Abstract
Objective:
To test the hypothesis that late-life participation in mentally stimulating activities affects subsequent cognitive health.
Methods:
Analyses are based on 1,076 older persons without dementia at study onset participating in a longitudinal cohort study. They completed annual clinical evaluations for a mean of 4.9 years. Each evaluation included administration of a self-report scale about participation in mentally stimulating activities and a battery of cognitive performance tests. Previously established measures of cognitively stimulating activity and cognitive function were derived. We assessed the temporal sequence of activity changes in relation to functional changes in a series of cross-lagged panel models adjusted for age, sex, and education.
Results:
During the observation period, cognitive activity participation (estimate of mean annual change = −0.066, SE = 0.005, p < 0.001) and cognitive functioning (estimate = −0.077, SE = 0.005, p < 0.001) declined at rates that were moderately correlated (r = 0.44, p < 0.001). The level of cognitive activity in a given year predicted the level of global cognitive function in the following year, but the level of global cognition did not predict the subsequent level of cognitive activity participation. Cognitive activity showed the same pattern of unidirectional associations with measures of episodic and semantic memory, but its associations with working memory were bidirectional.
Conclusions:
The results suggest that more frequent mental stimulation in old age leads to better cognitive functioning.
With few exceptions,1 longitudinal studies have found that more frequent participation in mentally stimulating activities is associated with decreased rate of cognitive decline2–7 and risk of dementia5–14 in old age. These observational data suggest that engagement in cognitive activities may somehow affect subsequent cognitive functioning.15,16 Alternatively, it is possible that a lower level of cognitive activity is simply a result of declining cognition.2,3 Distinguishing between these possibilities is likely to require a detailed understanding of the temporal sequence in which cognitive activity and cognitive function change as people age. However, most longitudinal studies that have addressed this issue were based on only 2–3 assessments per individual,1–3 limiting the opportunity to observe the interplay over time between change in cognitive activity and change in cognitive function.
The aim of the present study was to examine the temporal relationship between changes in cognitive activity and changes in cognitive function in more than 1,000 older persons. They completed a mean of 5.9 annual evaluations that included administration of a self-report scale about cognitive activity participation and a battery of cognitive performance tests. We used cross-lagged panel models to characterize change in participation in cognitively stimulating activity and performance on cognitive tests and to determine the association over time of activity with subsequent function and of function with subsequent activity.
METHODS
Participants.
Subjects are from the Rush Memory and Aging Project,17 an ongoing longitudinal clinical-pathologic cohort study of common chronic conditions of old age. The study began in 1997 and involves detailed annual clinical evaluations. Older persons were recruited from diverse retirement communities, subsidized housing facilities, local churches, and other social service agencies in and around Chicago.
At the time of these analyses, 1,400 people had enrolled in the study and completed the baseline evaluation, which included a medical history, complete neurologic examination, and assessment of cognitive function. We excluded 87 individuals who met the criteria for dementia18 and 60 who were younger than 65 years, leaving 1,259 eligible at baseline. Of these, 35 died before the first annual follow-up and another 76 had been in the study less than 1 year. Of the remaining 1,148 individuals, follow-up data were available for 1,076 people, 93.7% of those eligible. Analyses are based on this group. They had a mean baseline age of 80.4 years (SD = 6.5) and a mean of 14.5 years of education (SD = 3.2). At baseline, the mean Mini-Mental State Examination score was 27.9 (SD = 2.1), and 299 (29.7%) met the criteria for mild cognitive impairment (i.e., impairment in at least one cognitive domain in the absence of dementia19). A total of 74.2% were women and 89.5% were white and non-Hispanic. They had completed an average of 5.9 annual clinical evaluations (SD = 2.7) at the time of these analyses.
Standard protocol approvals, registrations, and patient consents.
After a presentation about the project, interested individuals met with study personnel who provided further information and obtained written informed consent. The project was approved by the institutional review board of Rush University Medical Center.
Assessment of participation in cognitively stimulating activities.
