Abstract
Objectives:
To investigate the characteristics and prevalence of poststroke depression (PSD) and poststroke emotional incontinence (PSEI) and the factors related to these conditions at admission and 3 months after stroke.
Methods:
We evaluated 508 consecutive patients with acute ischemic stroke for PSD and PSEI at admission and 3 months later. PSD was evaluated using the Beck Depression Inventory, and PSEI was evaluated using Kim's criteria. Blood samples were collected and genotyped for the promoter region of the serotonin transporter protein (5-HTTLPR) and the number of tandem repeats within intron 2 (STin2 VNTR). Perceived social support (the ENRICHD Social Support Inventory) was also measured.
Results:
PSD and PSEI were present in 13.7% and 9.4% of patients, respectively, at admission and in 17.7% and 11.7%, respectively, at 3 months after stroke. Multivariate analyses showed that PSD at admission was associated with the NIH Stroke Scale score at admission (p < 0.001), whereas PSD at 3 months was associated with the presence of microbleeds (p < 0.01) and perceived low social support (p < 0.001). In contrast, only lesion location (p = 0.022) was associated with PSEI at admission, whereas modified Rankin Scale score (p = 0.019), STin2 VNTR (p = 0.040), and low social support (p = 0.042) were related to PSEI 3 months after stroke.
Conclusions:
Diverse factors such as neurologic dysfunction, lesion location, microbleeds, genetic traits, and social support are differently related to acute and subacute emotional disturbances. Strategies to prevent or manage these problems should consider these differences.
Stroke patients often develop emotional disturbances, with poststroke depression (PSD) and poststroke emotional incontinence (PSEI) being relatively common.1 The social impact of these emotional changes can be distressing for both patients and their caregivers, negatively influencing patient quality of life and increasing caregiver burden.2,3
The relationship between PSD and PSEI is complex. Although these 2 emotional disturbances are closely related, they are considered different entities because of their associations with different anatomic locations1 and because many patients with PSEI do not have depression.1,4 The prevalence of factors related to and the natural course of these emotional dysfunctions remain unclear. The possible roles of neurotransmitter pathways, genetic predisposition, and social support have yet to be determined. We, therefore, evaluated the characteristics and prevalence of PSD and PSEI and the factors related to these conditions at admission and 3 months after stroke. Because these emotional disturbances have been shown to be related to alterations in the serotonin neurotransmitter system,5 we evaluated the relationships between SERT gene polymorphisms and PSD and PSEI in particular.
METHODS
Patients.
We evaluated 1,686 consecutive patients who were admitted to the Asan Medical Center with a diagnosis of acute stroke between March 2008 and February 2010. Diagnosis was confirmed by correlating CT or MRI findings within 7 days after stroke onset. We excluded patients with hemorrhagic stroke (n = 188), those with unusual causes such as dissection, venous infarction, or moyamoya disease (n = 136), those with TIA without progression to stroke (n = 226), those with communication problems (decreased consciousness, confusion, aphasia, dementia, or dysarthria) severe enough to preclude a reliable interview (n = 242), and those with very severe neurologic or medical (such as metastatic cancer) conditions (n = 98). We also excluded patients who scored ≤23 on the Mini-Mental State Examination6 (n = 47), patients diagnosed with depression or other psychiatric illnesses or treated with selective serotonin reuptake inhibitors before the onset of stroke (n = 28), patients who lived alone so that information from relatives was not available (n = 21), and patients who did not provide written informed consent (n = 192). Thus, 508 patients were finally enrolled.
Standard protocol approvals, registrations, and patient consents.
An institutional review board at Asan Medical Center approved the study. All participants provided written informed consent.
Study design.
This is a descriptive, cohort study conducted on stroke patients at admission and 3 months after stroke.
Procedures.
The first interview with each patient was conducted when the patient's condition became stabilized after the onset of stroke (mean 4.7 days), thus avoiding any possible effects of acute neurologic progression on patient's emotions. To increase the inter-rater reliability of assessments, 3 formal training sessions were held before interviews. Subsequently, each rater's initial interview sessions were supervised at the data collection site by one of the authors (S.C.-K.), and any disagreements were resolved by discussion. Questions arising during subsequent interviews were brought to a research team meeting to reach consensus on the appropriate answer. Most interviews were conducted in the presence of the patient's relatives, who confirmed the responses. When relatives were not present during the interview (n = 17), patient responses were confirmed by calling relatives who lived with the patient.
