Abstract
Objective:
To prospectively examine whether higher intakes of total flavonoids and their subclasses (flavanones, anthocyanins, flavan-3-ols, flavonols, flavones, and polymers) were associated with a lower risk of developing Parkinson disease (PD).
Methods:
In the current analysis, we included 49,281 men in the Health Professional Follow-up Study and 80,336 women from the Nurses' Health Study. Five major sources of flavonoid-rich foods (tea, berry fruits, apples, red wine, and orange/orange juice) were also examined. Flavonoid intake was assessed using an updated food composition database and a validated food frequency questionnaire.
Results:
We identified 805 participants (438 men and 367 women) who developed PD during 20–22 years of follow-up. In men, after adjusting for multiple confounders, participants in the highest quintile of total flavonoids had a 40%lower PD risk than those in the lowest quintile (hazard ratio [HR] = 0.60; 95% confidence interval 0.43, 0.83; p trend = 0.001). No significant relationship was observed in women (p trend = 0.62) or in pooled analyses (p trend = 0.23). In the pooled analyses for the subclasses, intakes of anthocyanins and a rich dietary source, berries, were significantly associated with a lower PD risk (HR comparing 2 extreme intake quintiles were 0.76 for anthocyanins and 0.77 for berries, respectively; p trend < 0.02 for both).
Conclusions:
Our findings suggest that intake of some flavonoids may reduce PD risk, particularly in men, but a protective effect of other constituents of plant foods cannot be excluded.
Flavonoids are widely distributed in many plant-based foods/beverages.1 Historically, their biological effects were attributed to antioxidant actions,2 but recent evidence suggests that this classic hydrogen-donating antioxidant activity cannot account for their in vivo bioactivity, particularly in the brain where they are found in low concentrations.3,4 Other relevant potential mechanisms include interactions with neuronal signaling pathways that are critical in controlling neuronal survival and differentiation and in modulating activity/expression of several oxidative-related enzymes (e.g., eNOS and SOD),4,5 and regulation of mitochondrial function or neuroinflammation.4–6 In experimental studies, administration of flavonoids or flavonoid-rich foods (e.g., berry fruits) protected dopamine neurons from oxidative damage and apoptosis and inhibited formation of α-synuclein fibrils.7–10
Because of these properties, it has been suggested that flavonoids (or their metabolites) that pass through the blood–brain barrier (BBB)11,12 could reduce Parkinson disease (PD) risk. However, the metabolic alteration of flavonoids following ingestion is considerable,1,13,14 and there are no convincing human data on whether diets high in flavonoids have neuroprotective effects. We therefore conducted the first prospective study to examine whether greater intakes of total flavonoid or their subclasses (i.e., flavanones, anthocyanins, flavan-3-ols, flavonols, flavones, and polymers) lowered risk of developing PD. We also examined the association between major flavonoid-rich foods and PD risk.
METHODS
Study population.
The study population included participants from the Nurses' Health Study (NHS) and the Health Professionals Follow-up Study (HPFS) cohorts. The NHS comprises 121,700 female registered nurses who were 30–55 years of age and resided in 1 of 11 US states at the time of enrollment in 1976. The HPFS is a prospective cohort study began in 1986 when 51,529 male health professionals, aged 40–75, answered a detailed mailed questionnaire including items on lifestyle practices and medical history. Both cohorts have been followed by means of biennial mailed questionnaires and the overall response rate is above 94% for each follow-up cycle. Dietary intake data have been collected since 1986 in the HFPS and since 1980 in the NHS. We used 1986 as baseline for the HFPS. For the NHS, we used 1984 as the baseline because the 1980 food frequency questionnaire (FFQ) did not include several major food items. Among 51,529 men and 97,510 women who returned the baseline questionnaires, we excluded participants who reported a baseline history of PD (128 men and 27 women), those (2,086 men and 16,846 women) who missed dietary information or reported daily caloric intake outside the plausible range (<500 or >3,500 kcal/day for women and <800 or >4,200 kcal/day for men), or those lost to follow-up or with missing information on important covariates such as age (34 men and 301 women), leaving 49,281 men and 80,336 women for the current analyses. Individuals who reported a PD diagnosis during the follow-up, but did not give consent to release their medical records (10.5% of men and 15.4% of women), were excluded from the main analyses. However, we conducted a sensitivity analysis including these individuals as incident PD cases.
Standard protocol approvals, registrations, and patient consents.
The study was approved by the Human Research Committees at the Harvard School of Public Health and the Brigham and Women's Hospital with receipt of each questionnaire accepted as participant's consent.
Assessment of dietary flavonoid intake and covariates.
Dietary intakes were assessed at baseline and every 4 years thereafter with a semiquantitative FFQ validated for use in these populations.15,16 Participants were asked how often on average over the previous year they had consumed a specific amount of each food item (servings, e.g., half cup of strawberries) with 9 possible responses ranging from “never” to “6 or more times per day.”
Assessment of flavonoid intake has been described elsewhere.17 Briefly, a database for assessment of intake of the different flavonoid subclasses was constructed by integrating data from the updated and expanded US Department of Agriculture flavonoid content of foods database18 together with additional information from an extensive European database (EUROFIR), and prominent literature sources. We calculated intakes of the 6 main subclasses commonly consumed in the US diet, specifically flavonols (quercetin, kaempferol, myricetin, and isorhamnetin), flavanones (eriodictyol, hesperetin, and naringenin), anthocyanins (cyanidin, delphinidin, malvidin, pelargonidin, petunidin, and peonidin), flavan-3-ols (catechins, epicatechins, gallocatechins, epigallocatechin, epicatechin 3 gallate, epigallocatechin 3 gallate), polymers (proanthocyanidins, theaflavins, thearubigins), and flavones (luteolin, apigenin). Because consumption of onion, a major source of dietary flavonols, was not asked in the 1986 HPFS FFQ, we used the onion intake data obtained in the 1990 questionnaire to calculate the baseline flavonols intake. The measure “total flavonoids” represents the sum of these 6 subclasses. Although the validity of values for flavonoid intake were not directly tested, we examined correlations between the questionnaire and the dietary records for the major food sources of flavonoids in subsamples of the HPFS and the NHS: the correlations were 0.70–0.80 for apple, 0.77–0.93 for tea, 0.30–0.38 for blueberry and strawberry, 0.83–0.90 for red wine, and 0.50–0.78 for orange and orange juice.15,16
In both cohorts, information on age, ethnicity, body weight, height, and smoking status was collected through biennial questionnaires. Body mass index (BMI) was calculated as weight (kg)/height (m)2.