At each annual evaluation, persons were asked to rate frequency of participation in 7 activities during the past year. Ratings were on a 5-point scale ranging from 1 for once a year or less to 5 for every day or about every day. Activities were chosen that involved information processing or retention and that had relatively few barriers to participation. They included reading the newspaper, writing letters, visiting a library, and playing games such as chess or checkers. As described previously,7 scores on individual items were averaged to yield a composite measure of frequency of participation in cognitively stimulating activities. A higher score on this measure at baseline has previously been associated with decreased risk of developing mild cognitive impairment and Alzheimer disease.7
Assessment of cognitive function.
Cognitive function was assessed at each annual evaluation with a battery of 20 individual performance tests. The Mini-Mental State Examination was used only to describe participants. The remaining 19 tests were used in analyses. There were 7 measures of episodic memory: Word List Memory, Word List Recall, and Word List Recognition plus immediate and delayed recall of Logical Memory Story A and the East Boston Story. Semantic memory was assessed with Verbal Fluency and 15-item versions of the Boston Naming Test and the National Adult Reading Test. Working memory was measured with Digit Ordering, Digit Span Forward, and Digit Span Backward. Perceptual speed was assessed with Number Comparison, Symbol Digit Modalities Test, and 2 measures from the Stroop Neuropsychological Screening Test. A 15-item form of Judgment of Line Orientation and a 16-item form of Standard Progressive Matrices were used to evaluate visuospatial ability. To minimize floor and ceiling artifacts and other forms of measurement error, we used composite measures of cognition in analyses. A composite measure of global cognition based on all 19 tests was the outcome in the initial analysis because it uses all tests and the tests are positively intercorrelated. Subsequent analyses used composite measures of episodic memory (7 tests), semantic memory (3 tests), working memory (3 tests), and perceptual speed (4 tests). In each case, raw scores on individual tests were converted to z scores, using the baseline mean and SD from all subjects, and then were averaged to yield the composite score. Further information on the individual tests and the derivation of the composite scores is contained in previous publications.20,21
Data analysis.
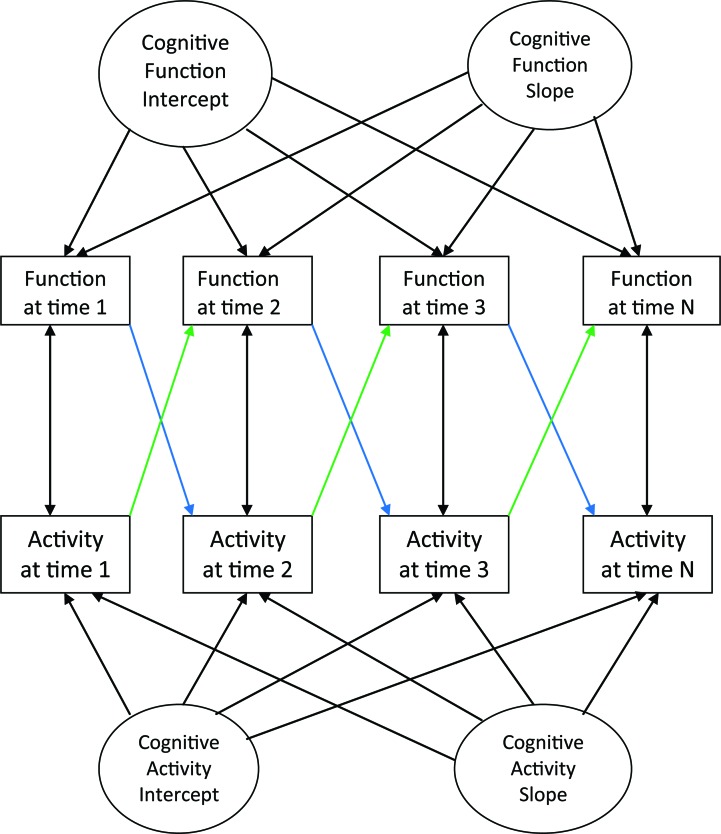
We examined change in cognitive function and change in cognitive activity and covariation between them over time in a series of cross-lagged panel models.22,23 Figure 1 shows a schematic version of the model. Each analysis included an across wave component that estimated rate of change in each outcome (function and activity in figure 1) from one wave of data collection to the next and a wave-specific component that characterized relations between outcomes from each specific wave. Latent growth curves were simultaneously fit to change in each outcome. The latent slopes of cognitive function and cognitive activity and their correlation were derived from the across wave component of the model. After these across wave components were accounted for, the wave-specific components of the model assessed whether the residual of one outcome at the current wave was related to the residual of the other outcome in the previous wave. This allowed us to establish the temporal relationship between cognitive activity and change in cognition on the one hand vs cognitive function and change in cognitive activity on the other hand, which is shown by the colored lines in figure 1, with the blue lines representing an association of function with subsequent activity and the green lines representing an association of activity with subsequent function.
