Abstract
Increasing evidence suggests that breast cancer is caused by cancer stem cells and the cure of breast cancer requires eradication of breast cancer stem cells. In this study, we established and characterized a sphere culture model derived from side population cells from the human breast cancer cell line MCF7. The sphere culture could be maintained long term and was enriched in cells expressing known breast cancer stem cell marker CD44+CD24−. These sphere cells showed higher colony formation ability in vitro and higher tumorigenicity in vivo than MCF7 cells, suggesting the enrichment of breast cancer stem/progenitor cells. To identify compounds that preferentially inhibit the sphere cells, we performed a compound library screening. Two lead compounds, NSC24076 and NSC125034 and an analog of NSC125034, 8-quinolinol (8Q), were identified as having preferential activity against the sphere cells. 8Q showed some antitumor activity alone but had much better therapeutic effect and relapse prevention when combined with paclitaxel than either 8Q or paclitaxel alone in both MCF7 and MDA-MB-435 xenograft models. We propose that compounds selectively targeting cancer stem/progenitor cells when combined with standard chemotherapy drugs may produce an improved treatment of cancer without significant relapse.
Keywords: Cancer stem cells, Sphere culture, 8-Quinolinol, Cancer therapy
Introduction
Cancer stem cells, defined as a small population of cancer cells that are capable of giving rise to a new tumor, were first demonstrated in acute myelogenous leukemia in 1994 by John Dick and colleagues [1-3]. However, cancer stem cell research did not draw much attention until the first solid tumor stem cells, breast cancer stem cells, were reported in 2003 by Clarke and colleagues [4]. These authors isolated a population of highly tumorigenic cells from human breast cancer clinical specimens [4]. This highly tumorigenic subpopulation expressed the CD44+CD24− surface markers and had a high capacity to form tumors in NOD/SCID mice. As few as one hundred CD44+CD24− cells were able to generate tumors, whereas tens of thousands of the CD44−/CD24+ cells failed to do so [4]. Cancer stem cells have since been identified in different tumors, including human brain tumor [5-8], multiple myeloma [9], colon cancer [10, 11], head and neck cancer [12] and pancreatic cancer [13].
The cancer stem cell research is important as it may provide new approaches for cancer treatment. The existing cancer chemotherapy drugs mainly inhibit the bulk growing tumor cells [2, 14-17] but have no or little effect on cancer stem cells due to quiescence of cancer stem cells and their high expression of ABC transporters, which are capable of extruding commonly used drugs [16, 18, 19]. It has been reported that both leukemia stem cells and glioblastoma stem cells have decreased sensitivity to commonly used clinical drugs [16, 19, 20]. Even imatinib (Gleevec), an effective drug for chronic myeloid leukemia (CML), was unable to eradiate CML stem cells [21, 22].
One approach to targeting cancer stem cells is to inhibit signaling pathways preferentially important for cancer stem cells. For instance, Jordan and colleague demonstrated a constitutive activation of nuclear factor-κB (NF-κB) pathway in primitive AML cells [23]. NF-κB pathway inhibitors, including parthenolide and its analog dimethylamino-parthenolide, MG-132, 4-benzyl, 2-methyl, 1,2,4-thiadiazolidine, 3,5 dione (TDZD-8), can selectively induce apoptosis in leukemia stem cells but not normal hematopoietic stem cells [23-27]. The importance of the NF-κB pathway has also been shown for breast cancer stem-like cells in our recent work [28]. In addition, our previous work also identified PTEN/mTOR/STAT3 pathway being important from breast cancer stem-like cells, which could be inhibited to preferentially target breast cancer stem-like cells [29]. Another promising approach is to screen small molecule drug library on cancer stem cells, but no such study has been reported so far.
Human breast cancer is the most common malignancy among women in Western countries [30, 31]. Sphere culture method was widely used in neural stem cell research and proved to enrich stem cells [32-34]. Sphere culture method has been successfully used to isolate and culture brain and colon cancer stem cells [5, 7, 11, 35]. Very recently, sphere culture method was also used to culture and enrich human normal breast stem cells [36-38] and breast cancer stem cells [39, 40]. In this study, we established a long-term sphere culture from side population (SP) cells, which are a rare population of cells with high efflux activity for Hoechst dye 33324 first defined by Goodell and colleagues in hematopoietic system [41] and are known to enrich stem cells in normal and cancer tissues or cancer cell lines [29, 41-47]. The sphere cells derived from the SP cells share characteristics of cancer stem cells, including higher expression of breast cancer stem cell surface markers CD44+CD24− and increased colony formation and tumorigenicity. Two lead compounds, NSC24076 and NSC125034, were identified that preferentially inhibit sphere cell proliferation. An analog of NSC125034, 8-quinolinol (8Q) was further evaluated and showed significant tumor growth inhibition and marked synergistic effects in combination with paclitaxel in tumor xenograft mouse models. Our study suggests that the sphere cells could be used as a model for identifying compounds that preferentially inhibit breast cancer stem/progenitor cells.
Materials and methods
Cell culture
Human breast cancer cell line MCF7 cells, MDA-MB-435 cells were obtained from ATCC (American Type Culture Collection). Cells were grown in DMEM medium (Invitrogen) supplemented with 10% fetal bovine serum (Invitrogen), 100 units/ml penicillin and 100 μg/streptomycin (Invitrogen), in a 37°C incubator containing 5% CO2. To culture sphere cells, SP cells were sorted first from MCF7 cells as described below. Sphere cell culture was performed according to published protocols with modifications [36, 40]. Briefly, single cells were plated in ultra-low attachment plates (Corning, NY) at a density of 20,000 viable cells/ml in primary culture and 1,000 cells/ml in subsequent passages. Cells were grown in a serum-free mammary epithelial growth medium without bovine pituitary extract (MEGM, BioWhittaker), supplemented with B27 (Invitrogen), 20 ng/ml and EGF and 20 ng/ml bFGF (BD Biosciences). In order to passage sphere cells, spheres were collected into 15 ml conic tube and allowed to settle for 15 min. Supernatant was removed. Sphere cells were dissociated with 0.05% trypsin, 0.5 mM EDTA (Invitrogen) and by a glass Pasteur pipette. The cells were passed through a 40-μm sieve and analyzed microscopically for single cells and subjected to experiments. All the sphere cells used in this study were within 20 generations.