PSD was considered to be present in patients with a Beck Depression Inventory score >13 or those who met the modified (omitting criteria on symptom duration of 2 weeks) criteria of the DSM-IV.1 PSEI was considered to be present in patients who exhibited excessive or inappropriate laughing or crying, compared with their premorbid state. When both the patient and relatives who lived with the patient agreed that excessive or inappropriate laughing or crying had occurred on at least 2 occasions, that patient was considered as having PSEI.1
Social support for patients was assessed using the ENRICHD Social Support Instrument, consisting of 6 items with scores of 1 to 5 each plus an item regarding marital status. This instrument measures structural, instrumental, and emotional support. Scores range from 8 to 34, with higher scores indicating greater social support. The Cronbach α (internal consistency) was 0.80 in this study.7,8
Each patient's NIH Stroke Scale (NIHSS) score at admission and neurologic findings were recorded by an experienced stroke neurologist. The location of each lesion on MRI was analyzed by one of the authors (J.S.K.) and characterized as anterior cortical if it was in the anterior cerebral artery territory, the frontal and parietal areas of the middle cerebral artery, or predominantly in the temporal lobe of the middle cerebral artery territory; posterior cortical, if it was in the occipital area or medial temporal area of the posterior cerebral artery territory; internal capsule/corona radiata/basal ganglia of the lenticulostriate artery territory; thalamus; pons, including a few patients with pure midbrain lesions; medulla; and cerebellum. White matter intensities (leukoaraiosis) on fluid-attenuated inversion recovery imaging were graded from 0 to 3 on the Fazekas scale of deep white matter changes, with scores of 2 and 3 indicating significant leukoaraiosis.9 Microbleeds were defined as unambiguous homogeneous round lesions of signal loss with diameters ≤5 mm, as determined on T2*-weighted gradient-echo imaging. Hypointense lesions within the subarachnoid space and symmetric hypointensities of the globus pallidus were regarded as pial blood vessels and calcifications, respectively.10
Blood samples were collected at admission., and genomic DNA was isolated using a QIAamp DNA Mini Kit (Qiagen Inc., Valencia, CA) to determine genotype according to the standard procedures, including the frequencies of the 5-HTT gene–linked functional polymorphic region (5-HTTLPR), S and L alleles in the promoter region, and the number of tandem repeat polymorphisms in intron 2 (STin2 VNTR).11,12
The second patient interview was performed between 3 and 4 months after the onset of stroke, when patients visited the outpatient clinic for follow-up. Each patient's score on the modified Rankin scale (mRS) was recorded by an experienced stroke neurologist and categorized as severe (3–6) or mild (0–2) for statistical purposes. The interviewer, interview procedure, and questionnaire were identical to those during the first interview.
Analysis.
Group differences were analyzed using descriptive statistics, Student t test, and χ2 test (SPSS version 11.5). Multiple logistic regression analysis was used to explore relationships among PSEI/PSD variables.
RESULTS
Prevalence of and factors related to PSD and PSEI.
Of the 508 participants enrolled, 469 completed their second interview at 3 months after stroke. The reasons for loss to follow-up at 3 months included deteriorations in a patient's physical (n = 7, 18%) or cognitive (n = 5, 12.8%) condition, refusal (n = 11, 25.6%), death (n = 5, 12.8%), and relocation (n = 12, 30.7%). The 469 patients followed up at 3 months and the 39 lost to follow-up did not differ significantly, except in mean age (61.6 ± 11.5 vs 66.8 ± 9.7 years, p = 0.008) and mean NIHSS score (2.58 ± 2.52 vs 3.58 ± 3.27, p = 0.02).
PSD was present in 13.7% of patients at admission and in 17.7% 3 months later. Female gender (p < 0.05), fewer years of education (p < 0.05), motor dysfunction (p < 0.01), sensory dysfunction (p < 0.01), and high NIHSS score (p < 0.01) were significantly related to PSD at admission (table 1), whereas fewer years of education (p < 0.05), the presence of microbleeds (p < 0.05), motor and sensory dysfunction at admission (p < 0.01 each), mRS score (p < 0.05), and low social support (p < 0.01) were related to PSD 3 months later (table 2). There were no differences in the distribution of 5-HTTLPR or STin2 VNTR between the patients with and without PSD, either at admission or after 3 months (tables 1 and 2).
Table 1.