Ascertainment of PD.
We identified new PD cases by biennial self-reported questionnaires.19–21 We then asked the treating neurologists to complete a questionnaire to confirm the PD diagnosis or to send a copy of the medical records. A case was confirmed if a diagnosis of PD was considered definite or probable by the treating neurologist or internist, or if the medical record included either a final diagnosis of PD made by a neurologist, or evidence of at least 2 of the 3 cardinal signs (rest tremor, rigidity, bradykinesia) in the absence of features suggesting other diagnoses. The review of medical records was conducted by a movement disorder specialist (M.A.S.), blind to the exposure status. Overall, the diagnosis was confirmed by the neurologist in >85% of the cases. We also requested the death certificates of the deceased study participants and identified PD diagnoses that were not reported in the regular follow-up (less than 2%). In this analysis, we used only definite and probable PD as we did previously.19–23
Statistical methods.
Participants contributed person-time of follow-up from the date of return of the baseline questionnaire to the date of PD onset, death, or end of follow-up (2006 for both cohorts). Energy-adjusted baseline dietary flavonoid intakes were categorized into quintiles and hazard ratios (HRs) and 95% confidence intervals (CIs) were obtained by use of the Cox proportional hazards models, adjusting for several potential cofounders: age, smoking status, BMI, use of nonsteroid anti-inflammatory drugs, and intake of total energy, caffeine, lactose, and alcohol. An advantage of this quintile approach is that it does not make assumptions on the dose-response relationship between exposures and confounders and risk of PD, which may be nonlinear. Linear trends were tested for significance by using the median value for each quintile of intake and treating this value as a continuous variable. A pooled HR was calculated by weighting the study-specific log HR by the inverse of its variance using a random effects model. We used the Q test to examine the heterogeneity between studies.
To take advantage of repeated measures of dietary intake, we calculated cumulative average of flavonoid intake to predict the incidence of PD in a 4-year lag analysis. For example, we used the average of flavonoid consumption in 1986, 1990, and 1994, in relation to incidence of PD between 1998 and 2002. We also conducted sensitivity analyses by restricting to PD cases confirmed by neurologists or further including cases not confirmed by treating physicians or medical record review. Finally, we evaluated whether the association between flavonoid consumption and risk of PD was modified by age (y), smoking status (ever vs never), caffeine intake (high vs lower, based on median value), and alcohol (none vs >0g/day). All p values are 2-tailed. All statistical analyses were performed using SAS software, version 9.0 (SAS Institute Inc., Cary, NC).
RESULTS
During 20–22 years of follow-up (mean follow-up was 20.0 years for men and 22.7 years for women), we documented 805 incident PD cases (438 men and 367 women). Participants with higher flavonoid intake were older and less likely to be current smokers, reported lower intakes of lactose and alcohol, reported higher intakes of vitamin C and beta-carotene, and had a healthier diet quality, as assessed by the alternate healthy eating index (table 1).
Table 1.
Age-adjusted lifestyle characteristics by quintile of baseline total flavonoid intake in the Health Professionals Follow-up Study (1986) and the Nurses' Health Study (1984)a
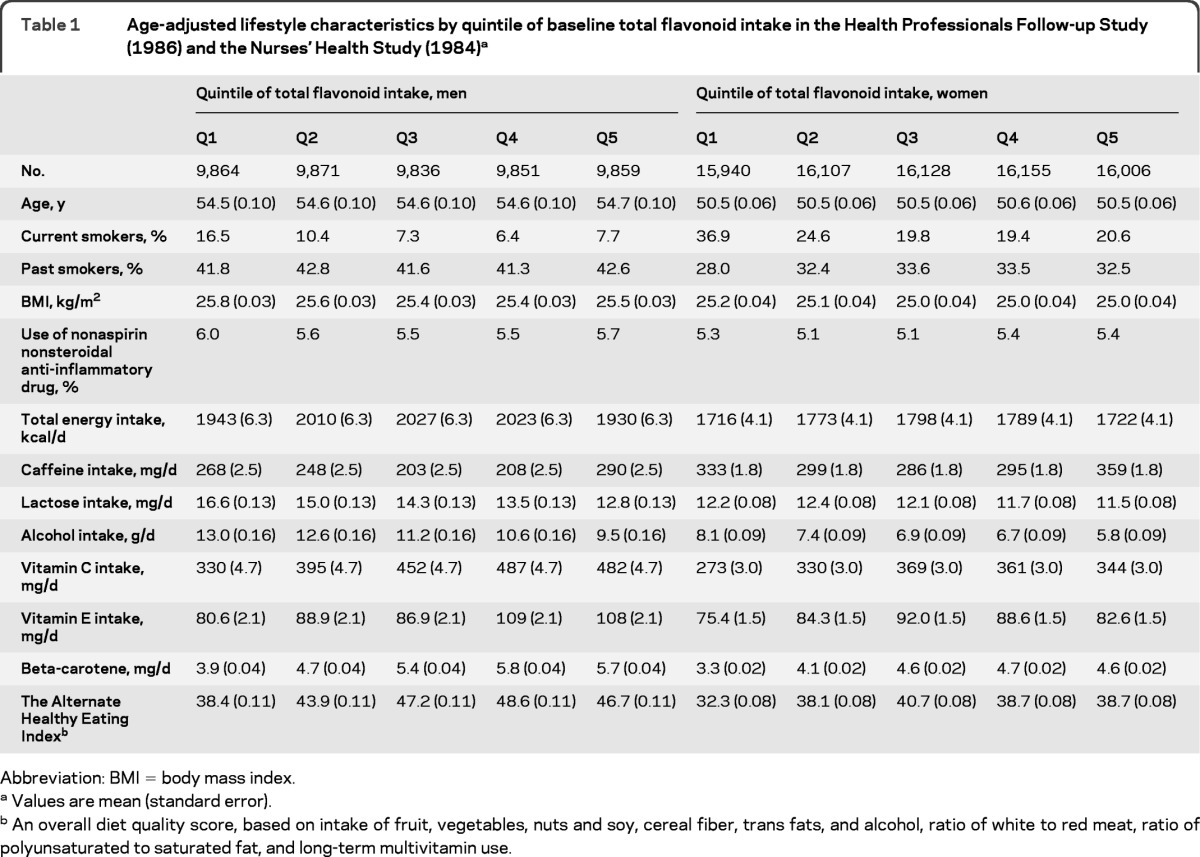
Abbreviation: BMI = body mass index.
