Abstract
This is a retrospective comparison of pregnant women with perinatally acquired HIV-infection (PAH) with a cohort of pregnant women with behaviorally acquired HIV-infection (BAH). PAH cases (11 women) included all pregnant adolescents followed at our HIV clinic from January 2000 to January 2009. BAH cases (27 women) were randomly selected from all deliveries within the study period at the same institution. Demographics, mode of delivery, CD4+ counts, and viral loads (VLs) before, during, and six months postpartum, as well as neonatal outcomes, were reviewed.
CD4 counts were significantly lower in the PAH group. VLs were statistically higher in the PAH group. VLs were undetectable at delivery in 60% of the PAH group compared with 88% of the BAH group. No cases of vertical transmission occurred.
PAH women may be at a higher risk for HIV-related disease progression. This may increase vertical transmission risks. Further studies and interventions with this growing population are warranted.
Keywords: vertical HIV-infection, behaviorally acquired HIV-infection, pregnancy, adolescent, young adult
Introduction
Advances in the management of HIV infection have enabled a growing group of young women with perinatally acquired HIV-infection (PAH) to enter their childbearing years. In the USA, approximately 10,000 perinatally infected children are now transitioning into adolescence and young adulthood; many of them may eventually become parents (Fowler et al., 2004; Rangel et al., 2006). In terms of sexual behaviors, PAH adolescents and young adults do not differ significantly from their uninfected peers (Zorrilla et al., 2003). In addition, overall reproductive health in HIV-infected individuals has mirrored the advances in antiretroviral (ARV) therapy and has led to an increase in the pregnancy rate (Blair et al., 2004; Brogly et al., 2007; Ventura et al., 2008). Similarly, the prevention of mother to child HIV transmission (MTCT) has improved dramatically over the last two decades (Fowler et al., 2004; Mofenson et al., 2006), and clinicians are more experienced and confident in the management of pregnant women with HIV infection. Guidelines on the management of such pregnancies are widely disseminated and are regularly updated. Currently, vertical HIV transmission rates in the USA are at 2% (Cooper et al., 2002). In 2006, strict adherence to New York State Department of Health guidelines, which mandate HIV counseling and testing of all pregnant women, coupled with a robust newborn screening protocol have further reduced transmission rates to 1.7% (New York State Department of Health, personal communication 2008).
Young women with PAH represent a growing population with unique issues related to pregnancy. The management of pregnancies in adolescents and young women with PAH is complicated by their extensive ARV treatment experience, the presence of multidrug-resistant (MDR) virus, histories of suboptimal adherence, limited therapeutic options, psychosocial issues, and others. Healthy teenagers struggle with a wide array of challenges relating to their physiological, emotional, and psychosocial development. For adolescents with PAH, this already difficult period is further exacerbated by the stigma of HIV infection, chronic illness, and inexorable psychosocial chaos. Pregnancy, often unplanned, adds another level of complexity and treatment challenges. There are limited published data specifically addressing pregnancies in women with PAH. This retrospective study reports systematically collected information on this unique population, and contrasted their outcomes with a historically well-described group of pregnant women with behaviorally acquired HIV-infection (BAH).
Methods
Our setting is an urban municipal medical center in a high-HIV seroprevalent area. PAH cases included all perinatally infected HIV adolescents and young adults followed at our institution for comprehensive HIV care who had given birth to live infants from January 2000 to January 2009. If a woman became pregnant more than once, each pregnancy was considered as a separate event. This approach was taken as multiple HIV-specific variables (clinical illness, viral load, and CD4 parameters), drug availability and resistance, adherence as well as psychosocial conditions may vary from pregnancy to pregnancy. An adequate age-matched control group of women with BAH was not available at our institution. To highlight the differences and unique qualities and challenges of the PAH group, we chose to use a comparison group randomly selected from a list of all pregnant women with BAH who delivered live infants during the same period and who had attended the adult outpatient HIV clinic at our institution. A ratio of approximately two BAH pregnancies for every PAH pregnancy was used to identify contrasting differences between these two groups.
