Abstract
This paper describes a paper-based microfluidic device that measures two enzymatic markers of liver function (alkaline phosphatase ALP, and aspartate aminotransferase AST) and total serum protein. A device consists of four components: i) a top plastic sheet, ii) a filter membrane, iii) a patterned paper chip containing the reagents necessary for analysis, and iv) a bottom plastic sheet. The device performs both the sample preparation (separating blood plasma from erythrocytes) and the assays; it also enables both qualitative and quantitative analysis of data. The data obtained from the paper-microfluidic devices show standard deviations in calibration runs and “spiked” standards that are acceptable for routine clinical use. This device illustrates a type of test useable for a range of assays in resource-poor settings.
Introduction
Typical tests conducted to diagnose disease and monitor health in patients in industrialized nations are expensive. In resource-poor settings the requirements of equipped clinical facilities, trained clinicians, and local laboratory staff often preclude the testing and monitoring of patients, and leaves only symptoms as the basis for diagnosis.1 Symptoms, however, may appear only after irreparable damage has occurred. There is, thus, a widely acknowledged need to develop low-cost, simple-to-use, point-of-care (POC) diagnostic methods for diagnosis, and monitoring the treatment of patients suffering from disease (especially infectious disease) in resource-poor settings. In 2004, the World Health Organization (WHO) established guidelines for developing POC diagnostic tests with characteristics required for resource-poor settings. The guidelines were summarized by the acronym ASSURED: Affordable, Sensitive, Specific, User-friendly, Rapid and robust, Equipment-free, and Delivered.2,3 Identifying patients using suitable diagnostic devices is important to ensure not only that sick patients are treated for the appropriate disease, but also to avoid treating individuals who are not infected and to prevent over-medicating, which could lead to drug-resistant organisms.4 The availability of medications in developing countries has increased due to the reduction in prices of medication and the launch of the Global Fund to Fight AIDS, Tuberculosis, and Malaria.5 Increasing use of medication has led to an increased incidence of medication-induced toxicity (especially problematic in drugs used against TB and AIDS). The lack of suitable means for monitoring medication-induced liver toxicity has led to a marked increase in deaths due to drug toxicity.6–8 The results of one study in the United States of patients taking anti-retroviral medicines for AIDS indicated that the risk of death due to drug toxicity was similar to that from AIDS itself.8 Risk factors in developing countries are generally much higher than those in developed countries.9 Monitoring patients for drug toxicity using diagnostic tests that follow the ASSURED guidelines will decrease morbidity and mortality from the treatment of disease.3,6
Many research groups are developing POC diagnostic tools and devices for the developing world. In the Whitesides lab, we have developed low-cost, POC diagnostic device platforms based on paper,10 thread,11 and magnetic levitation,12 which were configured for detecting simple analytes, such as protein,13 and glucose.14 Many groups are now working to develop paper-based POC diagnostics.15–18 Recently, two different groups reported low-cost devices for enzyme-linked immunosorbent assays (ELISA). The paper-based ELISA used a colorimetric output to detect and quantify antibodies to HIV.19 The microfluidic ELISA used silver reduction on gold nanoparticles as an amplified output to detect antibodies against HIV and Treponema pallidum (the causative agent of syphilis) in whole bood.20 Researchers from the Program for Appropriate Technology in Health (PATH) recently described a simple and inexpensive device that uses exothermic chemical reactions and phase change materials as a heater to achieve nucleic acid amplification (NAA).21 Although great strides have been made to develop simple, inexpensive diagnostic tools and devices, there is still much work that needs to be done.
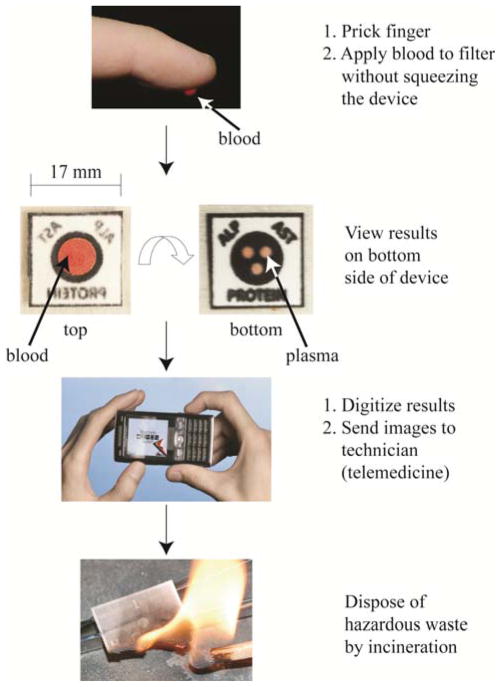
This article describes an inexpensive and portable system for measuring the levels of multiple analytes in a drop of blood obtained from a fingerstick (Figure 1). The system comprises a paper chip patterned into hydrophobic and hydrophilic regions, a filter, and self-adhesive laminating sheets; it serves four primary functions: it i) removes red blood cells from plasma; ii) distributes the resulting plasma into three regions within the paper; iii) runs three simultaneous colorimetric assays; and iv) displays the results of the colorimetric assays for quantitative analysis. Results digitized using, for example, a cellular phone, can be sent to an off-site technician (using established communications infrastructure) who can quantify the assays, provide a diagnosis, and recommend a treatment. Alternatively, on-chip color charts can give the results of the assays on-site. The diagnostic chip can be burned at the conclusion of the assay to dispose of biohazardous waste. We consider this system a prototype of a general platform for performing multiple bioassays using a single drop of blood. This paper demonstrates the construction and use of the device by quantitatively measuring markers of liver disease (aspartate aminotransferase (AST)22, alkaline phosphatase (ALP)23–25, and total serum protein26). Many other analytes (for example, alanine transaminase (ALT)27, gamma glutamyl transpeptidase (GGT)28, lactate dehydrogenase (LDH)29,30, or total bilirubin31) could also be measured.