Factors associated with poststroke depression and poststroke emotional incontinence at admission
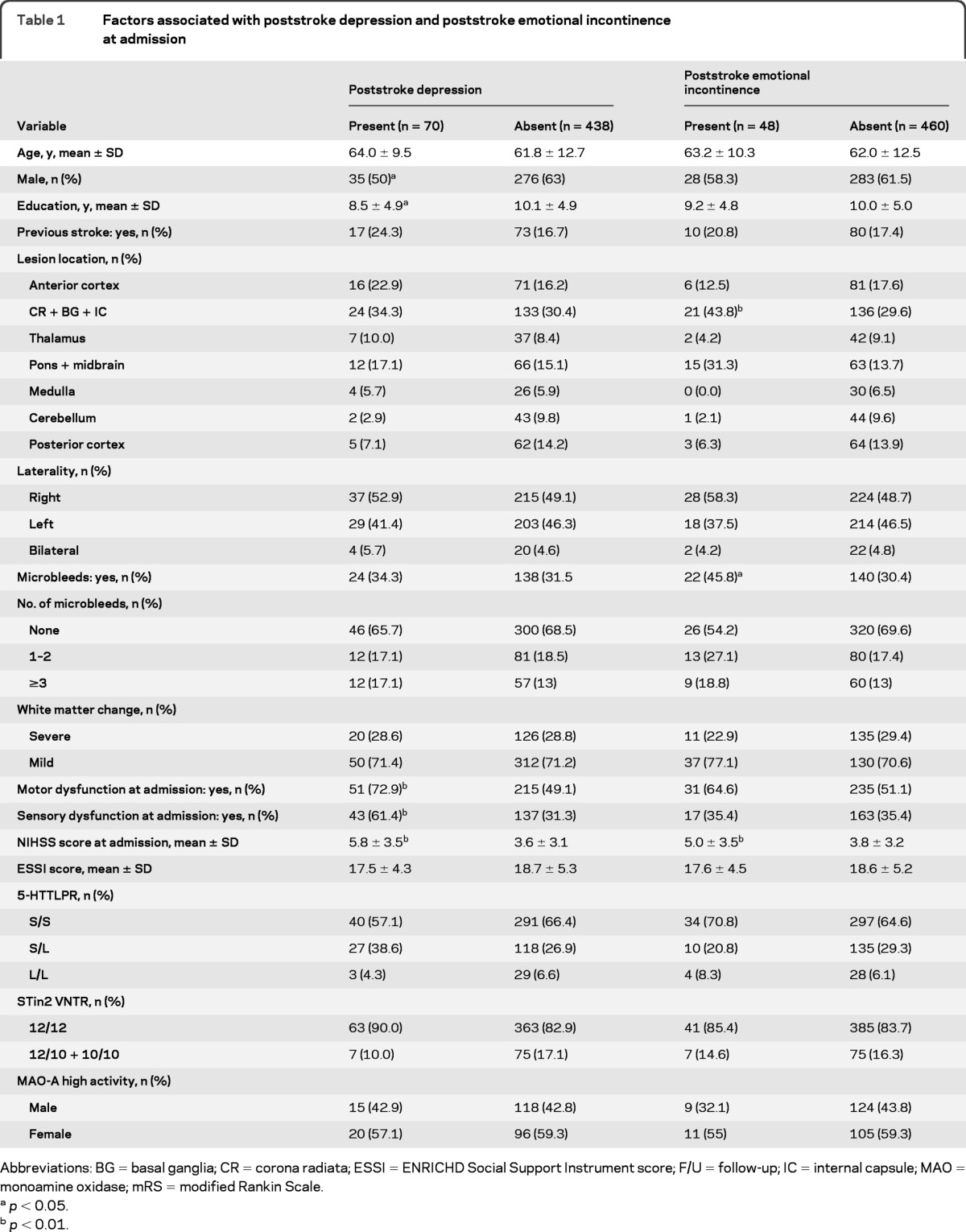
Abbreviations: BG = basal ganglia; CR = corona radiata; ESSI = ENRICHD Social Support Instrument score; F/U = follow-up; IC = internal capsule; MAO = monoamine oxidase; mRS = modified Rankin Scale.
p < 0.05.
p < 0.01.
Table 2.