Data abstracted from the medical records included demographics, mode of delivery, CD4 counts, viral load (VL), and ARV regimens before, during, and after pregnancy. CD4 counts and VLs were calculated as an average of all available values one year before, during the three trimesters of pregnancy, and six months postpartum. Newborn data included perinatal history, gestational age, weight at birth, and HIV-infection status.
In our institution we follow a Family Center Care Model, whereby weekly multidisciplinary perinatal meetings (attended by pediatric and adult clinicians, mental health providers, and case managers) are held to discuss comprehensive management plans for the pregnant women. Both PAH and BAH women received their obstetric care from the same personnel in the HIV Family Center Care. Supportive services not provided on site are referred to community-based organizations with which we have developed close working relationships.
Statistical analyses
Data distribution was tested by the Anderson-Darling normality test. Bivariate comparisons of CD4+ T lymphocyte counts and plasma VLs at different time points were assessed using the Mann-Whitney U test. Fisher exact test (two tailed) was used to compare the mode of delivery (cesarean section vs. vaginal) and VLs (undetectable at 400 copies/mL) at the time of birth (Minitab; Statistical Software, State College, PA).
Results
PAH cases included 11 women with 15 live births (four women had two pregnancies each). BAH cases were represented by 27 women, with 33 live births (six women had two pregnancies each). All of the women in this study were of Hispanic or African-American descent. As expected from the cohorts’ definition, the mean ages in the PAH and BAH groups were 20.8 (17.5–24.8) and 30.3 (19.3–36.8) years, respectively. The women in the PAH group were highly treatment experienced; during their pregnancies, 10 of 11 (14/15 pregnancies) were prescribed protease inhibitor (PI)-based combination antiretroviral therapy (cART) containing three to four drugs. In the BAH group, the women received cART that was either PI (23) or non-nucleoside reverse transcriptase inhibitor (NNRTI) (8) based (Table 1).
Table 1.
Demographic, clinical, and laboratory characteristics of women with behaviorally (BAH) and perinatally acquired HIV (PAH).
| Behaviorally acquired HIV (BAH) N = 27 subjects (n = 33 pregnancies) | Perinatally acquired HIV (PAH) N = 11 subjects (n = 15 pregnancies) | |
|---|---|---|
| Race | ||
| Hispanics | 6 | 7 (0.47%) |
| African Americans | 21 (78%) | 4 |
| Highly active antiretroviral therapy (HAART) | n = 33 | n = 15 |
| Nucleoside/nucleotide reverse transcriptase inhibitor (NRTI) | 31 | 15 |
| NNRTI | 8 | 1 |
| PI | 23 | 13 |
| HIV RNA levels (copies/ml at delivery) | n = 33 | n = 15 |
| <400 | 29 (88%) | 9 (60%) |
| 400–1000 | 1 | 1 |
| 1000–< 10,000 | 1 | 2 |
| > 10,000 | 2 | 3 (20%) |
| Birth weight (g) | n = 31 | n = 15 |
| 1500–2000 | 2 | 1 |
| 2000–2500 | 2 | 1 |
| 2500–3000 | 4 | 12 (80%) |
| > 3000 | 23 (66%) | 2 |
| Gestational age (weeks) | n = 31 | n = 15 |
| 39–40 | 20 (65%) | 1 |
| 37–38 | 9 (29%) | 12 (80%) |
| < 37 | 2 | 2 |
| Mode of delivery | n = 33 | n = 15 |
| Cesarean section | 13 (39%) | 12 (80%) |
| Normal spontaneous vaginal delivery (NSVD) | 20 | 3 |
All women received MTCT prophylaxis during labor and delivery, and all newborns were treated postnatally in accordance with established guidelines (US Department of Health and Human Services, 2009). Three of the newborns in the PAH group received a three-drug prophylaxis regimen because of heightened concerns about vertical transmission of resistant virus in the setting of persistent maternal drug-resistant viremia during the peripartum period. Three women in the PAH group also had chronic viral hepatitis, one with hepatitis B virus (HBV) and two with hepatitis C virus (HCV).