Figure 1.
System designed for the quantitative measurement of analyte levels in a drop of blood. A small device fabricated from paper patterned with wax, a filter membrane, and a plastic sheath processes and analyzes a drop of blood from a fingerstick. The multifunctional device, encased in two self-adhesive laminating sheets, uses the paper to store the reagents for the assays, and the filter on top of the paper to separate plasma from the red blood cells. A cell phone or desktop scanner digitizes the results from the assays, which can be analyzed off-site by trained personnel. Incineration of the devices easily disposes the bio-hazardous waste.
This bioanalytical system provides a complete assay, from processing blood to giving a digital read-out that operates with only minimal manipulation. We believe it has characteristics that will make it useful in programs of public health in resource-limited environments. (It may also be useful in military operations, border security, and environmental and agricultural applications). In particular, “innovative developing countries” (IDCs) may benefit from this technology. For these countries, cost, operational ease, and portability of an analytical system are just as important as sensitivity and specificity. In this paper, we present the development and performance of these devices. Although we used a scanner instead of a camera phone to digitize the devices and quantify these data as proof of principle, the principles of electronic analysis and communication apply across all classes of devices.
EXPERIMENTAL DESIGN
Monitoring Liver Function
Liver disease or dysfunction may be caused by several factors, including infections (especially viral infections such as: hepatitis A, B, C, delta, and E, HIV, malaria, and cancer), abuse of alcohol, and the overuse of or reaction to medications. As an example, drug-induced damage to the liver causes serious illness or even death, and occurs in 2–28% of patients taking medications (such as isoniazid, rifampicin, and pyrazinamide) for tuberculosis32, and 8–28% of patients using Nevirapine, a first-line treatment for HIV.33 The risk of hepatotoxicity increases for patients with co-infections.34,35 Therapies for diabetes and heart disease also affect the liver and require constant monitoring of liver function. These diseases are increasing in prevalence in developing regions.36 The initial onset of liver damage often goes unnoticed by patients, so screening for liver toxicity has become standard practice for many drug regimens in industrialized countries. In developing countries, however, the cost of monitoring toxicity associated with a treatment over an extended period is prohibitive.
Blood as the test medium
Blood contains the common markers of liver function that are not found in urine.
Obtaining Blood from a Fingerstick
Sticking a finger is probably the only kind of blood sampling that is routinely practical in resource-poor settings since it does not require a trained professional. It produces a small volume of blood (between 10 and 20 μL) from an average adult finger. This quantity can be applied directly to the device platform, and it requires minimal manipulation for successful analysis (ASSURED – User friendly). Small amounts of blood produce less waste than a vial of blood used for conventional liver function tests. Using blood from a fingerstick does have challenges. A lancet is required for the fingerstick (cheap, single use, disposable lancets are an option). The levels of analytes in capillary blood may not perfectly reflect the levels in veins.37 Hemolysis can occur from puncture with a lancet. The volume of blood obtained from infants (from a heel prick) is very low (~ 4 μL); this volume may be insufficient to perform the tests. These challenges can be overcome, for example, by adjusting the diagnostic levels of the analytes to those found in a fingerstick or generating tests specific for the volume obtained from an infant.