Figure 1. Schematic diagram of the cross-lagged panel model.
The colored lines represent the association of each outcome measure with subsequent level of the other one.
In preliminary analyses, wave-specific effects were either permitted to vary or were constrained to be the same over waves. Because model fits for the 2 approaches were comparable, we chose the constrained approach in the interest of parsimony. In addition, we examined the sensitivity of the model to the shape of the growth curve by fitting a flexible time-score curve24 instead of a straight line. Results were not sensitive to growth curve shape and so analyses are based on linear change.
The initial analysis used a composite measure of global cognition as the cognitive function outcome. We subsequently repeated the analysis, excluding persons with mild cognitive impairment at baseline, using a weighted version of the composite measure of global cognition with weights derived from a factor analysis, and using composite measures of specific cognitive functions, with separate models for each of 4 measures. All models included terms to control for the potentially confounding effects of age, sex, and education.
Model fit was assessed with 4 widely used measures. The root mean square error of approximation (values <0.08 indicate acceptable fit24) and standardized root mean square residual (values <0.06 indicate acceptable fit24) reflect the deviation of predicted results from a perfect fit, with values approaching zero indicating better model fit. The Tucker-Lewis index and comparative fit index (values >0.95 indicate acceptable fit24) reflect the difference between the predicted and observed covariance matrices, with values approaching 1 indicating better model fit.
RESULTS
At baseline, scores on the composite measure of global cognitive function ranged from a low of −1.83 to a high of 1.41 (mean = 0.09, SD = 0.53). Scores on the composite measure of participation in cognitively stimulating activities ranged from a low of 1.00 to a high of 5.00 (mean = 3.22, SD = 0.76). The measures of cognitive function and cognitive activity were approximately normally distributed and moderately correlated (r = 0.39, p < 0.001). Age was related to cognitive function (r = −0.28, p < 0.001) but not to cognitive activity (r = −0.06, p = 0.066), education was related to both cognitive function (r = 0.37, p < 0.001) and activity (r = 0.30, p < 0.001), and men and women did not differ in cognitive function (t[1,074] = 1.7, p = 0.086) or activity (t[568.3] = 0.5, p = 0.624).
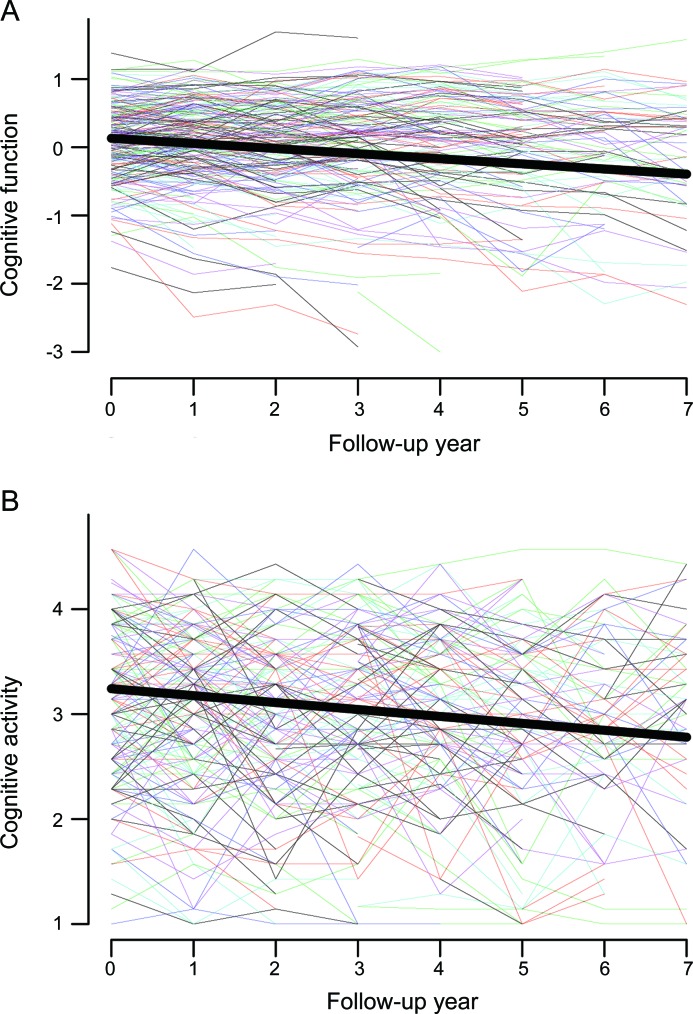
At annual intervals for a mean of 4.9 years, subjects underwent cognitive performance testing and rated their participation in cognitively stimulating activities. Figure 2 shows the crude paths of change (colored lines) in global cognitive performance (figure 2A) and cognitive activity participation (figure 2B) for a random sample of 200 to enhance visibility. In each case, considerable heterogeneity is evident.
Figure 2. Change in cognitive function and cognitive activity.