Hoechst 33342 staining and flow cytometry analysis/sorting, colony formation assay, tumorigenicity, cell proliferation assay, cell cycle analysis
The detailed procedures have been described in our recent paper [29].
Antibody staining
The antibody staining was performed after Hoechst dye staining. For sphere cell surface marker analysis, sphere cells were dissociated into single cells as above and cells were aliquoted, washed and resuspended with washing/staining buffer (PBS supplemented with 1% bovine serum albumin and 0.1% azide). Antibodies used in this study included CD44-PE-Cy5 (eBioscience, San Diego) and CD24-PE (eBioscience, San Diego). The antibody staining was performed on ice for 30 min, after which cells were washed with washing/staining buffer for 3 times. Cells stained with antibodies were kept on ice until flow cytometry analysis (Dako Cytomation, Fort Collins, CO. USA).
Compound library screen
Small-molecule library used in this study was from the NCI/NIH. The NCI Structural Diversity Set is a library consisting of 1,992 compounds selected from approximately 140,000-compound NCI drug depository. These compounds were selected based on various criteria including drug-like structure, uniqueness of pharmacophore, and anticancer activity as determined by cell growth inhibition assays against a panel of human tumor cell lines. Detailed data on the selection, structures, and activities of these diversity set compounds can be found on the NCI Developmental Therapeutics Program web site (http://dtp.nci.nih.gov/index.html). A fluorescence-based cell proliferation assay (CyQUANT Cell Proliferation Assay Kit, Molecular Probes) was used for cell proliferation determination in the screening experiments. Briefly, both MCF7 and sphere cells were seeded into 96-well culture plates at 500 cells per well in DMEM medium supplemented with 10% FBS. Cells were treated with compounds from the compound library overnight after seeding and further incubated for 72 h. Then, the cell proliferation was determined. Fluorescence signal was detected by a Bio-assay reader (Bio-Rad, USA) according to the manufacturer’s instructions.
Nuclear extract preparation and quantification of NF-κB activation
The detailed procedures were performed as described previously [28].
Antitumor assay in xenograft models
Female athymic nude mice (NCR-nu/nu, NCI) were housed under specific pathogen-free conditions. The in vivo experiments were performed in accordance with the guidelines of our institute. For the MCF7 xenograft study, mice were given injections of β-estradiol (Sigma) dissolved in pure sesame oil (0.1 mg per 0.05 ml sesame oil per mouse, subcutaneously over rump) 1 day before injection of MCF7 cells and then at weekly intervals [48, 49]. Mice were inoculated in fatpad with 2 × 107 MCF7 cells. For MDA-MB-435 xenograft study, mice were injected with 2 × 106 MDA-MB-435 tumor cells subcutaneously. When the tumor volumes reached 100–200 mm3, mice were randomly divided into groups such that each group harbored tumors of a similar size. Stock solutions of paclitaxel and 8-quinolinol (8Q) were prepared by dissolving the drugs in a vehicle solution (EtOH:cremophor, 50:50 v/v). Vehicle or paclitaxel alone, 8Q alone and 8Q in combination with paclitaxel stock solution were mixed with saline (10:90 v/v). 8Q at 20 mg/kg or paclitaxel at 15 mg/kg or the two in combination was given by tail vein injection twice weekly for 4 weeks. Tumor size measurements were done twice a week using traceable digital vernier calipers (Fisher). The tumor volumes were determined by measuring the length (l) and the width (w) and calculating the volume (V = lw2/2).
Statistical analysis
The growth inhibition effects were compared by Student’s t test (* P < 0.01; ** P < 0.001; *** P < 0.0001). One-way ANOVA analysis was performed to determine the statistical significance of treatment related changes in tumor volume in mice. Software R package was used for statistical calculation.
Results
Characterization of SP derived sphere cells
Sphere cells cultured from MCF7 cells were reported to enrich breast cancer stem/progenitor cells [39, 40]. We noted that sphere cells cultured directly from MCF7 cells often could not be passaged for more than 5 to 10 generations (data not shown). Since SP cells were more capable of forming sphere cells than bulk cells in normal breast stem cell study [36] and neurosphere cells cultured from SP cells were found to be enriched in more stem cells than neurosphere cells directly derived from bulk patient samples [33], we evaluated the possibility of obtaining long-term culturable sphere cells from SP cells. Thus, we isolated the MCF7 SP cells by flow cytometry and cultured sphere cells according to the reported protocols [36, 40] with some modifications as described in the Methods. Indeed, we successfully obtained long-term culturable sphere cells (Fig. 1a), which could be maintained in culture for more than 3 years. In contrast, sphere cells cultured from non-SP cells failed to passage for more than 5 generations. The sphere cells cultured using this method contained higher percentage of cells expressing the breast cancer stem cell surface marker CD44+CD24−(78.5% for sphere cells compared with 1.8% for MCF7 parental cells) (Fig. 1b). The sphere cells showed much higher colony formation ability by 10.9 (352.0 vs. 32.3) fold increase than MCF7 cells (Fig. 1c). The sphere cells also had higher tumorigenicity than MCF7 cells, indicating the sphere cells enrich cancer stem/progenitor cells. Four of five and three of five NOD/SCID mice had tumors 8 weeks after inoculation of the sphere cells (2nd generation) at 150,000 and 15,000, respectively, while only two of five and none of five mice that received the MCF7 parental cells at the same numbers gave rise to new tumors.
Fig. 1.
