SHP-2, and SHIP-1 (51, 52). SHIP and SHP molecules have been shown in vitro to dephosphorylate a variety of molecules involved in TCR, BCR, and co-stimulatory signaling cascades (53, 54). However, because these phosphatases can theoretically act on many different substrates, their direct in vivo targets remain largely elusive. In addition to the ITIM, many inhibitory receptor cytoplasmic domains also contain immunoreceptor tyrosine-based switch motifs (ITSMs). These six amino acid motifs are able to recruit both inhibitory and activating effector molecules in different contexts (55). Although the widespread mechanism of ITIM- and/or ITSM-mediated inhibition does not prevent the activation of adaptive immune cells, disrupting early signaling events allows inhibitory receptors to “fine-tune” the activation status of the cell.
Recent work by Quigley et al demonstrated that inhibitory receptors are also capable of upregulating genes involved in T cell dysfunction, suggesting a novel mechanism for inhibition (56). Using integrated genomic approaches, PD-1 signaling was shown to upregulate the expression of basic leucine zipper transcription factor, ATF-like (BATF) in exhausted T cells from humans and mice. Overexpression of BATF in primary human T cells reduced proliferation and IL-2 secretion upon stimulation. Conversely, effector functions could be rescued in exhausted T cells by shRNA-mediated silencing of BATF, further supporting an inhibitory function of BATF in T cells (56). These studies suggest that inhibitory receptors not only blunt positive signaling, but are also capable of inducing transcriptional pathways that actively regulate immune cell function. Since BATF might not only act as a dominant negative regulator of normal AP-1 activity, but also have unique transcriptional activity (57), this mechanism of inhibitory receptor activity suggests the potential to influence cellular differentiation. It will be interesting to determine if other inhibitory receptors use this mechanism of qualitatively altering gene expression.
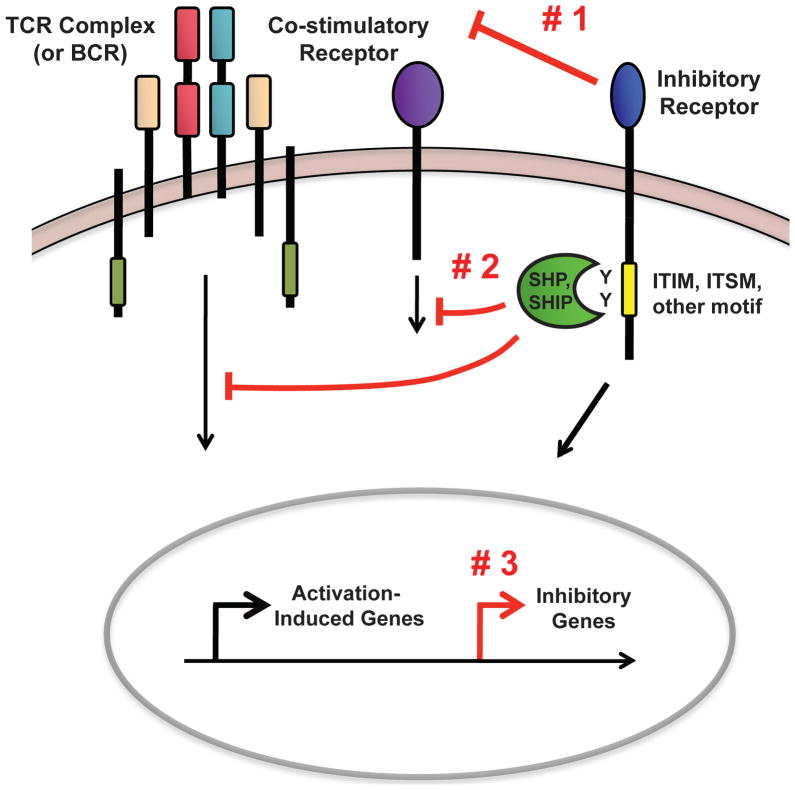
Overall, these data support the existence of three major mechanisms by which inhibitory receptors negatively regulate adaptive immune cell function (Figure 1). First, inhibitory receptors can sequester ligands for costimulatory molecules, preventing the cell from receiving the proper activation signals (Figure 1 #1). Second, inhibitory receptors can utilize intracellular motifs to disrupt the signaling cascades of activating receptors, such as the TCR, BCR, or co-stimulatory receptors. This inhibitory mechanism causes a global reduction in activation-induced gene expression (Figure 1 #2). Finally, recent work has indicated that inhibitory receptors can upregulate genes that inhibit immune cell function (Figure 1 #3). Whether this occurs by a direct or indirect molecular pathway remains unknown. Inhibitory receptors could use any combination of these mechanisms to inhibit T cell function and it is possible that other unknown mechanisms still exist. As we begin to understand inhibitory receptor pathways in more detail, new opportunities to therapeutically target these pathways are likely to emerge.
Figure 1. Major Inhibitory Receptor Mechanisms of Action.
Mechanism #1: Inhibitory receptors prevent T cells (or B cells) from receiving complete activation signals by sequestering the ligands for co-stimulatory receptors. Mechanism #2: Inhibitory sequence motifs, such as ITIMs or ITSMs, on the cytoplasmic tail of inhibitory receptors are phosphorylated upon cellular activation. These motifs then recruit intracellular phosphatases that dephosphorylate signaling molecules downstream of the TCR (or BCR) and co-stimulatory molecules, causing a broad, quantitative reduction in activation-induced gene expression. Mechanism #3: Inhibitory receptors have recently been demonstrated to upregulate genes that inhibit immune cell function; however, the pathways leading to this gene upregulation are not known.