Immunologic and virologic parameters were, in general, worse in the PAH group than in the BAH group. At all stages of pregnancy, median CD4 counts were consistently and significantly lower in the PAH group (Table 2 and Figure 1). The median VL before pregnancy, during the second and third trimesters of pregnancy, and in the postpartum period was statistically higher in the PAH group (Table 2 and Figure 2). Undetectable VLs (< 400 copies /mL) at delivery were achieved in 29 of 33 (88%) pregnancies in the BAH group as compared with 9 of 15 (60%) pregnancies in the PAH group (p = 0.03, Fisher exact test). During the post-partum period, persistent viral replication occurred more commonly in the PAH group (Table 2 and Figure 2). Cesarean section at term was the mode of delivery in 12 of 15 (80%) of the PAH women and 13 of 33 (39%) women of the BAH group, a statistically significant difference (p = 0.009; Fisher exact test). In terms of neonatal outcomes, there were four preterm deliveries, two in the BAH group (34 and 35 weeks of gestation) and two in the PAH group (32 and 34 weeks of gestation). The mean gestational ages for the PAH and BAH newborns were 37 and 40 weeks, respectively. The mean birth weight in the PAH group was 2688 g (1525–3440 g), significantly lower than 3117 g (1265–3920g) in the BAH group (p = 0.0027, Mann–Whitney U test) and not attributable to differences in gestational ages. Importantly, no maternal to child HIV transmission occurred in either group. While no cases of vertical HCV transmission were observed, perinatal HBV infection occurred in one child despite the administration of passive and active immunoprophylaxis.
Table 2.
Immunologie (CD4 cells/uL) and virologie (log10 copies/mL) parameters (median values) before, during, and after pregnancy. The groups were compared using Mann-Whitney U test; BAH (n = 33 pregnancies) and PAH (n = 15 pregnancies).
| CD4 absolute |
VL log10 |
|||||
|---|---|---|---|---|---|---|
| BAH | PAH | p-Value | BAH | PAH | p-Value | |
| Before pregnancy | 377 | 144 | 0.0001 | 2.6 | 3.62 | 0.017 |
| During pregnancy | ||||||
| First trimester | 367 | 128 | 0.0012 | 3.44 | 2.97 | 0.55 |
| Second trimester | 382 | 123 | 0.0003 | 2.35 | 3.24 | 0.003 |
| Third trimester | 424 | 158 | 0.0003 | 1.7 | 2.7 | 0.02 |
| Post partum period | ||||||
| At delivery | 422 | 169 | 0.0003 | 1.7 | 2.39 | 0.22 |
| Three months | 478 | 197 | 0.0001 | 2.39 | 3.89 | 0.0006 |
| Six months | 510 | 126 | 0.0006 | 2.29 | 3.93 | 0.001 |
Note: Values shown in bold are statistically significant.
Figure 1.
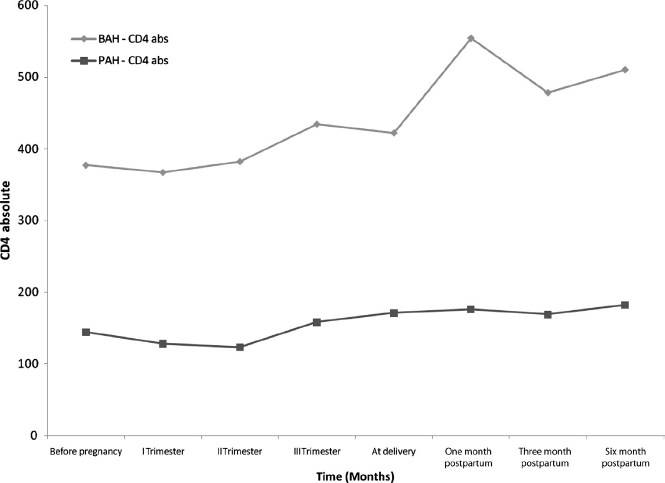
Longitudinal immunologic assessment of PAH and BAH groups before, during, and after pregnancy. Median values of CD4+ T lymphocyte counts (cells/uL) are depicted on the F axis and time in months on the Xaxis. Diamonds and squares represent the BAH and PAH groups, respectively.
