Abstract
Contractile actin-myosin networks generate forces that drive cell shape changes and tissue remodeling during development. These forces can also actively regulate cell signaling and behavior. Novel features of actin-myosin network dynamics, such as pulsed contractile behaviors and the regulation of myosin localization by tension, have been uncovered in recent studies of Drosophila. In vitro studies of single molecules and reconstituted protein networks reveal intrinsic properties of motor proteins and actin-myosin networks, while in vivo studies have provided insight into the regulation of their dynamics and organization. Analysis of the complex behaviors of actin-myosin networks will be crucial for understanding force generation in actively remodeling cells and the coordination of cell shape and movement at the tissue level.
Introduction
Cell movements and cell shape changes are responsible for massive transformations in tissue structure during development. The actin-myosin cytoskeleton plays a major role in generating the forces that drive these changes and determining the mechanical properties of cells and tissues [1-3]. The non-muscle myosin II motor protein is a hexamer of three subunits (two heavy chains, two regulatory light chains, and two essential light chains) that converts the energy from ATP hydrolysis into mechanical work [4,5]. When actin and myosin form an interconnected network at the cell cortex, processive assemblies of myosin motors pull on anti-parallel actin filaments to generate contractile tension that can deform cell shape (Figure 1) [6-8]. In epithelial cells, contractile actin-myosin networks are coupled to adherens junctions, which mediate the transmission of forces between neighboring cells and integrate single cell behaviors to produce tissue-level changes during morphogenesis [9,10].
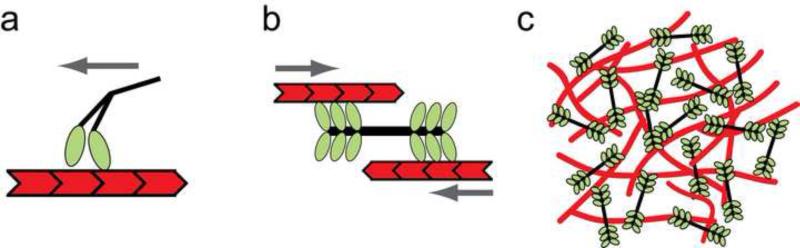
Figure 1. Contractile actin-myosin machinery.
(a) A single non-muscle myosin II motor translocates toward the plus end of an actin filament (left). However, it has a low duty ratio and thus spends only a small fraction of its time bound to the actin filament. Because of this, the motor is non-processive and does not move continuously along the actin filament for long distances. Gray arrow indicates the direction of motor movement. (b) Several myosin motors can assemble into a processive, bipolar filament that generates relative movement between two anti-parallel actin filaments. Gray arrows indicate the direction of actin filament movement. (c) A contractile network formed from many actin filaments and bipolar myosin filaments. Myosin motor activity causes the network to contract.
In addition to creating the forces that shape the embryo, the actin-myosin cytoskeleton is also a source of mechanical cues that regulate cell behavior, from cell differentiation and growth to cell shape and adhesion [11-15]. These forces are transduced into biochemical signals that influence cell behavior and modulate gene expression [12,13] and directly regulate the activity of motor proteins and the cytoskeleton [16-19].
In comparison to typical biochemical signals that travel by diffusion or are transported by vesicles or molecular motors, mechanical perturbations propagate as sound waves whose speed depends on the mechanical properties and density of the material. Mechanical signals therefore have the potential to propagate rapidly over large distances. In one example, mechanical stress applied to human smooth muscle cells in culture activates the Src tyrosine kinase within a few hundred milliseconds, while Src activation by a soluble growth factor requires more than 10 seconds [20], suggesting the possibility that mechanical stimuli can activate signaling pathways faster than chemical stimuli. Thus, mechanical signals may be ideal for coordinating cell behaviors over the length and time scales relevant to the developing embryo.
Here we describe recent advances in understanding the dynamics of contractile actin-myosin networks that drive cell shape changes and tissue remodeling during development. We focus on recent studies in Drosophila, which have uncovered novel aspects of actin-myosin dynamics and regulation within the context of epithelial tissues in vivo. We discuss how the organization and dynamics of the contractile machinery lead to distinct properties of tissue structure as well as the mechanisms by which mechanical forces in turn regulate cell signaling and cytoskeletal activity. We also address how in vitro studies of the intrinsic dynamics and force-generating properties of actin-myosin networks can provide insight into the in vivo outcome of contractile behavior.
Organization and dynamics of the actin-myosin contractile machinery in multicellular tissues
The localization of the actin-myosin contractile machinery within cells is a key factor in determining the outcome of contractile activity for cell and tissue structure. One prominent example is the apical constriction of invaginating cells [7,21]. Initiated by the transcription factor Snail [22], prospective mesoderm cells on the ventral surface of the Drosophila embryo constrict their apical surfaces (Figure 2a,c), which generates a bend in the tissue that causes the cells to invaginate to form a ventral furrow at gastrulation (Figure 2a) [23,24]. These cell shape changes are associated with an actin-myosin network that spans the apical cell surface (Figure 2c), which we refer to as a medial myosin network. The medial myosin network in apically constricting cells is connected through a second, junctional population that is anchored to adherens junctions at cell-cell contacts (Figure 2c) [9,10,25,26]. Without this coupling to junctions, the medial network can contract into a tight ball without decreasing the apical surface, suggesting that this connection is essential to translate contraction of the medial network into a change in cell shape [24]. Although the intrinsic contractile activity of the apical network is isotropic, global tissue mechanics inhibit constrictions parallel to the anterior-posterior axis of the embryo, so that apical cell surfaces primarily constrict along the dorsal-ventral axis to form a long, narrow furrow (Figure 2a) [27].