Immunoglobulin Superfamily
Of the three mechanisms described above, by far the most well-studied is the use of ITIMs and ITSMs. Many inhibitory receptors possess ITIMs or ITSMs and exert their inhibitory effects by recruiting SHP or SHIP molecules (Table 2), including a larger number in the immunoglobulin superfamily (IgSF). For example, two well-described inhibitory receptors, PD-1 and BTLA, attenuate TCR and/or co-stimulatory signaling via an ITIM followed by an ITSM in their cytoplasmic domains. SHP-1 and SHP-2 have been shown to bind PD-1 and BTLA; however, in the case of PD-1, SHP-1 may be recruited to the ITSM (58).
Table 2.
Inhibitory Receptors and Their Known Intracellular Mechanisms of Action
| Receptor | Ligands | Superfamily | Inhibitory Sequence Motif(s) | Recruited Effector Molecule(s) | Cellular Expression |
|---|---|---|---|---|---|
| PD-1 | PD-L1 PD-L2 |
IgSF | ITIM ITSM |
SHP-1 SHP-2 |
T cells, B cells, NKT cells, monocytes |
| BTLA | HVEM | IgSF | ITIM ITSM |
SHP-1 SHP-2 |
T cells, B cells, DCs |
| CD160 | HVEM MHC I (low affinity) |
IgSF | - | - | T cells, NKT cells, NK cells, IELs |
| 2B4 | CD48 | IgSF | 4 ITSMs | SHP-1 SHP-2 EAT-2 others |
T cells, NK cells, monocytes |
| CTLA-4 | B7-1 B7-2 |
IgSF | YxxM | SHP-2 PI3K others |
T cells |
| PD-L1 | B7-1 PD-1 |
IgSF | - | - | T cells, NKT cells, B cells, monocytes, DCs, endothelial cells, hepatocytes, etc. |
| Lag-3 | MHC class II | IgSF | KIEELE | - | T cells, NK cells, B cells |
| GP49B | αvβ3 integrin | IgSF | 2 ITIMs | SHP-1 SHP-2 |
T cells, NK cells, macrophages, mast cells, neutrophiles |
| Tim-3 | Galectin 9 Phosphatidylserine |
IgSF | Y235 Y242 |
- | T cells, NKT cells, macrophages, DCs, NK cells |
| Lair-1 | Collagen | IgSF | 2 ITIMs | SHP-1 SHP-2 Csk |
T cells, B cells, NK cells, monocytes, DCs |
| PirB | MHC class I | IgSF | 3 ITIMs (1 ITIM nonfunctional) | SHP-1 SHP-2 |
B cells, T cells, DCs |
| PECAM-1 | PECAM-1 αvβ3 integrin CD38 |
IgSF | ITIM | SHP-1 SHP-2 |
Platelets, monocytes, neutrophils, T cells, vascular endothelial cells |
| CD200R | CD200 | IgSF | NPxY motif | Dok1 Dok2 |
T cells, macrophages, DCs, neutrophils, basophils, mast cells |
| CD22 (Siglec 2) | Sialic Acid | IgSF | 3 ITIMs | SHP-1 SHIP |
B cells |
| Siglec 7 | Sialic Acid | IgSF (CD33-related Siglec) | ITIM ITSM |
SHP-1 | NK cells, DCs, monocytes, subpops of T cells |
| Siglec 9 | Sialic Acid | IgSF (CD33-related Siglec) | ITIM ITSM |
SHP-1 SHP-2 |
NK cells, DCs, monocytes, subpops of T cells |
| KLRG1 | E-Cadherin | C-type lectin | ITIM | SHIP-1 SHP-2 |
T cells, NK cells |
| ILT2 | MHC class I | HLA-A,-B, -G | 4 ITIMs | SHP-1 | B cells, T cells, NK cells |
| KIR2DL/3DL | HLA-C (2DL) HLA-B, -A (3DL) |
- | 2 ITIMs | - | T cells, NK cells |
| CD94-NKG2A | HLA-E | C-type lectin | 2 ITIMs | SHP-1 SHP-2 |
T cells, NK cells |
| CD72 | CD100 | C-type lectin | 2 ITIMs | - | B cells |
| Ly49 (mice) | MHC-a | C-type lectin | - | - | T cells, NK cells, monocytes, macrophages |
| CD5 | CD72 | Scavenger receptor | ITIM | SHP-1 | T cells, B cell subset |
For reviews on individual pathways, see (Keir et al Annual Reviews Immunology 2008, Greenwald et al Annual Reviews Immunology 2009, Cai et al Immunological Reviews 2009, Freeman et al Immunological Reviews 2010)
In some cases, the presence of an ITSM does not necessarily indicate an inhibitory function. The IgSF family member 2B4 harbors four ITSMs in its cytoplasmic tail, however, both inhibitory and activating roles have been demonstrated for 2B4 in NK cells and T cells (23, 59). Three major factors have been shown to contribute to 2B4 dual functionality, including the level of surface expression, degree of cross-linking, and abundance of different effector molecules (23). Thus, many IgSF family members utilize intracellular ITIMs or ITSMs as a widespread mechanism to attenuate adaptive immune cell activation. However, diversity in the number of inhibitory motifs expressed, as well as the intracellular effector proteins recruited, may indicate important differences in functions within this family of receptors.