Figure 2.
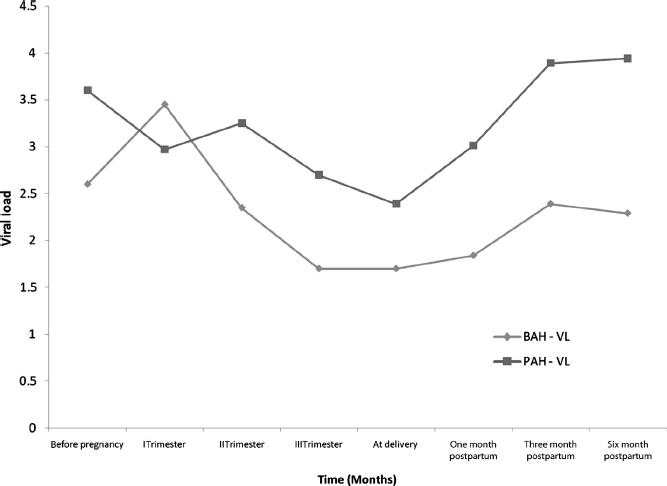
Longitudinal virologie assessment of PAH and BA groups before, during, and after pregnancy. Median values of log10 HIV RNA copies/mL are depicted on the Y axis and time in months on the X axis. Diamonds and squares represent the BAH and PAH groups, respectively.
Discussion
Epidemiologic trends predict that the incidence of pregnancy in women with PAH will rapidly increase over the next decade (Rangel et al., 2006). Study is among the initial comprehensive descriptions of neonatal outcomes and changes in HIV disease markers in a group of pregnant perinatally infected women.
As the HIV epidemic enters its third decade, clinicians continue to gain more experience in the management of pregnancy and prevention of MTCT in women with BAH who are naive or minimally exposed to ARVs (US Department of Health and Human Services, 2009). However, information on the management of pregnant women with extensive treatment experience and drug resistance is limited (Arrivé et al., 2007). The PAH cohort presents management challenges resulting from previous exposure to serial nonsuppressive regimens and the emergence of MDR-HIV. To overcome existent resistance, increasingly complex regimens with potential greater toxicities are prescribed, which may perpetuate the cycle of non-adherence and additional viral drug resistance.
Although no MTCT occurred in our cohort, the implications of poor immunologic and virologic markers in the PAH pregnant group require thoughtful prevention strategies to address this expanding population of pregnant adolescents and young adults. It is concerning that although the MTCT rates have fallen dramatically, the prevalence of drug resistance in the group of vertically infected infants in New York state increased 58% between 1998 and 2002 (Foster et al., 2006; Karchava et al., 2006). High rates of suboptimal virologic control close to the time of delivery led to more planned cesarean sections being done in the PAH group, and concerns regarding MDR HIV transmission prompted us to prescribe aggressive postnatal prophylaxis consisting of PI- or NNRTI-based cART to three infants born to mothers in the PAH cohort.
In addition to presenting with more complex treatment histories and drug resistance patterns, the women in the PAH cohort have distinct psychosocial and behavioral risks that set them apart from their BAH counterparts. These risks can in part be attributed to the normal developmental stages of adolescence but are compounded by the high prevalence of medical and psychiatric problems in this group (DeMatteo, Wells, Salter Goldie, & King, 2002; Karatzios et al., 2007). Three key issues affecting adolescents with PAH today are social stigma, sexuality, and mental health disorders (Ezeanolue et al., 2006; Mellins et al., 2006). Stigma is closely associated with disclosure of their HIV status to others and concerns about negative consequences in terms of relationships and choices related to sex and sexual orientation (Mellins et al., 2002). Studies have shown that approximately 60% of adolescents with PAH manifest evidence of a DSM IV diagnosis including depression, anxiety, impulsivity, and posttraumatic stress disorder (Mellins et al., 2009). These and other factors are associated with poor adherence and require special consideration when the teenager becomes pregnant (Bardeguez et al., 2008).
