Abstract
Objective:
To test the cognitive dedifferentiation hypothesis that cognitive abilities become increasingly correlated in late life.
Methods:
Participants are 174 older persons without dementia at the beginning of a longitudinal clinical-pathologic cohort study. At annual intervals for 6 to 15 years prior to death, they completed a battery of cognitive performance tests from which previously established composite measures of episodic memory, semantic memory, working memory, and perceptual speed were derived. At death, there was a uniform neuropathologic assessment and levels of diffuse plaques, neuritic plaques, and neurofibrillary tangles were summarized in a composite measure. Change in the 4 cognitive outcomes was analyzed simultaneously in a mixed-effects model that allowed rate of decline to accelerate at a variable point before death.
Results:
On average, cognitive decline before the terminal period was relatively gradual, and rates of change in different cognitive domains were moderately correlated, ranging from 0.25 (episodic memory–working memory) to 0.46 (episodic memory–semantic memory). By contrast, cognition declined rapidly during the terminal period, and rates of change in different cognitive functions were strongly correlated, ranging from 0.83 (working memory–perceptual speed) to 0.89 (episodic memory–semantic memory, semantic memory–working memory). Higher level of plaques and tangles on postmortem examination was associated with faster preterminal decline and earlier onset of terminal decline but not with rate of terminal decline or correlations between rates of change in different cognitive functions.
Conclusion:
In the last years of life, covariation among cognitive abilities sharply increases consistent with the cognitive dedifferentiation hypothesis.
According to a longstanding hypothesis, the functional organization of cognitive abilities shifts in old age with abilities becoming increasingly intercorrelated, or dedifferentiated.1,2 The phenomenon is assumed to reflect age-related neurobiologic processes that impair cognition in a general rather than domain-specific fashion. Despite years of research, however, knowledge about cognitive dedifferentiation in old age remains limited owing to several factors. First, much of the research consists of cross-sectional comparisons of the size of the correlations among cognitive abilities in younger vs older adults. For the most part, these studies have not found clear age group differences,3,4 but it is uncertain how relevant between-person correlations are to within-person correlations in rates of cognitive change. Second, some longitudinal studies suggest positive correlations between rates of change in different abilities,5–7 but it is unclear whether the correlations are changing with advancing age as hypothesized. Third, longitudinal studies have shown that late-life trajectories of cognitive decline tend to markedly accelerate in the last few years of life,8–12 but few studies of cognitive dedifferentiation have considered the effect of mortality.7
The present study examines cognitive dedifferentiation in old age. It differs from previous research in that we used death as the temporal reference point rather than birth or study baseline, and we divided each person's cognitive trajectory into preterminal and terminal components. Participants are older Catholic nuns, priests, and monks who had annual cognitive testing for 6 to 15 years before death and a brain autopsy and uniform neuropathologic examination from which a composite measure of plaques and tangles was derived. We constructed a mixed-effects model that simultaneously characterized change in 4 cognitive outcomes and let rates of decline accelerate at a variable point prior to death. This allowed us to test whether the correlations between rates of change in cognitive abilities shifted from the preterminal to terminal period and whether this shift was related to the pathologic burden of Alzheimer disease (AD).
METHODS
Participants.
Subjects are from the Religious Orders Study, a longitudinal clinical-pathologic study of aging and AD.13 All participants agreed to annual clinical evaluations and brain autopsy in the event of death. They were recruited from about 40 groups across the United States. Clinical evaluations began in 1994 and are continuing.
Eligibility for these analyses required absence of dementia at baseline and completion of at least 7 annual clinical evaluations prior to death to make it possible to observe a terminal shift in rate of cognitive decline. At the time of these analyses, 340 study participants without baseline dementia had died, and 174 of these had completed at least 7 clinical evaluations (mean = 10.2, SD = 2.0; range 7–16). Analyses are based on this group. Their mean age was 78.2 (SD = 6.1) at baseline and 88.3 (SD = 6.0) at death, they had a mean of 18.1 (SD = 3.2) years of education, 61.5% were women, 96.6% were white and non-Hispanic, and 27.6% had mild cognitive impairment (MCI) at baseline. The last clinical evaluation took place a mean of 0.56 (SD = 0.32) year prior to death. The 174 subjects included in analyses had an older age at death and a higher baseline level of cognitive function than the 166 subjects excluded because of insufficient longitudinal data, but the subgroups did not differ on other demographic variables, likelihood of developing dementia during the study, level of cognitive function proximate to death, or postmortem level of AD pathology (table e-1 on the Neurology® Web site at www.neurology.org).
Standard protocol approvals, registrations, and patient consents.
Following a thorough description of the study, written informed consent was obtained from all subjects. The study was approved by the institutional review board of Rush University Medical Center.
Assessment of cognitive function.