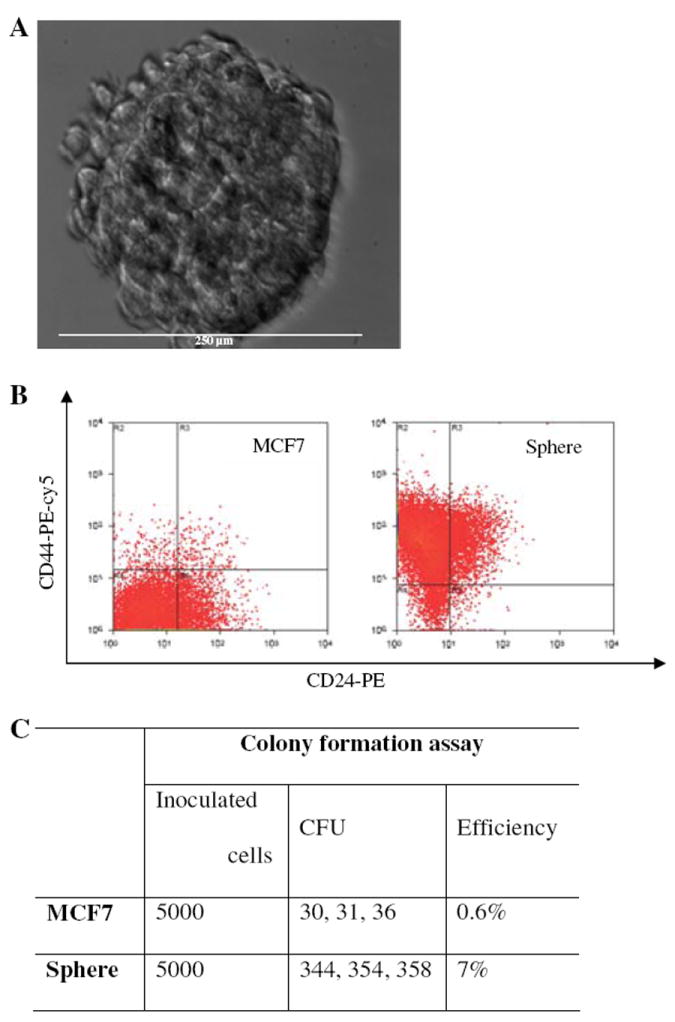
Morphology and characteristics of sphere cells. (a) Morphology of sphere cells. Single sphere of 14 day culture was photographed under bright field inverted microscope. The diameter of the sphere shown was about 250 micro meters. (b) Surface marker analysis of sphere cells. (c) Colony formation ability of sphere cells compared to MCF7 cells. Experiments were performed in triplicate and data was expressed as the original counts
Sphere cells are resistant to cancer drugs
Cancer stem cells are resistant to current cancer drugs, which are traditionally developed based on activity against bulk actively growing cancer cells [14-16, 19-22, 44, 47, 50]. To test the drug sensitivity of the sphere cells to cancer drugs, both MCF7 parental cells and sphere cells were seeded into 96 well plates and treated with adriamycin, mitomycin C, and paclitaxel, at indicated concentrations for 3 days. The growth inhibition effects were determined using MTT assay. As shown in Fig. 2, compared to MCF7 cells, the sphere cells were resistant to all the drugs tested. For instance, paclitaxel at a concentration of 2.5 nM inhibited MCF7 cells by 44.5% but by 6.1% for the sphere cells. Similar resistance in sphere cells was shown for adriamycin and mitomycin C (Fig. 2).
Fig. 2.
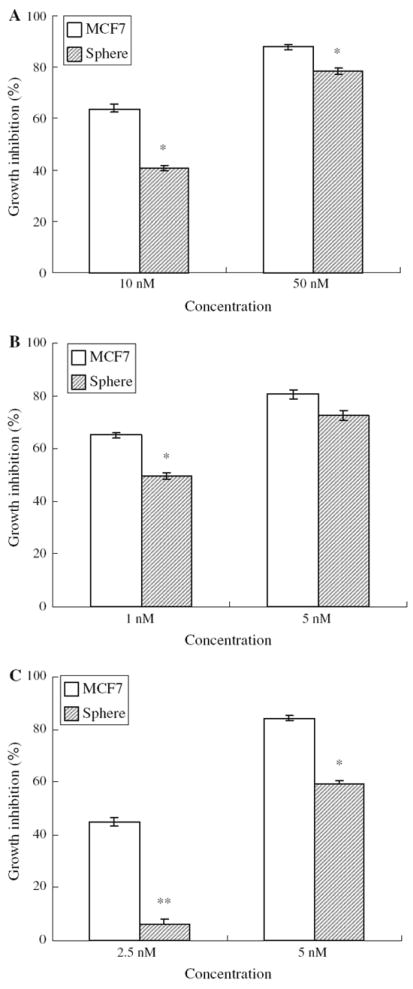
Susceptibility of sphere cells and MCF7 cells to cancer drugs, including adriamycin (a), mitomycin C (b) and paclitaxel (c). Statistics was calculated by Student t test. * P < 0.05; ** P < 0.005
Identification of compounds that preferentially inhibit sphere cells by compound library screening
To identify compounds that preferentially inhibit sphere cells over MCF7 cells, we screened the NCI diversity set compound library, which consists of about 2000 compounds. Interestingly, two lead compounds, NSC125034 and NSC24076 (Fig. 3a), were found to have better growth inhibition effect on the sphere cells than on MCF7 cells in a dose dependent manner. As shown in Fig. 3b, NSC125034 at 25 and 50 μM could inhibit sphere cell proliferation by 33.5 and 75.5%, respectively, compared with 0.1 and 26.6% for MCF7 cells. Another compound, NSC24076, showed even better effects, which could inhibit sphere cell proliferation by 13.0, 60.3 and 85.9% at concentrations of 0.5, 1 and 5 μM, respectively (Fig. 3c). In contrast, NSC24076 did not show inhibitory effects on MCF7 cells at 0.5 and 1 μM and only inhibited MCF7 cell proliferation by 22.7% at 5 μM (Fig. 3c). We further tested some analogs of NSC125034 and found 8-Quinolinol (8Q) had similar activity on the sphere cells as NSC125034 (Fig. 3a and d). We found 8Q at 5 μM inhibited the sphere cell proliferation by 86% but inhibited MCF7 cells by 30% (Fig. 3d). 8Q was chosen for further study below.