Values are mean (standard error).
An overall diet quality score, based on intake of fruit, vegetables, nuts and soy, cereal fiber, trans fats, and alcohol, ratio of white to red meat, ratio of polyunsaturated to saturated fat, and long-term multivitamin use.
Higher intakes of total flavonoids were significantly associated with a lower risk of PD in men (p trend = 0.001), but not in women (p trend = 0.62) (table 2). The multivariable adjusted HRs for the highest vs lowest quintile of total flavonoid intake were 0.60 (95% CI 0.43–0.83) for men and 1.01 (95% CI 0.70–1.44) for women. The pooled HR was 0.77 (95% CI 0.46–1.28; p trend = 0.23; p heterogeneity = 0.02). The associations between total flavonoids and PD risk were similar for those with relative younger onset of PD (<70 y) and those with onset 70+ y (data not shown). In the analyses of flavonoid subclasses (table 3), we found a significant association between greater anthocyanin intake and lower PD risk: the pooled HR comparing the 2 extreme intake quintiles was 0.76 (95% CI 0.61–0.96; p trend = 0.02; p heterogeneity = 0.83). Other flavonoid subclasses were not significantly associated with PD risk, with the exception of the polymeric forms in men. We also observed a significant trend toward greater consumption of flavonols and lower risk of PD in men (p trend = 0.01) although the HR comparing the 2 extreme intake quintiles was not significant. We then investigated PD risk according to intake of each individual flavonoid compound (table e-1 on the Neurology® Web site at www.neurology.org). In the pooled analyses, higher intake of the flavonol, quercetin, the anthocyanins, cyanidin, and pelargonidin and 2 flavan-3-ol monomers and polymers (epicatechin and proanthocyanidindimers) were significantly associated with lower risk of PD in the pooled analysis (p < 0.05 for all).
Table 2.
Hazard ratio and 95% confidence interval of Parkinson disease according to quintile of intake of total flavonoids in the Health Professionals Follow-up Study (1986) and the Nurses' Health Study (1984)a
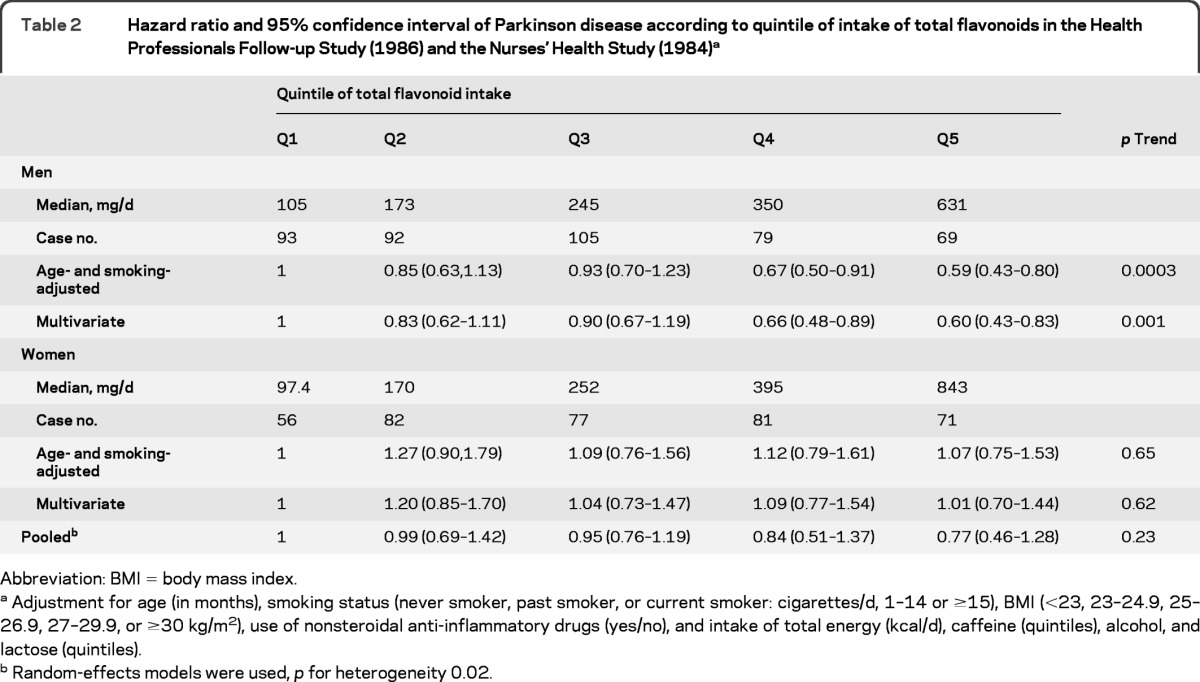
Abbreviation: BMI = body mass index
Adjustment for age (in months), smoking status (never smoker, past smoker, or current smoker: cigarettes/d, 1–14 or ≥15), BMI (<23, 23–24.9, 25–26.9, 27–29.9, or ≥30 kg/m2), use of nonsteroidal anti-inflammatory drugs (yes/no), and intake of total energy (kcal/d), caffeine (quintiles), alcohol, and lactose (quintiles).