Cognitive function was assessed at annual intervals with a battery of performance tests, as previously described.5,13 For the present analyses, we used 15 individual measures of 4 cognitive domains. Episodic memory was measured with 7 tests: Word List Memory, Word List Recall, Word List Recognition and immediate and delayed recall of the East Boston Story and Story A from Logical Memory. Semantic memory was evaluated with a 15-item form of the Boston Naming Test, Verbal Fluency, and a 15-item word recognition test. Working memory was measured with Digit Span Forward, Digit Span Backward, and Digit Ordering. Perceptual speed was assessed with Number Comparison and the oral version of the Symbol Digit Modalities Test. We used previously established composite measures of episodic memory (7 tests), semantic memory (3 tests), working memory (3 tests), and perceptual speed (2 tests) in analyses. Raw scores on individual tests were converted to z scores, using the baseline mean and SD of the entire cohort, and the z scores of component tests were averaged to yield composite scores. Previous publications contain further information about the individual tests and the derivation of the composite measures.5,13
Clinical classification.
Each annual evaluation also included a structured medical history and complete neurologic examination. On the basis of these data and the cognitive testing, an experienced clinician diagnosed dementia and AD using the criteria of the joint working group of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association.14 These criteria require a history of cognitive decline and impairment in a least 2 cognitive domains, one of which must be memory for a diagnosis of AD. Persons who had cognitive impairment but did not meet dementia criteria were classified as MCI. Persons meeting these MCI criteria have been shown to have intermediate levels of cognitive decline15,16 and plaques and tangles17 compared to persons with no cognitive impairment and dementia.
Neuropathologic assessment.
We used a standard protocol for brain removal, tissue sectioning and preservation, and quantifying pathologic results as previously described.18,19 Neuritic plaques, diffuse plaques, and neurofibrillary tangles were counted in 4 brain regions (entorhinal cortex, midfrontal gyrus, middle temporal gyrus, inferior parietal gyrus) with a modified Bielschowsky silver stain. Counts of each lesion in each brain region were standardized and then averaged to yield a composite AD pathologic index. Further information on the derivation of this composite index is published elsewhere.20
Data analysis.
Individual rates of change on the 4 cognitive outcome measures were estimated in a random change point model21 using a Bayesian Monte Carlo Markov Chain approach22 implemented in OpenBUGS software.23 Time was represented as years prior to death. The model allowed rate of decline on each measure to accelerate at some variable time before death. Age at death, sex, and education were modeled as fixed effects. Random effects were included to allow for variation in rate of cognitive decline before the change point (i.e., preterminal slope), the onset of the terminal period, rate of decline during the terminal period (i.e., terminal slope), and level of cognitive function just prior to death (i.e., intercept). The model provided estimates of the correlations between these random effects. To test the dedifferentiation hypothesis, we compared the correlations between preterminal slopes in different cognitive abilities with the correlations between terminal slopes in the same cognitive abilities. We then repeated the analysis with a term added to estimate the association of the AD pathologic index with cognitive trajectory components. Effects with p values of 0.05 or less were regarded as significant.
RESULTS
At baseline, the 4 composite cognitive measures had approximately normal distributions and were moderately correlated with one another (above the diagonal line in table 1). Participants completed annual cognitive testing 7 to 16 times prior to death. By the time of the last evaluation, mean performance had declined and variability had increased within each domain, and correlations between domain scores had increased (below the diagonal line in table 1).
Table 1.
Psychometric information on composite cognitive measures at baseline and at the last evaluation before death
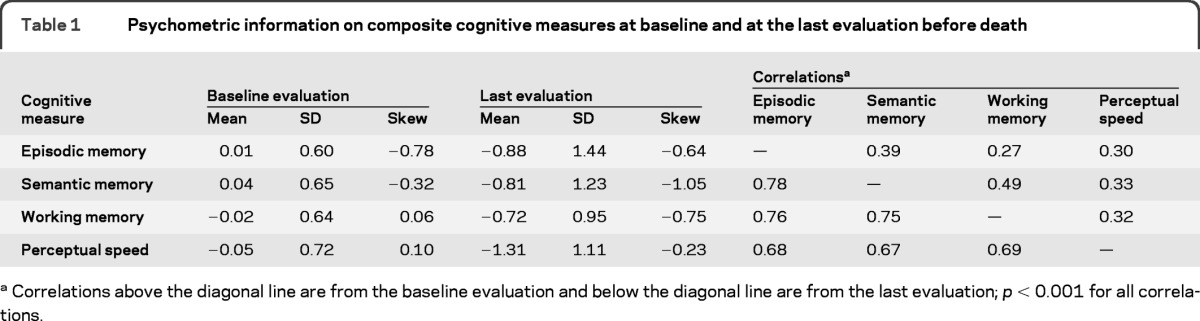
Correlations above the diagonal line are from the baseline evaluation and below the diagonal line are from the last evaluation; p < 0.001 for all correlations.
Cognitive dedifferentiation.
