Abstract
Purpose
Among long-term (≥5 years) colorectal cancer survivors with permanent ostomy or anastomosis, we compared the incidence of medical and surgical complications and examined the relationship of complications with health-related quality of life.
Background
The incidence and effects of complications on long-term health-related quality of life among colorectal cancer survivors are not adequately understood.
Methods
Participants (284 ostomy/395 anastomosis) were long-term colorectal cancer survivors enrolled in an integrated health plan. Health-related quality of life was assessed via mailed survey questionnaire in 2002–2005. Information on colorectal cancer, surgery, co-morbidities, and complications was obtained from computerized data and analyzed using survival analysis and logistic regression.
Results
Ostomy and anastomosis survivors were followed an average 12.1 and 11.2 years, respectively. Within 30 days of surgery, 19% of ostomy and 10% of anastomosis survivors experienced complications (p<0.01). From 31 days on, the percentages were 69% and 67% (after adjustment, p<0.001). Bleeding and post-operative infection were common early complications. Common long-term complications included hernia, urinary retention, hemorrhage, skin conditions, and intestinal obstruction. Ostomy was associated with long-term fistula (odds ratio 5.4; 95% CI 1.4–21.2), and among ostomy survivors, fistula was associated with reduced health-related quality of life (p<0.05).
Conclusions
Complication rates remain high despite recent advances in surgical treatment methods. Survivors with ostomy have more complications early in their survivorship period, but complications among anastomosis survivors catch up after 20 years, when the two groups have convergent complication rates. Among colorectal cancer survivors with ostomy, fistula has especially important implications for health-related quality of life.
INTRODUCTION
Approximately 148,800 new cases of colorectal cancer (CRC) will be diagnosed in 2008.1 Most of these require surgery, which may involve rectal excision. Permanent ostomy is most common with low rectal tumors, but may also be placed because of co-morbidities or poor anal sphincter function. In addition, anticipated temporary ostomy, placed to protect an anastomosis or in an emergency setting, may become permanent when performance status worsens or there is a procedure-related problem.
The Health-Related Quality of Life in Long-Term Colorectal Cancer Survivors Study was undertaken to elucidate experiences and correlates of HRQOL among long-term colorectal cancer survivors with ostomy compared to anastomosis.2 The study’s results provide the basis for developing and evaluating clinical interventions aimed at mitigating ostomy-related HRQOL deficits for these patients.3, 4 A previous report from the study reported HRQOL deficits in ostomates compared with anastomosis patients.5 Other reports from the study have examined sleep disruption and fatigue,6 dietary adjustments,7 male partner support in relation to female psychosocial adjustment,8 household income as a predictor of psychologic well being,9 and the greatest challenges faced by long-term CRC survivors with ostomy.10
The study we report here was undertaken to better understand the risk of medical complications in relation to CRC surgery. We also sought to understand the impact of complications on total HRQOL for CRC survivors. Only two previous reports have examined these issues using actuarial methods.11,12 By gaining a better understanding of surgical complications among CRC survivors and the impact on HRQOL, surgeons and others caring for these patients can anticipate issues and intervene when possible.
MATERIALS AND METHODS
Details of the study’s methods are described elsewhere.2 Briefly, the study was set among members of three Kaiser Permanente regions, with project coordination at the Southern Arizona Veterans Affairs Health Care System. Approval of institutional review boards from each site was obtained.
Study participants
The study included adult CRC 5-year survivors who were members of the Kaiser Permanente Medical Care Program during the recruitment period (2002–2005). In Northern California, the participants had been diagnosed with CRC at Kaiser Permanente. Northwest and Hawaii Kaiser Permanente participants included 19 patients diagnosed with CRC outside the healthplan. Patients were selected based on whether they had ostomy (cases) or anastomosis (controls) at the time of recruitment. Some of the ostomy patients had a prior anastomosis. However, none of the anastomosis patients had a temporary ostomy, as those patients were excluded from the study. Anastomosis controls were matched to ostomy cases on cancer site, coded as rectum (ICD-O codes C19.9 and C20.9) versus colon (C18.0-.9), time since cancer (within 5 years), age (within 10 years), and gender.
Data Collection
The modified City of Hope Quality of Life for Ostomates questionnaire (mCOH-QOL-Ostomy) was used to assess HRQOL,4 with modifications allowing survey of anastomosis patients. The mCOH-QOL-Ostomy has demographic, non-scaled, and scaled (0–10) items. The non-scaled items assess marital status, work, household income, health insurance, sexual activity, psychological support, and diet. The scaled items are mapped onto one of four HRQOL domains (physical, psychological, social, and spiritual well-being) based on psychometric analysis.4 The overall HRQOL score is calculated as the mean of items across all domains. The mCOH-QOL-Ostomy was modified for anastomosis subjects by referencing questions to their surgical treatment for CRC and by eliminating eight ostomy-specific items. As described in our earlier report,2 the reliability of the ostomy and anastomosis versions were very nearly identical. Convergent evidence for construct validity was demonstrated by correlating the two versions of the QOL scale with corresponding scales on the SF-36v2, with both the ostomy and anastomosis scales demonstrating similar Pearson product-moment.
Linkage to computerized data provided information on the characteristics of the cancer and its treatment, diabetes in the year prior to surgery, the patient’s Charlson-Deyo Comorbidity Index13 in the year prior to survey, and complications. Complications of interest (Appendix) were defined a priori. We counted as early complications those that were first recorded within 30 days of the surgery. We classified late complications as occurring ≥31 days after surgery. Only the first occurrence of each complication was counted. Body mass index (BMI), ascertained from the patient survey, was examined as a covariate in the analysis of poor HRQOL.
Statistical Analysis
All data analyses were performed using SAS® version 9.13 (SAS Institute, Inc, Cary, NC). For all cases and controls diagnosed with CRC while enrolled at Kaiser Permanente, follow-up for complications began on the date of the index ostomy or anastomosis surgery. For 19 patients who had been diagnosed with CRC outside the health-plan, follow-up began on the date of their first enrollment. For all patients, follow-up ended on the date of the patient’s disenrollment, death, or December 31, 2006. Gaps in enrollment were removed from follow-up.