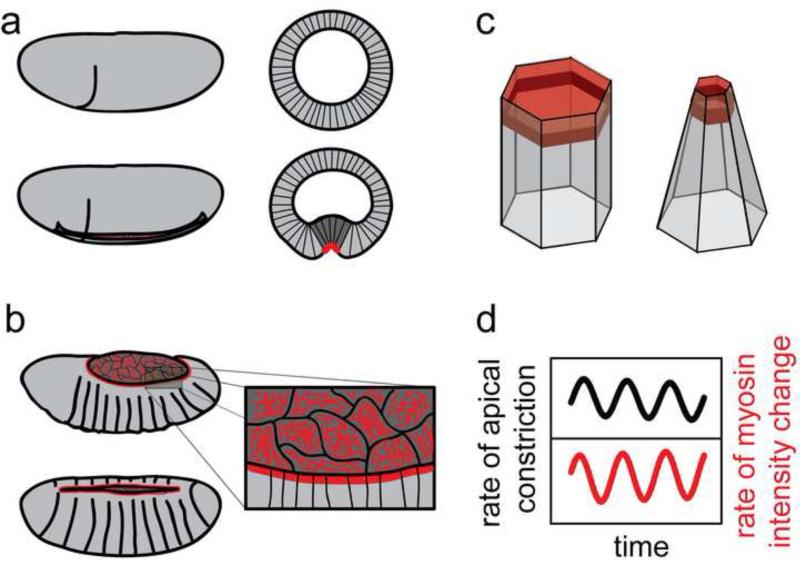
Figure 2. Pulsed contractile behaviors in apically constricting cells.
(a) Prospective mesoderm cells on the ventral surface of the Drosophila embryo constrict their apical surfaces. This generates a bend in the tissue that causes the cells to invaginate to form a ventral furrow (dark gray). These cell shape changes are associated with an apical actin-myosin network (red). Before (top) and during (bottom) furrow formation. Lateral views, anterior left, ventral down (left), cross-sections (right). (b) Apical actin-myosin networks (red) also drive apical constriction of amnioserosa cells (dark gray), which generates one force that pulls the lateral epidermis closed over the dorsal surface of the Drosophila embryo. Contraction of the leading edge cable (thick red line), amnioserosa cell death, and filopodial protrusions also contribute to dorsal closure. (c) A medial actin-myosin network that spans the apical cell surface (light red) is connected through a second, junctional population that is anchored to adherens junctions at cell-cell contacts (dark red). (d) Recent studies demonstrate that apical constriction occurs in brief pulses associated with fluctuations in the actin-myosin network. Apical constriction is closely correlated with bursts of myosin accumulation.
Surprisingly, instead of contracting in a uniform, steady progression, the medial myosin network in mesoderm cells contracts in brief pulses that are associated with bursts of myosin accumulation (Figure 2d) [22]. Cells undergo cycles of constriction and stabilization, which are translated into a net reduction in apical surface area by a ratchet-like mechanism that prevents complete relaxation of the cell surface and requires the Twist transcription factor [22].
Medial actin-myosin networks display similar pulsed contractile behaviors in the cells of the amnioserosa, a squamous epithelium on the dorsal surface of the Drosophila embryo (Figure 2b) [28-30]. Apical constriction of amnioserosa cells generates a force that pulls the lateral epidermis closed over the dorsal surface of the embryo. This process, together with contraction of the leading edge cable [30-33], amnioserosa cell death [34], and filopodial protrusions [35,36], accomplishes dorsal closure.
Cycles of apical constriction and relaxation in the amnioserosa are associated with dynamic assembly and disassembly of the medial actin-myosin network [28,37]. Actin-myosin assembly and disassembly are proposed to be converted into sustained apical constriction by an external ratchet-like structure provided by a contractile actin-myosin cable at the leading edge of the adjacent lateral epidermis [30] or an intrinsic constriction program in the amnioserosa cells [37]. Dynamic actin-myosin foci have been observed in cortical meshworks in other cell types that display contractile behaviors, such as the one-cell C. elegans embryo [38] and intercalating mesenchymal cells in the Xenopus notochord [39]. Cycles of actin-myosin contractile activity may be a common property of highly dynamic, force-generating cells.
Contractile forces regulate cellular signaling and behavior
Mechanical forces have long been appreciated to play important roles in regulating cell shape, cell adhesion, cytoskeletal organization, and cell fate [40-43]. Polarized actin-myosin contractility initiated by centrosomal cues within the one-cell C. elegans embryo drives cortical flows that distinguish the anterior and posterior cortical domains [38,44,45]. This results in the asymmetric division of this cell into two blastomeres with different fates [46]. Ectopic and endogenous forces also regulate gene expression in the Drosophila embryo, where they promote expression of the Twist transcription factor [47,48]. The lineage of human mesenchymal stem cells is specified toward neurons on soft matrices that resemble brain tissue, myoblasts on matrices of intermediate stiffness, and osteoblasts on stiff matrices that resemble bone [43]. In addition, culturing human mammary epithelial cells in a matrix of elevated stiffness characteristic of mammary tumors disrupts epithelial morphogenesis by clustering integrins, which can contribute to malignant behavior [49].
Focal adhesions are large protein complexes that mechanically couple the interior of the cell to the extracellular matrix and constitute sites where external mechanical signals are transduced into biochemical signals [11,12,50]. The assembly and growth of focal adhesions are responsive to mechanical inputs [51,52]. Several components of cell-matrix adhesion have been shown to be directly regulated by force and may contribute to the force-dependent regulation of these processes [12,13]. Applying mechanical stretch to cellular cytoskeletal networks after extraction of the overlying membrane of mouse fibroblasts leads to activation of the small GTPase Rap1 [53], and biophysical studies show that force can directly expose a phosphorylation site for Src family kinases on the Src substrate p130Cas [54]. Other protein-protein interactions at focal adhesions that have been shown to be regulated by tension include fibronectin matrix assembly [55-57] and the association of talin with vinculin [58].
Emerging evidence indicates that force may play an analogous role in regulating adherens junctions that mediate cell-cell adhesion [10,59]. A recent study found that adherens junction size correlates with the magnitude of forces exerted between human epithelial cells in culture [60]. Vinculin, an essential component of cell-matrix adhesions, is also recruited to E-cadherin complexes under tension [61,62]. Vinculin has been proposed to bind to a cryptic site in the core adherens junction protein α-catenin that is exposed by mechanical stretch [62]. These results suggest a potential mechanism linking actin-myosin contraction to adherens junction stabilization during epithelial morphogenesis.
Mechanical cues regulate myosin dynamics
In addition to its role in generating the forces that shape tissues during development, recent studies show that myosin activity itself is also regulated by mechanical force. Myosin is recruited to the cortex in isolated Dictyostelium cells exposed to an ectopic force from a micropipette [63-65], a process that has been proposed to regulate cell shape in response to external deformation. In multicellular tissues, applying a force to the Drosophila embryo is sufficient to recruit myosin to the cortex during mesoderm invagination and axis elongation [66,67], while using laser ablation to relax tension locally leads to a rapid loss of myosin from the cortex [66]. These results demonstrate that tension is necessary and sufficient for myosin cortical localization.