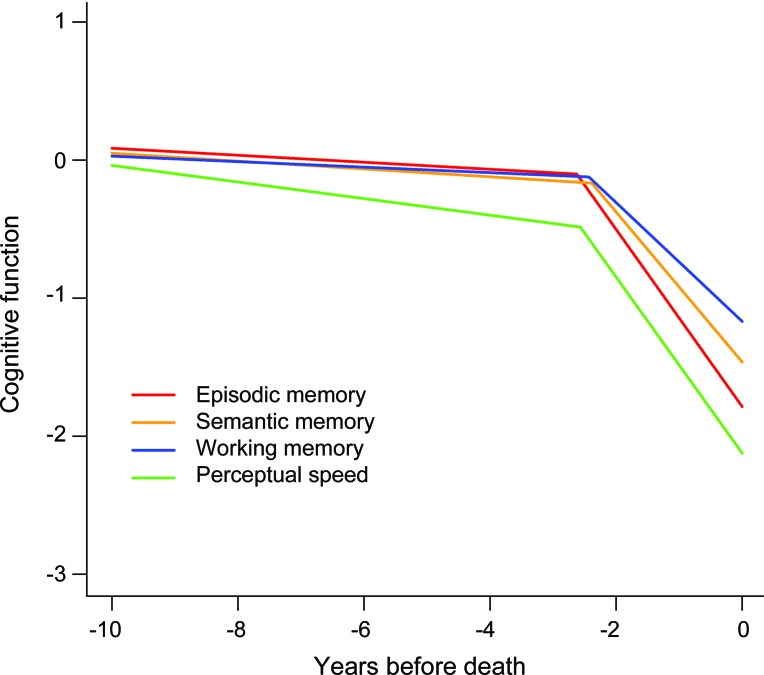
We simultaneously assessed change in the 4 cognitive outcomes in a mixed-effects model that allowed rate of cognitive decline to accelerate beginning at some variable time before death. In this analysis, performance declined in episodic memory before the terminal period at a rate of 0.031 unit per year (table 2). At an average of 2.62 years before death, annual rate of episodic memory decline increased to 0.535 unit. This represents a more than 15-fold increase in the rate of decline in the terminal period compared to the preterminal period. Further, rates of episodic memory decline in the 2 periods were not strongly correlated (r = 0.23, p = 0.10). Results were similar in the other 3 cognitive domains, with 8-fold to 17-fold increases in rate of decline during the terminal period compared to the preterminal period (table 2) and little correlation between preterminal and terminal rates of change (semantic memory r = 0.33, p = 0.01; working memory r = 0.29, p = 0.03; perceptual speed r = 0.18, p = 0.22). Figure 1 shows the predicted paths of change in the 4 cognitive domains for a typical subject who died after 10 years in the study. Decline in each domain is relatively gradual and then sharply accelerates about 2.5 years before death.
Table 2.
Trajectories of change in different cognitive domainsa