Crude paths of change in global cognitive function (A) and cognitive activity (B) for a random sample of 200 participants (colored lines) and the predicted paths for a typical participant (black line), adjusted for age, sex, and education.
We used a cross-lagged panel model to assess change in cognitive function and cognitive activity and determine the temporal sequence between change in function and change in activity over time. Model fit indices were satisfactory (root mean square error of approximation = 0.033, standardized root mean square residual = 0.023, Tucker-Lewis index = 0.980, and comparative fit index = 0.982). In the analysis, there was a mean annual decrease of 0.077 unit in the global cognitive measure (SE = 0.005, p < 0.001) (black line in figure 2A) and 0.066 unit in the cognitive activity measure (SE = 0.005, p < 0.001) (black line in figure 2B). In addition, rates of change in cognitive function and cognitive activity were positively correlated (r = 0.438, SE = 0.060, p < 0.001). Older age was associated with more rapid decline in cognitive function (estimate = −0.005, SE = 0.001, p < 0.001) and cognitive activity (estimate = −0.003, SE = 0.001, p < 0.001). Neither sex nor education was related to decline in either outcome.
With these across wave effects accounted for, the model indicated a wave-specific unidirectional association between cognitive activity in a given year and global cognitive function in the following year. A higher level of cognitive activity at time n predicted a higher level of cognitive function in the subsequent year (green lines in figure 1; estimate = 0.021, SE = 0.002, p < 0.001). In contrast, the level of global cognitive function at time n was not related to the level of cognitive activity in the subsequent year (blue lines in figure 1; estimate = 0.012, SE = 0.028, p = 0.667).
Prior research has suggested that mild cognitive impairment may affect the association between cognitive activity and cognitive function.25 Therefore, we repeated the model after excluding the 299 participants (29.7%) who met the criteria for mild cognitive impairment at baseline. In this analysis, model fit was satisfactory and results were essentially unchanged. A higher level of cognitive activity predicted a higher level of cognitive function in the following year (estimate = 0.018, SE = 0.003, p < 0.001), but level of cognitive function did not predict subsequent level of cognitive activity (estimate = 0.011, SE = 0.029, p = 0.706).
Because the composite measure of global cognition is the mean of standardized scores of the individual tests, each test makes an approximately equal contribution to the composite score. To determine whether this method biased results, we performed a factor analysis of the baseline test scores, derived weights for each test based on its factor loading and difficulty level, and repeated the analyses using this weighted composite measure of global cognition. Results were similar to those for the original analysis with cognitive activity predicting subsequent global cognition (estimate = 0.024, SE = 0.003, p < 0.001) but with global cognition unrelated to subsequent cognitive activity (estimate = 0.017, SE = 0.018, p = 0.355).