We computed the cumulative probability of developing ≥1 complication using Kaplan-Meier analysis.14 Cox proportional hazards analysis with allowance for late entry was used to compute the adjusted hazard ratios (HR) and 95% confidence interval (CI).15 Time since surgery was used as the time axis. P-values were obtained using the likelihood ratio test. Analyses were adjusted for age at survey, diabetes in the year prior to surgery, 16 Charlson-Deyo Comorbidity Index in the year before the first cancer surgery, tumor site (colon, rectum), and initial radiation therapy.
We dichotomized the overall HRQOL score and compared low and high HRQOL using logistic regression analysis.17 In addition to the variables in Cox proportional hazard model, BMI at the time of survey (≤26, 27–29, and ≥30 kg/m2)was included in the model.
Because of clinical differences between colon and rectal cancer, we performed subgroup analysis for all hypotheses for rectal cancer patients only, the number of colon cancer patients with ostomy being too small for meaningful interpretation.
RESULTS
Demographic Characteristics, Re-hospitalization, and Subsequent Surgeries
Response rates to the mailed questionnaire were 51% (Northern California), 62% (Northwest), and 37% (Hawaii), as detailed in an earlier publication.2 The denominator used in this calculation excludes incorrect addresses (<3%), ineligible patients, and deaths, but includes non-respondents after follow-up telephone calls and frank refusals. In total, 679 long-term CRC patients responded (284 ostomy and 395 anastomosis). Ostomy cases disproportionately had more rectal cancers than anastomosis controls (88% vs, 63%, p=0.0001), but did not differ on other characteristics (Table 1). Ostomy and anastomosis survivors were followed an average 12.1 and 11.2 years, respectively. Compared with anastomosis controls, a larger proportion of ostomy cases were Hispanic (7% vs 4%, p=0.05), had ≥2 co-morbid conditions (51% vs 40%, p=0.03), or received radiation therapy (36% vs 19%, p<0.0001) for their initial tumor.
Table 1.
Characteristics of the study sample of ostomy cases and anastomosis controls, %
| Characteristic | Category | Ostomy cases (N=284) | Anastomosis controls (N=395) | P-value |
|---|---|---|---|---|
| Tumor location | Colon | 12.3 | 37.5 | Reference |
| Rectum | 87.7 | 62.5 | <0.0001 | |
| Tumor stage | Localized | 48.6 | 47.6 | Reference |
| Regional | 39.1 | 50.4 | <0.0001 | |
| Distant metastases or systemic | 2.1 | 1.5 | 0.35 | |
| Unknown | 10.2 | 0.5 | 0.0001 | |
| Year of first cancer-directed surgery | ≤1989 | 23.0 | 17.8 | Reference |
| 1990–1994 | 21.9 | 27.4 | 0.05 | |
| 1995–1999 | 34.2 | 39.6 | 0.11 | |
| 2000–2001 | 20.9 | 15.2 | 0.09 | |
| Follow-up from the date of cancer surgery to the date of survey | 5–9 | 45.0 | 46.1 | Reference |
| 10–14 | 25.8 | 30.9 | 0.11 | |
| ≥15 | 29.2 | 23.0 | 0.06 | |
| Age at survey, yr | 39–59 | 14.1 | 16.2 | Reference |
| 60–69 | 22.2 | 27.3 | 0.19 | |
| 70–78 | 30.3 | 27.3 | 0.32 | |
| 79–96 | 33.5 | 29.1 | 0.19 | |
| Sex | Male | 58.8 | 59.0 | Reference |
| Female | 41.2 | 41.0 | 0.96 | |
| Race/ethnicity | Non-Hispanic White | 74.3 | 78.7 | Reference |
| Black/African-American | 3.5 | 3.5 | 0.67 | |
| Hispanic (all races) | 7.4 | 3.5 | 0.05 | |
| Asian/Pacific Islander | 9.5 | 8.4 | 0.96 | |
| Other | 5.3 | 5.8 | 0.41 | |
| BMI at survey completion, kg/m2 | ≤26 | 57.0 | 55.4 | Reference |
| 27–29 | 21.1 | 19.2 | 0.43 | |
| ≥30 | 21.9 | 25.4 | 0.27 | |
| Diabetes* recorded during follow-up | Yes | 6.3 | 4.6 | 0.31 |
| Charlson-Deyo Comorbidity | 0 | 34.5 | 45.6 | Reference |
| Index in the year before survey | 1 | 14.4 | 14.2 | 0.86 |
| 2+ | 51.1 | 40.3 | 0.03 | |
| Chemotherapy for initial tumor | Yes | 35.2 | 37.2 | 0.59 |
| Radiation therapy for initial tumor | Yes | 35.6 | 19.0 | <0.0001 |
Diabetes was determined from ICD9 code 250 in outpatient or inpatient diagnosis data
The risk of re-hospitalization within 30 days was similar among the ostomy (1.4%) and anastomosis (2.0%) groups (p=0.55) (Table 2). Over the course of follow-up, ostomy cases were more likely than anastomosis controls to have multiple surgeries (p<0.0001), and among those with multiple surgeries, the time from the first to the second procedure was longer in the ostomy cases (>1 year, 57% compared with 40%, p=0.008). Restriction to rectal cancer patients only did not change these findings (Table 3).
Table 2.
Rehospitalization and subsequent surgeries
| Characteristic | %
|
P-value | ||
|---|---|---|---|---|
| Ostomate | Anastomoses | |||
| N=284 | N=395 | |||
| Re-hospitalization within 30 days* | Yes | 1.4 | 2.0 | 0.55 |
| Total number of abdominal surgeries** | 1 | 60.6 | 83.3 | <0.0001 |
| 2 | 25.7 | 13.2 | ||
| 3 | 8.8 | 2.8 | ||
| ≥4 | 4.9 | 0.8 | ||
|
| ||||
| Among those with second surgery | N=112 | N=66 | ||
| Years between first and second surgery*** | <1 | 43.2 | 60.2 | 0.008 |
| 1 | 20.0 | 14.9 | ||
| 2 | 5.6 | 3.7 | ||
| ≥3 | 31.2 | 21.1 | ||
| Type of second surgery | Incision, excision, and anastomosis of intestine | 20.8 | 27.6 | 0.04 |
| Operation on rectum | 19.7 | 19.5 | 0.94 | |
| Operation on anus | 1.4 | 0.5 | 0.24 | |
| Other operation on intestine including colostomy | 21.8 | 2.3 | <0.0001 | |
Available for Northern California only.