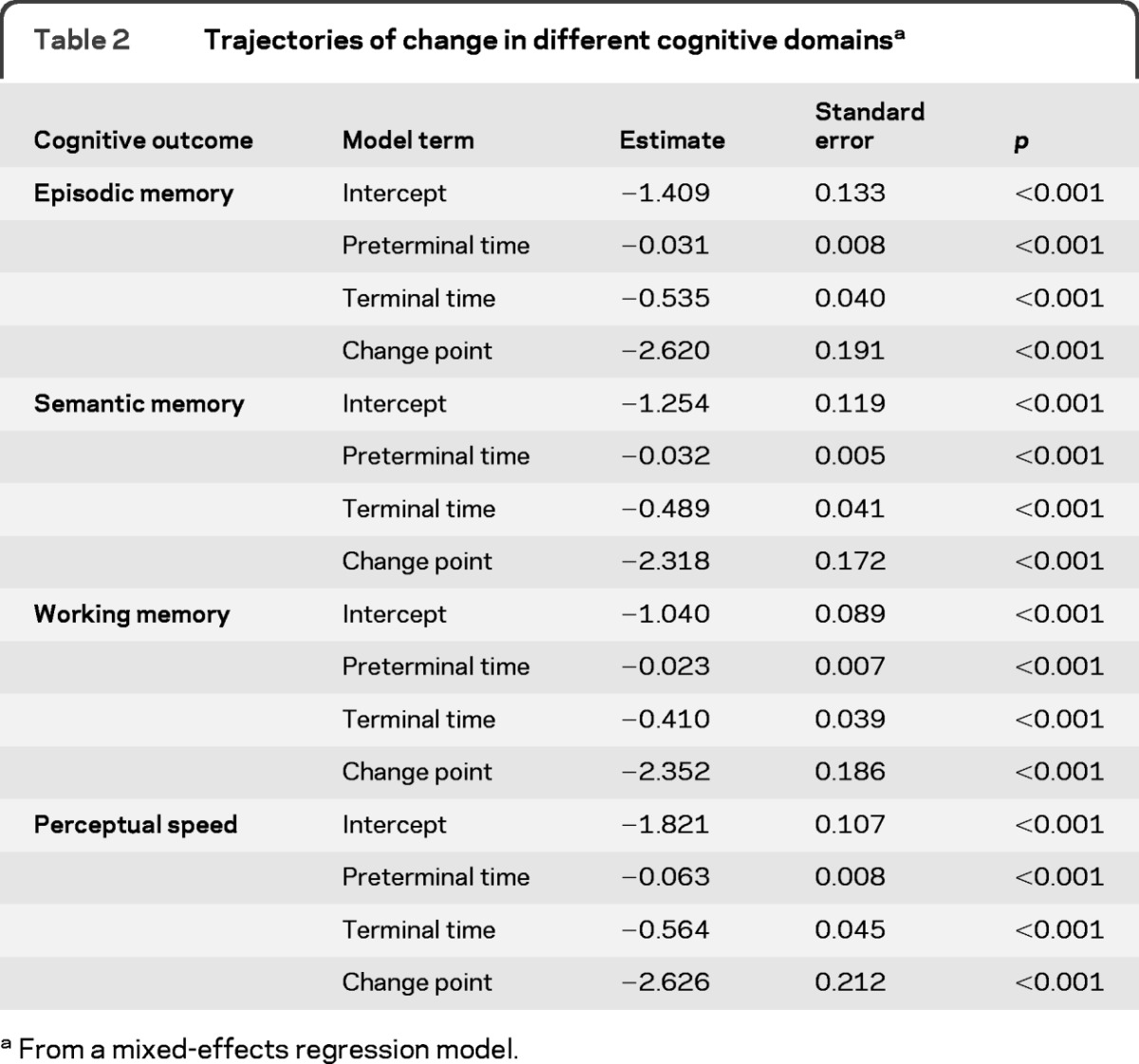
From a mixed-effects regression model.
Figure 1. Typical paths of change in different cognitive domains.
Predicted paths of change in different cognitive domains for a typical participant who died after 10 years in the study, adjusted for age at death, sex, and education.
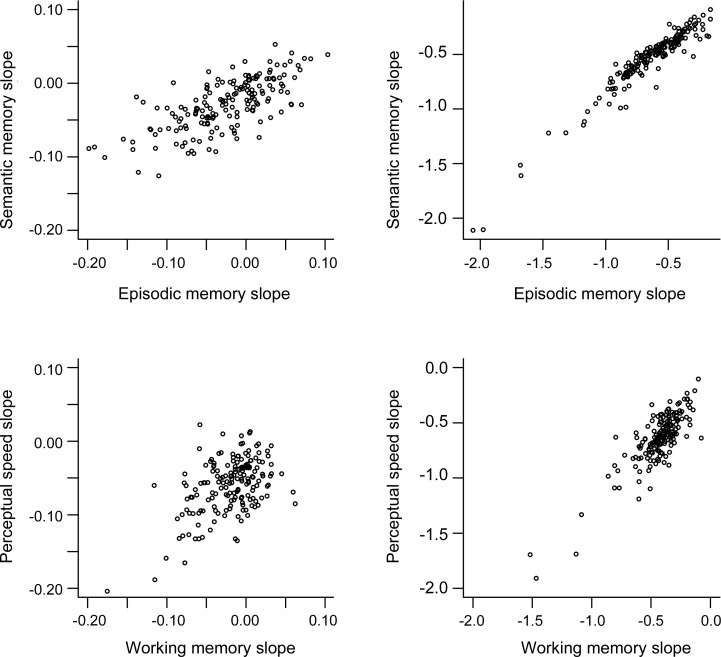
The mixed-effects model also provides estimates of the correlations between trajectories of change in different cognitive domains. As shown in table 3, the correlations between rates of change in episodic and semantic memory increased from 0.46 in the preterminal period to 0.89 during the terminal period. Figure 2 shows a scatterplot of these data: rates of decline in the 2 domains were moderately correlated before the terminal period and nearly perfectly correlated thereafter. Similar results were observed across the remaining cognitive domains, with correlations between slopes ranging from 0.25 to 0.33 in the preterminal period and from 0.83 to 0.89 in the terminal period (table 3, figure 2, figure e-1, figure e-2). These data indicate that the relationship between changes in different cognitive systems undergoes a dramatic shift during the last years of life. Cognitive decline is mostly domain-specific before the terminal period but mostly global during it.
Table 3.