To determine whether the relationship between cognitively stimulating activity and cognitive performance varied across functional domains, we repeated the analysis with composite measures of specific cognitive functions. In each case, model fit was satisfactory and comparable to the original analysis. As shown in the table, cognitive activity predicted the subsequent level of function in all 4 cognitive domains. In contrast, neither episodic nor semantic memory predicted the subsequent level of cognitive activity. However, level of working memory did predict subsequent level of cognitive activity, and there was a similar but not quite significant effect for perceptual speed.
Table.
Longitudinal relationship between cognitive activity and specific cognitive functionsa
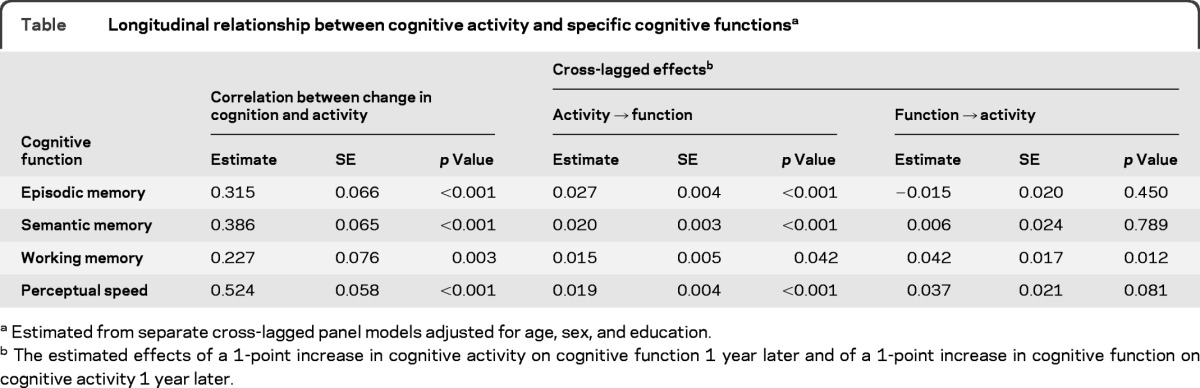
Estimated from separate cross-lagged panel models adjusted for age, sex, and education.
The estimated effects of a 1-point increase in cognitive activity on cognitive function 1 year later and of a 1-point increase in cognitive function on cognitive activity 1 year later.
DISCUSSION
At annual intervals for a mean of 4.9 years, more than 1,000 older persons without dementia at baseline rated frequency of participation in cognitively stimulating activities and completed a battery of cognitive function tests. We examined the temporal relationship between change in cognitive activity and change in cognitive function. A higher level of cognitive activity predicted better subsequent performance on a global measure of cognitive function and all domain specific measures as well. In contrast, only working memory function predicted subsequent level of cognitive activity. The results support the idea that late-life cognitive activity influences subsequent cognitive health.
A number of studies have reported an association between baseline level of cognitive activity and subsequent rate of cognitive change or risk of dementia.2–14 Despite rigorous sensitivity analyses, however, these studies have been unable to exclude the possibility of reverse causation, namely that lower cognitive activity is a consequence of prior cognitive decline, even among persons who do not yet meet the criteria for dementia or mild cognitive impairment.7,26,27 A few studies have investigated the direction of the association between cognitive activity and cognitive function using repeated measurement of each, but results have been mixed. One study found little evidence of an effect in either direction.1 Two studies concluded that the relationship was reciprocal, i.e., that cognitive activity influenced subsequent cognitive function and cognitive function influenced subsequent cognitive activity.2,3 Both studies had only 2 data collection points, however, making it difficult to separate change in either outcome from level or to determine the sequential relationship between their paths of change. The study most comparable to the present one used data from the Swiss Interdisciplinary Longitudinal Study on the Oldest Old.28 Participants aged 80–85 years at baseline were examined annually with up to 5 waves of data collection. Higher levels of cognitive activity predicted higher subsequent level of perceptual speed, consistent with the present results. However, cognitive activity did not predict subsequent level of verbal fluency, and cognitive performance had no effect on subsequent cognitive activity. In comparison, the present study had approximately twice the number of subjects, data collection points, and years of observation and used composite measures of cognitive function rather than individual tests, which are more subject to floor and ceiling effects. The resulting additional statistical power may have enhanced our ability to identify the global nature of the association of cognitive activity with subsequent cognitive function. The relatively weaker domain-specific association of cognitive function with subsequent cognitive activity was observed in a cognitive domain (i.e., working memory) that was not assessed in the previous study.