Adolescent pregnancy in the absence of HIV infection is recognized as a significant social issue and is frequently complicated by chronic poverty, unstable home environments, and other adverse academic and psychosocial circumstances (Grady & Bloom, 2004; Miller, 2000). Many pregnant adolescents have inconsistent prenatal care and are at a higher risk for pregnancy-related complications and infant morbidities such as premature birth and low birth weight (Klein & the Committee on Adolescence, 2005; Rees, Lederman, & Kiely, 1996). While much has been written on HIV, pregnancy, and the prevention of vertical infection, there is a paucity of literature addressing HIV infection in pregnant, vertically infected adolescents and young adults. While the mean age of our PAH group was 20.8 years (range 17–22 years), we did not observe higher birth weights, which may be attributed, in part, to elective cesarean section at no later than 37–38 weeks of gestation and to the overall impact of chronic illness.
Our observation on the impact of pregnancy on the immunologic status of young women with PAH merits comment. The PAH cohort exhibited median CD4 cell counts consistently below 200 cells/uL throughout pregnancy, which requires prescribing Pneumocystis jirovecii prophylaxis, further complicating the cART regimen. A subgroup of these women experienced robust CD4 count increases in response to their cART, suggesting that this population still retains the capacity for at least partial immune reconstitution. In contrast, the women in the BAH group had higher median CD4 counts at baseline, less advanced disease, and more robust increases in CD4 parameters during pregnancy, which persisted into the postpartum period. Importantly, all the women in the PAH group experienced sustained loss of virologic control during the postpartum period, suggesting that this population returns to its prepregnancy behavioral patterns, that is, nonstructured lifestyles and chronic inconsistent medication adherence. In addition, stressors associated with having a new infant and the abrupt change from their role from care-receiver to caregiver strains their already compromised coping skills. These new dynamics raise even higher psychological barriers that these young women must surmount in order to access and optimize their own healthcare. Poor postpartum adherence to ART regimens in the PAH group has significant adverse health consequences resulting in virologic and immunologic deterioration and more rapid disease progression or death after the birth of their child. Of great concern, within the PAH cohort, 3 of the 11 (27%) women died: one each at two, three, and four years post-partum. The underlying reasons for these deaths are undoubtedly multifactorial but may be related to stressors associated with first-time motherhood. Post-partum depression may have accentuated or triggered dormant or undiagnosed psychiatric disorders (Blaney et al., 2004; Mellins et al., 2009). The complex interplay between nonadherence, postpartum depression, lack of social support, and treatment fatigue related to the chronic nature of HIV infection contribute to bad virological and clinical outcomes in our population.
A limitation of this study is that we were unable to address the question whether the pregnancies were planned or not, an area that merits further consideration. Although some investigators have reported that 70% of female adolescents and young adults with PAH expressed a desire to have children, details of their motivations and reproductive decision making are not well understood (Ezeanolue et al., 2006). Attitudes toward parenting and future hopes, aspirations, and plans in this cohort also deserve additional exploration, especially given the fact that many of these young women grew up in broken households and were not exposed to appropriate parental role modeling.
This study raises important questions and emphasizes the urgent need for data collection and better understanding of the psychobiologic dynamics in the PAH population, so that creative approaches and evidence-based guidelines for the management of pregnancies and postpartum care can be drafted. The adverse health outcomes observed in the PAH group occurred despite the presence of a healthcare system providing intensive and integrated multidisciplinary medical and psychosocial care that is uniquely sensitive to their special needs during pregnancy and the postnatal periods. Multicenter studies addressing pregnancy management, monitoring, and long-term pediatric follow-up will help to identify intervention strategies targeted at PAH mothers and their children. In addition to guiding management in developed nations, these interventions may be adapted and implemented in resource-poor settings where a burgeoning cohort of young women with PAH with access to cART will be entering their childbearing years over the next few decades.