Correlation between trajectories of change in different cognitive domainsa
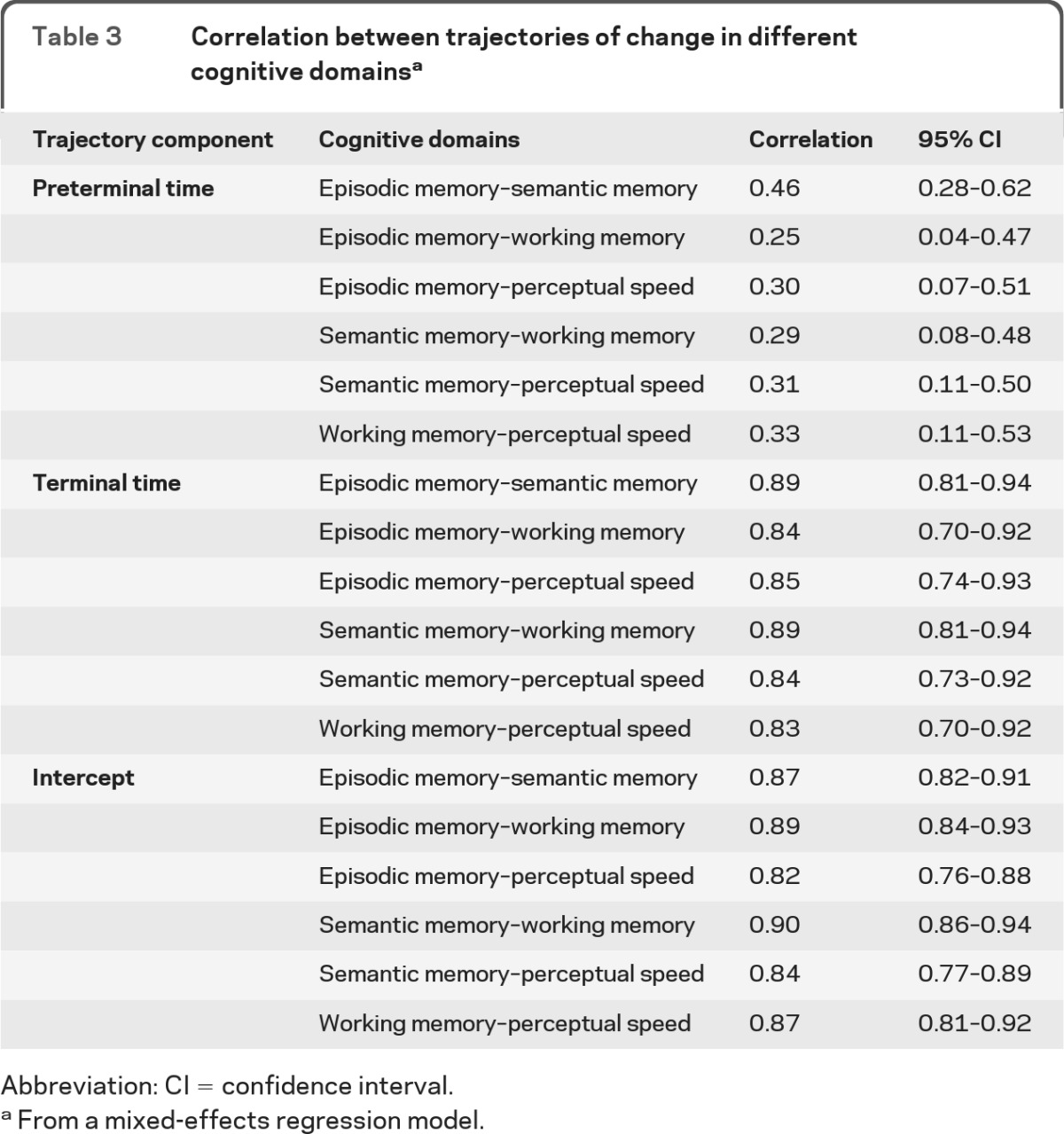
Abbreviation: CI = confidence interval
From a mixed-effects regression model.
Figure 2. Correlation between change in different cognitive domains.
Scatterplots of the association between rates of cognitive change in different cognitive domains before the terminal period (left side) and during it (right side), adjusted for age at death, sex, and education.
Table 3 also shows correlations between estimated intercepts. Because distance from death is the metric for time, the intercept is the estimated level of function just before death. The cross-sectional correlations between cognitive domains at the end of this terminal period (range 0.82–0.90) were comparable to the correlations between slopes during the terminal period, slightly larger than the correlations from the last evaluation (range 0.67–0.78), and substantially larger than the correlations at baseline (range 0.27–0.49).
AD and cognitive dedifferentiation.
To examine the contribution of AD to terminal changes in cognitive function, we repeated the analysis with the addition of a postmortem composite index of diffuse plaques, neuritic plaques, and neurofibrillary tangles. Pathologic scores ranged from a low of 0.003 to a high of 2.21 (mean = 0.63, SD = 0.53, skewness = 0.72). Higher AD pathologic burden was associated with more rapid preterminal decline in episodic and semantic memory (but not in working memory or perceptual speed) and earlier onset of terminal decline in all domains but not with rate of terminal decline (table e-2). Correlations between rates of change in different abilities were comparable to the original analysis (table e-3).
To provide a clinical context, we examined change in diagnosis. During the observation period, 81 individuals developed dementia (79 with clinical AD). Terminal episodic memory decline in these people began a median of 0.68 year (interquartile range: 1.70 to −1.51) before the diagnosis and nearly 2 years before terminal decline began in those who remained dementia free (mean onset = −3.59 years before death [SD = 1.99] vs −1.71 [SD = 0.91]; t [108.8] = 7.8, p < 0.001).