Recent cognitive training research provides some insight into how cognitively stimulating experience might influence subsequent cognitive functioning. In laboratory-based studies, a variety of narrowly designed interventions targeting executive control processes such as working memory or perceptual speed have been shown to improve a broad range of cognitive abilities.29–33 These training-based improvements in cognition are accompanied by changes in gray matter volume and white matter microstructure in regions that support the trained abilities.29,30,33,34 Similar findings have been reported with broad interventions that engage older people in stimulating real-world experiences such as working in an urban elementary school35 or taking classes in acting36 or reading and arithmetic.37 Thus, activity-based enhancement of neural systems underlying cognition might make older people less vulnerable to age-related neuropathologic changes, at least up to some threshold of pathologic severity.25
The present results also support the idea that lower cognitive activity participation can be a consequence of cognitive loss. That is, as cognition declines in old age, the ability and perhaps desire to participate in mentally challenging activities probably declines as well. However, this effect varied across cognitive abilities. Thus, a decline in executive abilities (i.e., working memory, nearly significant effect for perceptual speed) predicted decline in cognitive activity but decline in declarative memory (i.e., episodic and semantic memory) did not. This suggests that executive control processes are needed to support most cognitive activities and may be the main beneficiaries of such activities.6,20
Several methodologic features of the study make it likely that results are valid. Persons with dementia were excluded at baseline after a uniform evaluation and application of accepted criteria by an experienced clinician. Participation in follow-up by survivors was high, reducing the risk that attrition affected results. Both outcomes were assessed with previously established composite indices to minimize outcome measurement error. The availability of a mean of more than 5 annual observations per subject made it possible to reliability estimate person specific trajectories over time.
The principal study limitation is that the cohort was selected, and therefore the findings may not generalize to other groups. In addition, the results are based on self-reported cognitive activity and may not apply to cognitive activity assessed by other means.
ACKNOWLEDGMENT
The authors thank the many Illinois residents for participating in the Rush Memory and Aging Project; Traci Colvin, MPH, and Tracey Nowakowski, MA, for coordinating the study; Woojeong Bang, MS, for statistical programming; and John Gibbons, MS, and Greg Klein, MS, for data management.
AUTHOR CONTRIBUTIONS
Study concept or design: Dr. Wilson, Dr. Boyle, and Dr. Bennett. Analysis or interpretation of the data: Dr. Wilson, Dr. Segawa, Dr. Boyle, and Dr. Bennett. Drafting of the manuscript: Dr. Wilson, Dr. Segawa, Dr. Boyle, and Dr. Bennett. Statistical analysis: Dr. Segawa. Obtaining funding: Dr. Boyle and Dr. Bennett.
DISCLOSURE
Dr. Wilson serves as a Consulting Editor for Aging, Neuropsychology, and Cognition and Psychology and Aging; has served as a consultant for Pain Therapeutics, Inc.; and receives research support from the NIH/NIA. Dr. Segawa receives research support from the NIH/NIA. Dr. Boyle receives research support from the NIH/NIA. Dr. Bennett serves on the scientific advisory board for Vigorous Minds; serves on the editorial boards of Neurology®, Current Alzheimer Research, and Neuroepidemiology; serves/has served as a consultant to Nutricia, Wilmar Schwabe GmbH & Co., Eli Lilly and Company, Schlesinger Associates, Schering-Plough Corp., Medivation, Inc., and the Gerson Lehrman Group; and receives research support from Nutricia, Danone Research B.V., the NIH, and the Illinois Department of Public Health.