Fig. 3.
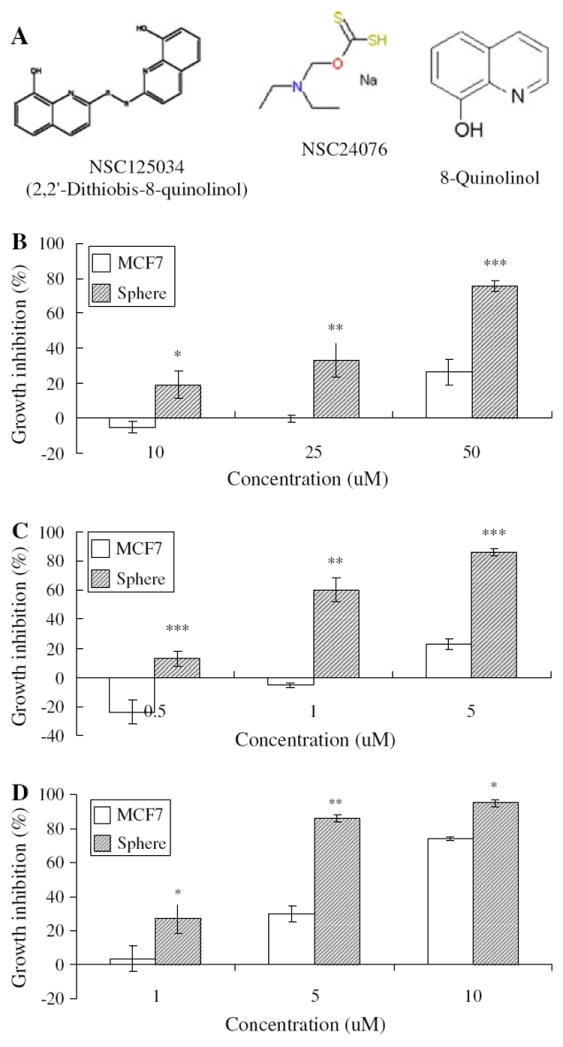
Preferential inhibition effects of compounds identified on sphere cells over MCF7 cells. (a) Chemical structures of NSC125034, NSC24076 and 8Q. (b) Susceptibility of sphere cells to compound NSC 125034 compared with MCF7 cells. (c) Susceptibility of sphere cells to compound NSC 24076 compared with MCF7 cells. (d) Susceptibility of sphere cells to compound 8Q compared with MCF7 cells. Statistics was calculated by Student t test. * P < 0.05; ** P < 0.005; *** P < 0.0005.
8Q inhibits the NF-κB activity within sphere cells
8Q was known to inhibit the NF-κB activation in RAW 264.7 cells [51]. It is possible that 8Q functions as a NF-κB inhibitor and inhibits the sphere cell proliferation through the NF-κB pathway. Thus, we determined the NF-κB activity in both MCF7 and sphere cells treated with 8Q. An ELISA-based method (Trans-AM NF-κB; ActiveMotif, Carlsbad, CA) was used because of its high sensitivity to detect and quantify NF-κB activation [52]. Indeed, 8Q significantly inhibited the NF-κB activity in both MCF7 and sphere cells. Treatment with 8Q at 25 μM was able to inhibit the NF-κB activity by 56.0% (0.29 vs. 0.70) for the sphere cells and 42.1% (0.30 vs. 0.53) for MCF7 cells.
Antitumor activity of 8Q in the mouse tumor xenograft models
We further evaluated the antitumor activity of 8Q in breast cancer xenograft models either alone or in combination with the clinical drug paclitaxel. The toxicity of the compound 8Q is modest according to U.S. National Toxicology Program acute toxicity studies (http://www.pesticideinfo.org/List_NTPStudies.jsp?Rec_Id=PC34299). 8Q when given through the tail vein at a dose of 20 mg/kg did not show significant toxicity as judged by lack of apparent symptoms and body weight loss (data not shown). As shown in Fig. 4a, b, 8Q alone had weak antitumor activity in both mouse models, but the 8Q + paclitaxel group had significantly better effect than either 8Q (P < 0.005) or paclitaxel alone (P < 0.005). In particular, the paclitaxel only treated group relapsed soon after treatment was stopped (Fig. 4a, b) whereas tumors in mice treated with combination of paclitaxel and 8Q had no apparent relapse or regrowth. These data suggest that, unlike paclitaxel which did not prevent relapse, cancer stem cell active compound 8Q had better therapeutic effect and more importantly reduced relapse when combined with paclitaxel.
Fig. 4.