Includes the initial procedure. Includes anastomosis of intestine (ICD-9 45.3x-45.9x), other operation on intestine (ICD-9 46), operation on rectum (ICD-9 48), and operation on anus (ICD-9 49).
Among patients who had second surgery only.
Table 3.
(Supplement to Table 2). RECTAL CANCER PATIENTS ONLY: Rehospitalization and subsequent surgeries.
| Characteristic | Ostomate | Anastomoses | P-value | |
|---|---|---|---|---|
| N=249 | N=247 | |||
| Re-hospitalization within 30 days* | Yes | 1.3 | 2.8 | 0.24 |
| Total number of abdominal surgeries** | 1 | 57 | 81 | Ref. |
| 2 | 27 | 15 | 0.52 | |
| 3 | 10 | 4 | 0.67 | |
| ≥4 | 6 | 0.8 | 0.05 | |
| Among those with second surgery | N=107 | N=47 | ||
| Years between first and second surgery*** | <1 | 44 | 61 | Ref. |
| 1–2 | 27 | 21 | 0.83 | |
| ≥3 | 30 | 19 | 0.66 0.40 |
|
| Type of second surgery | Incision, excision, and anastomosis of intestine | 20 | 22 | 0.40 |
| Operation on rectum | 23 | 30 | 0.07 | |
| Operation on anus | 2 | 1 | 0.39 | |
| Other operation on intestine including colostomy | 25 | 2 | <0.001 |
Available for Northern California only.
Includes the initial procedure. Includes anastomosis of intestine (ICD-9 45.3x-45.9x), other operation on intestine (ICD-9 46), operation on rectum (ICD-9 48), and operation on anus (ICD-9 49).
Among patients who had second surgery only.
Risk of Complications
During the early post-operative period (0–30 days), we observed the following risks in the ostomy cases: hemorrhage, 6.3%; peritonitis, 3.5%; urinary retention 3.2%; intraabdominal organ injury 2.1%, urinary tract infection, 1.8%; congestive heart failure (CHF), 1.4%; and fistula, 0.7%. Relative to anastomosis controls, ostomy cases had an increased risk of peritonitis and post-operative infection gastrointestinal hemorrhage (HR 2.5, 95% CI 1.1–5.8) and (HR 6.9, 95% CI 1.4–32.8) (Table 4). In the ostomy group, the risk of early complications of ostomy was 1.4%; in the anastomosis group, the risk of early complications of anastomosis was 5.8%. Other complications of interest were recorded in fewer than five survivors across both the ostomy and anastomosis groups during the early post-operative period. The risk of early complications overall was 19.0% among ostomy cases and 10.4% among anastomosis controls (HR 1.9, 95% CI 1.2–2.9, p=0.003). These findings were similar in an analysis restricted to rectal cancer patients only (Table 5), for whom intra-abdominal organ injury was identified more commonly as a complication of ostomy (HR 10.0, 95% CI 1.1–89).
Table 4.
Cumulative probability of complications among ostomates and anastomoses
| Complication* | Ostomy (N=284) | Anastomosis (N=395) | HR (95% CI)** | ||
|---|---|---|---|---|---|
| N | %** | N | %** | ||
| Early (0–30 days following surgery) | |||||
| Hemorrhage | 18 | 6.3 | 9 | 2.3§§ | 2.5 (1.1–5.8) |
| Peritonitis, post-op infection | 10 | 3.5 | 2 | 0.5§§ | 6.9 (1.4–32.8) |
| Urinary retention | 9 | 3.2 | 5 | 1.3 | 1.6 (0.5–5.1) |
| Intra-abdominal organ injury | 6 | 2.1 | 3 | 0.8 | 4.1 (0.9–17.7) |
| Urinary tract infection | 5 | 1.8 | 3 | 0.8 | 2.8 (0.6–13.4) |
| CHF, myocardial infarction | 4 | 1.4 | 2 | 0.5 | 3.2 (0.5–19.8) |
| Fistula | 2 | 0.7 | 1 | 0.3 | 5.3 (0.4–73.3) |
| Complications of ostomy | 4 | 1.4 | -- | -- | -- |
| Complications of anastomosis | -- | -- | 23 | 5.8 | 1.0 (0.5–2.0) |
| Any early complication*** | 54 | 19.0 | 41 | 10.4§§ | 1.9 (1.2–2.9) |
| Late (31 days post-surgery to the survey date)** | |||||
| Hernia of abdominal cavity | 41 | 31.7 | 53 | 37.2 | 1.0 (0.7–1.6) |
| Urinary retention | 20 | 22.5 | 8 | 2.8§§ | 2.7 (1.1–6.5) |
| Any skin condition | 18 | 20.2 | 15 | 15.0 | 1.2 (0.6–2.6) |
| Hemorrhage | 22 | 14.5 | 24 | 36.8 | 1.2 (0.6–2.2) |
| Urinary incontinence | 8 | 12.6 | 0 | 0 | -- |
| Intestinal obstruction w/o hernia | 22 | 9.8 | 13 | 3.8§§ | 2.0 (1.0–4.1) |
| Intestinal infections | 7 | 8.3 | 6 | 4.9 | 1.2 (0.4–4.0) |
| Fistula | 12 | 7.2 | 3 | 0.8§§ | 5.4 (1.4–21.2) |
| Radiation enterocolitis | 11 | 5.3 | 6 | 13.7 | 1.3 (0.4–3.6) |
| Peritonitis | 7 | 4.4 | 1 | 0.3§§ | 8.3 (0.9–79.6) |
| Complications of ostomy | 44 | 19.6 | -- | -- | -- |
| Complications of anastomosis | -- | -- | 26 | 9.0§ | 1.5 (0.8–2.6) |
| Any late complication*** | 126 | 68.7 | 112 | 67.1§§§ | 1.6 (1.2–2.1) |
Includes only complications from the Appendix that occurring in five or more study subjects.
For late complications, the cumulative probability of complications was calculated using life-table analysis. The average length of follow-up was 12.1 years (range, 5 to 33 years) in the ostomy cases and 11.2 years (range, 5 to 43) in the anastomosis controls.