During Drosophila axis elongation (Figure 3a), junctional myosin is localized to interfaces between anterior and posterior cells in the germband epithelium (Figure 3b), resulting in polarized cell rearrangements that elongate the body axis (Figure 3c) [68,69]. These cell rearrangements are driven by the contraction of single myosin-rich interfaces to promote local neighbor exchange [68] as well as the coordinated contraction of several connected cell boundaries to form multicellular rosette structures [70]. The anisotropic organization of myosin is associated with increased tension at interfaces between anterior and posterior cells [66,71], with the highest tension at the edges in multicellular contractile cables [66].
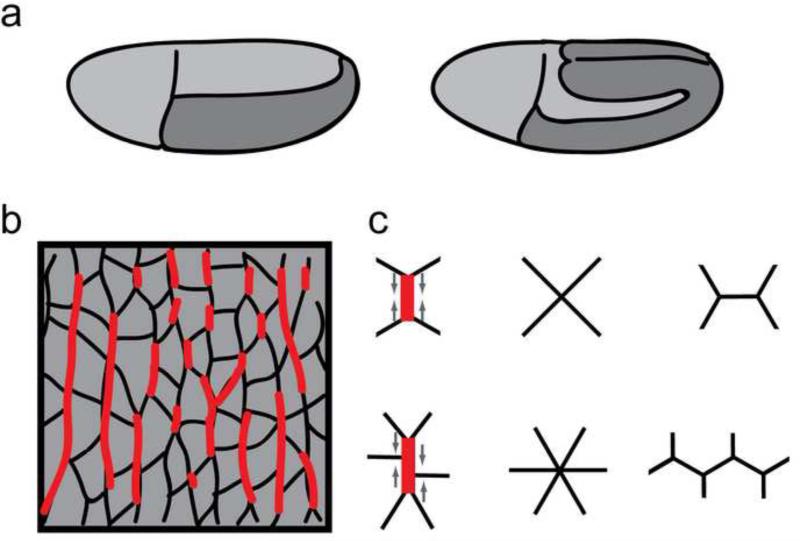
Figure 3. Role of actin-myosin in elongation of the Drosophila body axis.
(a) The germband epithelium (dark gray) lengthens and narrows to elongate the body axis. Before (left) and after (right) elongation. Lateral views, anterior left, ventral down. (b) Junctional myosin (red) is localized to vertical interfaces between anterior and posterior cells, including single cell interfaces and multicellular cables. Laser ablation experiments reveal that myosin-rich interfaces are under tension, with the highest tension in multicellular cables. (c) Polarized cell rearrangements contribute to elongation of the body axis. Contraction of a single myosin-rich interface promotes local neighbor exchange (top), and the coordinated contraction of several adjacent cell interfaces forms a multicellular rosette structure that promotes many-cell rearrangements (bottom). Mechanical tension promotes multicellular cable formation, recruiting myosin to the cortex in regions under high tension.
Fluorescence recovery after photobleaching experiments reveal that myosin dissociation from the cortex is selectively inhibited in regions of high tension [66]. In addition, multicellular actinmyosin cable formation in this tissue appears to be an active process that occurs at a higher frequency than expected by chance [66]. These results suggest a positive feedback loop in which the tension generated in one cell affects myosin dynamics in neighboring cells to promote multicellular contractile cable formation and efficient tissue elongation. Multicellular actinmyosin cables have been observed at the leading edge of the lateral epidermis during dorsal closure [31-33], at compartment boundaries [72-74], and during wound healing [75-78]. Mechanical signals may be important for the establishment and maintenance of these structures [79].
The recruitment of myosin by tension in vivo occurs within 1-3 minutes of applying force [64,66,67], suggesting that the response to force does not require transcription but instead involves a direct effect on myosin or its upstream regulators. In Dictyostelium, recruitment of the phosphoinositide phosphatase PTEN precedes myosin recruitment, and myosin accumulation in response to force is significantly reduced in pten mutants [80]. These results suggest that PTEN is part of a mechanosensory system that regulates myosin localization, perhaps by modifying properties of the plasma membrane. Consistent with this idea, the effects of applied tension in the Drosophila mesoderm are mimicked by inhibiting endocytosis [67].
Alternatively, myosin motor activity could also be directly regulated by force. During its ATPase cycle, non-muscle myosin II spends only a small fraction of the time attached to F-actin, resulting in a low duty ratio [5]. The binding of myosin to F-actin catalyzes phosphate release after ATP hydrolysis, which generates a strain in the motor that drives forward movement along the actin filament. Myosin remains strongly bound to F-actin after ATP hydrolysis, whereas subsequent ATP binding catalyzes the dissociation of myosin from F-actin. Single molecule measurements show that mechanical load stabilizes myosin II in the ADP-bound state when the motor is strongly bound to F-actin [81,82]. Indeed, in Dictyostelium the duty ratio of myosin was estimated to increase 5-10-fold under tension [65]. In the context of a contractile network, a fraction of myosin molecules will be attached to F-actin so that some motors actively pull on actin filaments while others mediate attachments between actin filaments. Increasing the duty ratio is predicted to increase the fraction of motors bound to F-actin, which could potentiate the contractile activity of the network within an active range. Further work will be required to determine how tension-dependent changes in the myosin duty ratio influence network dynamics, contraction, and force generation.
Intrinsic properties of contractile actin-myosin networks
Several properties of the actin cytoskeleton have been reconstituted with purified proteins, providing an opportunity to investigate the molecular and physical mechanisms underlying the behavior of complex protein networks [83]. For example, the behavior of protrusive F-actin networks that drive the forward movement of migrating cells and propel bacterial pathogens through the cytoplasm can be recapitulated with a small number of components including actin, ATP, and proteins involved in actin filament nucleation, capping, and depolymerization [84-86]. These studies have provided a detailed understanding of how assembly and disassembly of the protrusive F-actin network generates force and movement.