Design of the Device
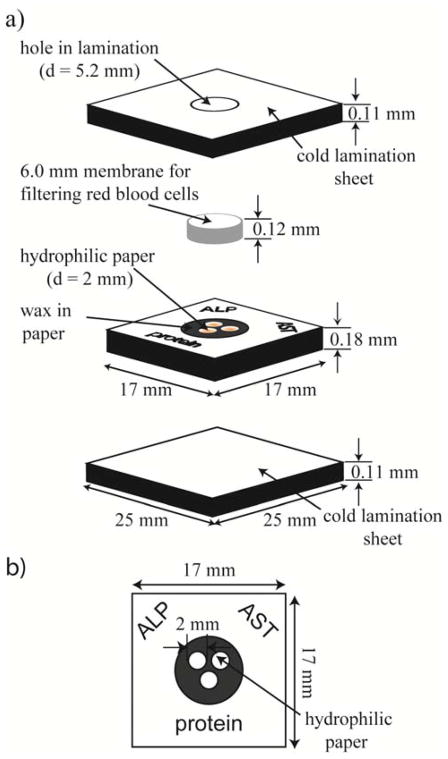
A vertical-flow device design has several advantages over a lateral-flow configuration: i) a smaller sample size is required, ii) the assay develops more quickly, iii) the device is simpler in construction, and iv) the overall weight of the device is lower. Capillary forces allow this device to function without the need of an external pump (ASSURED – Equipment-Free). The microsystem consisted of: i) a plastic sheath, ii) a filter capable of separating red blood cells from plasma, and iii) a patterned piece of paper with wax ink38 functioning as hydrophobic barriers, and creating three hydrophilic zones for running colorimetric assays.(Figure 2) Two plastic pieces formed a sheath around the device that served to protect it from excess blood and to limit evaporation of water from the plasma.(Figure 2a) The sheath also protected the reagents from contamination and the worker from contact with the blood of the patient. The lamination sheets encased a circular filter membrane and a piece of patterned paper in a vertical-flow configuration. Red blood cells applied to the device adsorbed on the filter and the plasma flowed vertically into the detection zones of the patterned paper. The removal of the red blood cells reduced their interference in the colorimetric read-out of the assay. Hydrophobic barriers patterned in the paper using a commercial wax printer defined hydrophilic zones. Patterning had the advantage that the colorimetric assays could be performed without cross-reactions or mixing of the coloring agents between hydrophilic zones. The hydrophobic areas also reduced the amount of plasma needed, since no plasma spread into the hydrophobic areas. We designed the size of the hydrophilic zones to have the largest colored spot for easy read-out, while keeping the plasma volume needed to fill them within the range available from a fingerstick. A microsystem fabricated from paper lowers the overall cost of the device (ASSURED – Affordable), minimizes its weight and volume (characteristics that are especially relevant to portability and for shipping large quantities) (ASSURED - Deliverable), and allows the bioharzardous waste to be incinerated.
Figure 2.
Design of a vertical-flow device for detecting and quantifying i) the levels of total serum protein (Protein), ii) alkaline phosphatase (ALP), and iii) aspartate aminotransferase (AST) in blood obtained from a fingerstick. (a) An oblique view of the assembled device. The device comprises three inexpensive, lightweight, and readily available components: Fellows® single-sided, self-adhesive cold lamination sheets (the top and bottom sheets used to encase the paper holding the reaction zones), Whatman Grade 1 Chromatography paper (patterned to create three reaction zones), and a Pall plasma separation membrane (PSM) (Vivid GX PSM for filtering red blood cells). b) Paper patterned with three circular hydrophilic zones (d ~ 2 mm) surrounded by a hydrophobic wax barrier.
Choice of Assays
Colorimetric assays are attractive because they are generally complete in a short time (~30 minutes) and they provide easily interpretable results as colors, which can be read without sophisticated equipment. Results can be evaluated i) on-site by comparison with a color chart, or digital scanning and analyzing using software, or ii) off-site if integrated with telemedicine (ASSURED – Equipment Free).
Choice of Analytes for Measuring Liver Function
A liver-function panel consists of several different assays, which typically include: aspartate aminotransferase (AST), alkaline phosphatase (ALP), alanine aminotransferase (ALT), bilirubin, albumin, and total serum protein. Other analytes that can provide diagnostically useful information are gamma glutamyl transferase (GGT) and lactate dehydrogenase (LDH). The prothrombin time (PT) is also valuable.39 In this paper, we describe the development of three of these assays, AST,22 ALP,23–25 and total serum protein,26 on the paper-microfluidics diagnostic microsystem. These three assays have simple colorimetric tests and in combination can give a first indication on the liver function of a patient.
Aspartate Aminotransferase (AST)
The blood of a healthy adult contains low concentrations of AST (5–40 U/L), while liver cells contain high concentrations. A diseased or damaged liver releases AST into the bloodstream and elevates its concentrations there; this elevation can be detected and monitored using the AST test. For example, patients with acute hepatitis will have ten times the normal level of AST in their blood (~400 U/L), whereas those with chronic hepatitis will have four times the normal level (~160 U/L).39
Alkaline Phosphatase (ALP)
The blood of a healthy adult has low concentrations of ALP (30–120 U/L). The liver (as well as bone, the intestines and kidneys) generates ALP. ALP released into the bloodstream from damaged or diseased livers causes elevated concentrations. High levels of blood ALP can indicate liver conditions such as blocked bile ducts.39
Total Serum Protein (Protein)
A Protein test measures the total amount of protein in the blood. The blood contains two main groups of proteins: albumin and globulins. Albumin is present in higher concentrations and it helps maintain blood pressure; globulins are important for the immune system. Normal levels range from 60–83 g/L. Low levels of total serum protein can indicate, for example, liver disorders, kidney disorders, or malnutrition.39
Details for preparing, running, and analyzing the assays are described in the supplemental information.