Adjusted for age at survey (categorical), diabetes (yes, no), Charlson-Deyo Comorbidity Index in the year before the first cancer surgery (none, 1, 2+), tumor site (colon, rectum), and initial radiation therapy (yes, no). Rank test on the difference between ostomy and anastomosis where
denotes p<0.05,
denotes p<0.01 and
denotes p<0.001.
Includes complications occurring in fewer than five study subjects.
Table 5.
(Supplement to Table 4). RECTAL CANCER PATIENTS ONLY: Cumulative probability of complications among ostomates and anastomoses
| Complication* | Ostomy (N=249) | Anastomosis (N=247) | HR (95% CI)** | ||
|---|---|---|---|---|---|
| N | %** | N | %** | ||
| Early (0–30 days following surgery) | |||||
| Hemorrhage | 15 | 5.3 | 8 | 2.0§§ | 2.2 (0.9–5.3) |
| Peritonitis, post-op infection | 10 | 3.5 | 1 | 0.3§§ | 15.1 (1.9–120) |
| Urinary retention | 9 | 3.2 | 4 | 1.0 | 2.1 (0.6–7.1) |
| Intra-abdominal organ injury | 5 | 1.8 | 1 | 0.3 | 10.0 (1.1–89) |
| Urinary tract infection | 4 | 1.4 | 1 | 0.3 | 4.4 (0.5–41) |
| Complications of ostomy | 3 | 1.1 | -- | -- | -- |
| Complications of anastomosis | -- | -- | 17 | 4.3 | 1.0 (0.5–2.2) |
| Any early complication*** | 42 | 14.8 | 29 | 7.3§§ | 1.8 (1.1–3.0) |
| Late (31 days post-surgery to the survey date)** | |||||
| Hernia of abdominal cavity | 33 | 11.6 | 37 | 9.4 | 1.1 (0.7–1.9) |
| Urinary retention | 17 | 6.0 | 5 | 1.3§§ | 3.9 (1.3–10.8) |
| Any skin condition | 18 | 20.2 | 15 | 15.0 | 1.2 (0.6–2.6) |
| Hemorrhage | 17 | 6.0 | 16 | 4.1 | 1.2 (0.6–2.4) |
| Urinary incontinence | 6 | 2.1 | 0 | 0 | -- |
| Intestinal obstruction w/o hernia | 20 | 7.0 | 10 | 2.5§§ | 2.4 (1.1–5.2) |
| Intestinal infections | 5 | 1.8 | 5 | 1.3 | 1.1 (0.3–3.9) |
| Fistula | 9 | 3.2 | 3 | 0.8§§ | 2.9 (0.8–11.4) |
| Radiation enterocolitis | 9 | 3.2 | 6 | 1.5 | 1.1 (0.4–3.2) |
| Peritonitis | 5 | 1.8 | 1 | 0.3§§ | 6.5 (0.7–59.6) |
| Complications of ostomy | 38 | 13.4 | -- | -- | -- |
| Complications of anastomosis | -- | -- | 22 | 5.6§ | 1.4 (0.8–2.6) |
| Any late complication*** | 105 | 37.0 | 77 | 19.5§§§ | 1.8 (1.3–2.5) |
Includes only complications from the Appendix that occurring in five or more study subjects.
Cumulative probability of complications was calculated using life-table analysis. The average length of follow-up was 12.1 years (range, 5 to 33 years) in the ostomy cases and 11.2 years (range, 5 to 43) in the anastomosis controls.
Adjusted for age at survey (categorical), diabetes (yes, no), Charlson-Deyo Comorbidity Index in the year before the first cancer surgery (none, 1, 2+), tumor site (colon, rectum), and initial radiation therapy (yes, no). Rank test on the difference between ostomy and anastomosis where
denotes p<0.05,
denotes p<0.01 and
denotes p<0.001.
Includes complications occurring in fewer than five study subjects.
During the average 12 years of late post-operative (≥31 days following hospitalization) follow-up among the ostomy cases, we observed hernia in 31.7%; urinary retention 22.5%; skin conditions, 20.2%; gastrointestinal hemorrhage 14.5%; urinary incontinence, 12.6%; intestinal obstruction without hernia, 9.8%; intestinal infection, 8.3%; fistula 7.2%; radiation enterocolitis, 5.3%; and peritonitis, 4.4%. In ostomy cases, the cumulative probability of developing a late complication of ostomy was 19.6%. During the average 11 years of late post-operative follow-up among the anastomosis controls, the cumulative probability of a late anastomosis complication was 9.0%. Compared with anastomosis controls, ostomy cases had an increased risk of intestinal obstruction without hernia (HR 2.0, 95% CI 1.0–4.1), fistula (HR 5.4, 95% CI 1.4–21.2), and urinary incontinence (HR 2.7, 95% CI 1.1–6.5). The cumulative probability of late complications overall was 68.7% among ostomy cases and 67.1% among anastomosis controls (HR 1.6, 95% CI 1.2–2.2).
In analysis of rectal cancer patients only, the frequency of several complications was notably lower (Table 5). Less common were hernia of the abdominal cavity (ostomy 11.6%, anastomosis 9.4%), urinary retention (ostomy 6%, anastomosis 1.3%), hemorrhage (ostomy 6.0%, anastomosis 4.1%), urinary incontinence (ostomy 2.1%, anastomosis 0.0%), and intestinal infection (ostomy 1.8%, anastomosis 1.3%). Nonetheless, the relationship of ostomy with these late complications did not change substantially, although the confidence intervals widened. Among rectal cancer patients, any late complication was identified in 37.0% of ostomy and 19.5% of anastomosis patients (HR 1.8, 95% CI 1.3–2.5).
Figure 1 provides the cumulative probability of complication-free survival for ostomy and anastomosis separately for colon and rectal cancer patients. The risk of late complications in ostomy cases was 41.8% at 10 years and 54.8% at 20 years; for anastomosis controls it was 24.7% at 10 years and 54.0% at 20 years. Over the follow-up period, ostomy cases developed more late complications than anastomosis controls (p<0.0001).
Figure 1.