Random-effects models were used, p for heterogeneity 0.02.
Table 3.
Hazard ratio and 95% confidence interval of Parkinson disease according to quintile of intake of each subclass of flavonoidsa
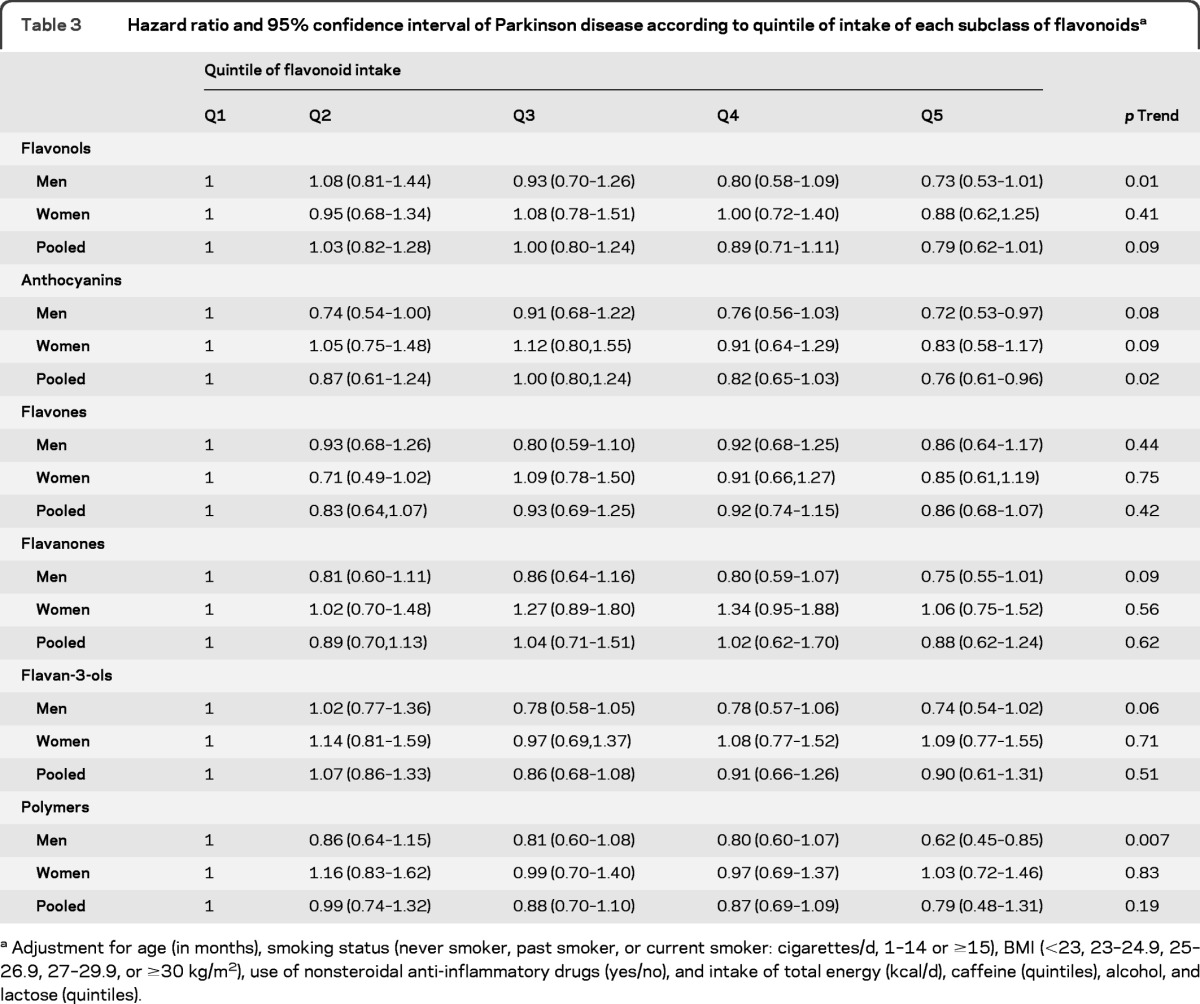
Adjustment for age (in months), smoking status (never smoker, past smoker, or current smoker: cigarettes/d, 1–14 or ≥15), BMI (<23, 23–24.9, 25–26.9, 27–29.9, or ≥30 kg/m2), use of nonsteroidal anti-inflammatory drugs (yes/no), and intake of total energy (kcal/d), caffeine (quintiles), alcohol, and lactose (quintiles).
We further analyzed the top 5 major flavonoid-rich foods in relation to PD risk (table 4). Consistent with our subclass analyses, greater intake of berries, which are rich in anthocyanins, but not of tea, red wine, and orange/orange juice, were significantly associated with a lower risk of developing PD (p trend = 0.007) in the pooled analyses. Apple intake was also associated with a decreased risk in men (p trend < 0.0001) but not in women (p trend = 0.31).
Table 4.