RESULTS AND DISCUSSION
Design of the device
Patterned Paper
A device that performs multiple assays in parallel requires distinct reaction zones for each assay. Martinez et. al., previously reported a simple, inexpensive method to generate multiple reaction zones.13,40 They described the use of paper as a substrate and reaction zones created by making parts of that paper hydrophobic with SU-8. Carrilho et al., described the same features using commercial wax-based ink to pattern paper.38 Using wax-based ink is cheaper and more scalable than SU-8 (even “home-made” photoresist broadly similar to SU-8).10 In our system, devices with wax-based hydrophobic barriers showed better wetting of the reagents and plasma in the hydrophilic zones than did those in devices made using SU-8 as the hydrophobic barriers; complete removal of residual uncrosslinked SU-8 photoresist from the patterned paper in the reaction zones was difficult and required many rinsing steps using organic solvents (e.g., propylene glycol methyl ether acetate (PGMEA), toluene, and hexanes). Simply printing wax-based ink onto the desired paper, and then heating it briefly, allowed the wax to spread vertically through the whole thickness of the paper. This vertical spreading generated hydrophobic barriers around hydrophilic zones. The wax also spread laterally when heated;38 the diameter of the circles for the hydrophilic reaction zones in the printed sheets therefore had to be larger than the target size. We established empirically that the optimal diameter for these zones was 3 mm; these dimensions shrank to ~2 mm after heating. The minimal amount of blood needed to develop the assays dictated the final size and spacing of the zones.
The Filter or Plasma Separation Membrane (PSM)
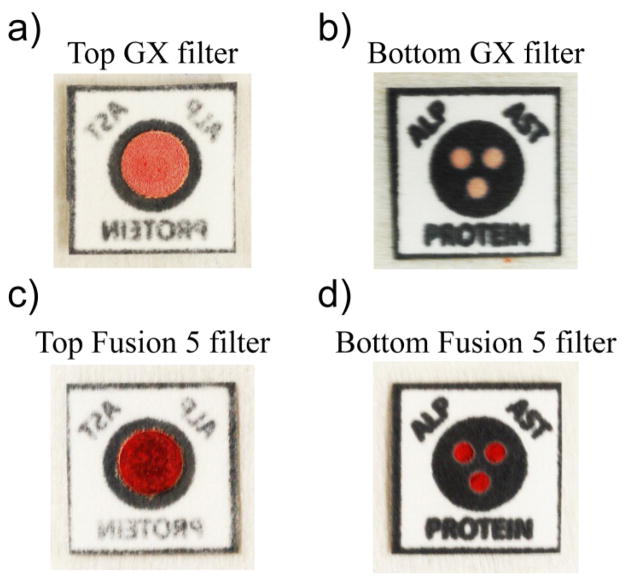
The red color of whole blood samples can interfere with the interpretation of colorimetric assays. A filter placed on top of the patterned paper removed the red blood cells and allowed only colorless plasma to flow into the detection zones. We tested seven commercially available filters (from Pall Corporation and Whatman) for their ability to separate red blood cells from plasma in a vertical configuration. We added 15 μL of whole blood to the top of assembled devices (Figure 3, the reaction zones were reagent-free). After 15 minutes, each of the three hydrophilic zones was assessed to determine if: i) plasma completely or partially wetted the zone, and ii) if blood —red coloration—was present in the zones. We considered a filter functional if all three zones were wetted with blood plasma only. A filter failed if: i) any of the three zones were only partially filled with blood plasma, ii) any of the three zones were not wet at all, or iii) any of the three zones contained red blood cells. Table 1 summarizes the effectiveness of each filter type. Figures 3a and 3b show the results from a fully functional device assembled with a GX PSM; blood plasma wetted all three zones. Figures 3c and 3d show a failed device assembled with a Fusion 5 filter; all three zones contain red blood cells. Both GX and GR membranes proved to be suitable for reliably filtering red blood cells from plasma in a vertical-flow configuration.41 GX PSMs were designed to work with smaller volumes of blood than the GR PSMs. We chose to continue using GX PSMs in all remaining experiments.
Figure 3.
Images of two Liver Function Test (LFT) devices constructed with a Vivid GX PSM (top) and a Fusion 5 filter (bottom). Top: a) The topside of the GX PSM with adsorbed red blood cells. b) The bottom side of the GX PSM device showing all three hydrophilic zones wetted with blood plasma; this device passed as fully functional. Bottom: c) The topside of the Fusion 5 showing adsorbed red blood cells. d) The bottom side of the Fusion 5 device showing all three zones containing red blood cells; this device failed for ineffective plasma separation.
Table 1. Assessment of red blood cell filtration of various filter types.
Evaluation results of the ability of each filter to separate erythrocytes from plasma and wet all three zones on the device. Both GX and GR plasma filters (Pall Corporation) could reliably filter red blood cells from plasma in a vertical-flow configuration.
| Filter | Supplier | Zones Filled | % Zones Filled | Functional Devices |
|---|---|---|---|---|
| GX | Pall | 24/24 | 100 | 8/8 |
| GR | Pall | 24/24 | 100 | 8/8 |
| GF | Pall | 21/24 | 88 | 5/8 |
| VF1 | Whatman | 9/24 | 38 | 0/8 |
| VF2 | Whatman | 4/24 | 17 | 0/8 |
| MF1 | Whatman | 0/24 | 0 | 0/8 |
| Fusion 5 | Whatman | 0/24 | 0 | 0/8 |
Alignment and assembly
Efficient transfer of the blood plasma required careful alignment of the filter above the reaction zones. Fellows® cold lamination sheets protected the reagents and held the PSM in place on the paper devices.