Similarly, the in vitro reconstitution of actin-myosin networks has helped to elucidate physical principles that govern the dynamics of the contractile machinery. Processive assemblies of bipolar myosin filaments move actin filaments in vitro in two-dimensional filament gliding assays [87] and three-dimensional networks [88]. The ability of myosin to produce macroscopic network contraction depends on the concentration of actin crosslinking proteins [89,90]. In reconstituted systems containing only myosin, F-actin, and the actin crosslinking protein filamin, a minimum level of crosslinks is required for network contraction, while high concentrations inhibit contraction, presumably because the motors cannot generate sufficient force to contract the highly crosslinked network [90]. Because a fraction of myosin motors mediate attachments between actin filaments, contraction could in principle be varied by tuning the myosin duty ratio as well as the crosslink concentration.
Myosin motors can drive the formation of complex structures such as rings, asters, and active networks, depending on the relative concentrations of actin, myosin, and crosslinks [91]. High concentrations of myosin can also lead to the disassembly of these same structures [91], and myosin has been shown to promote actin depolymerization in vitro [92] and network disassembly in several cell types in culture [93-95]. These studies suggest that properties of cytoskeletal organization and dynamics can be intrinsically controlled by the molecular composition of contractile networks, a regulatory mechanism that may also influence cytoskeletal behavior in vivo.
Conclusions
The mechanical integration of forces generated by contractile actin-myosin networks and transmitted through cell-cell junctions is essential for the dynamic rearrangements that drive morphogenesis in actively remodeling cell populations. Here we describe several features of myosin networks revealed by studies in vivo, such as pulsed contractile behaviors during apical constriction and the recruitment of myosin by tension in single cells and multicellular tissues. It remains to be seen whether these behaviors represent intrinsic properties of contractile networks or if they are actively regulated by tissue-specific biochemical and mechanical cues. The transcription factors Snail and Twist are required to initiate pulses of contraction and stabilize the apical surface between contractions in the Drosophila mesoderm, respectively [22] and several targets of Twist are essential for mesoderm invagination [24,96,97]. In the amnioserosa, Baz/Par-3 stabilizes the actin-myosin network in a contracted state while Par-6 and aPKC regulate the interval between pulses [28]. Identifying the relevant transcriptional targets of Snail and Twist and the mechanisms by which Baz/Par-3, Par-6 and aPKC influence contractile behavior are challenges for future studies. Detailed investigation of the composition, architecture, and dynamics of cellular contractile networks in vivo and comparison with the behavior of reconstituted networks in vitro will help to determine to what extent intrinsic properties are sufficient to account for network dynamics and force generation.
It is clear that properties of contractile networks influence the dynamics of mesoderm invagination, cell intercalation, and dorsal closure, but it is not known if these properties are essential for the ultimate outcome of morphogenesis. A mechanical feedback loop that recruits myosin to the cortex in regions of increased tension is predicted to increase the number of cells engaged in contractile behavior, converting local neighbor exchange events into multicellular rosette structures and producing greater tissue elongation during development [66]. This mechanism may also reinforce myosin activity in other multicellular contractile structures such as compartment boundaries [72-74,79]. The small, incremental changes afforded by pulsed contractile behavior during mesoderm invagination may allow the contractile machinery to adapt as cells change shape during tissue remodeling [22]. Transient or pulsed actin-myosin contractile behaviors are also observed in other contexts, such as in spreading and migrating mouse embryonic fibroblasts, where periodic myosin-dependent contractions of the lamellipodium may help to probe the local mechanical environment [98,99]. The combination of single molecule studies, reconstituted actin-myosin networks, and in vivo studies of myosin dynamics and cell behavior will uncover how intrinsic properties of contractile networks, together with the mechanical and biochemical cues that regulate cytoskeletal activity, contribute to cell shape and tissue morphogenesis during development.
Acknowledgments
We thank Rodrigo Fernandez-Gonzalez, Masako Tamada, and Richard Zallen for critical reading of the manuscript. J.A.Z. is supported by a Burroughs Wellcome Fund Career Award in the Biomedical Sciences, a W. M. Keck Foundation Distinguished Young Scholar in Medical Research Award, and NIH/NIGMS R01 grant GM079340. J.A.Z. is an Early Career Scientist of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kasza KE, Rowat AC, Liu J, Angelini TE, Brangwynne CP, Koenderink GH, Weitz DA. The cell as a material. Curr Opin Cell Biol. 2007;19:101–107. doi: 10.1016/j.ceb.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Cai Y, Sheetz MP. Force propagation across cells: mechanical coherence of dynamic cytoskeletons. Curr Opin Cell Biol. 2009;21:47–50. doi: 10.1016/j.ceb.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou J, Kim HY, Davidson LA. Actomyosin stiffens the vertebrate embryo during crucial stages of elongation and neural tube closure. Development. 2009;136:677–688. doi: 10.1242/dev.026211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vale RD, Milligan RA. The way things move: looking under the hood of molecular motor proteins. Science. 2000;288:88–95. doi: 10.1126/science.288.5463.88. [DOI] [PubMed] [Google Scholar]
- 5.Howard J. Mechanics of Motor Proteins and the Cytoskeleton. 2001 [Google Scholar]
- 6.Cowan CR, Hyman AA. Acto-myosin reorganization and PAR polarity in C. elegans. Development. 2007;134:1035–1043. doi: 10.1242/dev.000513. [DOI] [PubMed] [Google Scholar]