REFERENCES
- 1. Aartsen MJ, Smits CH, van Tilburg T, Knipscheer KC, Deeg DJ. Activity in older adults: cause or consequence of cognitive functioning? A longitudinal study on everyday activities and cognitive performance in older adults. J Gerontol B Psychol Sci Soc Sci 2002; 57: P153– P162 . [DOI] [PubMed] [Google Scholar]
- 2. Bosma H, van Boxtel MP, Ponds RW, et al. Engaged lifestyle and cognitive function in middle and old-aged, non-demented persons: a reciprocal association? Z Gerontol Geriatr 2002; 35: 575– 581 . [DOI] [PubMed] [Google Scholar]
- 3. Hultsch DF, Hertzog C, Small BJ, Dixon RA. Use it or lose it: engaged lifestyle as a buffer of cognitive decline in aging? Psychol Aging 1999; 14: 245– 263 . [DOI] [PubMed] [Google Scholar]
- 4. Schooler C, Mulatu MS. The reciprocal effects of leisure time activities and intellectual functioning in older people: a longitudinal analysis. Psychol Aging 2001; 16: 466– 482 . [DOI] [PubMed] [Google Scholar]
- 5. Wilson RS, Bennett DA, Bienias JL, Mendes de Leon CF, Morris MC, Evans DA. Cognitive activity and cognitive decline in a biracial community population. Neurology 2003; 61: 812– 816 . [DOI] [PubMed] [Google Scholar]
- 6. Wilson RS, Mendes de Leon CF, Barnes LL, et al. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA 2002; 287: 742– 748 . [DOI] [PubMed] [Google Scholar]
- 7. Wilson RS, Scherr PA, Schneider JA, Tang Y, Bennett DA. The relation of cognitive activity to risk of developing Alzheimer disease. Neurology 2007; 69: 1911– 1920 . [DOI] [PubMed] [Google Scholar]
- 8. Wilson RS, Bennett DA, Bienias JL, et al. Cognitive activity and incident AD in a population-based sample of older persons. Neurology 2002; 24: 1910– 1914 . [DOI] [PubMed] [Google Scholar]
- 9. Crowe M, Andel R, Pedersen NL, Johansson B, Gatz M. Does participation in leisure activities lead to reduced risk of Alzheimer's disease? A prospective study of Swedish twins. J Gerontol B Psychol Sci Soc Sci 2003; 58: P249– P255 . [DOI] [PubMed] [Google Scholar]
- 10. Scarmeas N, Levy G, Tang MX, Manly J, Stern Y. Influence of leisure activity on the incidence of Alzheimer's disease. Neurology 2001; 57: 2236– 2242 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Verghese J, Lipton RB, Katz MJ, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med 2003; 348: 2508– 2516 . [DOI] [PubMed] [Google Scholar]
- 12. Wang HX, Karp A, Winblad B, Fratiglioni L. Late-life engagement in social and leisure activities is associated with a decreased risk of dementia: a longitudinal study from the Kungsholmen Project. Am J Epidemiol 2002; 155: 1081– 1087 . [DOI] [PubMed] [Google Scholar]
- 13. Akbaraly TN, Portet F, Fustinoni S, et al. Leisure activities and the risk of dementia in the elderly: results from the Three-City Study. Neurology 2009; 73: 854– 861 . [DOI] [PubMed] [Google Scholar]
- 14. Carlson MC, Helms MJ, Steffens DC, Burke JR, Potter GG, Plassman BL. Midlife activity predicts risk of dementia in older male twin pairs. Alzheimers Dement 2008; 4: 324– 331 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wilson RS, Bennett DA. Cognitive activity and risk of Alzheimer's disease. Curr Dir Psychol Sci 2003; 12: 87– 91 . [Google Scholar]
- 16. Hertzog C, Kramer AF, Wilson RS, Lindenberger U. Enrichment effects on adult cognitive development: can the functional capacity of older adults be preserved and enhanced? Psychol Sci Pub Int 2009; 9: 1– 65 . [DOI] [PubMed] [Google Scholar]
- 17. Bennett DA, Schneider JA, Buchman AS, Mendes de Leon CF, Bienias JL, Wilson RS. The Rush Memory and Aging Project: study design and baseline characteristics of the study cohort. Neuroepidemiology 2005; 25: 163– 175 . [DOI] [PubMed] [Google Scholar]
- 18. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984; 34: 939– 944 . [DOI] [PubMed] [Google Scholar]