Assays on paper
The colorimetric assays for each analyte reached saturation after a given amount of time, which depended on the concentration of reagents stored in the reaction zones. A lower concentration of substrate reagents resulted in a faster development of the colorimetric assay, but at the cost of a decrease in the sensitivity of the assay to analyte concentration. Optimization of the assays on paper required a balance between sensitivity and time for development of each assay.
Calibration Curves
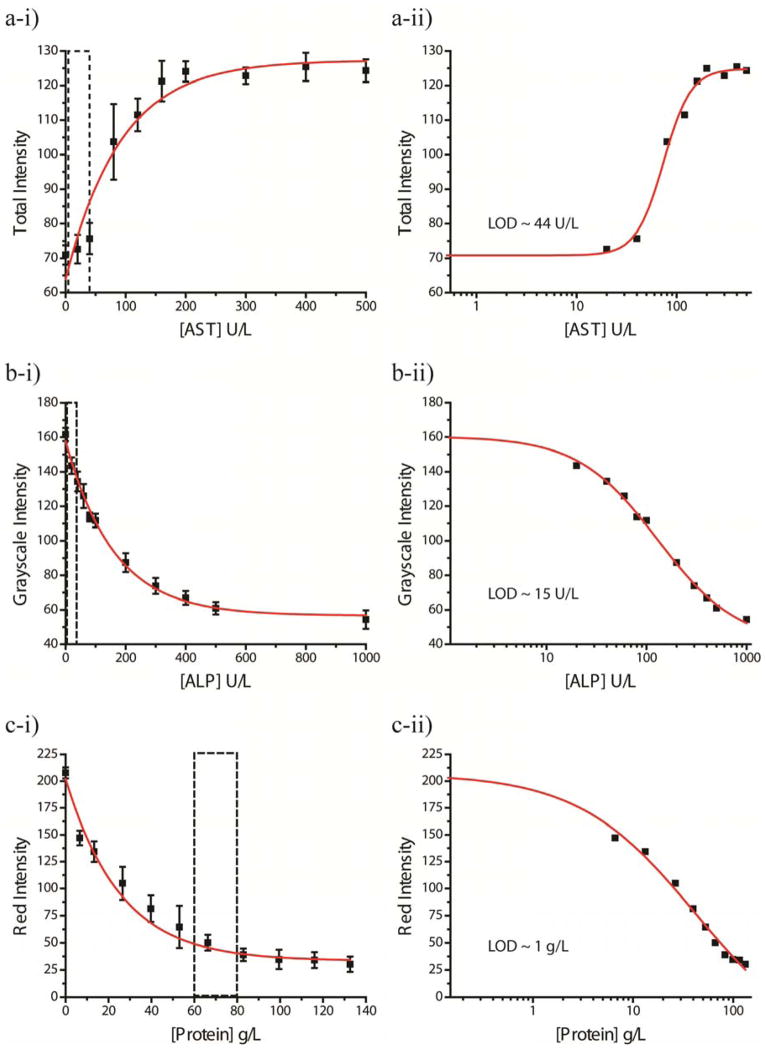
We generated calibration curves by spotting all three reaction-zones with the reagents for one assay and assembling devices with the PSM and plastic sheath. These conditions approximated those of actual device usage. Solutions of each analyte (from zero to ten times the upper limit of the normal range for AST and ALP, and from zero to ~two times the lower limit for Protein) prepared in an artificial blood plasma42 (ABP) buffer for a series of concentrations spanning the entire clinical range were warmed to 37°C and added to the device. We scanned the devices in five-minute intervals as the assays developed, and analyzed each image for the total color, grayscale, red, green, and blue color intensity channels. The image that provided the best dynamic range of intensities over the different concentrations determined the best time and color channel with which to generate the calibration curve. For AST, the total color intensity channel analyzed at 20 minutes provided the largest range (zero concentration to saturation) of 71–125; for ALP, grayscale intensity analyzed at 30 minutes provided the largest range of 144-54; for Protein, red intensity analyzed at 30 minutes provided the largest range of 207-30. Overall, the test takes ~35 minutes to complete, from obtaining a sample of blood, to assay read-out to enable assessment and treatment within one visit (ASSURED - Rapid). Figures 4a-i, b-i, c-i plot the calibration curves for AST, ALP, and Protein, respectively. Exponential curves fitted the calibration curves to give equations relating concentration and color intensity (Table S1). It is important that the color of the assay at different levels of concentrations be sufficiently different to make an accurate interpretation of the results. For example, the color for the normal concentration range of AST (5–40 U/L) should be different from two-three times the upper normal limit (80–120 U/L) and ten times the upper limit (400 U/L) to differentiate normal levels of enzyme from slightly elevated and dangerously elevated levels of enzyme.43 The calibration curve for AST indicates a sufficient difference in the color intensity for each of these ranges (the intensity at 40 U/L is ~76 ± 4, at 80 U/L is ~103 ± 11, and at 400 U/L is ~125 ± 4). The color intensity for the normal concentration range of ALP (~50 U/L) should be different from the value just above the upper normal limit (150 U/L) and ten times the upper limit (400 U/L).44 The calibration curve for ALP indicates a sufficient difference in the color intensity for each of these ranges (the intensity at 50 U/L is ~112 ± 4, at 200 U/L is ~87 ± 6, and at 400 U/L is ~67 ± 4). The Protein assay is more sensitive to low levels of total serum protein than to high levels of total serum protein. To be clinically relevant, intensity values for concentrations lower than 45 g/L should be different from the lower normal limit (60 g/L).44 The calibration curve for Protein indicates a sufficient difference in the color intensity for these concentrations (the intensity at 40 U/L is ~82 ± 12, and at 60 U/L is ~50 ± 7).The sufficient differences between normal range and high or low ranges for the assays meet the criteria for sensitive devices (ASSURED - Sensitive). The sizeable error bars for some of the analyte concentrations can be attributed to two sources: i) variation in the quantity of spotted reagents in the reaction-zones due to manual fabrication of the