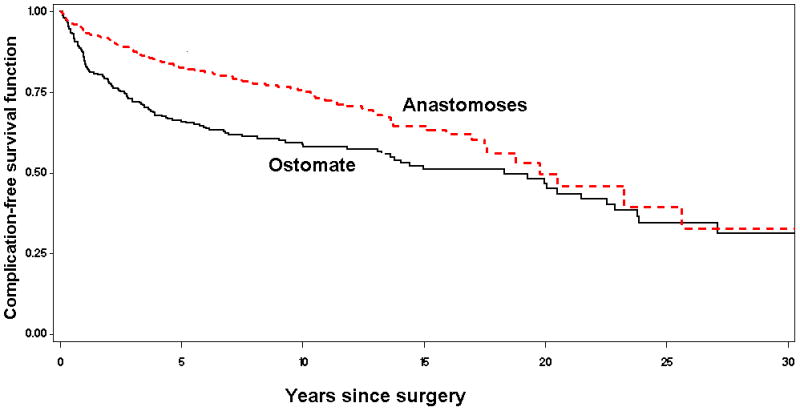
Kaplan-Meier plot of late complication-free survival after ostomy for colorectal cancer.
Risk Factors for Complications
A Charlson-Deyo Comorbidity Index of ≥2 was associated with early and late complications, while diabetes in the year prior to surgery was associated with late complications (Table 6). After adjustment, ostomy was related to a 90% (95% CI, 1.2–2.9) increased risk of early complications and 60% (95% CI, 1.2–2.2) increased risk of late complications. When we examined rectal cancer patients only (Table 7), comorbidities including diabetes were less predictive of early and late complications, while radiation therapy increased the risk of both early and late complications by 80% (95% CI: early 1.1–2.9; late 1.3–2.5). Ostomy was related to an 80% increased risk of early (95% CI, 1.1–3.0) and late (95% CI, 1.3–2.5) complications.
Table 6.
Hazard ratio and 95% CI for the relationship between various risk factors and early and late complications (any, none).
| Risk factor | Timing of complication
|
|
|---|---|---|
| Early (≤30 days) | Late (31 days-survey completion) | |
| Age at survey | ||
| 14–59 | 1.0 (reference) | 1.0 (reference) |
| 60–69 | 1.0 (0.5–1.8) | 0.7 (0.4–1.1) |
| 70–78 | 1.1 (0.6–2.0) | 0.8 (0.5–1.2) |
| 79–96 | 0.9 (0.4–1.7) | 0.7 (0.4–1.0) |
| Diabetes before the cancer diagnosis | 1.0 (0.5–2.4) | 1.8 (1.1–3.0)* |
| Charlson-Deyo Comorbidity Index=1 | 0.7 (0.3–1.5) | 1.4 (0.9–2.1) |
| Charlson-Deyo Comorbidity Index=2+ | 1.7 (1.1–2.6)* | 1.4 (1.1–1.9)* |
| Tumor site, rectum vs colon | 0.9 (0.5–1.5) | 1.2 (0.9–1.7) |
| Radiation therapy for initial tumor | 1.2 (0.7–1.9) | 1.1 (0.8–1.5) |
| Ostomy | 1.9 (1.2–2.9)** | 1.6 (1.2–2.2)** |
P<0.05
P<0.01
Table 7.
(Supplement to Table 6). RECTAL CANCER PATIENTS ONLY (N=496): Hazard ratio and 95% CI for the relationship between various risk factors and early and late complications (any, none).
| Risk factor | Timing of complication
|
|
|---|---|---|
| Early (≤30 days) | Late (31 days-survey completion) | |
| Age at survey | ||
| 14–59 | 1.0 (reference) | 1.0 (reference) |
| 60–69 | 0.7 (0.4–1.4) | 1.0 (0.6–1.6) |
| 70–78 | 0.8 (0.4–1.5) | 1.1 (0.7–1.8) |
| 79–96 | 0.6 (0.3–1.3) | 0.9 (0.5–1.5) |
| Diabetes before the cancer diagnosis | 0.5 (0.1–2.0) | 1.2 (0.6–2.3) |
| Charlson-Deyo Comorbidity Index=1 | 0.8 (0.3–1.9) | 1.5 (1.0–2.3) |
| Charlson-Deyo Comorbidity Index=2+ | 1.4 (0.8–2.3) | 1.2 (0.9–1.7) |
| Radiation therapy for initial tumor | 1.8 (1.1–2.9) | 1.8 (1.3–2.5) |
| Ostomy | 1.8 (1.1–3.0) | 1.8 (1.3–2.5) |
Relationship of Late Complications with HRQOL
Overall, 33% of CRC survivors (42% for ostomates and 27% for anastomoses) had low HRQOL. Enterocutaneous fistula, recorded in 4.2% of ostomy cases and less than 1% of anastomosis controls, was associated with lower overall HRQOL among ostomy cases (Odds Ratio [OR] 4.8, 95% CI 1.2–19.2) (Table 8). Ostomy patients with any late complication had lower overall HRQOL (OR 1.5, 95% CI 0.9–2.6), although the confidence interval included 1.0. This was not the case for anastomosis patients (OR 0.9, 95% CI 0.5–1.5). Among rectal cancer patients alone, complications of ostomy (OR 2.0, 95% CI 1.0–4.1), but not anastomsis (OR 1.0, 95% CI, 0.4–2.8), were associated with reduced HRQOL (Table 9).
Table 8.