C-type Lectin Family
The C-type lectin family of inhibitory receptors, like the IgSF, can mediate inhibition of cellular function via ITIM-mediated phosphatase recruitment. Two ITIM-containing members of the C-type lectin family, CD94/NKG2 and KLRG1, inhibit CD8+ T cell cytotoxicity and cytokine production (60, 61). Recent work has demonstrated in primary CD8+ T cells that a mechanism for KLRG1 inhibition is reduced Akt phosphorylation by SHIP-1 recruitment (61).
The mouse Ly49 family of inhibitory receptors has been well characterized in NK cells as ITIM-containing molecules that recruit SHP-1 to inhibit cytotoxity. While Ly49 family members are expressed by T cells, their role in this setting remains less well understood (62). The functional equivalents of Ly49 receptors in humans are inhibitory KIRs and LIRs. These inhibitory receptors are present primarily on NK cells and CD8+ T cells and have been shown to negatively regulate cytokine production and cytotoxicity through SHP-1 recruitment to ITIMs (63). Thus, both IgSF and C-type lectin family members utilize ITIMs and ITSMs as a mechanism to inhibit adaptive immune cell function. These families of inhibitory receptors, with distinct ligand specificities and expression patterns, might diversify how inhibitory receptors can be used to regulate T cell responses.
Since the first description of an ITIM in FcγRIIB over 15 years ago, many inhibitory receptors have been discovered by the presence of intracellular inhibitory motifs. Recent advances in genomic and proteomic informatics allowed for the identification of a large number of novel ITIM-containing molecules (64, 65). These complementary studies revealed over 800 previously unidentified ITIM-bearing molecules in the human genome. It is likely that these databases contain some false positives, emphasizing the need for functional and biological analysis of these molecules. However, these ITIM databases provide the field with a basic foundation for future work on the functional roles of novel ITIM-containing molecules. It will be interesting to extend these types of approaches to other motifs, such as ITSMs, in the future.
Inhibitory Receptors without ITIMs/ITSMs
Much of what is known about inhibitory receptor mechanisms of action has been obtained through studies of cytoplasmic inhibitory motifs, specifically ITIMs and ITSMs. However, several well-known inhibitory receptors, including CTLA-4, Tim-3, Lag-3, and CD160, do not mediate their inhibitory effects through classical ITIMs or ITSMs. For example, the well-studied inhibitory receptor CTLA-4 lacks an ITIM or ITSM. Some work has suggested that a phosphorylated YxxM motif indirectly recruits SHP-2 to the intracellular tail of CTLA-4. However, this issue remains controversial since CTLA-4 has been shown to mediate inhibitory effects without SHP-2 recruitment (66). It is, of course, possible that CTLA-4 uses more than one mechanism of T cell inhibition. Nevertheless, these observations clearly demonstrate inhibitory receptor function in the absence of an ITIM or ITSM.
Although the TIM gene family members do not contain classical ITIMs, these molecules contain intracellular tyrosine-kinase phosphorylation motifs (67). Tim-3, which has recently received attention as an important regulator of CD8+ T cells during infection, contains a highly conserved tyrosine residue that is phosphorylated upon binding to Galectin-9 (68). Inhibitory motifs other than ITIMs and ITSMs have also been identified in some inhibitory receptors. For example, the IgSF member Lag-3 negatively regulates the homeostatic expansion of T cells via a KIEELE motif in its cytoplasmic tail (12, 69). Relatively little is known about how Lag-3 mediates these inhibitory effects through its KIEELE motif. Finally, CD160, a glycoylphosphatidylinosital (GPI)-anchored receptor, inhibits T cell activation by reducing phosphorylation of CD3ξ (70). However, the signaling cascade that leads to this inhibitory effect remains elusive, given the absence of an intracellular tail (70).
The ability of many different inhibitory receptors to disrupt proximal TCR/costimulatory signaling events emphasizes the importance of this widespread mechanism of cell inhibition. However, the intricacies of these inhibitory pathways continue to be defined. Many studies on inhibitory receptor signaling have been performed in limited cell types and often under non-physiological conditions. As a result, a major gap in knowledge is the cell-specific effects of inhibitory receptor ligation. This issue is especially important for those inhibitory receptors known to recruit SHP and SHIP molecules, since it has been proposed that baseline expression of these phosphatases can vary in different cell types and at different stages of immune responses (58, 71).
Targeting Inhibitory Receptor Pathways Therapeutically
Enhancing inhibitory receptor activity might help prevent the activation or function of autoreactive and alloreactive immune cells, while blockade of inhibitory receptor pathways has shown promise in partially reversing T cell exhaustion during chronic infections and cancer. However, it is not yet clear why blockade of some inhibitory receptors is beneficial while disrupting others has little effect. Moreover, while some combined blockades are beginning to show promise, it remains unclear which combinations of inhibitory receptor blockades can synergize for the most optimal benefit or how this synergy works mechanistically. For example, blockade of PD-1/PD-L1 alone, but not Lag-3 alone, restores function of exhausted CD8+ T cells during chronic LCMV infection, while blockade of both the PD-1 and Lag-3 pathways provides synergistic recovery of effector T cell functions and viral control (36). Similarly, blockade of PD-1 and Lag-3 also dramatically improves T and B cell responses during Plasmodium infection in mice, which results in accelerated parasite clearance (39). In addition, combined blockade of PD-1 with Tim-3 also synergistically increases the function of exhausted CD8+ T cells during chronic infection and cancer (37, 72).