Hazard ratio and 95% confidence interval of Parkinson disease according to intake of flavonoid-rich foodsa
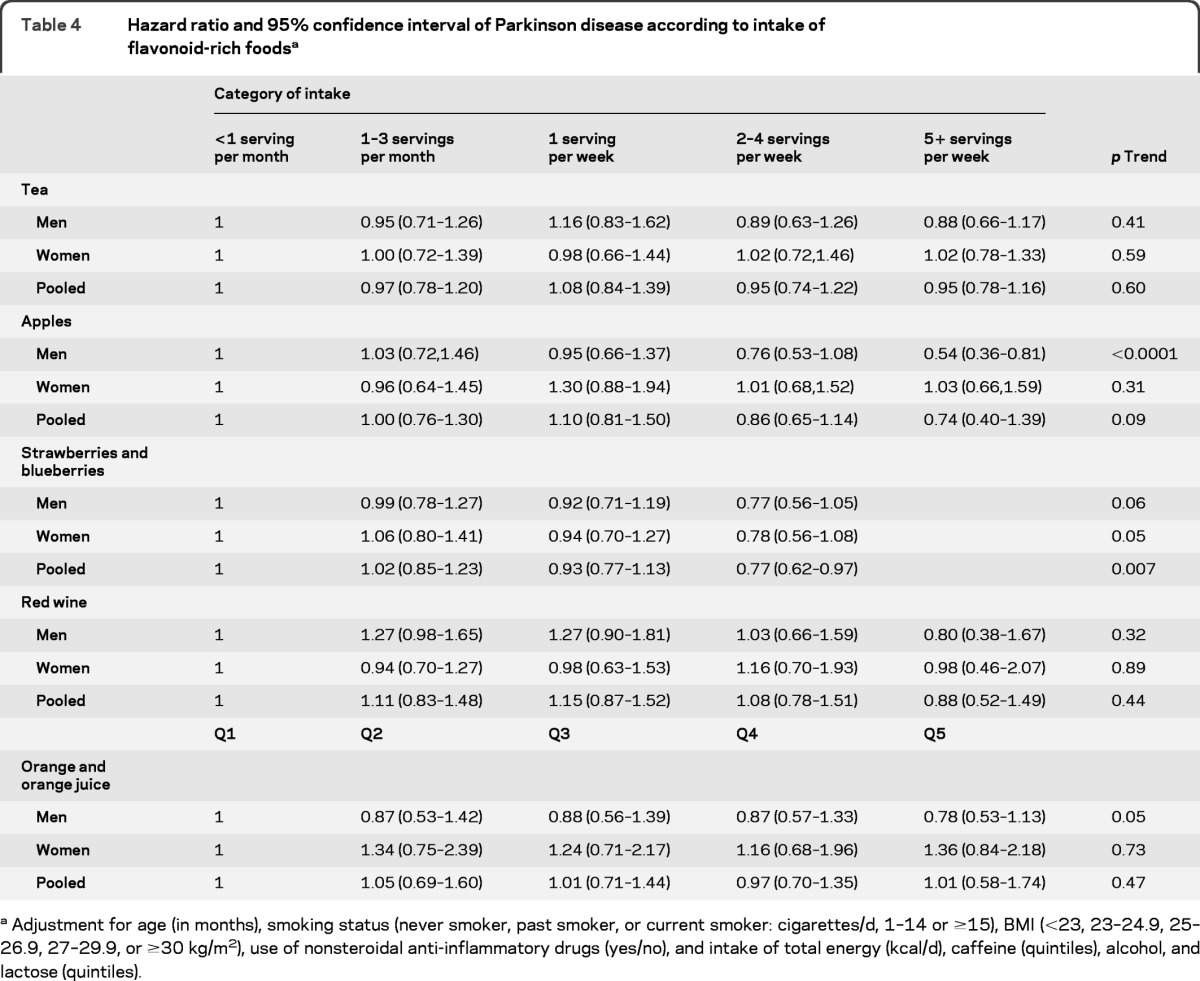
Adjustment for age (in months), smoking status (never smoker, past smoker, or current smoker: cigarettes/d, 1–14 or ≥15), BMI (<23, 23–24.9, 25–26.9, 27–29.9, or ≥30 kg/m2), use of nonsteroidal anti-inflammatory drugs (yes/no), and intake of total energy (kcal/d), caffeine (quintiles), alcohol, and lactose (quintiles).
Similar results were obtained in all sensitivity analyses. When we used the cumulative average intake of flavonoids as exposures, the multivariate HRs were 0.65 (95% CI 0.46–0.91; p trend = 0.01) for total flavonoids in men and 1.08 (95% CI 0.74–1.56; p trend = 0.64) in women. The pooled HR was 0.69 (95% CI 0.54–0.88; p trend = 0.002) for anthocyanin intake. Even when we further adjusted for the Alternate Healthy Eating Index (comprised of fruit, vegetables, nuts and soy, and other dietary components)20 and the dietary urate index (comprised of intake of fructose, vitamin C, alcohol, and dairy protein),23 the associations did not change materially: the HRs were 0.61 (95% CI 0.44–0.85; p trend = 0.003) for total flavonoids in men and 1.04 (95% CI 0.72–1.51; p trend = 0.68) in women and the pooled HR was 0.80 (95% CI 0.63–1.02; p trend = 0.05) for anthocyanin intake. Restricting to neurologist-diagnosed cases did not materially change the results (p trend < 0.05 for total flavonoids in men and antocyanin in the pooled analysis). Further including PD cases not confirmed by treating physicians or review of medical record attenuated the associations: the HRs were 0.67 (p trend = 0.005) for total flavonoids in men and 1.05 (p trend = 0.95) in women and the pooled HR was 0.90 (p trend = 0.16) for anthocyanin intake. Using the original continuous dietary intake variables for trend test generated similar results (data not shown). Finally, we observed no significant interactions between total flavonoids and age, smoking, caffeine, or alcohol intake (p interaction > 0.3 for all).