devices, and ii) reagents that rendered the paper hydrophobic and made wetting of the zones with aqueous blood plasma difficult; insufficient wetting of the reaction-zones by the blood plasma caused incomplete or no assay development, and hence incomplete or no color change.45 Automating the spotting of reagents during device manufacture would likely decrease the variability from manual fabrication. Addition of surfactants to the reaction-zones helped to increase their hydrophilicity, which improved the wetting of the zones with plasma. Hill Plots46 determined the limit of detection (LOD) for each assay (Figures 4a-ii, b-ii, c-ii). The LOD was determined to be in the normal range for both the AST and ALP assays (~44 U/L and ~15 U/L, respectively); the LOD for the Protein assay was determined to be at the low end of the range (~1.0 g/L).
Figure 4.
Calibration (left) and Limit of Detection (LOD) Curves (right) for a) AST, b) ALP, and c) Protein. The boxes outlined in dotted lines on the calibration curves indicate the normal range for each analyte. The total color intensity analyzed at 20 minutes for each concentration generated the AST calibration curve. Grayscale intensity analyzed at 30 minutes for each concentration generated the ALP calibration. Red intensity analyzed at 30 minutes for each concentration generated the Protein Calibration. The limits of detection were determined to be ~44 U/L, ~15 U/L, ~1 g/L for AST, ALP, and Protein, respectively.
Shelf-Life
Viable devices must still function after storage in different environments for at least one year.4 Drying of the reagents on paper helped to stabilize them during storage, as did additives such as trehalose (AST assay).47 We tested devices containing ALP and AST assays that had been stored at room temperature for up to three months; the ALP assay still developed as expected, while the AST reaction zone already showed discoloration before the assay was performed (results not shown). AST assays were stable for at least one month at room temperature and for at least 3 months when stored at 4 °C. Extensive testing in different environments (cool and hot, dry and humid) would be required to determine the shelf-life of these devices.
Cross-Reactivity
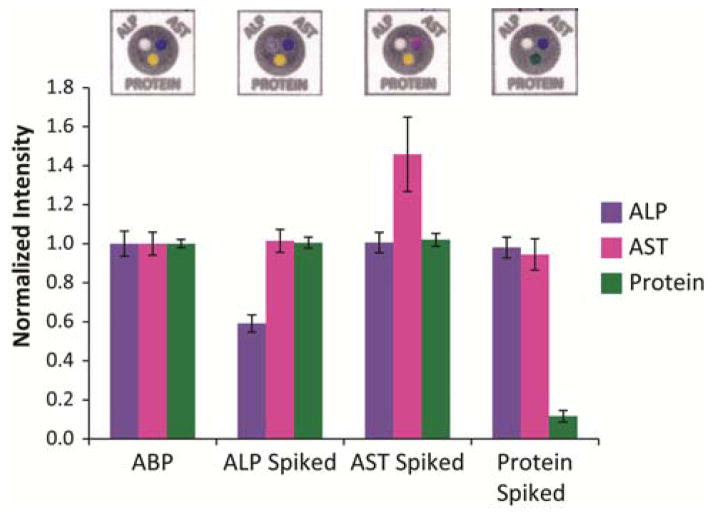
The colorimetric assays we chose for the markers of liver function are specific enough to prevent cross-reactivity with the other analytes tested on the device. We tested this hypothesis by assembling regular test devices (each of the three test zones contained the reagents for the different assays) and applying ABP spiked with only one analyte to the top of the device (unspiked ABP was used as a control). Sufficiently high concentrations of the analytes in ABP (AST (780 U/L), ALP (1200 U/L), or BSA (150 g/L) elicited a response in the reaction zone containing the corresponding assay. Figure 5 summarizes the results from this cross-reactivity experiment. A sample device is shown above the intensity bars for each solution tested. We analyzed the intensities of all of the zones for each assay at the appropriate time and on the appropriate color channel (as determined from the calibration curves) and normalized the intensities against those from the control devices (unspiked ABP solution). The control devices treated with ABP solution showed no response in any of the assays, i.e., the ALP reaction zone remained colorless, the AST reaction zone remained dark blue, and the Protein reaction zone remained yellow. The devices treated with AST only showed a response in the AST assay; it turned pink, whereas the ALP zone remained colorless and the Protein zone remained yellow. Similarly, the ALP solution elicited a response only in the ALP assay (it turned purple), and the BSA solution elicited a response only in the Protein assay (it turned green). These results indicate that cross-reactivity between assays and analytes did not occur; each assay is specific for its intended analyte (ASSURED - Specific).