In addition to targeting multiple inhibitory receptor pathways, recent work has demonstrated the potential of coordinately targeting inhibitory receptors and other types of immune regulatory pathways. For example, PD-1 blockade has been successfully combined with IL-10 blockade or low dose anti-4-1BB agonistic antibody to enhance anti-viral T cell responses and lower viral load (73, 74). Thus, combining blockade of inhibitory receptors with blockade of suppressive cytokines or stimulation of positive regulatory pathways may be a promising approach for enhancing immunity to chronic infections or cancers.
Targeting of multiple inhibitory receptor pathways may be a potent way to boost immune responses during infection and cancer, but the potential risk of autoimmunity is a concern when considering these treatments for clinical use. CTLA-4 blockade has been associated with autoimmune events in some human subjects, though most of these side effects subside with appropriate clinical management (75). Similar concerns will exist for most inhibitory receptor blockades in humans. In addition to modulating self-tolerance, one must also consider potential effects on immunopathology. Although PD-1 and PD-L1 deficient mice tolerate many acute infections quite well, these mice succumb to chronic LCMV infection within 7–10 days and PD-1 deficient mice also have reduced survival after M.tb infection due to uncontrolled inflammation (26, 76). Several themes have emerged that might allow for the prediction of immunopathology when blocking inhibitory pathways. The number and location of immune cells in the body at the time of treatment may influence the development of pathology. For instance, blockade of the PD-1/PD-L pathway during chronic LCMV and M. tb infections only causes pathological outcomes in the acute phase of infection when T cell numbers and function, as well as pathogen load, are very high. At later time points, blockade of the PD-1/PD-L pathway does not result in severe immunopathology (26, 36). High antigen load and a robust immune response in one or more sensitive tissues, such as the lungs or central nervous system, may also cause severe pathology upon inhibitory receptor blockade. Other factors that may affect immunopathology include the activation status of the target cell and other regulatory pathways in effect (i.e. co-stimulatory pathways, Tregs, etc). An important question in the field is how much enhancement of T and B cell function can be tolerated in different infections or cancer before detrimental pathological events occur.
Recent work in animal models and humans has already demonstrated that the benefits of targeting inhibitory receptor pathways can outweigh the risks in some settings. For example, two antagonistic inhibitory receptor antibodies have been successfully translated from experimental studies in mice to the clinical treatment of human cancers. In March 2011, the FDA approved a humanized CTLA-4 blocking antibody (Yervoy/Ipilimumab) for the treatment of late-stage melanoma and several clinical trials are underway for the use of anti-CTLA-4 antibodies in other cancer settings (75, 77). Humanized anti-PD-1 antibodies (CT-011 and MDX-1106) were also recently developed. In a phase 1 clinical trial for patients with advanced hematologic malignancies, CT-011 had clinical benefit in 33% of patients with one patient now in complete remission (78). Notably, in addition to a positive impact on tumors, these treatments were largely well-tolerated. There are currently over 32 clinical trials underway for the use of antibodies targeting PD-1, CTLA-4, and Lag-3 in various settings of disease (ClinicalTrials.gov). These exciting clinical examples provide support and motivation for continued research on inhibitory receptor mechanisms of action.
In addition to blocking inhibitory receptor pathways, several studies in mice demonstrated the beneficial effects of treatment with agonistic inhibitory receptor antibodies during autoimmunity (1). To successfully translate these types of therapies into human use, the field exploited an important mechanism of CTLA-4 inhibition: binding and sequestration of CD80 and CD86. This knowledge led to the design of CTLA-4-Ig, a fusion protein that blocks the engagement of CD28 with its ligands and prevents autoreactive T cell activation. In 2005, the FDA approved the use of CTLA-4-Ig (Abatacept) for the treatment of moderate-to-severe rheumatoid arthritis (79). The development of Abatacept is a prime example of how the design of effective therapeutics can be aided by our understanding of inhibitory receptor mechanisms of action.
Conclusion
The successes of CTLA-4 and PD-1 monoclonal antibodies are at the forefront of inhibitory receptor clinical applications. Given the important roles of inhibitory receptors in autoimmunity and the prevention of immunopathology, the effects that administration of blocking antibodies have on other cell types and healthy tissues must also be carefully examined. As the field begins to better grasp how inhibitory receptors function individually and synergistically on distinct cell types, it may be possible to design more specific therapeutic and prophylactic treatment strategies.
Acknowledgments
We thank Alison Crawford, Michael Paley, and Jonathan Johnnidis from the Wherry lab for critical reading and helpful insights.
Footnotes
Studies in the Wherry lab are supported by the NIH (AI083022, AI095608, AI082630, AI078897 and HHSN266200500030C) and T32 HIV Pathogenesis Training Grant to P.M.O.