DISCUSSION
In this large prospective study, we found that participants with greater consumption of anthocyanins, quercetin, epicatechin, and some proanthocyanidins were less likely to develop PD during 20–22 years of follow-up. Higher intakes of anthocyanin-rich foods, such as berries, were associated with lower PD risk. When we combined all individual flavonoids together, total flavonoid intake was also associated with a significantly lower PD risk in men but not in women.
Our observation that a higher intake of anthocyanins and anthocyanins-rich foods were associated with a lower risk of PD are consistent with a previous case-control study, where PD patients (n = 81) were found to be ∼40%–60% less likely to consume blueberries or strawberries, compared to controls.24 Experimental studies have shown that after oral administration of a blueberry or strawberry extract, anthocyanin concentrations were significantly increased in the brain, reaching concentrations that were much higher than in peripheral tissues.10 Oral administration of blueberry or strawberry extract in 11 animal studies showed consistent favorable neuroprotective effects including increasing dopamine release, alleviating oxidative stress, and suppressing neuroinflammation.10 Anthocyanins have been found to induce detoxifying enzymes which may play an important role in PD etiology.25 In a recent animal study of PD, intake of the anthocyanin pelargonidin dose-dependently attenuated behavioral and structural abnormalities due to 6-OHDA toxicity and decreased lipid peroxidation.26
It is established that anthocyanins can cross the BBB in rodents and in pigs following ingestion of anthocyanins-rich diets.27 In vitro and animal studies have also shown that the flavanones and their in vivo metabolites can cross the BBB and oral administration of tea to mice identified levels of epicatechin metabolites in brain tissue.28 However, different flavonoid subclasses differ in their ability to cross the BBB and these differences depend in part on the lipophilicity and polarity of the flavonoid compound.12,29 During absorption they are extensively metabolized, with chemical transformations resulting in O-methylation and glucuronidation during phase II metabolism which may have a significant impact on flavonoid bioavailability to the brain. Further transformation has been reported in the colon where the intestinal microflora degrade flavonoids to simple phenolic acids.1,13 It is therefore possible that the less polar O-methylated metabolites, for example O-methylated epicatechin metabolites, which are formed in the small intestine and liver, may be more bioavailable to the brain than their parent aglycones.12
We observed that greater intakes of epicatechin and proanthocyanidindimers (i.e., dimers of the monomeric flavan-3-ol subclass) were associated with lower PD risks. Previous experimental studies reported that dietary epicatechin stimulated the phosphorylation of the transcription factor cAMP-response element binding protein, a regulator of neuronal viability and synaptic plasticity, and inhibited NADPH oxidase activity.30,31 Recent data suggest that the neuroprotective mechanisms of the flavan-3-ol, epigallocatechin-3-gallate, relate to regulation of neuroinflammation and modulation of genes involved in cell survival and death by decreasing intracellular calcium levels and controlling NO production.32 Oral administration of proanthocyanidins in rats significantly increased brain dopamine concentrations, inhibited monoamine oxidase-A activity, and attenuated the 6-OHDA-induced dopaminergic loss.33,34 Interestingly, proanthocyanidins may not be able to cross the BBB due to their high molecular weight, but following ingestion they are extensively metabolized, with degradation to simple phenolic acids accruing in the large intestine,13,14 and recently these simple phenolic acids have been shown to protect neurons in vitro against injury induced by 5-s-cysteinyl-dopamine at a similar magnitude to the effects of the aglycones.6 It is thus important to examine in future proanthocyanidin studies the relative importance of the range of in vivo metabolites on neuroprotection.
We did not find a significant association between consumption of flavonols and flavones and PD risk. However, greater intake of the flavonol quercetin may be associated with lower PD risk. In previous animal and in vitro studies, quercetin has been found to exert a neuroprotective effect against oxidative stress and neuron loss induced by MPP+ or 6-OHDA,35,36 although high dose could be neurotoxic.37 However, no human studies have been conducted to examine effects of quercetin on PD risk to date. In a population-based study, patients with PD (n = 31) and controls reported similar dietary intake of flavonols and flavones (27.5 vs 28.5 mg/day).38 However, this study did not differentiate quercetin from other flavonoids and was limited by its cross-sectional design and small sample size. Further studies are therefore needed to replicate our observations.
The association between total flavonoid intake and PD risk was more pronounced in men than women. This difference seems unlikely to be due to the higher intake of flavonoid in women, because we observed a similar nonsignificant result in women when we regrouped them using the men's quintile cutoff points for flavonoid intakes (data not shown). In previous studies, a similar gender difference has been reported for several risk/protective factors of PD. For example, the significant inverse associations between caffeine intake, plasma urate, and risk of PD have been observed only in men, but not in women.19,23,39,40 However, it is unclear if this gender difference in the effects of flavonoids on PD risk reflects a true biological difference or is driven by chance because we did not observe such gender difference when we examined the subclasses of flavonoid in relation to risk of PD. Further, the interaction between total flavonoids and use of estrogen was not significant in women (p interaction = 0.63).
Because of our prospective design, our study is unlikely to be affected by recall and selection biases. Conversely, misclassification of flavonoid intake is inevitable, because of errors in reporting food consumption, and incompleteness of existing databases that only list the most common among several hundreds of flavonoids. However, there are currently no reliable biomarkers of flavonoid intake to integrate intake with the extensive metabolism that occurs following ingestion. Because the study population was primarily white health professionals, the results might not be generalizable to all populations. However, this homogeneity of social educational status helps to reduce some confounding and enhances the internal validity. Finally, as with any observational study, we cannot exclude the possibility of residual confounding. Although we controlled for a range of diet and lifestyle factors, it remains possible that other constituents of fruits and vegetables confound the association between flavonoids and PD risk. Because of these limitations, the results presented here should be interpreted cautiously and need to be confirmed in other large prospective studies, preferably in populations with wide heterogeneity in flavonoid content.