Acknowledgements
These data were presented in part at the 15th Conference on Retroviruses and Opportunistic Infections (CROI 2008), 2–6 February 2008, Boston, MA; and published in the Program and Abstracts page 280, number 605.
References
- Arrivé E., Newell M.L., Ekouevi D.K., Chaix M.L., Thiebaut R., Masquelier B. Ghent Group on HIV in Women and Children. Prevalence of resistance to nevirapine in mothers and children after single-dose exposure to prevent vertical transmission of HIV-1: A meta-analysis. International Journal of Epidemiology. 2007;36:1009–1021. doi: 10.1093/ije/dym104. [DOI] [PubMed] [Google Scholar]
- Bardeguez A.D., Lindsey J.C., Shannon M., Tuomala R.E., Cohn S.E., Smith E. PACTG 1025 Protocol Team. Adherence to antiretrovirals among US women during and after pregnancy. Journal of Acquired Immune Deficiency Syndrome. 2008;48:408–417. doi: 10.1097/QAI.0b013e31817bbe80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair J.M., Hanson D.L., Jones J.L., Dworkin M.S. Trends in pregnancy rates among women with human immunodeficiency virus. Obstetrics and Gynecology. 2004;103:663–668. doi: 10.1097/01.AOG.0000117083.33239.b5. [DOI] [PubMed] [Google Scholar]
- Blaney N.T., Fernandez M. I., Ethier K.A., Wilson T.E., Walter E., Koenig L.J. Perinatal Guidelines Evaluation Project Group. Psychosocial and behavioral correlates of depression among HIV-in-fected pregnant women. AIDS Patient Care STDs. 2004;18:405–415. doi: 10.1089/1087291041518201. [DOI] [PubMed] [Google Scholar]
- Brogly S.B., Watts D.H., Ylitalo N., Franco E.L., Seage G.R., III, Oleske J., Van Dyke R. Reproductive health of adolescent girls perinatally infected with HIV. American Journal of Public Health. 2007;97:1047–1052. doi: 10.2105/AJPH.2005.071910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper E.R., Charurat M., Mofenson L., Hanson I.C., Pitt J., Diaz C. Women and Infants’ Transmission Study Group. Combination antiretroviral strategies for the treatment of pregnant HIV-1 infected women and prevention of perinatal vertical transmission. Journal of Acquired Immune Deficiency Syndrome. 2002;29:484–494. doi: 10.1097/00126334-200204150-00009. [DOI] [PubMed] [Google Scholar]
- DeMatteo D., Wells L.M., Salter Goldie R., King S.M. The ‘family’ context of HIV: A need for comprehensive health and social policies. AIDS Care. 2002;14:261–278. doi: 10.1080/09540120120076940. [DOI] [PubMed] [Google Scholar]
- Ezeanolue E.E., Wodi A.P., Patel R., Dieudonne A., Oleske J.M. Sexual behaviors and procreational intentions of adolescents and young adults with perinatally acquired human immunodeficiency virus infection: Experience of an urban tertiary center. Journal of Adolescent Health. 2006;38:719–725. doi: 10.1016/j.jadohealth.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Foster C., Mackie N., Seery P., Walters S., Tudor-Williams G., Lyall H. Emerging multi-drug resistance in children with perinatally acquired HIV-1. 2006. International Congress Drug Therapy HIV, [Abstract Number P360]. Glasgow, UK. Nov 12–16.