Figure 5.
Results from testing the cross-reactivity of the enzymes prepared in artificial blood plasma. Samples were prepared with sufficient quantities of one analyte (AST (780 U/L), ALP (1200 U/L), or BSA (150 g/L) to elicit a response in each assay. Analysis of each assay on the devices at the appropriate time and color channel determined the intensity of each analyte. Normalized intensities of each assay against the control ABP solution shows that each series of devices only elicited a response (i.e. an increase or decrease in intensity)48 between the analyte and their respective assay, indicating that each assay was specific for its analyte and that no cross-reactivity occurred.
Analysis in whole blood
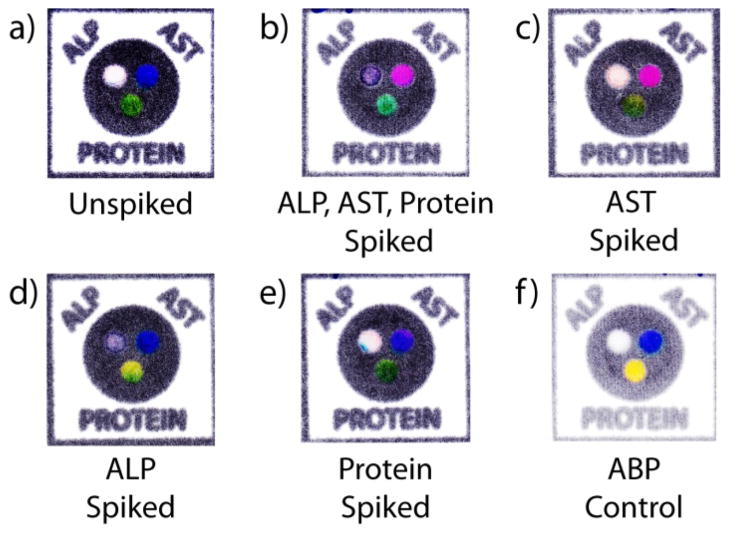
We have established the performance of the devices using artificial blood plasma as a surrogate for whole blood. To validate that the new blood testing platform is a viable prototype, we demonstrated its ability to process whole blood samples and still reliably develop the assays. Five different samples of blood were assessed: an unspiked sample of blood obtained from a fingerstick of a human subject, a sample of human blood purchased from a blood bank and spiked with: i) all three analytes (AST, ALP, and BSA), ii) AST only, iii) ALP only, and iv) BSA only. A device spotted with ABP served as a control in which no reaction would occur. Figure 6 shows the devices from this experiment. The blood from a fingerstick gave results consistent with the colors within the normal range for AST and ALP analyte compared to a control using ABP; the Protein assay developed green, which is consistent with protein being present in blood. The blood sample spiked with all three analytes produced a response in all three assays; AST turned pink, ALP turned purple and Protein turned green. The blood sample spiked with only AST registered a response in the AST assay. The sample spiked with ALP registered a response in the ALP assay. Both AST-spiked and ALP-spiked samples also registered a response for Protein which is normal since the blood of a healthy person should measure between 60 and 83 g/L of protein. The sample of blood spiked with BSA registered a response only in the Protein assay.
Figure 6.
Samples of devices tested using whole blood samples after 30 minutes. a) An unspiked sample from a fingerstick. A sample of whole blood spiked with sufficient quantities to elicit a response in each assay: b) all three analytes, c) AST only, d) ALP only, e) BSA only. f) A control with ABP without any analytes.
The actual concentrations of the analytes in the spiked whole blood samples were not measured. Based on the estimated quantity of enzyme added from the stock solutions, the theoretical concentrations in the samples were ~4000 U/L for AST, ~26000 U/L for ALP, and/or ~50 g/L for Protein. The concentrations calculated based on the color intensity for the samples analyzed at the specified times for each analyte are summarized in Table S2. The values calculated for AST and ALP in the spiked samples were lower than the theoretical values. The discrepancies could be the result of one or a combination of several factors. i) We generated the calibration curves using a surrogate artificial blood plasma (ABP) and not real blood plasma, which could affect the measured color intensities. The presence of a background color from the red blood cells on the top side of the filter could affect the color intensity of the developed assay. ii) The activities of the enzymes could have been lower than the measured value on the bottle, perhaps due to aging of the stock solution. iii) The variation in the quantity of spotted reagents in the reaction-zones due to manual fabrication of the devices could potentially alter the rate of color development for a particular assay. The platform, overall, is effective for separating blood plasma and developing three separate assays simultaneously.