- 7.Martin AC. Pulsation and stabilization: contractile forces that underlie morphogenesis. Dev Biol. 2009;341:114–125. doi: 10.1016/j.ydbio.2009.10.031. [DOI] [PubMed] [Google Scholar]
- 8.Quintin S, Gally C, Labouesse M. Epithelial morphogenesis in embryos: asymmetries, motors and brakes. Trends Genet. 2008;24:221–230. doi: 10.1016/j.tig.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Nishimura T, Takeichi M. Remodeling of the adherens junctions during morphogenesis. Curr Top Dev Biol. 2009;89:33–54. doi: 10.1016/S0070-2153(09)89002-9. [DOI] [PubMed] [Google Scholar]
- 10.Harris TJ, Tepass U. Adherens junctions: from molecules to morphogenesis. Nat Rev Mol Cell Biol. 2010;11:502–514. doi: 10.1038/nrm2927. [DOI] [PubMed] [Google Scholar]
- 11.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 12.Vogel V, Sheetz M. Local force and geometry sensing regulate cell functions. Nat Rev Mol Cell Biol. 2006;7:265–275. doi: 10.1038/nrm1890. [DOI] [PubMed] [Google Scholar]
- 13.Brown AE, Discher DE. Conformational changes and signaling in cell and matrix physics. Curr Biol. 2009;19:R781–789. doi: 10.1016/j.cub.2009.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wozniak MA, Chen CS. Mechanotransduction in development: a growing role for contractility. Nat Rev Mol Cell Biol. 2009;10:34–43. doi: 10.1038/nrm2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mammoto T, Ingber DE. Mechanical control of tissue and organ development. Development. 2010;137:1407–1420. doi: 10.1242/dev.024166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan S, Sheetz MP. Force effects on biochemical kinetics. Annu Rev Biochem. 1997;66:785–805. doi: 10.1146/annurev.biochem.66.1.785. [DOI] [PubMed] [Google Scholar]
- 17.Purcell TJ, Sweeney HL, Spudich JA. A force-dependent state controls the coordination of processive myosin V. Proc Natl Acad Sci U S A. 2005;102:13873–13878. doi: 10.1073/pnas.0506441102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas WE, Vogel V, Sokurenko E. Biophysics of catch bonds. Annu Rev Biophys. 2008;37:399–416. doi: 10.1146/annurev.biophys.37.032807.125804. [DOI] [PubMed] [Google Scholar]
- 19.Howard J. Mechanical signaling in networks of motor and cytoskeletal proteins. Annu Rev Biophys. 2009;38:217–234. doi: 10.1146/annurev.biophys.050708.133732. [DOI] [PubMed] [Google Scholar]
- 20•.Na S, Collin O, Chowdhury F, Tay B, Ouyang M, Wang Y, Wang N. Rapid signal transduction in living cells is a unique feature of mechanotransduction. Proc Natl Acad Sci U S A. 2008;105:6626–6631. doi: 10.1073/pnas.0711704105. [In this study, activation of the Src tyrosine kinase by mechanical and biochemical signals was compared using a FRET-based reporter of Src activity. Mechanical deformation of a cultured smooth muscle cell induced Src activation in less than 0.3 s while activation in response to soluble growth factor took more than 10 seconds, demonstrating that mechanical signals can propagate quickly over long distances and rapidly be transduced into a biochemical change.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Odell GM, Oster G, Alberch P, Burnside B. The mechanical basis of morphogenesis. I. Epithelial folding and invagination. Dev Biol. 1981;85:446–462. doi: 10.1016/0012-1606(81)90276-1. [DOI] [PubMed] [Google Scholar]
- 22••.Martin AC, Kaschube M, Wieschaus EF. Pulsed contractions of an actin-myosin network drive apical constriction. Nature. 2009;457:495–499. doi: 10.1038/nature07522. [This study reveals that apical constriction of ventral furrow cells in the Drosophila embryo occurs in pulses associated with bursts of myosin accumulation in a medial actin-myosin network. Constriction proceeds in a ratchet-like manner where pulses of constriction are followed by pauses during which the constricted state is stabilized. This may allow the contractile actin-myosin network to optimize force generation as the cells constrict.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leptin M, Grunewald B. Cell shape changes during gastrulation in Drosophila. Development. 1990;110:73–84. doi: 10.1242/dev.110.1.73. [DOI] [PubMed] [Google Scholar]
- 24.Dawes-Hoang RE, Parmar KM, Christiansen AE, Phelps CB, Brand AH, Wieschaus EF. folded gastrulation, cell shape change and the control of myosin localization. Development. 2005;132:4165–4178. doi: 10.1242/dev.01938. [DOI] [PubMed] [Google Scholar]
- 25.Halbleib JM, Nelson WJ. Cadherins in development: cell adhesion, sorting, and tissue morphogenesis. Genes Dev. 2006;20:3199–3214. doi: 10.1101/gad.1486806. [DOI] [PubMed] [Google Scholar]
- 26.Yap AS, Crampton MS, Hardin J. Making and breaking contacts: the cellular biology of cadherin regulation. Curr Opin Cell Biol. 2007;19:508–514. doi: 10.1016/j.ceb.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27•.Martin AC, Gelbart M, Fernandez-Gonzalez R, Kaschube M, Wieschaus EF. Integration of contractile forces during tissue invagination. J Cell Biol. 2010;188:735–749. doi: 10.1083/jcb.200910099. [This study demonstrates that tissue-level tension in the ventral furrow of the Drosophila embryo is higher along the anterior-posterior axis and that when cell adhesion is reduced, cells tend to constrict isotropically. These findings indicate that global mechanics of the embryo constrain cells to constrict primarily along the dorsal-ventral axis to produce a long narrow furrow.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28•.David DJ, Tishkina A, Harris TJ. The PAR complex regulates pulsed actomyosin contractions during amnioserosa apical constriction in Drosophila. Development. 2009;137:1645–1655. doi: 10.1242/dev.044107. [This study demonstrates that pulsed apical constrictions of amnioserosa cells in the Drosophila embryo are associated with cycles of assembly and disassembly of an apical actin-myosin network. Baz/Par-3, Par-6 and aPKC regulate distinct phases of the actin-myosin network dynamics.] [DOI] [PubMed] [Google Scholar]