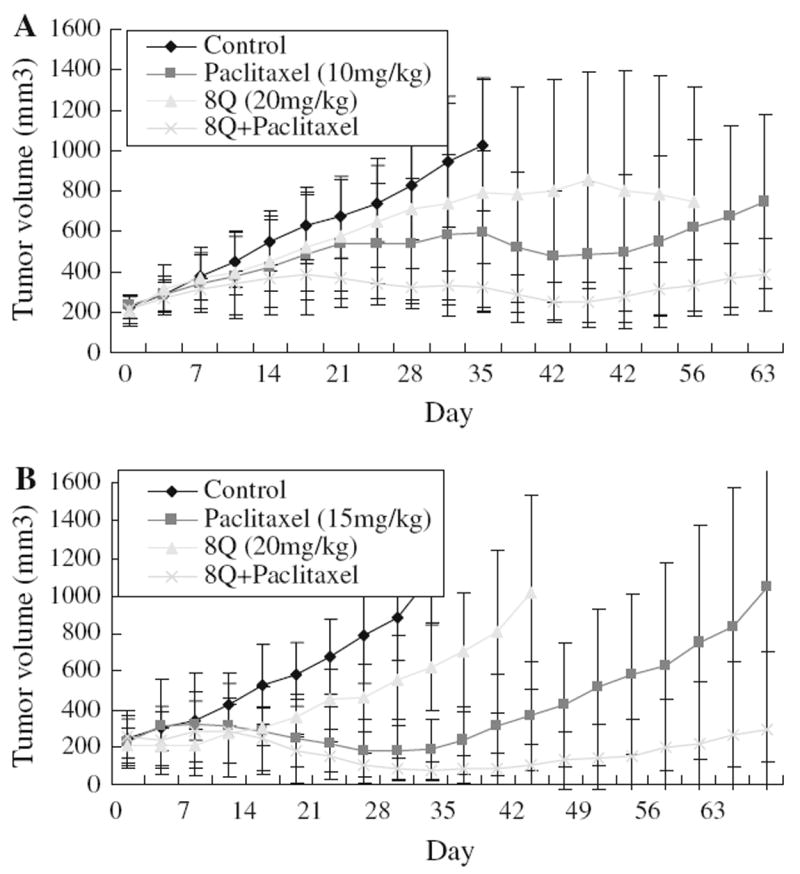
Nude mouse xenograft assays of 8Q. (a) In vivo effect of 8Q (20 mg/kg) on MCF7 xenograft. Nude mouse xenograft assay using MCF7 cells was carried out for evaluation of 8Q or paclitaxel (10 mg/kg) alone or a combination of the two compounds on tumor growth. Mice were treated twice weekly for 4 weeks. Treatment was indicated in the figure as arrows. Tumor sizes were measured twice weekly. (b) In vivo effect of 8Q (20 mg/kg) on MDA-MB-435 xenograft. Mouse treatment was the same as the treatment on MCF7 xenograft except the dose of paclitaxel is 15 mg/kg. Mice were treated twice weekly for 3 weeks
Discussion
Recent studies suggest that cancer stem cells are the root of cancer and long term remission requires the eradication of cancer stem cells [2, 14-17]. The existing chemotherapy drugs, which were traditionally developed on bulk actively growing cancer cells, may not be able to eradicate cancer stem cells efficiently [16, 19-22, 44, 47, 50]. New approaches including feasible cancer stem cell models are needed for cancer stem cell research. Currently, most cancer stem cells were identified in primary patient samples, which are of limited supply and pose significant difficulty for cancer stem cell research. However, cancer stem/progenitor cells from some exiting cell lines may provide a useful source for cancer stem cell research because of its unlimited availability and ease of handling. In this study, by culturing sphere cells from MCF7 SP cells instead of bulk cells, we obtained long-term culturable sphere cells, which could be passaged and maintained in culture for up to 3 years. In contrast, we failed to culture sphere cells derived from bulk MCF7 cells for more than 5–10 generations. Similar to the reported sphere cells [39, 40], the sphere cells cultured from SP cells were enriched in cells expressing known breast cancer stem cell surface markers CD44+CD24- and had higher tumorigenicity in mice (Fig. 1), suggesting the sphere cells enrich breast cancer stem/progenitor cells.
In this study the sphere cells were found to be resistant to different cancer drugs, a finding that is consistent with previous observations on other cancer stem cells [16, 19-22, 44, 47, 50]. Here we performed a compound library screening and identified two lead compounds, NSC24076, NSC125034 and 8Q, an analog of NSC125034, which showed better inhibition effect on the sphere cells than MCF7 cells (Fig. 3). We further investigated the mechanism of action of 8Q. 8Q is known to be a metal chelator [53], which can form a complex with copper and induce apoptosis in cancer cells through NF-κB signaling [51, 53]. NF-κB pathway is a major mediator of cell survival and is often inactive in most cell types [23, 54]. However, upon activation by various stimuli, the regulatory molecule IkBa is degraded and NF-κB is translocated to the nucleus and forms an active transcriptional complex which leads to transcription of various downstream genes [55]. Interestingly, our recent study suggested that some NF-κB pathway specific inhibitors including parthenolide (PTL), pyrrolidinedithiocarbamate (PDTC) and diethyldithiocar-bamate (DETC) can selectively inhibit breast cancer stem/progenitor cells [28]. These studies suggest that the NF-κB pathway might be preferentially important for sphere cell proliferation and survival. This finding is consistent with the previous observation that the NF-κB pathway is essential for leukemia stem cell survival [23-27]. However, we found that while 8Q inhibited the NF-κB activity within sphere cells, its inhibitory effect on NF-kB is only slightly higher for sphere cells (56%) than for MCF7 cells (42%) (P = 0.09). Since epigenetic regulation is important for cancer stem cells [56-58], it is possible that 8Q may act as a histone deacetylase inhibitor to selectively inhibit breast cancer stem/progenitor cells [59]. Further studies are needed to confirm the mechanism of action of 8Q on cancer stem cells.
It is interesting that 8Q alone showed some weak anti-tumor effects but the combination of 8Q and paclitaxel produced much better antitumor effects than either 8Q or paclitaxel alone in both MCF7 and MDA-MB-435 xenograft models. It is possible that cancer stem cells are a minor population of cells among bulk tumor cells thus cancer stem cell active compound 8Q alone may not show significant antitumor effects in the treatment experiment. In contrast, the current clinical cancer drugs like paclitaxel, which are developed based extensively on activity for bulk replicating cancer cells, have significant effects on reducing tumor size. However, tumor in paclitaxel alone treatment group relapsed soon after treatment was discontinued (Fig. 4a, b). This is most likely because paclitaxel, while killing bulk replicating cancer cells, can not eliminate cancer stem cells, as shown in our recent study [28], a finding that is also consistent with recent clinical studies that paclitaxel does not have clinical benefit for metastatic breast cancer [60]. Therefore, combination of the current chemotherapy drugs with drugs that specifically target cancer stem cells will not only produce better chemother-apeutic effects than either drug alone but also prevent tumor relapse after treatment is stopped. This study suggests combination of current therapy with compounds that target cancer stem cells may be a more effective approach to eradicating tumors.