Supplementary Material
GLOSSARY
- BBB
blood–brain barrier
- BMI
body mass index
- CI
confidence interval
- FFQ
food frequency questionnaire
- HPFS
Health Professionals Follow-up Study
- HR
hazard ratio
- NHS
Nurses' Health Study
- PD
Parkinson disease
Footnotes
Editorial, page 1112
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Dr. Gao: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, statistical analysis. Dr. Cassidy: drafting/revising the manuscript, study concept or design, analysis or interpretation of data. Dr. Schwarzschild: drafting/revising the manuscript, analysis or interpretation of data, acquisition of data, obtaining funding. Dr. Rimm: drafting/revising the manuscript, study concept or design, acquisition of data. Dr. Ascherio: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, statistical analysis, study supervision, obtaining funding.
DISCLOSURE
Dr. Gao serves on the Committee on Nutrition, Trauma, and the Brain, Institute of Medicine; has received research support from the NIH/NINDS; and has served as a consultant for Teva Pharmaceutical Industries Ltd. Dr. Cassidy has received research support from Unilever and Diabetes UK. Dr. Schwarzschild has received research support from the NIH/NINDS, the US Department of Defense, the Michael J. Fox Foundation, the Parkinson Disease Foundation, the RJG Parkinson's Disease Foundation, the American Parkinson Disease Association, and the American Federation for Aging Research. Dr Rimm serves on the editorial boards of American Journal of Clinical Nutrition and American Journal of Epidemiology; and receives research support from the NIH. Dr. Ascherio serves on a scientific advisory board for the Michael J. Fox Foundation; serves as Associate Editor for Multiple Sclerosis and Related Disorders and the Journal of Parkinson Disease and on the editorial boards of Neurology® and Annals of Neurology; has received speaker honoraria from Merck Serono; and receives research support from the NIH, the US Department of Defense, the Michael J. Fox Foundation, and the National Multiple Sclerosis Society.
REFERENCES
- 1. Manach C, Scalbert A, Morand C, Remesy C, Jimenez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr 2004; 79: 727– 747 [DOI] [PubMed] [Google Scholar]
- 2. Waterhouse AL, Shirley JR, Donovan JL. Antioxidants in chocolate. Lancet 1996; 348: 834 [DOI] [PubMed] [Google Scholar]
- 3. Kroon PA, Clifford MN, Crozier A, et al. How should we assess the effects of exposure to dietary polyphenols in vitro? Am J Clin Nutr 2004; 80: 15– 21 [DOI] [PubMed] [Google Scholar]
- 4. Spencer JP. Flavonoids: modulators of brain function? Br J Nutr 2008; 99 (E Suppl 1): ES60– E77 [DOI] [PubMed] [Google Scholar]
- 5. Ramassamy C. Emerging role of polyphenolic compounds in the treatment of neurodegenerative diseases: a review of their intracellular targets. Eur J Pharmacol 2006; 545: 51– 64 [DOI] [PubMed] [Google Scholar]
- 6. Vafeiadou K, Vauzour D, Spencer JP. Neuroinflammation and its modulation by flavonoids. Endocr Metab Immune Disord Drug Targets 2007; 7: 211– 224 [DOI] [PubMed] [Google Scholar]
- 7. Meng X, Munishkina LA, Fink AL, Uversky VN. Molecular mechanisms underlying the flavonoid-induced inhibition of alpha-synuclein fibrillation. Biochemistry 2009; 48: 8206– 8224 [DOI] [PubMed] [Google Scholar]
- 8. Mercer LD, Kelly BL, Horne MK, Beart PM. Dietary polyphenols protect dopamine neurons from oxidative insults and apoptosis: investigations in primary rat mesencephalic cultures. Biochem Pharmacol 2005; 69: 339– 345 [DOI] [PubMed] [Google Scholar]
- 9. Zhu M, Rajamani S, Kaylor J, Han S, Zhou F, Fink AL. The flavonoid baicalein inhibits fibrillation of alpha-synuclein and disaggregates existing fibrils. J Biol Chem 2004; 279: 26846– 26857 [DOI] [PubMed] [Google Scholar]
- 10. Balk E, Chung M, Raman G, et al. B vitamins and berries and age-related neurodegenerative disorders. Evid Rep Technol Assess (Full Rep) 2006: 1– 161 [PMC free article] [PubMed] [Google Scholar]
- 11. Dreiseitel A, Oosterhuis B, Vukman KV, et al. Berry anthocyanins and anthocyanidins exhibit distinct affinities for the efflux transporters BCRP and MDR1. Br J Pharmacol 2009; 158: 1942– 1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Youdim KA, Qaiser MZ, Begley DJ, Rice-Evans CA, Abbott NJ. Flavonoid permeability across an in situ model of the blood-brain barrier. Free Radic Biol Med 2004; 36: 592– 604 [DOI] [PubMed] [Google Scholar]
- 13. Williamson G, Manach C. Bioavailability and bioefficacy of polyphenols in humans: II: review of 93 intervention studies. Am J Clin Nutr 2005; 81: 243S– 255S [DOI] [PubMed] [Google Scholar]
- 14. Erdman JW, Jr, Balentine D, Arab L, et al. Flavonoids and heart health: proceedings of the ILSI North America Flavonoids Workshop, May 31–June 1, 2005, Washington, DC. J Nutr 2007; 137: 718S– 737S [DOI] [PubMed] [Google Scholar]
- 15. Feskanich D, Rimm EB, Giovannucci EL, et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc 1993; 93: 790– 796 [DOI] [PubMed] [Google Scholar]
- 16. Salvini S, Hunter DJ, Sampson L, et al. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol 1989; 18: 858– 867 [DOI] [PubMed] [Google Scholar]