Factors associated with poststroke depression and poststroke emotional incontinence 3 months after stroke
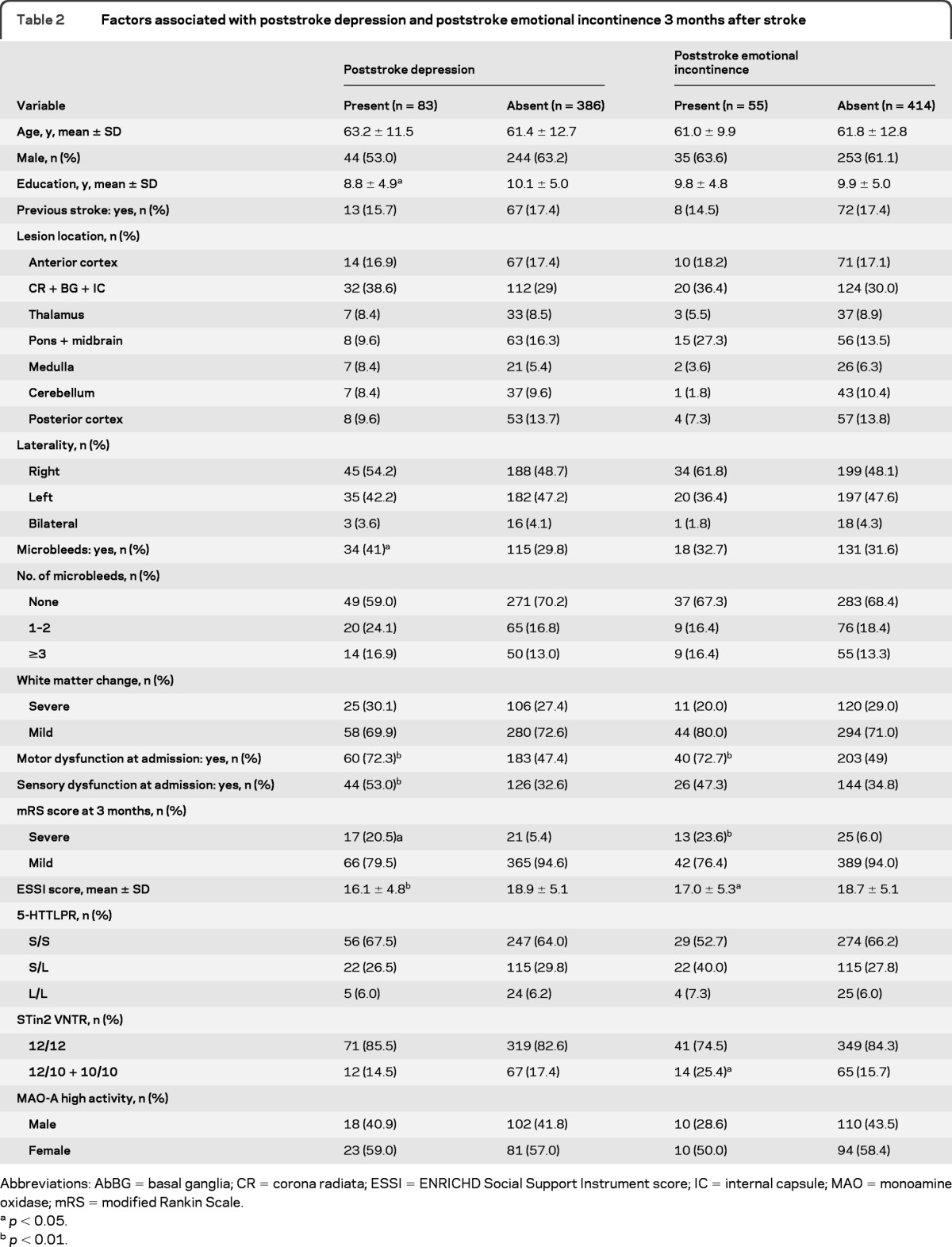
Abbreviations: AbBG = basal ganglia; CR = corona radiata; ESSI = ENRICHD Social Support Instrument score; IC = internal capsule; MAO = monoamine oxidase; mRS = modified Rankin Scale.
p < 0.05.
p < 0.01.
PSEI was present in 9.4% of patients at admission and in 11.7% 3 months later. We found that lesion location (p < 0.01), the presence of microbleeds (p < 0.05), and NIHSS score (p < 0.05) at admission were related to PSEI at admission (table 1), whereas lesion location (p < 0.05), motor dysfunction at admission (p < 0.001), mRS score (p < 0.001), and low social support (p < 0.05) were related to PSEI 3 months after stroke (table 2). Genotype frequencies of the STin2 VNTR polymorphism differed significantly 3 months after stroke between patients with and without PSEI (table 2), with the STin2 10/10 and 12/10 genotypes being more common among patients with PSEI. There were no differences, however, in the distribution of 5-HTTLPR between patients with and without PSEI at admission and 3 months after stroke.
PSD was significantly correlated with PSEI, both at admission and after 3 months (p < 0.01 each). Of the patients with PSD at admission, 20 (28.6%) had PSEI at admission and 24 (28.9%) had PSEI 3 months later. Among the patients with PSEI at admission, 20 (41.7%) had PSD at admission and 24 (43.6%) had PSD 3 months after stroke.
Our multivariate logistic regression analysis included age, gender, and factors significant in univariate analysis (p < 0.05) (table 3). NIHSS score at admission and mRS score at 3 months were included in place of individual neurologic deficits because of their overlap with general neurologic deficits. We found that NIHSS score (p < 0.001) was independently associated with PSD at admission, whereas the presence of microbleeds (p = 0.009) and lack of social support (p = 0.001) were independently related to PSD 3 months later. Lesion location (p = 0.022) was the only independent factor associated with PSEI at admission, whereas mRS score (p = 0.019), STin2 VNTR (p = 0.040), and low social support (p = 0.042) were independently associated with PSEI at 3 months after stroke.