There are some limitations of this study. MCF7 cells have been propagated in vitro for many years and may not accurately reflect the behavior of human breast cancer in patients. In addition, the current breast cancer stem cell models using CD44+/CD24- surface markers, SP and sphere culture have limitations as each model probably only captures one subpopulation of the cancer stem cells and thus, these methods have not been universally accepted. However, no breast cancer stem cell research can be performed at this time without the use of these models. Furthermore, the sphere cells are heterogeneous, and this study may underestimate the differences between breast cancer stem cells and normal cancer cells [39] and the drug screens using the sphere model may also have some limitations. Further validation on cancer stem cells from patient specimens is needed in future studies.
In summary, this study has demonstrated that the sphere cells derived from SP cells are enriched in cancer stem/progenitor cells and could be used as a model to identify compounds that preferentially inhibit cancer stem/progenitor cells. An important finding of this study is that although cancer stem cell active compound 8Q by itself had limited anticancer activity, the combination of 8Q with the current cancer drug paclitaxel produced a better therapeutic effect with no apparent relapse than either agent alone. Based on this encouraging finding, we propose a new treatment concept of combining cancer stem cell active compounds with current cancer drugs that inhibit or kill bulk growing cancer cells for improved treatment of different cancers. This concept needs to be further evaluated and validated in future clinical studies.
Acknowledgments
The work was supported in part by NIH grant AI44063, Ho Ching Yang Memorial Faculty Fellowship in Cancer Prevention, and the Johns Hopkins Center for AIDS Research. We are grateful to NCI Developmental Therapeutics Program for the provision of the compound library.
Abbreviations
- 8Q
8-Quinolinol
- SP
Side population
- NF-κB
Nuclear factor-κB
Contributor Information
Jiangbing Zhou, Department of Molecular Microbiology and Immunology, Bloomberg School of Public Health, The Johns Hopkins University, 615 N. Wolfe Street, Baltimore, MD 21205, USA.
Hao Zhang, Department of Molecular Microbiology and Immunology, Bloomberg School of Public Health, The Johns Hopkins University, 615 N. Wolfe Street, Baltimore, MD 21205, USA.
Peihua Gu, Department of Molecular Microbiology and Immunology, Bloomberg School of Public Health, The Johns Hopkins University, 615 N. Wolfe Street, Baltimore, MD 21205, USA.
Joseph B. Margolick, Department of Molecular Microbiology and Immunology, Bloomberg School of Public Health, The Johns Hopkins University, 615 N. Wolfe Street, Baltimore, MD 21205, USA
Deling Yin, Department of Internal Medicine, James Quillen College of Medicine, East Tennessee State University, P.O. Box 70622, Johnson City, TN 37614, USA.
Ying Zhang, Department of Molecular Microbiology and Immunology, Bloomberg School of Public Health, The Johns Hopkins University, 615 N. Wolfe Street, Baltimore, MD 21205, USA yzhang@jhsph.edu.
References
- 1.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hema-topoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 2.Behbod F, Rosen JM. Will cancer stem cells provide new therapeutic targets? Carcinogenesis. 2004;26:703–711. doi: 10.1093/carcin/bgh293. [DOI] [PubMed] [Google Scholar]
- 3.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 4.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 6.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 7.Hemmati HD, Nakano I, Lazareff JA, Masterman-Smith M, Geschwind DH, Bronner-Fraser M, Kornblum HI. Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci USA. 2003;100:15178–15183. doi: 10.1073/pnas.2036535100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, Fiocco R, Foroni C, Dimeco F, Vescovi A. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 9.Matsui W, Huff CA, Wang Q, Malehorn MT, Barber J, Tanhehco Y, Smith BD, Civin CI, Jones RJ. Characterization of clonogenic multiple myeloma cells. Blood. 2004;103:2332–2336. doi: 10.1182/blood-2003-09-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 11.Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 12.Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF, Ailles LE. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci USA. 2007;104:973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 14.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 15.Al-Hajj M, Becker MW, Wicha M, Weissman I, Clarke MF. Therapeutic implications of cancer stem cells. Curr Opin Genet Dev. 2004;14:43–47. doi: 10.1016/j.gde.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 16.Jones RJ, Matsui WH, Smith BD. Cancer stem cells: are we missing the target? J Natl Cancer Inst. 2004;96:583–585. doi: 10.1093/jnci/djh095. [DOI] [PubMed] [Google Scholar]
- 17.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 18.Donnenberg VS, Donnenberg AD. Multiple drug resistance in cancer revisited: the cancer stem cell hypothesis. J Clin Pharmacol. 2005;45:872–877. doi: 10.1177/0091270005276905. [DOI] [PubMed] [Google Scholar]
- 19.Guzman ML, Jordan CT. Considerations for targeting malignant stem cells in leukemia. Cancer Control. 2004;11:97–104. doi: 10.1177/107327480401100216. [DOI] [PubMed] [Google Scholar]
- 20.Costello RT, Mallet F, Gaugler B, Sainty D, Arnoulet C, Gastaut JA, Olive D. Human acute myeloid leukemia CD34+/CD38− progenitor cells have decreased sensitivity to chemotherapy and Fas-induced apoptosis, reduced immunogenicity, and impaired dendritic cell transformation capacities. Cancer Res. 2000;60:4403–4411. [PubMed] [Google Scholar]