- 17. Cassidy A, O'Reilly EJ, Kay C, et al. Habitual intake of flavonoid subclasses and incident hypertension in adults. Am J Clin Nutr 2011; 93: 338– 347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bhagwat SA, Gebhardt SE, Haytowitz DB, Holden JM, Harnly J. USDA database for the flavonoid content of selected foods. Available at: http://www.nal.usda.gov/fnic/foodcomp/Data/Flav/Flav02.pdf Accessed October 10, 2010
- 19. Ascherio A, Zhang SM, Hernan MA, et al. Prospective study of caffeine consumption and risk of Parkinson's disease in men and women. Ann Neurol 2001; 50: 56– 63 [DOI] [PubMed] [Google Scholar]
- 20. Gao X, Chen H, Fung TT, et al. Prospective study of dietary pattern and risk of Parkinson disease. Am J Clin Nutr 2007; 86: 1486– 1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gao X, Simon KC, Han J, Schwarzschild MA, Ascherio A. Family history of melanoma and Parkinson disease risk. Neurology 2009; 73: 1286– 1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gao X, Simon KC, Han J, Schwarzschild MA, Ascherio A. Genetic determinants of hair color and Parkinson's disease risk. Ann Neurol 2009; 65: 76– 82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gao X, Chen H, Choi HK, Curhan G, Schwarzschild MA, Ascherio A. Diet, urate, and Parkinson's disease risk in men. Am J Epidemiol 2008; 167: 831– 838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Golbe LI, Farrell TM, Davis PH. Case-control study of early life dietary factors in Parkinson's disease. Arch Neurol 1988; 45: 1350– 1353 [DOI] [PubMed] [Google Scholar]
- 25. Ajiboye TO, Salawu NA, Yakubu MT, Oladiji AT, Akanji MA, Okogun JI. Antioxidant and drug detoxification potentials of Hibiscus sabdariffa anthocyanin extract. Drug Chem Toxicol 2011; 34: 109– 115 [DOI] [PubMed] [Google Scholar]
- 26. Roghani M, Niknam A, Jalali-Nadoushan MR, Kiasalari Z, Khalili M, Baluchnejadmojarad T. Oral pelargonidin exerts dose-dependent neuroprotection in 6-hydroxydopamine rat model of hemi-parkinsonism. Brain Res Bull 2010; 82: 279– 283 [DOI] [PubMed] [Google Scholar]
- 27. Milbury PE, Kalt W. Xenobiotic metabolism and berry flavonoid transport across the blood-brain barrier. J Agric Food Chem 2010; 58: 3950– 3956 [DOI] [PubMed] [Google Scholar]
- 28. Abd El Mohsen MM, Kuhnle G, Rechner AR, et al. Uptake and metabolism of epicatechin and its access to the brain after oral ingestion. Free Radic Biol Med 2002; 33: 1693– 1702 [DOI] [PubMed] [Google Scholar]
- 29. Milbury P. Transport of flavonoids into the brain In: Packer L, Sies H, Eggersdorfer M, Cadenas E. eds. Micronutrients and Brain Health, 1st ed. London: CRC Press; 2009: 432 [Google Scholar]
- 30. Schroeter H, Bahia P, Spencer JP, et al. (-)Epicatechin stimulates ERK-dependent cyclic AMP response element activity and up-regulates GluR2 in cortical neurons. J Neurochem 2007; 101: 1596– 1606 [DOI] [PubMed] [Google Scholar]
- 31. Steffen Y, Schewe T, Sies H. (-)-Epicatechin elevates nitric oxide in endothelial cells via inhibition of NADPH oxidase. Biochem Biophys Res Commun 2007; 359: 828– 833 [DOI] [PubMed] [Google Scholar]
- 32. Jang S, Jeong HS, Park JS, et al. Neuroprotective effects of (-)-epigallocatechin-3-gallate against quinolinic acid-induced excitotoxicity via PI3K pathway and NO inhibition. Brain Res 2010; 1313: 25– 33 [DOI] [PubMed] [Google Scholar]
- 33. Datla KP, Zbarsky V, Rai D, et al. Short-term supplementation with plant extracts rich in flavonoids protect nigrostriatal dopaminergic neurons in a rat model of Parkinson's disease. J Am Coll Nutr 2007; 26: 341– 349 [DOI] [PubMed] [Google Scholar]
- 34. Xu Y, Li S, Chen R, et al. Antidepressant-like effect of low molecular proanthocyanidin in mice: involvement of monoaminergic system. Pharmacol Biochem Behav 2010; 94: 447– 453 [DOI] [PubMed] [Google Scholar]
- 35. Bournival J, Quessy P, Martinoli MG. Protective effects of resveratrol and quercetin against MPP+ induced oxidative stress act by modulating markers of apoptotic death in dopaminergic neurons. Cell Mol Neurobiol 2009; 29: 1169– 1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang ZJ, Cheang LC, Wang MW, Lee SM. Quercetin exerts a neuroprotective effect through inhibition of the iNOS/NO system and pro-inflammation gene expression in PC12 cells and in zebrafish. Int J Mol Med 2011; 27: 195– 203 [DOI] [PubMed] [Google Scholar]
- 37. Kaariainen TM, Piltonen M, Ossola B, et al. Lack of robust protective effect of quercetin in two types of 6-hydroxydopamine-induced parkinsonian models in rats and dopaminergic cell cultures. Brain Res 2008; 1203: 149– 159 [DOI] [PubMed] [Google Scholar]
- 38. de Rijk MC, Breteler MM, den Breeijen JH, et al. Dietary antioxidants and Parkinson disease: The Rotterdam Study. Arch Neurol 1997; 54: 762– 765 [DOI] [PubMed] [Google Scholar]
- 39. O'Reilly EJ, Gao X, Weisskopf MG, et al. Plasma urate and Parkinson's disease in women. Am J Epidemiol 2010; 172: 666– 670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Weisskopf MG, O'Reilly E, Chen H, Schwarzschild MA, Ascherio A. Plasma urate and risk of Parkinson's disease. Am J Epidemiol 2007; 166: 561– 567 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