References
- 1.Francisco L, Sage P, Sharpe A. The PD-1 pathway in tolerance and autoimmunity. Immunological Reviews. 2010:1–24. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.del Rio ML, Buhler L, Gibbons C, Tian J, Rodriguez-Barbosa JI. PD-1/PD-L1, PD-1/PD-L2, and other co-inhibitory signaling pathways in transplantation. Transplant International. 2008 doi: 10.1111/j.1432-2277.2008.00726.x. [DOI] [PubMed] [Google Scholar]
- 3.Peggs K, Quezada S, Allison J. Cancer immunotherapy: co-stimulatory agonists and co-inhibitory antagonists. Clinical & Experimental Immunology. 2009;157:9–19. doi: 10.1111/j.1365-2249.2009.03912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wherry EJ. T cell exhaustion. Nature Immunology. 2011;131:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 5.Shik D, Munitz A. Regulation of allergic inflammatory responses by inhibitory receptors. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. 2010;40:700–9. doi: 10.1111/j.1365-2222.2010.03501.x. [DOI] [PubMed] [Google Scholar]
- 6.Linsley P, Tepper M. Immunosuppression in Vivo by a Soluble Form of the CTLA-4 T Cell Activation Molecule. Science. 2008:1–5. doi: 10.1126/science.1496399. [DOI] [PubMed] [Google Scholar]
- 7.Lenschow D, Bluestone J. Long-Term Survival of Xenogeneic Pancreatic Islet Grafts Induced by CTLA41g. Science. 2008:1–5. doi: 10.1126/science.1323143. [DOI] [PubMed] [Google Scholar]
- 8.Zhu Y, Yao S, Chen L. Cell Surface Signaling Molecules in the Control of Immune Responses: A Tide Model. Immunity. 2011;34:466–478. doi: 10.1016/j.immuni.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Telcian A, Laza-Stanca V, Edwards M, Harker J, Wang H, Bartlett N, Mallia P, Zdrenghea M, Kebadze T, Coyle A, Openshaw P, Stanciu L, Johnston S. RSV-Induced Bronchial Epithelial Cell PD-L1 Expression Inhibits CD8+ T Cell Nonspecific Antiviral Activity. Journal of Infectious Diseases. 2011;203:85–94. doi: 10.1093/infdis/jiq020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lafon M, Wiendl H. Detrimental Contribution of the Immuno-Inhibitor B7-H1 to Rabies Virus Encephalitis. The Journal of Immunology. 2008:1–11. doi: 10.4049/jimmunol.180.11.7506. [DOI] [PubMed] [Google Scholar]
- 11.Lázár-Molnár E, Gácser A, Freeman GJ, Almo SC, Nathenson SG, Nosanchuk JD. The PD-1/PD-L costimulatory pathway critically affects host resistance to the pathogenic fungus Histoplasma capsulatum. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:2658–2663. doi: 10.1073/pnas.0711918105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Workman C, Vignali D. Negative Regulation of T Cell Homeostasis by Lymphocyte Activation Gene-3 (CD223) The Journal of Immunology. 2004:1–9. doi: 10.4049/jimmunol.174.2.688. [DOI] [PubMed] [Google Scholar]
- 13.Iwai Y, Terawaki S, Ikegawa M, Okazaki T, Honjo T. PD-1 Inhibits Antiviral Immunity at the Effector Phase in the Liver. Journal of Experimental Medicine. 2003;198:39–50. doi: 10.1084/jem.20022235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Talay O, Shen CH, Chen L, Chen J. B7-H1 (PD-L1) on T cells is required for T-cell-mediated conditioning of dendritic cell maturation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:2741–2746. doi: 10.1073/pnas.0813367106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rowe J, Sing Way S. PDL-1 Blockade Impedes T Cell Expansion and Protective Immunity Primed by Attenuated Listeria monocytogenes. The Journal of Immunology. 2008:1–6. doi: 10.4049/jimmunol.180.11.7553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamazaki T, Okumura K. Blockade of B7-H1 on Macrophages Suppresses CD4 + T Cell Proliferation by Augmenting IFN-γ-Induced Nitric Oxide Production. The Journal of Immunology. 2005:1–8. doi: 10.4049/jimmunol.175.3.1586. [DOI] [PubMed] [Google Scholar]
- 17.Seo S, Jeong H, Park S, Lee S, Choi I, Chen L. Blockade of endogenous B7-H1 suppresses antibacterial protection after primary Listeria monocytogenes infection. Immunology. 2008;123:90–99. doi: 10.1111/j.1365-2567.2007.02708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown KE, Freeman GJ, Wherry EJ, Sharpe AH. Role of PD-1 in regulating acute infections. Current Opinion in Immunology. 2010;22:397–401. doi: 10.1016/j.coi.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raue H, Slifka M. Pivotal Advance: CTLA-4+ T cells exhibit normal antiviral functions during acute viral infection. Journal of Leukocyte Biology. 2007;81:1165–1175. doi: 10.1189/jlb.0806535. [DOI] [PubMed] [Google Scholar]
- 20.Snelgrove R, Goulding J, Didierlaurent A, Lyonga D, Vekaria S, Edwards L, Gwyer E, Sedgwick J, Barclay AN, Hussell T. A critical function for CD200 in lung immune homeostasis and the severity of influenza infection. Nature Immunology. 2008;9:1074–1083. doi: 10.1038/ni.1637. [DOI] [PubMed] [Google Scholar]
- 21.McMahon C, Raulet D. Viral and Bacterial Infections Induce Expression of Multiple NK Cell Receptors in Responding CD8 + T Cells. The Journal of Immunology. 2002:1–10. doi: 10.4049/jimmunol.169.3.1444. [DOI] [PubMed] [Google Scholar]