- 29•.Gorfinkiel N, Blanchard GB, Adams RJ, Martinez Arias A. Mechanical control of global cell behaviour during dorsal closure in Drosophila. Development. 2009;136:1889–1898. doi: 10.1242/dev.030866. [This study reveals that the constriction rate of amnioserosa cells during dorsal closure of the Drosophila embryo varies spatially and temporally in a manner suggesting that these cell behaviors may be responsive to mechanical cues.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30••.Solon J, Kaya-Copur A, Colombelli J, Brunner D. Pulsed forces timed by a ratchet-like mechanism drive directed tissue movement during dorsal closure. Cell. 2009;137:1331–1342. doi: 10.1016/j.cell.2009.03.050. [This study provides evidence that the pulsed apical constriction and relaxation of amnioserosa cells is converted to net apical constriction coincident with formation of the leading edge actinmyosin cable during dorsal closure in the Drosophila embryo, suggesting a ratchet-like mechanism by which the amnioserosa and the leading edge cable collaborate to generate forces that drive dorsal closure.] [DOI] [PubMed] [Google Scholar]
- 31.Franke JD, Montague RA, Kiehart DP. Nonmuscle myosin II generates forces that transmit tension and drive contraction in multiple tissues during dorsal closure. Curr Biol. 2005;15:2208–2221. doi: 10.1016/j.cub.2005.11.064. [DOI] [PubMed] [Google Scholar]
- 32.Jacinto A, Wood W, Woolner S, Hiley C, Turner L, Wilson C, Martinez-Arias A, Martin P. Dynamic analysis of actin cable function during Drosophila dorsal closure. Curr Biol. 2002;12:1245–1250. doi: 10.1016/s0960-9822(02)00955-7. [DOI] [PubMed] [Google Scholar]
- 33.Kiehart DP, Galbraith CG, Edwards KA, Rickoll WL, Montague RA. Multiple forces contribute to cell sheet morphogenesis for dorsal closure in Drosophila. J Cell Biol. 2000;149:471–490. doi: 10.1083/jcb.149.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toyama Y, Peralta XG, Wells AR, Kiehart DP, Edwards GS. Apoptotic force and tissue dynamics during Drosophila embryogenesis. Science. 2008;321:1683–1686. doi: 10.1126/science.1157052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jacinto A, Wood W, Balayo T, Turmaine M, Martinez-Arias A, Martin P. Dynamic actin-based epithelial adhesion and cell matching during Drosophila dorsal closure. Curr Biol. 2000;10:1420–1426. doi: 10.1016/s0960-9822(00)00796-x. [DOI] [PubMed] [Google Scholar]
- 36.Millard TH, Martin P. Dynamic analysis of filopodial interactions during the zippering phase of Drosophila dorsal closure. Development. 2008;135:621–626. doi: 10.1242/dev.014001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37•.Blanchard GB, Murugesu S, Adams RJ, Martinez Arias A, Gorfinkiel N. Cytoskeletal dynamics and supracellular organisation of cell shape fluctuations during dorsal closure. Development. 2010;137:2743–2752. doi: 10.1242/dev.045872. [This study shows that fluctuations in the apical shape of amnioserosa cells in Drosophila decrease in amplitude and duration as dorsal closure progresses. These changes are associated with an increase in medial and junctional myosin and are not strongly dependent on the leading edge cable in the lateral epidermis, providing an alternative model for the mechanical interaction between the amnioserosa and lateral epidermis during dorsal closure.] [DOI] [PubMed] [Google Scholar]
- 38.Munro E, Nance J, Priess JR. Cortical flows powered by asymmetrical contraction transport PAR proteins to establish and maintain anterior-posterior polarity in the early C. elegans embryo. Dev Cell. 2004;7:413–424. doi: 10.1016/j.devcel.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 39.Skoglund P, Rolo A, Chen X, Gumbiner BM, Keller R. Convergence and extension at gastrulation require a myosin IIB-dependent cortical actin network. Development. 2008;135:2435–2444. doi: 10.1242/dev.014704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276:1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 41.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 42.Nelson CM, Jean RP, Tan JL, Liu WF, Sniadecki NJ, Spector AA, Chen CS. Emergent patterns of growth controlled by multicellular form and mechanics. Proc Natl Acad Sci U S A. 2005;102:11594–11599. doi: 10.1073/pnas.0502575102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 44.Cowan CR, Hyman AA. Centrosomes direct cell polarity independently of microtubule assembly in C. elegans embryos. Nature. 2004;431:92–96. doi: 10.1038/nature02825. [DOI] [PubMed] [Google Scholar]
- 45.Mayer M, Depken M, Bois JS, Julicher F, Grill SW. Anisotropies in cortical tension reveal the physical basis of polarizing cortical flows. Nature. 2010;467:617–621. doi: 10.1038/nature09376. [DOI] [PubMed] [Google Scholar]
- 46.Cowan CR, Hyman AA. Asymmetric cell division in C. elegans: cortical polarity and spindle positioning. Annu Rev Cell Dev Biol. 2004;20:427–453. doi: 10.1146/annurev.cellbio.19.111301.113823. [DOI] [PubMed] [Google Scholar]
- 47.Desprat N, Supatto W, Pouille PA, Beaurepaire E, Farge E. Tissue deformation modulates twist expression to determine anterior midgut differentiation in Drosophila embryos. Dev Cell. 2008;15:470–477. doi: 10.1016/j.devcel.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 48.Farge E. Mechanical induction of Twist in the Drosophila foregut/stomodeal primordium. Curr Biol. 2003;13:1365–1377. doi: 10.1016/s0960-9822(03)00576-1. [DOI] [PubMed] [Google Scholar]
- 49.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 50.Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol. 2009;10:21–33. doi: 10.1038/nrm2593. [DOI] [PubMed] [Google Scholar]
- 51.Balaban NQ, Schwarz US, Riveline D, Goichberg P, Tzur G, Sabanay I, Mahalu D, Safran S, Bershadsky A, Addadi L, et al. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat Cell Biol. 2001;3:466–472. doi: 10.1038/35074532. [DOI] [PubMed] [Google Scholar]