Table 3.
Factors associated with poststroke depression and poststroke emotional incontinence on multiple logistic regression analysis
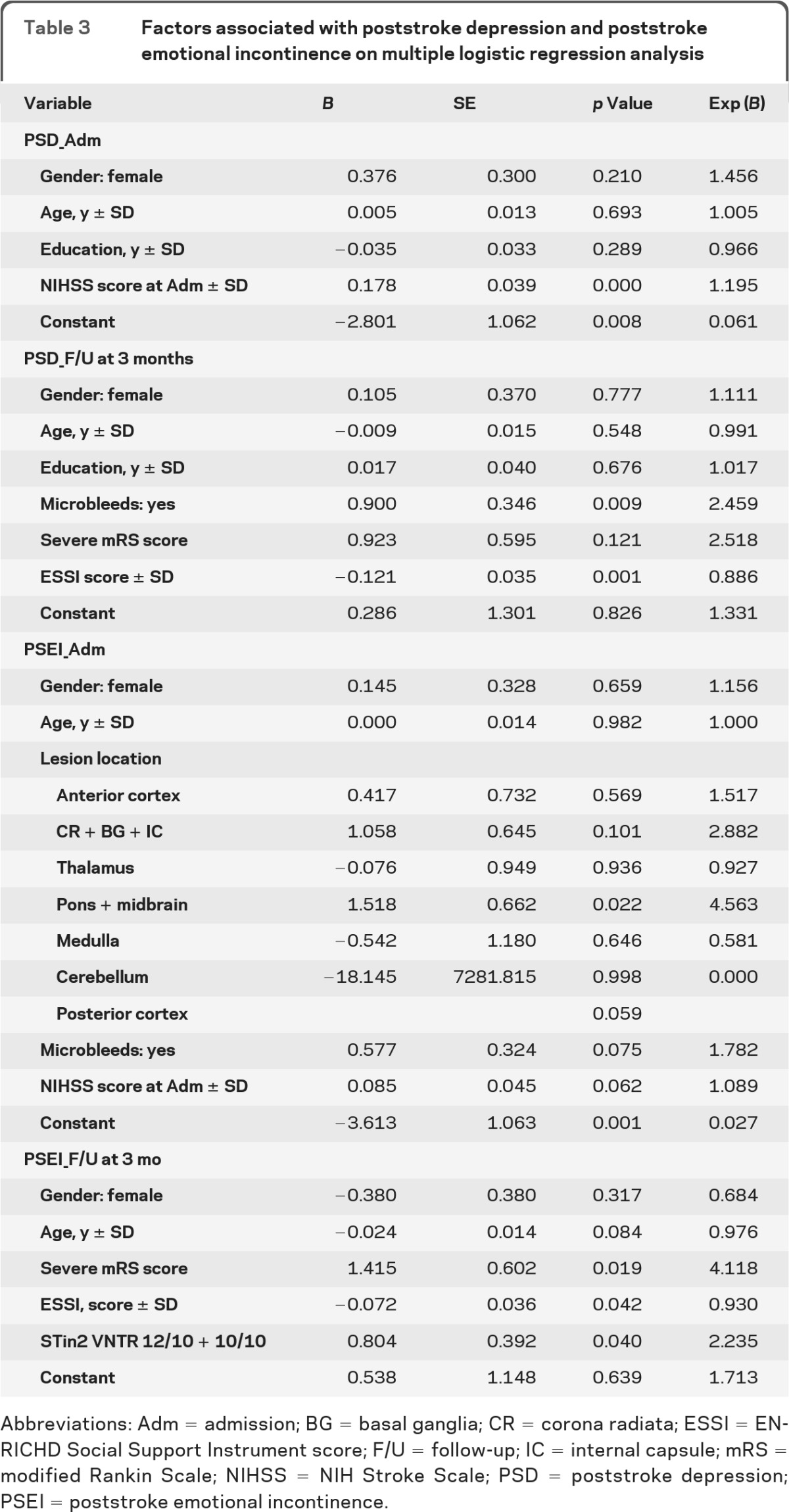
Abbreviations: Adm = admission; BG = basal ganglia; CR = corona radiata; ESSI = ENRICHD Social Support Instrument score; F/U = follow-up; IC = internal capsule; mRS = modified Rankin Scale; NIHSS = NIH Stroke Scale; PSD = poststroke depression; PSEI = poststroke emotional incontinence.