- 22.West E, Youngblood B, Tan W, Jin H, Araki K, Alexe G, Konieczny B, Calpe S, Freeman G, Terhorst C, Haining W, Ahmed R. Tight Regulation of Memory CD8+ T Cells Limits Their Effectiveness during Sustained High Viral Load. Immunity. 2011:1–14. doi: 10.1016/j.immuni.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chlewicki L, Kumar V. Molecular Basis of the Dual Functions of 2B4 (CD244) The Journal of Immunology. 2008:1–10. doi: 10.4049/jimmunol.180.12.8159. [DOI] [PubMed] [Google Scholar]
- 24.Virgin H, Wherry J, Ahmed R. Redefining Chronic Viral Infection. Cell. 2009:1–21. doi: 10.1016/j.cell.2009.06.036. [DOI] [PubMed] [Google Scholar]
- 25.Keir M, Butte M, Freeman GJ, Sharpe AH. PD-1 and Its Ligands in Tolerance and Immunity. Annual Review of Immunology. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barber D, Wherry E, Masopust D, Zhu B, Allison J, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 27.Day C, Kaufmann D, Kiepiela P, Brown J, Moodley E, Reddy S, Mackey E, Miller J, Leslie A, Depierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry E, Coovadia H, Goulder P, Klenerman P, Ahmed R, Freeman GJ, Walker B. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 28.Boni C, Fisicaro P, Valdatta C, Amadei B, Di Vincenzo P, Giuberti T, Laccabue D, Zerbini A, Cavalli A, Missale G, Bertoletti A, Ferrari C. Characterization of Hepatitis B Virus (HBV)-Specific T-Cell Dysfunction in Chronic HBV Infection. Journal of Virology. 2007;81:4215–4225. doi: 10.1128/JVI.02844-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Urbani S, Amadei B, Tola D, Massari M, Schivazappa S, Missale G, Ferrari C. PD-1 Expression in Acute Hepatitis C Virus (HCV) Infection Is Associated with HCV-Specific CD8 Exhaustion. Journal of Virology. 2006;80:11398–11403. doi: 10.1128/JVI.01177-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Velu V, Titanji K, Zhu B, Husain S, Pladevega A, Lai L, Vanderford T, Chennareddi L, Silvestri G, Freeman GJ, Ahmed R, Amara R. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458:206–210. doi: 10.1038/nature07662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jurado J, Garcia V. Programmed death (PD)-1:PD-ligand 1/PD-ligand 2 pathway inhibits T cell effector functions during human tuberculosis. The Journal of Immunology. 2008:1–11. doi: 10.4049/jimmunol.181.1.116. [DOI] [PubMed] [Google Scholar]
- 32.Wu Y, Lin C, Cheng K, Wang Y, Lin I, Chou Y, Hsu P. Increased programmed death-ligand-1 expression in human gastric epithelial cells in Helicobacter pylori infection. Clinical & Experimental Immunology. 2010;161:551–559. doi: 10.1111/j.1365-2249.2010.04217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhadra R, Gigley J, Weiss L, Khan I. Control of Toxoplasma reactivation by rescue of dysfunctional CD8+ T-cell response via PD-1-PDL-1 blockade. Proceedings of the National Academy of Sciences. 2011:1–6. doi: 10.1073/pnas.1015298108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joshi T, Rodriguez S, Perovic V, Cockburn IA, Stäger S. B7-H1 blockade increases survival of dysfunctional CD8(+) T cells and confers protection against Leishmania donovani infections. PLoS pathogens. 2009;5:e1000431. doi: 10.1371/journal.ppat.1000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith P, Walsh CM, Mangan NE, Fallon RE, Sayers JR, McKenzie AN, Fallon PG. Schistosoma mansoni worms induce anergy of T cells via selective up-regulation of programmed death ligand 1 on macrophages. Journal of immunology (Baltimore, Md: 1950) 2004;173:1240–1248. doi: 10.4049/jimmunol.173.2.1240. [DOI] [PubMed] [Google Scholar]
- 36.Blackburn SD, Shin H, Haining W, Zou T, Workman C, Polley A, Betts M, Freeman GJ, Vignali D, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nature Immunology. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jin HT, Anderson AC, Tan WG, West EE, Ha SJ, Araki K, Freeman GJ, Kuchroo V, Ahmed R. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:14733–14738. doi: 10.1073/pnas.1009731107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakamoto N, Cho H, Shaked A, Olthoff K, Valiga M, Kaminski M, Gostick E, Price D, Freeman GJ, Wherry E, Chang K. Synergistic Reversal of Intrahepatic HCV-Specific CD8 T Cell Exhaustion by Combined PD-1/CTLA-4 Blockade. PLoS Pathogens. 2009:5. doi: 10.1371/journal.ppat.1000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Butler NS, Moebius J, Pewe LL, Traore B, Doumbo OK, Tygrett LT, Waldschmidt TJ, Crompton PD, Harty JT. Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection. Nature Immunology. 2011:13. doi: 10.1038/ni.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moir S, Ho J, Malaspina A, Wang W, Dipoto A, O’shea M, Roby G, Kottilil S, Arthos J, Proschan M, Chun T, Fauci A. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. Journal of Experimental Medicine. 2008;205:1797–1805. doi: 10.1084/jem.20072683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clatworthy MR, Willcocks L, Urban B, Langhorne J, Williams TN, Peshu N, Watkins NA, Floto RA, Smith KG. Systemic lupus erythematosus-associated defects in the inhibitory receptor FcgammaRIIb reduce susceptibility to malaria. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:7169–7174. doi: 10.1073/pnas.0608889104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Terawaki S, Chikuma S, Shibayama S, Hayashi T, Yoshida T, Okazaki T, Honjo T. IFN-α directly promotes programmed cell death-1 transcription and limits the duration of T cell-mediated immunity. Journal of immunology. 2011;186:2772–2779. doi: 10.4049/jimmunol.1003208. [DOI] [PubMed] [Google Scholar]