- 52.Riveline D, Zamir E, Balaban NQ, Schwarz US, Ishizaki T, Narumiya S, Kam Z, Geiger B, Bershadsky AD. Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J Cell Biol. 2001;153:1175–1186. doi: 10.1083/jcb.153.6.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tamada M, Sheetz MP, Sawada Y. Activation of a signaling cascade by cytoskeleton stretch. Dev Cell. 2004;7:709–718. doi: 10.1016/j.devcel.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 54.Sawada Y, Tamada M, Dubin-Thaler BJ, Cherniavskaya O, Sakai R, Tanaka S, Sheetz MP. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell. 2006;127:1015–1026. doi: 10.1016/j.cell.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhong C, Chrzanowska-Wodnicka M, Brown J, Shaub A, Belkin AM, Burridge K. Rho-mediated contractility exposes a cryptic site in fibronectin and induces fibronectin matrix assembly. J Cell Biol. 1998;141:539–551. doi: 10.1083/jcb.141.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baneyx G, Baugh L, Vogel V. Fibronectin extension and unfolding within cell matrix fibrils controlled by cytoskeletal tension. Proc Natl Acad Sci U S A. 2002;99:5139–5143. doi: 10.1073/pnas.072650799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gao M, Craig D, Lequin O, Campbell ID, Vogel V, Schulten K. Structure and functional significance of mechanically unfolded fibronectin type III1 intermediates. Proc Natl Acad Sci U S A. 2003;100:14784–14789. doi: 10.1073/pnas.2334390100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.del Rio A, Perez-Jimenez R, Liu R, Roca-Cusachs P, Fernandez JM, Sheetz MP. Stretching single talin rod molecules activates vinculin binding. Science. 2009;323:638–641. doi: 10.1126/science.1162912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maruthamuthu V, Aratyn-Schaus Y, Gardel ML. Conserved F-actin dynamics and force transmission at cell adhesions. Curr Opin Cell Biol. 2010;22:583–588. doi: 10.1016/j.ceb.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60•.Liu Z, Tan JL, Cohen DM, Yang MT, Sniadecki NJ, Ruiz SA, Nelson CM, Chen CS. Mechanical tugging force regulates the size of cell-cell junctions. Proc Natl Acad Sci U S A. 2010;107:9944–9949. doi: 10.1073/pnas.0914547107. [This study demonstrates that myosin-dependent tugging forces regulate adherens junction assembly between cultured endothelial cells by using a system of microfabricated force sensors to make quantitative force measurements.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61•.le Duc Q, Shi Q, Blonk I, Sonnenberg A, Wang N, Leckband D, de Rooij J. Vinculin potentiates E-cadherin mechanosensing and is recruited to actin-anchored sites within adherens junctions in a myosin II-dependent manner. J Cell Biol. 2010;189:1107–1115. doi: 10.1083/jcb.201001149. [This study shows that application of force to a cultured cell by manipulation of an E-cadherin coated bead leads to force-dependent stiffening of E-cadherin junctions and that vinculin is required for this mechanosensitive response.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62•.Yonemura S, Wada Y, Watanabe T, Nagafuchi A, Shibata M. alpha-Catenin as a tension transducer that induces adherens junction development. Nat Cell Biol. 2010;12:533–542. doi: 10.1038/ncb2055. [This study shows that α-catenin recruits vinculin to adherens junctions through a force-dependent conformational change in α-catenin, providing a mechanism by which mechanical forces can regulate adherens junction assembly.] [DOI] [PubMed] [Google Scholar]
- 63.Merkel R, Simson R, Simson DA, Hohenadl M, Boulbitch A, Wallraff E, Sackmann E. A micromechanic study of cell polarity and plasma membrane cell body coupling in Dictyostelium. Biophys J. 2000;79:707–719. doi: 10.1016/S0006-3495(00)76329-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Effler JC, Kee YS, Berk JM, Tran MN, Iglesias PA, Robinson DN. Mitosis-specific mechanosensing and contractile-protein redistribution control cell shape. Curr Biol. 2006;16:1962–1967. doi: 10.1016/j.cub.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65••.Ren Y, Effler JC, Norstrom M, Luo T, Firtel RA, Iglesias PA, Rock RS, Robinson DN. Mechanosensing through cooperative interactions between myosin II and the actin crosslinker cortexillin I. Curr Biol. 2009;19:1421–1428. doi: 10.1016/j.cub.2009.07.018. [This study shows that the F-actin crosslinker cortexillin I is required to recruit myosin II to cortical regions of Dictyostelium cells deformed by micropipette aspiration. Force-dependent myosin recruitment depends on the length of the lever arm of the myosin motor, suggesting that myosin itself may act as a mechanosensor.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66••.Fernandez-Gonzalez R, Simoes S, Roper JC, Eaton S, Zallen JA. Myosin II dynamics are regulated by tension in intercalating cells. Dev Cell. 2009;17:736–743. doi: 10.1016/j.devcel.2009.09.003. [This study shows that multicellular actin-myosin cables during Drosophila axis elongation form through the active recruitment of myosin to the cortex by mechanical tension, suggesting that the mechanical regulation of myosin dynamics contributes to contractile force generation and tissue morphogenesis.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67•.Pouille PA, Ahmadi P, Brunet AC, Farge E. Mechanical signals trigger Myosin II redistribution and mesoderm invagination in Drosophila embryos. Sci Signal. 2009;2:ra16. doi: 10.1126/scisignal.2000098. [This study shows that mechanical deformation of Drosophila embryos recruits myosin to the apical cortex of mesodermal cells and that Fog signaling is involved in this force-dependent myosin redistribution.] [DOI] [PubMed] [Google Scholar]
- 68.Bertet C, Sulak L, Lecuit T. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature. 2004;429:667–671. doi: 10.1038/nature02590. [DOI] [PubMed] [Google Scholar]
- 69.Zallen JA, Wieschaus E. Patterned gene expression directs bipolar planar polarity in Drosophila. Dev Cell. 2004;6:343–355. doi: 10.1016/s1534-5807(04)00060-7. [DOI] [PubMed] [Google Scholar]
- 70.Blankenship JT, Backovic ST, Sanny JS, Weitz O, Zallen JA. Multicellular rosette formation links planar cell polarity to tissue morphogenesis. Dev Cell. 2006;11:459–470. doi: 10.1016/j.devcel.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 71.Rauzi M, Verant P, Lecuit T, Lenne PF. Nature and anisotropy of cortical forces orienting Drosophila tissue morphogenesis. Nat Cell Biol. 2008;10:1401–1410. doi: 10.1038/ncb1798. [DOI] [PubMed] [Google Scholar]
- 72.Major RJ, Irvine KD. Localization and requirement for Myosin II at the dorsal-ventral compartment boundary of the Drosophila wing. Dev Dyn. 2006;235:3051–3058. doi: 10.1002/dvdy.20966. [DOI] [PubMed] [Google Scholar]