Odds ratio and 95% CI for the relationship between late complications (31 days post surgery to survey date) and low HRQOL
| Number (%) with the complication | Odds ratio** | 95% CI | |
|---|---|---|---|
|
Complications
| |||
| Among ostomy patients (N=284) | |||
| Hernia of abdominal cavity | 41 (14.4%) | 1.5 | 0.7–3.2 |
| Intestinal obstruction without hernia | 22 (7.7%) | 1.9 | 0.7–5.0 |
| Fistula | 12 (4.2%) | 4.8 | 1.2–19.2 |
| Hemorrhage | 22 (7.7%) | 0.8 | 0.3–2.1 |
| Skin complications | 18 (6.3%) | 2.4 | 0.8–7.1 |
| Urinary retention | 20 (7.0%) | 1.5 | 0.6–4.3 |
| Complications of ostomy | 44 (15.5) | 1.7 | 0.9–3.5 |
| Any late complication | 126 (44.4%) | 1.5 | 0.9–2.6 |
| Among anastomosis patients (N=395) | |||
| Hernia of abdominal cavity | 53 (13.4%) | 0.8 | 0.4–1.6 |
| Intestinal obstruction without hernia | 13 (3.3%) | 1.0 | 0.3–3.5 |
| Fistula | 3 (0.8%) | Not estimable | Not estimable |
| Hemorrhage | 24 (6.1%) | 1.1 | 0.4–2.8 |
| Skin complications | 15 (3.8%) | 1.7 | 0.5–5.6 |
| Urinary retention | 8 (2.0%) | 0.8 | 0.2–4.3 |
| Complications of anastomosis | 26 (6.6%) | 1.0 | 0.4–2.5 |
| Any late complication | 112 (28.4%) | 0.9 | 0.5–1.5 |
| Among ostomy and anastomosis patients combined (N=679)*** | |||
| Hernia of abdominal cavity | 94 (13.8%) | 1.1 | 0.7–1.8 |
| Intestinal obstruction without hernia | 35 (5.2%) | 1.4 | 0.7–3.0 |
| Fistula | 15 (2.2%) | 2.5 | 0.8–7.6 |
| Hemorrhage | 46 (6.8%) | 0.9 | 0.5–1.8 |
| Skin complications | 33 (4.9%) | 1.8 | 0.8–3.8 |
| Urinary retention | 28 (4.1%) | 1.1 | 0.5–2.7 |
| Complications of ostomy | 44 (6.5%) | 1.8 | 0.9–3.5 |
| Complications of anastomosis | 57 (8.4%) | 1.4 | 0.8–2.4 |
| Any late complication | 238 (35.1%) | 1.2 | 0.8–1.6 |
We dichotomized the overall COH-QOL-Ostomy score by 0–6 (low) and 7–10 (high). The odds ratio and 95% confidence interval for the relationship between complications and low HRQOL, compared with high HRQOL, was assessed separately for the ostomy and anastomosis groups, and for the two groups combined, using logistic regression analysis.
Adjusted for age at survey (categorical), diabetes (yes, no), Charlson-Deyo Comorbidity Index in the year before the first cancer surgery (none, 1, 2+), tumor site (colon, rectum), and initial radiation therapy (yes, no) and BMI (≤26, 27–29, and ≥30 kg/m2), with HRQOL as the dependent variable modeling the probability of the total COH-QOL-Ostomy score ≤6.
In addition to the variables listed above, we further adjusted for ostomy status.
Table 9.
(Supplement to Table 8). RECTAL CANCER PATIENTS ONLY: Odds ratio and 95% CI for the relationship between late complications (31 days post surgery to survey date) and low HRQOL
| Complications | Number (%) with the complication | Odds ratio** | 95% CI |
|---|---|---|---|
| Among ostomy patients (N=249) | |||
| Hernia of abdominal cavity | 33 (14.0%) | 2.0 | 0.9–4.5 |
| Intestinal obstruction without hernia | 20 (8.5%) | 1.8 | 0.7–4.8 |
| Fistula | 9 (3.8%) | 2.8 | 0.6–12.1 |
| Hemorrhage | 17 (7.2%) | 0.7 | 0.2–2.1 |
| Skin complications | 15 (6.4%) | 1.7 | 0.5–5.5 |
| Urinary retention | 17 (7.2%) | 1.7 | 0.6–4.9 |
| Complications of ostomy | 38 (16.2) | 2.0 | 0.9–4.1 |
| Complications of anastomosis | 26 (11.1%) | 1.4 | 0.6–3.4 |
| Any late complication | 105 (44.7%) | 1.8 | 1.0–3.1 |
| Among anastomosis patients (N=247) | |||
| Hernia of abdominal cavity | 37 (15.0%) | 0.7 | 0.3–1.9 |
| Intestinal obstruction without hernia | 10 (4.0%) | 1.0 | 0.2–4.1 |
| Fistula | 3 (1.2%) | Not est. | Not est. |
| Hemorrhage | 16 (6.5%) | 1.1 | 0.3–3.7 |
| Skin complications | 9 (3.6%) | 1.4 | 0.3–6.5 |
| Urinary retention | 5 (2.0%) | 2.0 | 0.3–13 |
| Complications of anastomosis | 22 (8.9%) | 1.0 | 0.4–2.8 |
| Any late complication | 77 (31.2%) | 1.0 | 0.5–1.9 |
| Among ostomy and anastomosis patients combined (N=496)*** | |||
| Hernia of abdominal cavity | 70 (14.5%) | 1.2 | 0.7–2.2 |
| Intestinal obstruction without hernia | 30 (6.2%) | 1.4 | 0.7–3.1 |
| Fistula | 12 (2.5%) | 1.6 | 0.5–5.4 |
| Hemorrhage | 33 (6.8%) | 0.9 | 0.4–2.0 |
| Skin complications | 24 (5.0%) | 1.5 | 0.6–3.6 |
| Urinary retention | 22 (4.6%) | 1.7 | 0.7–4.2 |
| Complications of ostomy | 38 (7.9%) | 2.0 | 1.0–4.1 |
| Complications of anastomosis | 48 (10.0%) | 0.7 | 0.7–2.4 |
| Any late complication | 182 (37.8%) | 0.9 | 0.9–2.1 |
We dichotomized the overall COH-QOL-Ostomy score by 0–6 (low) and 7–10 (high). The odds ratio and 95% confidence interval for the relationship between complications and low HRQOL, compared with high HRQOL, was assessed separately for the ostomy and anastomosis groups, and for the two groups combined, using logistic regression analysis.
Adjusted for age at survey (categorical), diabetes (yes, no), Charlson-Deyo Comorbidity Index in the year before the first cancer surgery (none, 1, 2+), tumor site (colon, rectum), and initial radiation therapy (yes, no) and BMI (≤26, 27–29, and ≥30 kg/m2), with HRQOL as the dependent variable modeling the probability of the total COH-QOL-Ostomy score ≤6.
In addition to the variables listed above, we further adjusted for ostomy status.