Changes in PSD and PSEI over time.
Of the 70 patients who developed PSD during the acute stage of stroke, 40 had recovered and 26 still had PSD after 3 months. In comparison, 57 patients without PSD at admission had developed PSD at 3 months. We found that a low NIHSS score at admission (p = 0.051) was marginally related to recovery from PSD, whereas low social support was related to the development of PSD 3 months after stroke (p = 0.012). Of the 48 patients who developed PSEI during the acute stage of stroke, 24 had recovered, and 15 still had PSEI after 3 months, whereas 40 patients without PSEI at admission had developed PSEI at 3 months. We found that mRS score (p = 0.012) and STin2 VNTR (p = 0.03) were significantly related to the new development of PSEI at 3 months.
Relationship between PSD/PSEI and functional outcomes.
To determine whether the presence of PSEI and PSD at admission could predict functional outcomes at 3 months, we analyzed the correlation between these emotional disturbances at admission and mRS score at 3 months. We found that poorer mRS score at 3 months was significantly associated with the presence of PSD and PSEI at admission (p < 0.01 each).
DISCUSSION
In this study, we investigated PSD and PSEI in both the acute and subacute stage and comprehensively evaluated the neurologic, socioenvironmental, and genetic factors related to these emotional disturbances.
Of our patients, 13.7% and 9.4% had PSD and PSEI, respectively, at admission, with 17.7% and 11.7%, respectively, having these emotional disturbances after 3 months. We found that some patients spontaneously recover from PSD and PSEI, whereas others newly develop these symptoms at 3 months. Moreover, factors related to PSD and PSEI differed according to the timing of assessment. These findings illustrate the fact that emotional symptoms after stroke may be caused by multiple factors, including lesion location, genetic predisposition, and changeable items such as functional deficits and social/environmental situation. When we further analyzed the factors related to the recovery or new occurrence of PSD and PSEI after 3 months, we found that the lack of social support and NIHSS score at admission were associated with the development of PSD at 3 months, whereas mRS score and STin2 VNTR 10/10 genotypes were associated with the development of PSEI at 3 months.
Not surprisingly, we found that PSEI and PSD were closely associated, both at admission and after 3 months. However, the factors related to each type of emotional disturbance at different times differed. During the acute stage, overall neurologic dysfunction, as assessed by NIHSS score, was closely associated with PSD, whereas only lesion location was independently related to PSEI. In agreement with previous findings,1 locations closely related to PSEI were the basal ganglia/internal capsule and the pons, which contain abundant serotonergic fibers. These observations suggest that, at least during the acute stage of stroke, PSD is closely associated with the severity of neurologic dysfunction, whereas PSEI is more closely related to neurochemical changes associated with damage to specific brain regions. Our data are in agreement with a recent PET study, in which the brain of patients with poststroke pathologic crying had low baseline serotonin binding potentials.13
We also found that polymorphisms in the serotonin gene, the STin2 10/10 genotypes, were independently related to PSEI at 3 months after stroke. Although an association was reported between the STin2.10 allele and anxiety-related traits (neuroticism and harm avoidance) in a healthy Russian population,14 our data highlight a relationship between PSEI and serotonin gene polymorphism in stroke patients. The reason that STin2 polymorphisms are related to PSEI at 3 months but not during the acute stage remains unclear. Perhaps genetic traits may play a more important role during the subacute than the acute stage, when patients are likely to be emotionally triggered by confrontation with the surrounding environment (gene-environment interaction).15 Our results therefore suggest that, in addition to damaged serotonergic neuronal fibers, genetic traits in individual patients are involved in the development of PSEI.
However, serotonin gene polymorphism was not related to PSD in our result, a finding at odds with previous studies reporting positive relationships between PSD and the 5-HTTLPR S/S and STin2 VNTR 9/12 genotypes.12,15,16 This discrepancy may have been due to differences in inclusion criteria, sample size, or criteria for PSD or ethnicity-associated differences in the distribution of 5-HTTLPR gene variants.16 However, our results could not completely exclude the possibility of an association between 5-HTTLPR and PSD, because we did not evaluate the functional single nucleotide polymorphism in the promoter region rs25531 of the lower expressing allele (LG) which, together with another lower expressing allele (SA), has been reported to be related to PSD.15
Markers of small vessel diseases, such as leukoaraiosis and microbleeds, have been shown to be associated with cognitive decline17 and possibly with depression.18 Although we observed no association between leukoaraiosis and either PSD or PSEI, microbleeds were associated with PSD at 3 months. These findings are in partial agreement with those of recent Chinese studies, which reported that microbleeds were associated with both PSD and PSEI 3 months after stroke.19,20 Therefore, microbleeds seem to affect poststroke emotional disturbances more than white matter ischemic changes. Further studies are needed to more clearly elucidate the relationship between small vessel diseases and emotional disturbances.