- 73.Landsberg KP, Farhadifar R, Ranft J, Umetsu D, Widmann TJ, Bittig T, Said A, Julicher F, Dahmann C. Increased cell bond tension governs cell sorting at the Drosophila anteroposterior compartment boundary. Curr Biol. 2009;19:1950–1955. doi: 10.1016/j.cub.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 74.Monier B, Pelissier-Monier A, Brand AH, Sanson B. An actomyosin-based barrier inhibits cell mixing at compartmental boundaries in Drosophila embryos. Nat Cell Biol. 2010;12:60–65. doi: 10.1038/ncb2005. sup pp 61-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Martin P, Lewis J. Actin cables and epidermal movement in embryonic wound healing. Nature. 1992;360:179–183. doi: 10.1038/360179a0. [DOI] [PubMed] [Google Scholar]
- 76.Bement WM, Forscher P, Mooseker MS. A novel cytoskeletal structure involved in purse string wound closure and cell polarity maintenance. J Cell Biol. 1993;121:565–578. doi: 10.1083/jcb.121.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bement WM, Mandato CA, Kirsch MN. Wound-induced assembly and closure of an actomyosin purse string in Xenopus oocytes. Curr Biol. 1999;9:579–587. doi: 10.1016/s0960-9822(99)80261-9. [DOI] [PubMed] [Google Scholar]
- 78.Rodriguez-Diaz A, Toyama Y, Abravanel DL, Wiemann JM, Wells AR, Tulu US, Edwards GS, Kiehart DP. Actomyosin purse strings: renewable resources that make morphogenesis robust and resilient. Hfsp J. 2008;2:220–237. doi: 10.2976/1.2955565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vincent JP, Irons D. Developmental biology: tension at the border. Curr Biol. 2009;19:R1028–1030. doi: 10.1016/j.cub.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 80.Pramanik MK, Iijima M, Iwadate Y, Yumura S. PTEN is a mechanosensing signal transducer for myosin II localization in Dictyostelium cells. Genes Cells. 2009;14:821–834. doi: 10.1111/j.1365-2443.2009.01312.x. [DOI] [PubMed] [Google Scholar]
- 81.Veigel C, Molloy JE, Schmitz S, Kendrick-Jones J. Load-dependent kinetics of force production by smooth muscle myosin measured with optical tweezers. Nat Cell Biol. 2003;5:980–986. doi: 10.1038/ncb1060. [DOI] [PubMed] [Google Scholar]
- 82.Kovacs M, Thirumurugan K, Knight PJ, Sellers JR. Load-dependent mechanism of nonmuscle myosin 2. Proc Natl Acad Sci U S A. 2007;104:9994–9999. doi: 10.1073/pnas.0701181104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu AP, Fletcher DA. Biology under construction: in vitro reconstitution of cellular function. Nat Rev Mol Cell Biol. 2009;10:644–650. doi: 10.1038/nrm2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Loisel TP, Boujemaa R, Pantaloni D, Carlier MF. Reconstitution of actin-based motility of Listeria and Shigella using pure proteins. Nature. 1999;401:613–616. doi: 10.1038/44183. [DOI] [PubMed] [Google Scholar]
- 85.Akin O, Mullins RD. Capping protein increases the rate of actin-based motility by promoting filament nucleation by the Arp2/3 complex. Cell. 2008;133:841–851. doi: 10.1016/j.cell.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.van der Gucht J, Paluch E, Plastino J, Sykes C. Stress release drives symmetry breaking for actin-based movement. Proc Natl Acad Sci U S A. 2005;102:7847–7852. doi: 10.1073/pnas.0502121102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kron SJ, Spudich JA. Fluorescent actin filaments move on myosin fixed to a glass surface. Proc Natl Acad Sci U S A. 1986;83:6272–6276. doi: 10.1073/pnas.83.17.6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Humphrey D, Duggan C, Saha D, Smith D, Kas J. Active fluidization of polymer networks through molecular motors. Nature. 2002;416:413–416. doi: 10.1038/416413a. [DOI] [PubMed] [Google Scholar]
- 89.Bendix PM, Koenderink GH, Cuvelier D, Dogic Z, Koeleman BN, Brieher WM, Field CM, Mahadevan L, Weitz DA. A quantitative analysis of contractility in active cytoskeletal protein networks. Biophys J. 2008;94:3126–3136. doi: 10.1529/biophysj.107.117960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Janson LW, Kolega J, Taylor DL. Modulation of contraction by gelation/solation in a reconstituted motile model. J Cell Biol. 1991;114:1005–1015. doi: 10.1083/jcb.114.5.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Backouche F, Haviv L, Groswasser D, Bernheim-Groswasser A. Active gels: dynamics of patterning and self-organization. Phys Biol. 2006;3:264–273. doi: 10.1088/1478-3975/3/4/004. [DOI] [PubMed] [Google Scholar]
- 92.Haviv L, Gillo D, Backouche F, Bernheim-Groswasser A. A cytoskeletal demolition worker: myosin II acts as an actin depolymerization agent. J Mol Biol. 2008;375:325–330. doi: 10.1016/j.jmb.2007.09.066. [DOI] [PubMed] [Google Scholar]
- 93.Medeiros NA, Burnette DT, Forscher P. Myosin II functions in actin-bundle turnover in neuronal growth cones. Nat Cell Biol. 2006;8:215–226. doi: 10.1038/ncb1367. [DOI] [PubMed] [Google Scholar]
- 94.Skwarek-Maruszewska A, Hotulainen P, Mattila PK, Lappalainen P. Contractility-dependent actin dynamics in cardiomyocyte sarcomeres. J Cell Sci. 2009;122:2119–2126. doi: 10.1242/jcs.046805. [DOI] [PubMed] [Google Scholar]
- 95.Wilson CA, Tsuchida MA, Allen GM, Barnhart EL, Applegate KT, Yam PT, Ji L, Keren K, Danuser G, Theriot JA. Myosin II contributes to cell-scale actin network treadmilling through network disassembly. Nature. 2010;465:373–377. doi: 10.1038/nature08994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Leptin M. twist and snail as positive and negative regulators during Drosophila mesoderm development. Genes Dev. 1991;5:1568–1576. doi: 10.1101/gad.5.9.1568. [DOI] [PubMed] [Google Scholar]
- 97.Kolsch V, Seher T, Fernandez-Ballester GJ, Serrano L, Leptin M. Control of Drosophila gastrulation by apical localization of adherens junctions and RhoGEF2. Science. 2007;315:384–386. doi: 10.1126/science.1134833. [DOI] [PubMed] [Google Scholar]
- 98.Giannone G, Dubin-Thaler BJ, Dobereiner HG, Kieffer N, Bresnick AR, Sheetz MP. Periodic lamellipodial contractions correlate with rearward actin waves. Cell. 2004;116:431–443. doi: 10.1016/s0092-8674(04)00058-3. [DOI] [PubMed] [Google Scholar]
- 99.Giannone G, Dubin-Thaler BJ, Rossier O, Cai Y, Chaga O, Jiang G, Beaver W, Dobereiner HG, Freund Y, Borisy G, et al. Lamellipodial actin mechanically links myosin activity with adhesion-site formation. Cell. 2007;128:561–575. doi: 10.1016/j.cell.2006.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]