DISCUSSION
We assessed multiple domains of cognition in older people at annual intervals for up to 15 years prior to death. On average, decline in each domain was relatively gradual before accelerating rapidly in the final 2 to 3 years of life. Rates of change in different cognitive domains were much more strongly correlated during the terminal period than before it. The results support the cognitive dedifferentiation hypothesis.
Most research on cognitive dedifferentiation has involved comparing correlations among cognitive abilities in different age groups. With some exceptions24 these cross-sectional studies have not found evidence that correlations among abilities are higher in old people than in younger people.3,4 However, the cognitive dedifferentiation hypothesis refers to within-person correlations among rates of cognitive change. Thus, it remains unclear whether examination of between-person correlations among levels of cognitive abilities represents an adequate test of the dedifferentiation hypothesis.4
Longitudinal studies have examined the cognitive dedifferentiation hypothesis in different ways. One approach has been to compare ability intercorrelations at each wave of data collection to see if they increase as participants age. These studies have not suggested a systematic shift in the intercorrelations.25,26 By contrast, in the present study correlations between cognitive abilities were substantially larger proximate to death than at study entry, suggesting that cognitive dedifferentiation may be associated with mortality rather than aging. Another approach has been to test whether change in a particular cognitive ability such as processing speed is a leading indicator of change in other cognitive domains, possibly thereby contributing to cognitive dedifferentiation.6,27–29 To date, however, these studies have not clarified whether cognitive dedifferentiation occurs in old age. A third approach has been to directly examine correlations between rates of change in cognitive function. In 2 of these studies,5,7 including one of the Religious Orders Study cohort published about a decade ago,5 a single factor explained approximately 60% of the variance in rates of change in diverse cognitive abilities. This observation supports the cognitive dedifferentiation hypothesis and suggests that age-related cognitive change is mostly diffuse rather than domain specific. However, neither study addressed whether correlations among rates of change in cognitive abilities shift over time as people age. The present longitudinal results build on existing knowledge by showing that cognitive dedifferentiation is an evolving process and that it is related to impending death rather than advancing age.
A persistent question in research on terminal cognitive decline has been whether the effect is global or domain specific.30,31 Some prior studies have found evidence of terminal decline in some cognitive measures and not others,32–34 but the pattern has not been consistent from study to study, making interpretation difficult. The present study more directly assessed the issue by calculating correlations between rates of change in different abilities during the terminal period and between ability levels proximate to death. That the correlations approached 1 demonstrates that terminal cognitive decline is predominantly a global process.
Previous estimates of the duration of terminal cognitive decline have varied from about 3 years8 to more than a decade.12 This variability partly reflects the difficulty of precisely dating the onset of a continuous process. It also reflects other factors including differences in study design (e.g., number and spacing of observations, duration of observation period, proximity of observations to death), study operations (e.g., follow-up participation rate), and analytic approach (e.g., fixed vs variable change point). We used a model that allowed rates of cognitive decline to accelerate at a variable point prior to death because it is likely that such approaches optimally capture the natural history of cognitive aging. Further, the development of dementia is related to terminal cognitive decline, and it has been difficult to disentangle the influence of impending death vs dementia. In this study, many individuals met dementia criteria after the onset of terminal decline, but some met criteria before it and most never met criteria. More importantly, in analyses that directly examined the key pathologic lesions underlying dementia, we found that pathology was not associated with rates of terminal change in different cognitive abilities and did not modify the correlations among those rates. These findings suggest that terminal dedifferentiation of cognitive abilities is not driven by the pathologic processes known to cause dementia in old age.
The present results in conjunction with previous research indicate that cognitive change during the terminal period differs in important ways from preterminal cognitive change. Thus, rate of terminal cognitive decline is weakly correlated with preterminal rate of decline and is substantially more rapid and global in nature. Further, the lesions most strongly linked with late-life cognitive impairment and dementia (i.e., plaques and tangles) were associated with rate of cognitive decline in the preterminal but not terminal period. These and previous35 data suggest that novel biological mechanisms are contributing to terminal cognitive decline. It may be that pathologic processes traditionally associated with dementia initiate a cascade of pathologic events and that downstream components of this cascade are primarily responsible for the temporal course of terminal cognitive decline. In addition, unmeasured pathologic lesions such as TDP-43 may be involved. An implication of these data is that treatments that target the pathologic processes thought to underlie AD should focus on early mild cognitive change not only because the pathologic changes are less severe but also because they are more tightly linked to cognitive changes during the preterminal period than is the case during the terminal period. These results also imply that understanding early domain-specific changes in cognitive function will be difficult without data on mortality.