- 21.Graham SM, Jorgensen HG, Allan E, Pearson C, Alcorn MJ, Richmond L, Holyoake TL. Primitive, quiescent, Philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro. Blood. 2002;99:319–325. doi: 10.1182/blood.v99.1.319. [DOI] [PubMed] [Google Scholar]
- 22.Angstreich GR, Matsui W, Huff CA, Vala MS, Barber J, Hawkins AL, Griffin CA, Smith BD, Jones RJ. Effects of imatinib and interferon on primitive chronic myeloid leukaemia progenitors. Br J Haematol. 2005;130:373–381. doi: 10.1111/j.1365-2141.2005.05606.x. [DOI] [PubMed] [Google Scholar]
- 23.Guzman ML, Neering SJ, Upchurch D, Grimes B, Howard DS, Rizzieri DA, Luger SM, Jordan CT. Nuclear factor-kappaB is constitutively activated in primitive human acute myelogenous leukemia cells. Blood. 2001;98:2301–2307. doi: 10.1182/blood.v98.8.2301. [DOI] [PubMed] [Google Scholar]
- 24.Guzman ML, Swiderski CF, Howard DS, Grimes BA, Rossi RM, Szilvassy SJ, Jordan CT. Preferential induction of apoptosis for primary human leukemic stem cells. Proc Natl Acad Sci USA. 2002;99:16220–16225. doi: 10.1073/pnas.252462599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guzman ML, Rossi RM, Karnischky L, Li X, Peterson DR, Howard DS, Jordan CT, Liesveld JL, Phillips GL, 2nd, Swiderski CF, Grimes BA, Szilvassy SJ, Neering SJ, Upchurch D, Grimes B, Rizzieri DA, Luger SM, Lemischka IR, Pettigrew AL, Meyerrose T, Rossi R, Phillips GL. The sesquiterpene lactone parthenolide induces apoptosis of human acute myelogenous leukemia stem and progenitor cells. Blood. 2005;16:708–712. doi: 10.1182/blood-2004-10-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guzman ML, Rossi RM, Neelakantan S, Li X, Corbett CA, Hassane DC, Becker MW, Bennett JM, Sullivan E, Lachowicz JL, Vaughan A, Sweeney CJ, Matthews W, Carroll M, Liesveld JL, Crooks PA, Jordan CT. An orally bioavailable parthenolide analog selectively eradicates acute myelogenous leukemia stem and progenitor cells. Blood. 2007;110:4427–4435. doi: 10.1182/blood-2007-05-090621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guzman ML, Li X, Corbett CA, Rossi RM, Bushnell T, Liesveld JL, Hebert J, Young F, Jordan CT. Rapid and selective death of leukemia stem and progenitor cells induced by the compound 4-benzyl, 2-methyl, 1, 2, 4-thiadiazolidine, 3, 5 dione (TDZD-8) Blood. 2007;110:4436–4444. doi: 10.1182/blood-2007-05-088815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou J, Zhang H, Gu P, Bai J, Margolick JB, Zhang Y. NF-kappaB pathway inhibitors preferentially inhibit breast cancer stem-like cells. Breast Cancer Res Treat. 2007 Oct 27; doi: 10.1007/s10549-007-9798-y. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou J, Wulfkuhle J, Zhang H, Gu P, Yang Y, Deng J, Margolick JB, Liotta LA, Petricoin E, 3rd, Zhang Y. Activation of the PTEN/mTOR/STAT3 pathway in breast cancer stem-like cells is required for viability and maintenance. Proc Natl Acad Sci USA. 2007;104:16158–16163. doi: 10.1073/pnas.0702596104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang M, Rosen JM. Stem cells in the etiology and treatment of cancer. Curr Opin Genet Dev. 2005;16:60–64. doi: 10.1016/j.gde.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 31.Edwards BK, Brown ML, Wingo PA, Howe HL, Ward E, Ries LA, Schrag D, Jamison PM, Jemal A, Wu XC, Friedman C, Harlan L, Warren J, Anderson RN, Pickle LW. Annual report to the nation on the status of cancer, 1975–2002, featuring population-based trends in cancer treatment. J Natl Cancer Inst. 2005;97:1407–1427. doi: 10.1093/jnci/dji289. [DOI] [PubMed] [Google Scholar]
- 32.Suslov ON, Kukekov VG, Ignatova TN, Steindler DA. Neural stem cell heterogeneity demonstrated by molecular phenotyping of clonal neurospheres. Proc Natl Acad Sci USA. 2002;99:14506–14511. doi: 10.1073/pnas.212525299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim M, Morshead CM. Distinct populations of forebrain neural stem and progenitor cells can be isolated using side-population analysis. J Neurosci. 2003;23:10703–10709. doi: 10.1523/JNEUROSCI.23-33-10703.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okano H. Neural stem cells: progression of basic research and perspective for clinical application. Keio J Med. 2002;51:115–128. doi: 10.2302/kjm.51.115. [DOI] [PubMed] [Google Scholar]
- 35.Singh SK, Clarke ID, Hide T, Dirks PB. Cancer stem cells in nervous system tumors. Oncogene. 2004;23:7267–7273. doi: 10.1038/sj.onc.1207946. [DOI] [PubMed] [Google Scholar]
- 36.Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, Wicha MS. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253–1270. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dontu G, Jackson KW, McNicholas E, Kawamura MJ, Abdallah WM, Wicha MS. Role of Notch signaling in cell-fate determination of human mammary stem/progenitor cells. Breast Cancer Res. 2004;6:R605–R615. doi: 10.1186/bcr920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Youn BS, Sen A, Kallos MS, Behie LA, Girgis-Gabardo A, Kurpios N, Barcelon M, Hassell JA. Large-scale expansion of mammary epithelial stem cell aggregates in suspension bio-reactors. Biotechnol Prog. 2005;21:984–993. doi: 10.1021/bp050059f. [DOI] [PubMed] [Google Scholar]
- 39.Phillips TM, McBride WH, Pajonk F. The response of CD24(−/low)/CD44+ breast cancer-initiating cells to radiation. J Natl Cancer Inst. 2006;98:1777–1785. doi: 10.1093/jnci/djj495. [DOI] [PubMed] [Google Scholar]