- Fowler M.G., Garcia P., Hanson C., Sansom S. Progress in preventing perinatal HIV transmission in the United States [conference summary] Emerging Infectious Diseases; 2004. Retrieved from http://www.cdc.gov/ncidod/EID/vol10no11/04-0622_02.htm. [Google Scholar]
- Grady M.A., Bloom K.C. Pregnancy outcomes of adolescents enrolled in a centering pregnancy program. Journal of Midwifery & Women's Health. 2004;49:412–420. doi: 10.1016/j.jmwh.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Karatzios C., Marin M.Y., Wilkinson J.D., Garcia A., Willen E., Illa L., Scott G.B. The clinical and psychosocial characteristics of adolescent behavior with HIV infection acquired early in life. 2007. 45th annual meeting of the Infectious Diseases Society of America [Abstract Number 943]. San Diego, CA.
- Karchava M., Pulver W., Smith L., Philpott S., Sullivan T.J., Wethers J., Parker M.M. Prevalence of drug-resistance mutations and non-subtype B strains among HIV-infected infants from New York State. Journal of Acquired Immune Deficiency Syndrome. 2006;42:614–619. doi: 10.1097/01.qai.0000225871.87456.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein D.J. the Committee on Adolescence. Adolescent pregnancy: Current trends and issues. Pediatrics. 2005;116:281–286. doi: 10.1542/peds.2005-0999. [DOI] [PubMed] [Google Scholar]
- Mellins C.A., Brackis-Cott E., Dolezal C., Richards A., Nicholas S.W., Abrams E.J. Patterns of HIV status disclosure to perinatally HIV-infected children and subsequent mental health outcomes. Clinical Child Psychology and Psychiatry. 2002;7:101–114. [Google Scholar]
- Mellins C.A., Brackis-Cott E., Dolezal C., Abrams E.J. Psychiatric disorders in youth with perinatally acquired human immunodeficiency virus infection. Pediatric Infectious Disease Journal. 2006;25:432–437. doi: 10.1097/01.inf.0000217372.10385.2a. [DOI] [PubMed] [Google Scholar]
- Mellins C.A., Brackis-Cott E., Leu C.S., Elkington K.S., Dolezal C., Wiznia A., Abrams E.J. Rates and types of psychiatric disorders in perinatally human immunodeficiency virus-infected youth and seroreverters. Journal of Child Psychology and Psychiatry. 2009;50:1131–1138. doi: 10.1111/j.1469-7610.2009.02069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller F.C. Impact of adolescent pregnancy as we approach the new millennium. Journal of Pediatric Adolescent Gynecology. 2000;13:5–8. doi: 10.1016/s1083-3188(99)00035-2. [DOI] [PubMed] [Google Scholar]
- Mofenson L., Taylor A.W., Rogers M., Campsmith M., Ruffo N.M., Clark J., Sansom S. Achievements in public health: Reduction in perinatal transmission of HIV infection — United States, 1985–2005. MMWR. 2006;55:592–597. [PubMed] [Google Scholar]
- Rangel M.C., Gavin L., Reed C., Fowler M.G., Lee L.M. Epidemiology of HIV and AIDS among adolescents and young adults in the United States. Journal of Adolescent Health. 2006;39:156–163. doi: 10.1016/j.jadohealth.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Rees J.M., Lederman S.A., Kiely J.L. Birth weight related to lowest neonatal mortality in infants born to adolescent and adult mothers. Pediatrics. 1996;98:1161–1166. [PubMed] [Google Scholar]
- US Department of Health and Human Services. Recommendations for the use of antiretroviral drugs for pregnant HIV-1 infected women for maternal health and interventions to reduce perinatal HIV-1 transmission in the United States. 2009. Retrieved from http://www.aidsinfo.nih.gov.
- Ventura S.J., Abma J.C., Mosher W.D., Henshaw S.K. Estimated pregnancy rates by outcome for the United States, 1990–2004. National Vital Statistics Report. 2008;56:1–26. [PubMed] [Google Scholar]
- Zorrilla C., Febo I., Ortiz I., Orengo J.C., Miranda S., Rodriguez A., McConnell M. Pregnancy in the perinatally HIV infected adolescents and young adults in Puerto Rico 2002. MMWR. 2003;52:149–151. [PubMed] [Google Scholar]