This study has important strengths and limitations. Multiple domains of cognition were assessed with previously established composite measures. In addition, there was a mean of about 10 years of annual cognitive testing available including the period most proximate to death with a high rate of participation in follow-up and brain autopsy. These factors enhanced our ability to reliably characterize individual differences in the onset of the terminal period and in rates of cognitive decline before and after that point and to correlate these cognitive elements with a postmortem index of disease burden. The main limitation is that results are based on a selected group of highly educated older persons. So the generalizability of these findings remains to be determined.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the many Catholic nuns, priests, and monks who have participated in the Religious Orders Study; Traci Colvin, MPH, for coordinating the study; Woojeong Bang, MS, for statistical programming; and John Gibbons, MS, and Greg Klein, MS, for data management.
GLOSSARY
- AD
Alzheimer disease
- MCI
mild cognitive impairment
Footnotes
Editorial, page 1110
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Study concept or design: Drs. Wilson, Boyle, and Bennett. Analysis or interpretation of the data: Drs. Wilson, Segawa, Boyle, and Bennett. Drafting of the manuscript: Drs. Wilson, Segawa, Boyle, and Bennett, and L.P. Hizel. Acquisition of data: L.P. Hizel. Statistical analysis: Dr. Segawa. Obtaining funding: Drs. Boyle and Bennett.
DISCLOSURE
Dr. Wilson serves as a Consulting Editor for Aging, Neuropsychology, and Cognition and Psychology and Aging; has served as a consultant for Pain Therapeutics, Inc.; and receives research support from the NIH/NIA. Dr. Segawa receives research support from the NIH/NIA. L.P. Hizel receives research support from the NIH/NIA. Dr. Boyle receives research support from the NIH/NIA. Dr. Bennett serves on the scientific advisory board for Vigorous Minds; serves on the editorial boards of Neurology®, Current Alzheimer Research, and Neuroepidemiology; serves/has served as a consultant to Nutricia, Wilmar Schwabe GmbH & Co., Eli Lilly and Company, Schlesinger Associates, Schering-Plough Corp., Medivation, Inc., and the Gerson Lehrman Group; and receives research support from Nutricia, Danone Research B.V., the NIH, and the Illinois Department of Public Health.
REFERENCES
- 1. Baltes PB, Cornelius SW, Spiro A, Nesselroade JR, Willis SL. Integration vs. differentiation of fluid-crystallized intelligence in old age. Dev Psychol 1980; 16: 625– 635 . [Google Scholar]
- 2. Cunningham AR, Clayton V, Overton W. Fluid and crystallized intelligence in young and old adulthood. J Gerontol 1975; 30: 53– 55 . [DOI] [PubMed] [Google Scholar]
- 3. Tucker-Drob EM, Salthouse TA. Adult age trends in the relations among cognitive abilities. Psychol Aging 2008; 23: 453– 460 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tucker-Drob EM. Differentiation of cognitive abilities across the lifespan. Dev Psychol 2009; 45: 1097– 1118 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wilson RS, Beckett LA, Barnes LL, et al. Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging 2002; 17: 179– 193 . [PubMed] [Google Scholar]
- 6. Hertzog C, Dixon RA, Hultsch DF, MacDonald SWS. Latent change models of adult cognition: are changes in processing speed and working memory associated with changes in episodic memory? Psychol Aging 2003; 18: 755– 769 . [DOI] [PubMed] [Google Scholar]
- 7. Lindenberger U, Ghisletta P. Cognitive and sensory declines in old age: gauging the evidence for a common cause. Psychol Aging 2009; 24: 1– 16 . [DOI] [PubMed] [Google Scholar]
- 8. Wilson RS, Beckett LA, Bienias JL, Evans DA, Bennett DA. Terminal decline in cognitive function. Neurology 2003; 60: 1782– 1787 . [DOI] [PubMed] [Google Scholar]
- 9. Sliwinski MJ, Stawski RS, Hall CB, et al. Distinguishing preterminal and terminal cognitive decline. Eur Psychol 2006; 11: 172– 181 . [Google Scholar]
- 10. Laukka EJ, MacDonald SWS, Backman L. Contrasting cognitive trajectories of impending death and preclinical dementia in the very old. Neurology 2006; 66: 833– 838 . [DOI] [PubMed] [Google Scholar]
- 11. Wilson RS, Beck TL, Bienias JL, Bennett DA. Terminal cognitive decline: accelerated loss of cognition in the last years of life. Psychsom Med 2007; 69: 131– 137 . [DOI] [PubMed] [Google Scholar]
- 12. Thorvaldsson V, Hofer SM, Berg S, Skoog I, Sacuiu S, Johansson B. Onset of terminal decline in cognitive abilities in persons without dementia. Neurology 2008; 71: 882– 887 . [DOI] [PubMed] [Google Scholar]