DISCUSSION
We evaluated the risk of complications, risks factors for complications, and the role of late complications in HRQOL among long-term CRC survivors. Study strengths included: (1) community-based setting, (2) restriction to CRC survivors, (3) comparison of ostomy to anastomosis, (4) large sample size, (5) long follow-up, (6) multivariate Cox modeling, and (7) clearly defined list of complications. In addition, the study was novel in examining ostomy complications in relation to HRQOL. In our previous report, both male and female ostomy patients had significantly worse social well-being compared to anastomosis patients (men, 7.2 vs 8.2; women, 7.2 vs. 8.5; both p<0.001), while only female ostomates showed significantly worse overall HRQOL (7.0 vs 7.8, p=0.002) and psychological well-being (6.8 vs 7.8, p<0.001).5
Comparison of our findings with earlier studies is challenging because of differences in the reason for ostomy, in the ascertainment and classification of complications, in length of follow-up, and in the analysis. In comparing our study with earlier reports, we give emphasis to studies that had complete follow-up or used actuarial methods. This study did not have information on the American Society of Anesthesiologists score, a predictor of immediate postoperative complications, although we did adjust for the Charlson-Deyo comorbidity score in the year prior to the surgery. Neither did we know the exact location of the tumor relative to the anal verge. Two other features of our study design should be considered while interpreting the results. First, by design, all participants in our study were alive at the time of survey. Thus, in contrast to earlier reports, this study represents long-term CRC survivors. Second, our ascertainment of complications was from computerized clinical data recorded by the physician. An important limitation of the study, inherent with computerized clinical data, is the presence of random coding errors that would lead to a diminution of the associations. Also, less serious conditions might have been under-coded when patients presented with more serious concerns. Computerized data is also less detailed; most notable in our study was the lack of detailed information on complications related directly to the ostomy, which could include poor stoma location, stoma necrosis, and prolapse, for example. Nor could we identify pouchitis as a complication of anastomosis. In a United Kingdom study with prospective data collection, an appreciable proportion of patients had stomal complications at 12 months, including retractions (24%), poor sitings (19%), skin excoriation (12%), and appliance detachments (12%).16 The response rate of 51% should further be considered in interpreting our study; it is possible that non-respondents differed from respondents in their rate of complications and in their HRQOL. If so, we may have under- or over-estimated rates of complication. Any difference that was systematic between ostomy and anastomosis patients may have biased the results to some degree.
Early complications
A previous study from our health plan18 that included patients with a variety of underlying indications undergoing ostomy during 1992–2002, observed a 30-day re-hospitalization risk of 5.2%, compared to this study in which the risk among ostomy survivors was 1.4%. A possible explanation for this difference is the more common emergency hospitalization of cases of inflammation and infection, with greater risk for early re-hospitalizations. During the early post-operative period (0–30 days), we observed a 2.5-fold difference in hemorrhage and a 6.9-fold difference in the risk of peritonitis and post-operative infection between ostomy cases and anastomosis controls. The earlier report from our health plan11 estimated the risk of CHF to be 2.9%, compared with 1.4% in this report, and infection to be 5.2%, compared with 3.5% observed here. Risk of infection was appreciably higher, 14.6%, among 137 rectal cancer patients undergoing anastomosis in a Mexican surgical oncology department.19
A retrospective study of 132 ostomy patients ascertained in 1998–2005 at an Italian surgical center, and followed for a mean of 4 months, observed complications including dermatitis (32%), parastoma hernia (22%), leakage (12%), and stenosis (15%).20 These were recorded in our computerized databases in less than 1% of our ostomy and anastomosis subjects during the first 30 days post-surgery, with the period of follow-up and method of ascertainment of these complications providing a possible explanation for the difference between the two studies.
A study from St. Marks Hospital, London followed 289 patients with a variety of underlying conditions undergoing surgery for 12 months during 1971–1980.11 Twelve percent of ostomy patients developed parastomal hernia at one year.16 A study that followed 408 ostomy and ileostomy patients for two years reported parastomal hernia among 40% of colostomy patients, with 10% being “major” in that they affected the appliance.21
Late complications
In this study, the average length of follow-up was 12.1 years in the ostomy cases and 11.2 years in the anastomosis controls. The St. Marks study11 included 289 anal and rectal cancer patients with permanent end-sigmoid colostomies performed during 1971–1980 and observed for 10–13 years. Actuarial risks were as follows: hernia, 37% at 10 years (versus 32% in this study); skin disorder, 17% at 11 years (this study, 20%); obstruction, 14% at 13 years (13%); with only two patients observed with fistula (this study, 12 cases observed; actuarial risk, 7.2%). In our analysis of rectal cancer patients only, risks were lower than those in the St. Marks study, possibly because of improvements in outcomes over time. In the Swedish Rectal Cancer Trial (1987–1990) of 908 patients, the actuarial risk of bowel obstruction at 10 years was 17% among those not receiving and 7% among those receiving pre-operative radiation therapy,12 compared with 9.8% in this study (7.0% among rectal cancer cases only).
With respect to late complications, older survivors and survivors with severe systemic disease (American Society for Anesthesiologists grade 3) were at increased risk of hernia and other complications in two earlier reports.11, 22 We did not observe age to be a predictor of ostomy complications after adjusting for comorbidity.
We have previously reported on the multiple complications stemming from surgical treatment for colorectal cancer, including bleeding, anastomotic leak, and myocardial infarction, as well as longer-term complications, such as hernia, fistula, bowel obstruction, and sexual dysfunction. In addition, there may be ostomy-specific complications such as peri-stomal hernia, retraction, prolapse, stenosis, and skin irritation.23 Despite recent technical advances, risk of complications from ostomy remain high. We recommend further research to improve prevention and management. Particularly fistula, but indeed nearly all of the complications evaluated in our study were associated with lower HRQOL. This pattern was observed among ostomy but not anastomosis survivors, and to our knowledge has not previously been reported. Increasing clinical awareness of the implications of complications on HRQOL should lead to greater use of resources in this patient population.
Supplementary Material
Acknowledgments
Stephen Joel Coons, M. Jane Mohler, Carol M. Baldwin, Eric Matayoshi, and Sylvan B. Green (deceased) made substantive contributions to the study design and execution.
This research was performed by the SAVAHCS/Kaiser Permanente Collaborative Research Group and was made possible by Grant Number R01 CA106912, HRQOL in Colorectal Cancer Survivors with Stomas, and Arizona Cancer Center support grant P30 CA23074, from the National Cancer Institute, National Institutes of Health in collaboration with resources and the use of facilities provided at the Southern Arizona VA Health Care System, Tucson, Arizona and Kaiser Permanente.