- 43.Kinter A, Fauci A. The Common γ-Chain Cytokines IL-2, IL-7, IL-15, and IL-21 Induce the Expression of Programmed Death-1 and Its Ligands. The Journal of Immunology. 2008:1–10. doi: 10.4049/jimmunol.181.10.6738. [DOI] [PubMed] [Google Scholar]
- 44.Matloubian M, Ahmed R. CD4+ T Cells Are Required To Sustain CD8+ Cytotoxic T-Cell Responses during Chronic Viral Infection. Journal of Virology. 2011:1–8. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fuse S, Tsai CY, Molloy MJ, Allie SR, Zhang W, Yagita H, Usherwood EJ. Recall responses by helpless memory CD8+ T cells are restricted by the up-regulation of PD-1. Journal of immunology. 2009;182:4244–4254. doi: 10.4049/jimmunol.0802041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Youngblood B, Oestreich K, Ha S, Duraiswamy J, Akondy R, West E, Wei Z, Lu P, Austin J, Riley J, Boss J, Ahmed R. Chronic Virus Infection Enforces Demethylation of the Locus that Encodes PD-1 in Antigen-Specific CD8+ T Cells. Immunity. 2011:1–14. doi: 10.1016/j.immuni.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Egen J, Allison J. CTLA-4: New Insights into its Biological Function and Use in Tumor Immunotherapy. Nature Immunology. 2002:1–8. doi: 10.1038/ni0702-611. [DOI] [PubMed] [Google Scholar]
- 49.Butte M, Freeman G. Programmed Death-1 Ligand 1 Interacts Specifically with the B7-1 Costimulatory Molecule to Inhibit T Cell Responses. Immunity. 2011:1–12. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vivier E, Daeron M. Immunoreceptor tyrosine-based inhibition motifs. Immunology Today. 2002:1–6. doi: 10.1016/s0167-5699(97)80025-4. [DOI] [PubMed] [Google Scholar]
- 51.Maeda A, Kurosaki T. Requirement of SH2-containing Protein Tyrosine Phosphatases SHP-1 and SHP-2 for Paired Immunoglobulin-like Receptor B (PIR-B)–mediated Inhibitory Signal. The Journal of Experimental Medicine. 1998:1–6. doi: 10.1084/jem.187.8.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sweeney M, Pei D. Decoding Protein-Protein Interactions through Combinatorial Chemistry: Sequence Specificity of SHP-1, SHP-2, and SHIP SH2 Domains. Biochemistry. 2011:1–16. doi: 10.1021/bi051408h. [DOI] [PubMed] [Google Scholar]
- 53.Rohrschneider L, Wolf I. Structure, function, and biology of SHIP proteins. Genes and Development. 2000:1–17. [PubMed] [Google Scholar]
- 54.Lorenz U. SHP-1 and SHP-2 in T cells: two phosphatases functioning at many levels. Immunological Reviews. 2009:1–18. doi: 10.1111/j.1600-065X.2008.00760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shlapatska LM, Mikhalap SV, Berdova AG, Zelensky OM, Yun TJ, Nichols KE, Clark EA, Sidorenko SP. CD150 association with either the SH2-containing inositol phosphatase or the SH2-containing protein tyrosine phosphatase is regulated by the adaptor protein SH2D1A. Journal of immunology. 2001;166:5480–5487. doi: 10.4049/jimmunol.166.9.5480. [DOI] [PubMed] [Google Scholar]
- 56.Quigley M, Pereyra F, Nilsson B, Porichis F, Fonseca C, Eichbaum Q, Julg B, Jesneck JL, Brosnahan K, Imam S, Russell K, Toth I, Piechocka-Trocha A, Dolfi D, Angelosanto J, Crawford A, Shin H, Kwon DS, Zupkosky J, Francisco L, Freeman GJ, Wherry EJ, Kaufmann D, Walker B, Ebert B, Haining W. Transcriptional analysis of HIV-specific CD8+ T cells shows that PD-1 inhibits T cell function by upregulating BATF. Nature Medicine. 2010;16:1147–1151. doi: 10.1038/nm.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuroda S, Yamazaki M, Abe M, Sakimura K, Takayanagi H, Iwai Y. Basic leucine zipper transcription factor, ATF-like (BATF) regulates epigenetically and energetically effector CD8 T-cell differentiation via Sirt1 expression. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:14885–9. doi: 10.1073/pnas.1105133108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Riley J. PD-1 signaling in primary T cells. Immunological Reviews. 2009:1–12. doi: 10.1111/j.1600-065X.2009.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tangye SG, Lazetic S, Woollatt E, Sutherland GR, Lanier LL, Phillips JH. Cutting edge: human 2B4, an activating NK cell receptor, recruits the protein tyrosine phosphatase SHP-2 and the adaptor signaling protein SAP. Journal of immunology (Baltimore, Md: 1950) 1999;162:6981–6985. [PubMed] [Google Scholar]
- 60.Moser J, Gibbs J, Jensen P, Lukacher A. CD94-NKG2A receptors regulate antiviral CD8+ T cell responses. Nature Immunology. 2002;3:189–195. doi: 10.1038/ni757. [DOI] [PubMed] [Google Scholar]