- 13. Wilson RS, Bienias JL, Evans DA, Bennett DA. Religious Orders Study: overview and change in cognitive and motor speed. Aging Neuropsychol Cogn 2004; 11: 280– 303 . [Google Scholar]
- 14. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984; 34: 939– 944 . [DOI] [PubMed] [Google Scholar]
- 15. Bennett DA, Wilson RS, Schneider JA, et al. Natural history of mild cognitive impairment in older persons. Neurology 2002; 59: 198– 205 . [DOI] [PubMed] [Google Scholar]
- 16. Wilson RS, Aggarwal NT, Barnes LL, Mendes de Leon CF, Hebert LE, Evans DA. Cognitive decline in incident Alzheimer disease in a community population. Neurology 2010; 74: 951– 955 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bennett DA, Schneider JA, Bienias JL, Evans DA, Wilson RS. Mild cognitive impairment is related to Alzheimer disease pathology and cerebral infarctions. Neurology 2005; 64: 834– 841 . [DOI] [PubMed] [Google Scholar]
- 18. Schneider JA, Aggarwal NT, Barnes LL, Boyle P, Bennett DA. The neuropathology of older persons with and without dementia from community versus clinic cohorts. J Alzheimers Dis 2009; 18: 691– 701 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schneider JA, Arvantakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer's disease and mild cognitive impairment. Ann Neurol 2009; 66: 200– 208 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wilson RS, Evans DA, Bienias JL, Mendes de Leon CF, Schneider JA, Bennett DA. Proneness to psychological distress is associated with risk of Alzheimer's disease. Neurology 2003; 61: 1479– 1485 . [DOI] [PubMed] [Google Scholar]
- 21. Laird N, Ware J. Random effects models for longitudinal data. Biometrics 1982; 38: 963– 974 . [PubMed] [Google Scholar]
- 22. Gelman A, Carli JB, Stern HS, Rubin DB. Bayesian Data Analysis. New York: Chapman and Hall; 2004. [Google Scholar]
- 23. Lunn D, Spiegelhalter D, Thomas A, Best N. The BUGS project: evolution, critique and future directions (with discussion). Stat Med 2009; 28: 3049– 3082 . [DOI] [PubMed] [Google Scholar]
- 24. Baltes PB, Lindenberger U. Emergence of a powerful connection between sensory and cognitive functions across the adult life span: a new window to the study of cognitive aging. Psychol 1977; 12: 12– 21 . [DOI] [PubMed] [Google Scholar]
- 25. Anstey KJ, Hofer SM, Luszcz MA. Cross-sectional and longitudinal patterns of dedifferentiation in late-life cognitive and sensory function: the effects of age, ability, attrition, and occasion of measurement. J Exp Psychol: Gen 2003; 132: 470– 487 . [DOI] [PubMed] [Google Scholar]
- 26. Zelinski EM, Lewis KL. Adult age differences in multiple cognitive functions: differentiation, dedifferentiation, or process-specific change? Psychol Aging 2003; 18: 727– 745 . [DOI] [PubMed] [Google Scholar]
- 27. Ghisletta P, Lindenberger U. Age based structural dynamics between perceptual speed and knowledge in the Berlin Aging Study: direct evidence for ability dedifferentiation in old age. Psychol Aging 2003; 18: 696– 713 . [DOI] [PubMed] [Google Scholar]
- 28. Ghisletta P, Lindenberger U. A dynamic investigation of cognitive dedifferentiation with control for retest: evidence from the Swiss Interdisciplinary Longitudinal Study on the oldest old. Psychol Aging 2005; 20: 671– 682 . [DOI] [PubMed] [Google Scholar]
- 29. Finkel D, Reynolds CA, McArdle JJ, Pedersen NL. Age changes in processing speed as a leading indicator of cognitive aging. Psychol Aging 2007; 22: 558– 588 . [DOI] [PubMed] [Google Scholar]
- 30. White N, Cunningham WR. Is terminal drop pervasive or specific? J Gerontol Psychol Sci 1988; 43: 141– 144 . [DOI] [PubMed] [Google Scholar]
- 31. Small BJ, Backman L. Time to death and cognitive performance. Curr Directions Psychol Sci 1999; 8: 168– 172 . [Google Scholar]
- 32. Bosworth HB, Schaie KW, Willis SL. Cognitive and sociodemographic risk factors for mortality in the Seattle Longitudinal Study. J Gerontol Psychol Sci 1999; 54: 273– 282 . [DOI] [PubMed] [Google Scholar]
- 33. Anstey KJ, Luszcz MA, Giles LC, Andrews GR. Demographic, health, cognitive, and sensory variables as predictors of mortality in very old adults. Psychol Aging 2001; 16: 3– 11 . [DOI] [PubMed] [Google Scholar]
- 34. Johansson B, Hofer SM, Allaire JC, et al. Change in cognitive capabilities in the oldest old: the effects of proximity to death in genetically related individuals over a 6 year period. Psychol Aging 2004; 19: 145– 156 . [DOI] [PubMed] [Google Scholar]
- 35. Wilson RS, Leurgans SE, Boyle PA, Schneider JA, Bennett DA. Neurodegenerative basis of age related cognitive decline. Neurology 2010; 75: 1070– 1078 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.