- 19. Bennett DA, Wilson RS, Schneider JA, et al. Natural history of mild cognitive impairment in older persons. Neurology 2002; 59: 198– 205 . [DOI] [PubMed] [Google Scholar]
- 20. Wilson RS, Barnes LL, Bennett DA. Assessment of lifetime participation in cognitively stimulating activities. J Clin Exp Neuropsychol 2003; 25: 634– 642 . [DOI] [PubMed] [Google Scholar]
- 21. Wilson RS, Barnes LL, Krueger KR, Hoganson G, Bienias JL, Bennett DA. Early and late life cognitive activity and cognitive systems in old age. J Int Neuropsychol Soc 2005; 11: 400– 407 . [PubMed] [Google Scholar]
- 22. Bollen KA, Curran PJ. Latent Curve Models: A Structural Equation Perspective. Hoboken, NJ: John Wiley & Sons; 2006 [Google Scholar]
- 23. Curran PJ, Bollen KA. The best of both worlds. In: Collins LM, Sayer AG, eds. New Methods for the Analysis of Change. Washington, DC: American Psychological Association; 2001 [Google Scholar]
- 24. Muthén BO. Mplus Technical Appendices. Los Angeles, CA: Muthén & Muthén; 1994–2004 [Google Scholar]
- 25. Wilson RS, Barnes LL, Aggarwal NT, et al. Cognitive activity and the cognitive morbidity of Alzheimer disease. Neurology 2010; 75: 990– 996 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Verghese J, LeValley A, Derby C, et al. Leisure activities and the risk of amnestic mild cognitive impairment in the elderly. Neurology 2006; 66: 821– 827 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang JY, Zhou DH, Li J, et al. Leisure activity and risk of cognitive impairment: the Chongqing aging study. Neurology 2006; 66: 911– 913 . [DOI] [PubMed] [Google Scholar]
- 28. Ghisletta P, Bickel JF, Lövdén M. Does activity engagement protect against cognitive decline in old age? Methodological and analytical considerations. J Gerontol B Psychol Sci Soc Sci 2006; 61: P253– P261 . [DOI] [PubMed] [Google Scholar]
- 29. Takeuchi H, Sekiguchi A, Taki Y, et al. Training of working memory impacts structural connectivity. J Neurosci 2010; 30: 3297– 3303 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Takeuchi H, Taki Y, Hashizume H, et al. Effects of training of processing speed on neural systems. J Neurosci 2011; 31: 12139– 12148 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Borella E, Carretti B, Riboldi F, De Beni R. Working memory training in older adults: evidence of transfer and maintenance effects. Psychol Aging 2010; 25: 767– 778 . [DOI] [PubMed] [Google Scholar]
- 32. Basak C, Boot WR, Voss MW, Kramer AF. Can training in a real-time strategy video game attenuate cognitive decline in older adults? Psychol Aging 2008; 23: 765– 777 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jaeggi SM, Buschkuehl M, Jonides J, Perrig WJ. Improving fluid intelligence with training on working memory. Proc Natl Acad Sci USA 2008; 105: 6829– 6833 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lövdén M, Bodammer NC, Kühn S, et al. Experience-dependent plasticity of white-matter microstructure extends into old age. Neuropsychologia 2010; 48: 3878– 3883 . [DOI] [PubMed] [Google Scholar]
- 35. Carlson MC, Erickson KI, Kramer AF, et al. Evidence for neurocognitive plasticity in at-risk older adults: the experience corps program. J Gerontol A Biol Sci Med Sci 2009; 64: 1275– 1282 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Noice H, Noice T. An arts intervention for older adults living in subsidized retirement homes. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 2009; 16: 56– 79 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Uchida S, Kawashima R. Reading and solving arithmetic problems improves cognitive functions of normal aged people: a randomized controlled study. Age 2008; 30: 21– 29 . [DOI] [PMC free article] [PubMed] [Google Scholar]