There were certain factors shared by PSD and PSEI. For example, a lack of social support was associated with both PSD and PSEI after 3 months, suggesting that psychosocial-behavioral intervention may reduce emotional disturbances during the subacute stage of stroke.21 In addition, a high NIHSS score at admission was related to PSD and severe mRS score at 3 months to PSEI, suggesting that physical dysfunction is related to both PSD and PSEI. Finally, we found that PSD and PSEI at admission were both closely related to poorer mRS score at 3 months, a finding in agreement with the previous result that PSD at admission had a negative effect on patient recovery.22 Thus, the relationships between emotional disturbances and functional outcomes may be bidirectional.
Our study has limitations. The prevalence of PSD and PSEI was generally lower than that in previous studies.23,24 We excluded patients with severe neurologic conditions, aphasia, and cognitive impairment and those who lived alone. In addition, the 42 patients who dropped out of the study before 3 months were older and had more severe neurologic dysfunction at admission, suggesting that occurrence of emotional disturbances may have been underestimated. Our prevalence results, therefore, may not be generalizable, and interpretation of data should be made cautiously. Nevertheless, our data show that diverse factors such as neurologic dysfunction, lesion location, genetic traits, and social support are differently related to acute and subacute emotional disturbances. Further studies are needed to confirm our preliminary results and to find appropriate strategies to prevent or manage these important problems in stroke patients.
GLOSSARY
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders, 4th edition
- mRS
modified Rankin scale
- NIHSS
NIH Stroke Scale
- PSD
poststroke depression
- PSEI
poststroke emotional incontinence
AUTHOR CONTRIBUTIONS
Dr. Choi-Kwon: drafting/revising the manuscript for content, including medical writing for content, analysis or interpretation of data, study concept or design, statistical analysis, obtaining funding. Dr. Han: drafting/revising the manuscript for content, including medical writing for content, acquisition of data. S. Choi: drafting/revising the manuscript for content, including medical writing for content, acquisition of data. Dr. Suh: drafting/revising the manuscript for content, including medical writing for content, acquisition of data. Dr. Kim: drafting/revising the manuscript for content, including medical writing for content, acquisition of data. Dr. Song: drafting/revising the manuscript for content, including medical writing for content, acquisition of data. Dr. Cho: drafting/revising the manuscript for content, including medical writing for content, acquisition of data. Dr. Nah: drafting/revising the manuscript for content, including medical writing for content, acquisition of data. Dr. Kwon: drafting/revising the manuscript for content, including medical writing for content, acquisition of data. Dr. Kang: drafting/revising the manuscript for content, including medical writing for content, acquisition of data. Dr. Kim: drafting/revising the manuscript for content, including medical writing for content, study concept or design, analysis or interpretation of data, study supervision or coordination, obtaining funding.
DISCLOSURE
Prof. Choi-Kwon, Dr. Han, S. Choi, Dr. Suh, Dr. Y.-J. Kim, Dr. Song, Prof. Cho, Dr. Nah, Prof. Kwon, and Dr. Kang report no disclosures. Dr. J.S. Kim serves as an Associate Editor for International Journal of Stroke and on the editorial boards of Stroke, Cerebrovascular Diseases, European Journal of Neurology, and Neurocritical Care.