Footnotes
This paper was presented as a poster without the rectal cancer subanalysis at the Society of Surgical Oncology 62nd Annual Meeting, Phoenix, AZ, March 5–8, 2009.
Contribution of each author
Liu: study design and execution, data analysis, manuscript preparation
Herrinton: study design and execution, data analysis, manuscript preparation
Hornbrook: study design and execution, data analysis, manuscript approval
Wendel: data analysis, manuscript approval
Grant: study design and execution, data analysis, manuscript approval
Krouse: study design and execution, data analysis, manuscript approval
References
- 1.American Cancer Society. [Accessed May 27, 2008.];Overview: Colon and Rectum Cancer. http://www.cancer.org/docroot/CRI/content/CRI_2_2_1X_How_Many_People_Get_Colorectal_Cancer.asp.
- 2.Mohler MJ, Coons SJ, Hornbrook MC, et al. The health-related quality of life in long-term colorectal cancer survivors study: objectives, methods and patient sample. Curr Med Res Opin. 2008;24:2059–70. doi: 10.1185/03007990802118360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grant M. Quality of life issues in colorectal cancer. Develop Support Care. 1999;3:4–9. [Google Scholar]
- 4.Grant M, Ferrell B, Dean G, et al. Revision and psychometric testing of the City of Hope Quality of Life-Ostomy questionnaire. Qual Life Res. 2004;13:1445–57. doi: 10.1023/B:QURE.0000040784.65830.9f. [DOI] [PubMed] [Google Scholar]
- 5.Krouse RS, Herrinton LJ, Grant M, et al. Health-related quality of life among long-term colorectal cancer survivors with an ostomy: Manifestations by gender. J Clin Oncol. doi: 10.1200/JCO.2008.20.9502. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baldwin CM, Grant M, Wendel C, et al. Gender differences in sleep disruption and fatigue among persons with ostomies. J Clin Sleep Med. (in press) [PMC free article] [PubMed] [Google Scholar]
- 7.Grant M, Krouse R, McMullen C, et al. Dietary adjustments reported by colorectal cancer (CRC) survivors with permanent ostomies. J Cancer Educ. 2007;22:33. [Google Scholar]
- 8.Altschuler A, Ramirez M, Grant M, et al. The influence of husbands’ or male partners’ support on women’s psychosocial adjustment to having an ostomy resulting from colorectal cancer. J Wound Ostomy Continence Nurs. 2009;36:299–305. doi: 10.1097/WON.0b013e3181a1a1dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lundy JJ, Coons SJ, Wendel C, et al. Exploring household income as a predictor of psychological well-being among long-term colorectal cancer. Qual Life Res. 2009;18:157–61. doi: 10.1007/s11136-008-9432-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McMullen CK, Hornbrook MC, Grant M, et al. The greatest challenges reported by long-term colorectal cancer survivors with ostomies. J Support Oncol. 2008;6:175–82. [PubMed] [Google Scholar]
- 11.Londono-Schimmer EE, Leong AP, Phillips RK. Life table analysis of stomal complications following colostomy. Dis Colon Rectum. 1994;37:916–20. doi: 10.1007/BF02052598. [DOI] [PubMed] [Google Scholar]
- 12.Birgisson H, Pahlman L, Gunnarsson U, Glimelius B. Late gastrointestinal disorders after rectal cancer surgery with and without postoperative radiation therapy. Br J Surg. 2008;95:206–13. doi: 10.1002/bjs.5918. [DOI] [PubMed] [Google Scholar]
- 13.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 14.Cutler SJ, Ederer EF. Maximum utilization of the life table method in analyzing survival. J Chronic Dis. 1958;8:699–712. doi: 10.1016/0021-9681(58)90126-7. [DOI] [PubMed] [Google Scholar]
- 15.Cox DR. Regression models and life-tables. J R Stat Soc. 1972;B34:187–220. [Google Scholar]
- 16.Arumugam PJ, Bevan L, Macdonald L, et al. A prospective audit of stomas-analysis of risk factors and complications and their management. Colorectal Dis. 2003;5:49–52. doi: 10.1046/j.1463-1318.2003.00403.x. [DOI] [PubMed] [Google Scholar]
- 17.Krouse R, Grant M, Ferrell B, Dean G, Nelson R, Chu D. Quality of life outcomes in 599 cancer and non-cancer patients with colostomies. J Surg Res. 2007;138:79–87. doi: 10.1016/j.jss.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 18.Bosshardt TL. Outcomes of ostomy procedures in patients aged 70 years and older. Arch Surg. 2003;138:1077–82. doi: 10.1001/archsurg.138.10.1077. [DOI] [PubMed] [Google Scholar]
- 19.Luna-Perez P, Rodriguez-Ramirez S, Vega J, Sandoval E, Labastida S. Morbidity and mortality following abdominaperineal resection for low rectal adenocarcinoma. Rev Invest Clin. 2001;53:388–95. [PubMed] [Google Scholar]
- 20.Caricato M, Ausania F, Ripetti V, Bartolozzi F, Campoli G, Coppola R. Restrospective analysis of long-term defunctioning stoma complications after colorectal surgery. Colorectal Dis. 2007;9:559–61. doi: 10.1111/j.1463-1318.2006.01187.x. [DOI] [PubMed] [Google Scholar]
- 21.Robertson I, Leung E, Hughes D, et al. Prospective analysis of stoma-related complications. Colorectal Dis. 2005;7:279–85. doi: 10.1111/j.1463-1318.2005.00785.x. [DOI] [PubMed] [Google Scholar]
- 22.Saghir JH, Mckenzie FD, Leckie DM, et al. Factors that predict complications after construction of a stoma: A retrospective study. Eur J Surg. 2001;167:531–34. doi: 10.1080/110241501316914911. [DOI] [PubMed] [Google Scholar]
- 23.Pittman J, Rawl SM, Schmidt CM, et al. Demographic and clinical factors related to ostomy complications and quality of life in veterans with stomas. J Wound Ostomy Continence Nurs. 2008;35:493–503. doi: 10.1097/01.WON.0000335961.68113.cb. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