- 40.Ponti D, Costa A, Zaffaroni N, Pratesi G, Petrangolini G, Coradini D, Pilotti S, Pierotti MA, Daidone MG. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65:5506–5511. doi: 10.1158/0008-5472.CAN-05-0626. [DOI] [PubMed] [Google Scholar]
- 41.Goodell MA, Rosenzweig M, Kim H, Marks DF, DeMaria M, Paradis G, Grupp SA, Sieff CA, Mulligan RC, Johnson RP. Dye efflux studies suggest that hematopoietic stem cells expressing low or undetectable levels of CD34 antigen exist in multiple species. Nat Med. 1997;3:1337–1345. doi: 10.1038/nm1297-1337. [DOI] [PubMed] [Google Scholar]
- 42.Welm B, Behbod F, Goodell MA, Rosen JM. Isolation and characterization of functional mammary gland stem cells. Cell Prolif. 2003;36(Suppl 1):17–32. doi: 10.1046/j.1365-2184.36.s.1.3.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clayton H, Titley I, Vivanco M. Growth and differentiation of progenitor/stem cells derived from the human mammary gland. Exp Cell Res. 2004;297:444–460. doi: 10.1016/j.yexcr.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 44.Szotek PP, Pieretti-Vanmarcke R, Masiakos PT, Dinulescu DM, Connolly D, Foster R, Dombkowski D, Preffer F, Maclaughlin DT, Donahoe PK. Ovarian cancer side population defines cells with stem cell-like characteristics and Mullerian Inhibiting Substance responsiveness. Proc Natl Acad Sci USA. 2006;103:11154–11159. doi: 10.1073/pnas.0603672103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chiba T, Kita K, Zheng YW, Yokosuka O, Saisho H, Iwama A, Nakauchi H, Taniguchi H. Side population purified from hepatocellular carcinoma cells harbors cancer stem cell-like properties. Hepatology. 2006;44:240–251. doi: 10.1002/hep.21227. [DOI] [PubMed] [Google Scholar]
- 46.Kondo T, Setoguchi T, Taga T. Persistence of a small subpopulation of cancer stem-like cells in the C6 glioma cell line. Proc Natl Acad Sci USA. 2004;101:781–786. doi: 10.1073/pnas.0307618100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hirschmann-Jax C, Foster AE, Wulf GG, Nuchtern JG, Jax TW, Gobel U, Goodell MA, Brenner MK. A distinct ‘‘side population’’ of cells with high drug efflux capacity in human tumor cells. Proc Natl Acad Sci USA. 2004;101:14228–14233. doi: 10.1073/pnas.0400067101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kasukabe T, Okabe-Kado J, Kato N, Sassa T, Honma Y. Effects of combined treatment with rapamycin and cotylenin A, a novel differentiation-inducing agent, on human breast carcinoma MCF-7 cells and xenografts. Breast Cancer Res. 2005;7:R1097–R1110. doi: 10.1186/bcr1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hardman WE, Moyer MP, Cameron IL. Fish oil supplementation enhanced CPT-11 (irinotecan) efficacy against MCF7 breast carcinoma xenografts and ameliorated intestinal side-effects. Br J Cancer. 1999;81:440–448. doi: 10.1038/sj.bjc.6690713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haraguchi N, Utsunomiya T, Inoue H, Tanaka F, Mimori K, Barnard GF, Mori M. Characterization of a side population of cancer cells from human gastrointestinal system. Stem Cells. 2006;24:506–513. doi: 10.1634/stemcells.2005-0282. [DOI] [PubMed] [Google Scholar]
- 51.Kim YH, Woo KJ, Lim JH, Kim S, Lee TJ, Jung EM, Lee JM, Park JW, Kwon TK. 8-Hydroxyquinoline inhibits iNOS expression and nitric oxide production by down-regulating LPS-induced activity of NF-kappaB and C/EBPbeta in Raw 264.7 cells. Biochem Biophys Res Commun. 2005;329:591–597. doi: 10.1016/j.bbrc.2005.01.159. [DOI] [PubMed] [Google Scholar]
- 52.Renard P, Ernest I, Houbion A, Art M, Le Calvez H, Raes M, Remacle J. Development of a sensitive multi-well colorimetric assay for active. Nucleic Acids Res. 2001;29:E21. doi: 10.1093/nar/29.4.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Daniel KG, Chen D, Orlu S, Cui QC, Miller FR, Dou QP. Clioquinol and pyrrolidine dithiocarbamate complex with copper to form proteasome inhibitors and apoptosis inducers in human breast cancer cells. Breast Cancer Res. 2005;7:R897–R908. doi: 10.1186/bcr1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mayo MW, Baldwin AS. The transcription factor NF-kappaB: control of oncogenesis and cancer therapy resistance. Biochim Biophys Acta. 2000;1470:M55–M62. doi: 10.1016/s0304-419x(00)00002-0. [DOI] [PubMed] [Google Scholar]
- 55.Baldwin AS., Jr The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 56.Feinberg AP, Ohlsson R, Henikoff S. The epigenetic progenitor origin of human cancer. Nat Rev Genet. 2006;7:21–33. doi: 10.1038/nrg1748. [DOI] [PubMed] [Google Scholar]
- 57.Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128:683–692. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ohm JE, McGarvey KM, Yu X, Cheng L, Schuebel KE, Cope L, Mohammad HP, Chen W, Daniel VC, Yu W, Berman DM, Jenuwein T, Pruitt K, Sharkis SJ, Watkins DN, Herman JG, Baylin SB. A stem cell-like chromatin pattern may predispose tumor suppressor genes to DNA hypermethylation and heritable silencing. Nat Genet. 2007;39:237–242. doi: 10.1038/ng1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martirosyan A, Leonard S, Shi X, Griffith B, Gannett P, Strobl J. Actions of a histone deacetylase inhibitor NSC3852 (5-nitroso-8-quinolinol) link reactive oxygen species to cell differentiation and apoptosis in MCF-7 human mammary tumor cells. J Pharmacol Exp Ther. 2006;317:546–552. doi: 10.1124/jpet.105.096891. [DOI] [PubMed] [Google Scholar]
- 60.Gennari A, Amadori D, De Lena M, Nanni O, Bruzzi P, Lorusso V, Manzione L, Conte PF. Lack of benefit of maintenance paclitaxel in first-line chemotherapy in metastatic breast cancer. J Clin Oncol. 2006;24:3912–3918. doi: 10.1200/JCO.2006.06.1812. [DOI] [PubMed] [Google Scholar]