- 61.Tessmer M, Fugere C, Stevenaert F, Naidenko O, Chong H, Leclercq G, Brossay L. KLRG1 binds cadherins and preferentially associates with SHIP-1. International Immunology. 2007;19:391–400. doi: 10.1093/intimm/dxm004. [DOI] [PubMed] [Google Scholar]
- 62.Peacock C, Welsh R. Dynamics of Ly49 expressing cytotoxic lymphocyte subsets in response to virus infection. Microbes and Infection. 2002:1–10. doi: 10.1016/s1286-4579(02)00031-x. [DOI] [PubMed] [Google Scholar]
- 63.Anfossi N, Vivier E. Expansion and Function of CD8+ T Cells Expressing Ly49 Inhibitory Receptors Specific for MHC Class I Molecules. The Journal of Immunology. 2004:1–10. doi: 10.4049/jimmunol.173.6.3773. [DOI] [PubMed] [Google Scholar]
- 64.Staub E, Rosenthal A, Hinzman B. Systematic identification of immunoreceptor tyrosine-based inhibitory motifs in the human proteome. Cellular SIgnaling. 2003:1–22. doi: 10.1016/j.cellsig.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 65.Daeron M, Vivier E. Immunoreceptor tyrosine-based inhibition motifs: a quest in the past and future. Immunological Reviews. 2008:1–33. doi: 10.1111/j.1600-065X.2008.00666.x. [DOI] [PubMed] [Google Scholar]
- 66.Rudd C, Schneider H. CD28 and CTLA-4 coreceptor expression and signal transduction. Immunological Reviews. 2009;229:12–26. doi: 10.1111/j.1600-065X.2009.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kuchroo V, Umetsu D, Dekruyff R, Freeman GJ. The TIM gene family: emerging roles in immunity and disease. Nature Reviews Immunology. 2003;3:454–462. doi: 10.1038/nri1111. [DOI] [PubMed] [Google Scholar]
- 68.van de Weyer P, Kuehn EW. A highly conserved tyrosine of Tim-3 is phosphorylated upon stimulation by its ligand galectin-9. Biochemical and Biophysical Research Communications. 2006;351:571–576. doi: 10.1016/j.bbrc.2006.10.079. [DOI] [PubMed] [Google Scholar]
- 69.Hannier S, Triebel F. CD3/TCR Complex-Associated Lymphocyte Activation Gene-3 Molecules Inhibit CD3/TCR Signaling. The Journal of Immunology. 1998:1–8. [PubMed] [Google Scholar]
- 70.Cai G, Anumanthan A, Brown J, Greenfield E, Zhu B, Freeman GJ. CD160 inhibits activation of human CD4+ T cells through interaction with herpesvirus entry mediator. Nature Immunology. 2008;9:176–185. doi: 10.1038/ni1554. [DOI] [PubMed] [Google Scholar]
- 71.Feinerman O, Veiga J, Dorfman J, Germain R, Altan-Bonnet G. Variability and Robustness in T Cell Activation from Regulated Heterogeneity in Protein Levels. Science. 2008;321:1081–1084. doi: 10.1126/science.1158013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo V, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. The Journal of Experimental Medicine. 2010;207:2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brooks DG, Ha SJ, Elsaesser H, Sharpe AH, Freeman GJ, Oldstone MB. IL-10 and PD-L1 operate through distinct pathways to suppress T-cell activity during persistent viral infection. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:20428–20433. doi: 10.1073/pnas.0811139106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vezys V, Penaloza-Macmaster P, Barber D, Ha SJ, Konieczny B, Freeman GJ, Mittler RS, Ahmed R. 4-1BB Signaling Synergizes with Programmed Death Ligand 1 Blockade To Augment CD8 T Cell Responses during Chronic Viral Infection. Journal of immunology. 2011;187:1634–1642. doi: 10.4049/jimmunol.1100077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lipson EJ, Drake CG. Ipilimumab: an Anti-CTLA-4 Antibody for Metastatic Melanoma. Clinical cancer research: an official journal of the American Association for Cancer Research. 2011 doi: 10.1158/1078-0432.CCR-11-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lázár-Molnár E, Chen B, Sweeney KA, Wang EJ, Liu W, Lin J, Porcelli SA, Almo SC, Nathenson SG, Jacobs WR. Programmed death-1 (PD-1)-deficient mice are extraordinarily sensitive to tuberculosis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:13402–13407. doi: 10.1073/pnas.1007394107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hodi F. Overcoming immunological tolerance to melanoma: Targeting CTLA-4. Asia-Pacific Journal of Clinical Oncology. 2010;6:S16–S23. doi: 10.1111/j.1743-7563.2010.01271.x. [DOI] [PubMed] [Google Scholar]
- 78.Berger R, Rotem-Yehudar R, Slama G, Landes S, Kneller A, Leiba M, Koren-Michowitz M, Shimoni A, Nagler A. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clinical cancer research: an official journal of the American Association for Cancer Research. 2008;14:3044–3051. doi: 10.1158/1078-0432.CCR-07-4079. [DOI] [PubMed] [Google Scholar]
- 79.Schiff M. Abatacept treatment for rheumatoid arthritis. Rheumatology. 2011;50:437–449. doi: 10.1093/rheumatology/keq287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Allen SJ, Mott KR, Zandian M, Ghaisi H. Immunization with different viral antigens alters the pattern of T cell exhaustion and latency in herpes simplex virus type 1-infected mice. Journal of Virology. 2010;84:12315–12324. doi: 10.1128/JVI.01600-10. [DOI] [PMC free article] [PubMed] [Google Scholar]