REFERENCES
- 1. Kim JS, Choi-Kwon S. Poststroke depression and emotional incontinence: correlation with lesion location. Neurology 2000;54:1805–1810 [DOI] [PubMed] [Google Scholar]
- 2. Kim JS, Choi-Kwon S, Kwon SU, Lee HJ, Park KA, Seo YS. Factors affecting the quality of life after ischemic stroke: young versus old patients. J Clin Neurol 2005;1:59–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Choi-Kwon S, Kim HS, Kwon SU, Kim JS. Factors affecting the burden on caregivers of stroke survivors in South Korea. Arch Phys Med Rehabil 2005;86:1043–1048 [DOI] [PubMed] [Google Scholar]
- 4. Calvert T, Knapp P, House A. Psychological associations with emotionalism after stroke. J Neurol Neurosurg Psychiatry 1998;65:928–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Choi-Kwon S, Han SW, Kwon SU, Kang DW, Choi JM, Kim JS. Fluoxetine treatment in poststroke depression, emotional incontinence, and anger proneness: a double-blind, placebo-controlled study. Stroke 2006;37:156–161 [DOI] [PubMed] [Google Scholar]
- 6. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198 [DOI] [PubMed] [Google Scholar]
- 7. Mitchell PH, Powell L, Blumenthal J, et al. A short social support measure for patients recovering from myocardial infarction: the ENRICHD Social Support Inventory. J Cardiopulm Rehabil 2003;23:398–403 [DOI] [PubMed] [Google Scholar]
- 8. Choi-Kwon S, Chung CK, Lee SK, Choi J, Han K, Lee EH. Quality of life after epilepsy surgery in Korea. J Clin Neurol 2008;4:116–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kapeller P, Barber R, Vermeulen RJ, et al. Visual rating of age-related white matter changes on magnetic resonance imaging: scale comparison, interrater agreement, and correlations with quantitative measurements. Stroke 2003;34:441–445 [DOI] [PubMed] [Google Scholar]
- 10. Jeon SB, Kwon SU, Cho AH, Yun SC, Kim JS, Kang DW. Rapid appearance of new cerebral microbleeds after acute ischemic stroke. Neurology 2009;73:1638–1644 [DOI] [PubMed] [Google Scholar]
- 11. Kim SJ, Kim YS, Choi NK, Hong HJ, Lee HS, Kim CH. Serotonin transporter gene polymorphism and personality traits in a Korean population. Neuropsychobiology 2005;51:243–247 [DOI] [PubMed] [Google Scholar]
- 12. Kohen R, Cain KC, Mitchell PH, et al. Association of serotonin transporter gene polymorphisms with poststroke depression. Arch Gen Psychiatry 2008;65:1296–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moller M, Andersen G, Gjedde A. Serotonin 5HT1A receptor availability and pathological crying after stroke. Acta Neurol Scand 2007;116:83–90 [DOI] [PubMed] [Google Scholar]
- 14. Kazantseva AV, Gaysina DA, Faskhutdinova GG, Noskova T, Malykh SB, Khusnutdinova EK. Polymorphisms of the serotonin transporter gene (5-HTTLPR, A/G SNP in 5-HTTLPR, and STin2 VNTR) and their relation to personality traits in healthy individuals from Russia. Psychiatr Genet 2008;18:167–176 [DOI] [PubMed] [Google Scholar]
- 15. Ramasubbu R, Tobias R, Bech-Hansen NT. Extended evaluation of serotonin transporter gene functional polymorphisms in subjects with post-stroke depression. Can J Psychiatry 2008;53:197–201 [DOI] [PubMed] [Google Scholar]
- 16. Fang J, Yan W, Jiang GX, Li W, Cheng Q. Serotonin transporter gene polymorphism in Chinese patients with poststroke depression: a case-control study. Stroke 2011;42:1461–1463 [DOI] [PubMed] [Google Scholar]
- 17. Werring DJ, Gregoire SM, Cipolotti L. Cerebral microbleeds and vascular cognitive impairment. J Neurol Sci 2010;299:131–135 [DOI] [PubMed] [Google Scholar]
- 18. Alexopoulos GS, Meyers BS, Young RC, Campbell S, Silbersweig D, Charlson M. ‘Vascular depression' hypothesis. Arch Gen Psychiatry 1997;54:915–922 [DOI] [PubMed] [Google Scholar]
- 19. Tang WK, Chan SS, Chiu HF, Ungvari GS, Wong KS, Kwok TC. Emotional incontinence in Chinese stroke patients: diagnosis, frequency, and clinical and radiological correlates. J Neurol 2004;251:865–869 [DOI] [PubMed] [Google Scholar]
- 20. Tang WK, Chen YK, Lu JY, et al. Cerebral microbleeds and depression in lacunar stroke. Stroke 2011;42:2443–2446 [DOI] [PubMed] [Google Scholar]
- 21. Mitchell PH, Veith RC, Becker KJ, et al. Brief psychosocial-behavioral intervention with antidepressant reduces poststroke depression significantly more than usual care with antidepressant: living well with stroke: randomized, controlled trial. Stroke 2009;40:3073–3078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Parikh RM, Robinson RG, Lipsey JR, Starkstein SE, Fedoroff JP, Price TR. The impact of poststroke depression on recovery in activities of daily living over a 2-year follow-up. Arch Neurol 1990;47:785–789 [DOI] [PubMed] [Google Scholar]
- 23. Hackett ML, Yapa C, Parag V, Anderson CS. Frequency of depression after stroke: a systematic review of observational studies. Stroke 2005;36:1330–1340 [DOI] [PubMed] [Google Scholar]
- 24. Schiffer R, Pope LE. Review of pseudobulbar affect including a novel and potential therapy. J Neuropsychiatry Clin Neurosci 2005;17:447–454 [DOI] [PubMed] [Google Scholar]