Conclusions
This paper presents a new platform for measuring analytes in blood obtained from a fingerstick that consists of three simple components: paper, a plasma filter, and a plastic sheath. The platform satisfies all aspects of the ASSURED guidelines developed by the WHO for POC diagnostic devices suitable for low-resource settings. It offers several advantages including: i) the device only requires microliter volumes of fluid which can be obtained from a fingerstick and alleviates the challenge of specimen collection, ii) sample preparation and processing is all done on one device; the use of a single chip simplifies the challenge of specimen processing—reagents are all stored on chip, iii) multiple assays develop simultaneously, iv) the device is inexpensive, light weight, portable, energy efficient (no pump or external equipment is needed to run the assays), robust, and contains no integrated sharps, v) the device can be integrated with telemedicine for quantitative off-site diagnosis of disease, and vi) the device can be incinerated for easy disposal.
Although this paper presents significant initial work towards a complete point-of-care liver function device, several issues need to be resolved before the device would be truly suitable for distribution in low-resource settings. For instance, more in-depth stability analyses – particularly at elevated temperatures, and studies of other potential substances that could interfere with the assays. A manufacturability assessment will also need to be addressed before field-testing. The calibration curves presented here do not account for background color contributed by blood plasma. This problem can be addressed by either generating calibration curves using whole blood samples with known concentrations of analytes, or including an extra zone on the device as a control that can be used to subtract the background color from the plasma. Additionally, while we have demonstrated the performance of our device on spiked samples of whole blood, a more rigorous analysis of device performance on clinical specimens, especially in the hands of minimally trained users, is needed. These studies will be performed in future work.
Current methods of monitoring health based on symptoms in developing countries are often times ineffective. The introduction of a zero-cost diagnostic platform for monitoring liver function establishes a new level of health care currently unavailable in resource-poor settings. Diagnostics for All, Inc. (DFA), a non-profit organization, is working towards commercializing a version of the liver function device described in this paper.49
Supplementary Material
Acknowledgments
This work was funded by Bill and Melinda Gates Foundation Award Number 51308 and by DARPA through the MF3 Micro/nano Fluidics Fundamentals Focus Center, Natural Sciences and Engineering Research Council (NSERC) of Canada Postdoctoral Fellowhip (S.J.V.), a pre-doctoral fellowship from NSF (A.W.M.), the Damon Runyon Cancer Research Foundation (DRG-1805-04) (S.T.P.), NIH Ruth L. Kirschstein NRSA Postdoctoral Fellowhip (S.T.P).
Footnotes
Supporting Information Available
Additional information as noted in text is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Yager P, Domingo GJ, Gerdes J. Annu Rev Biomed Eng. 2008;10:107–144. doi: 10.1146/annurev.bioeng.10.061807.160524. [DOI] [PubMed] [Google Scholar]
- 2.Kettler H, White K, Hawkes S. Mapping the landscape of diagnostics for sexually transmitted infections. World Health Organization; Geneva, SW: 2004. pp. 1–37. [Google Scholar]
- 3.Mabey D, Peeling RW, Ustianowski A, Perkins MD. Nat Rev Microbiol. 2004;2:231–240. doi: 10.1038/nrmicro841. [DOI] [PubMed] [Google Scholar]
- 4.Udea M, Penny LA, Olmsted SS, Giovanni MY, Kaspar P, Shepherd A, Wilson P, Dahl CASBGM, Burgess DCH. Nature. 2006;444:73–79. doi: 10.1038/nature05448. [DOI] [PubMed] [Google Scholar]
- 5.Fitzgerald DW, Pape JW, Wasserheit JN, Counts GW, Corey L. The Lancet. 2003;362:993–994. doi: 10.1016/S0140-6736(03)14372-3. [DOI] [PubMed] [Google Scholar]
- 6.Kent DM, McGrath D, Ioannidis JPA, Bennish ML. Clinical Infectious Diseases. 2003;37:S13–S24. doi: 10.1086/375368. [DOI] [PubMed] [Google Scholar]
- 7.Jambroes M, Dieleman J, van der Weil M, van Sighem A, Reiss P, Gijssens I, Brinkman K, Lange J, de Wolf F. Changing Mortality in an HIV-Infected Population on HAART. 9th Conference on Retroviruses and Opportunistic Infections; Alexandria, VA. 2002. p. Abstract 754-W. [Google Scholar]
- 8.Reisler R, Han C, Burman W, Tedaldi E, Neaton J. Incidence of grade IV event, AIDS, and mortality in a large multicenter cohort receiving HAART. 9th Conference on Retroviruses and Opportunistic Infections; Seattle, WA. 2002. p. Abstract 36. [Google Scholar]
- 9.Mathers CGS, Mascarenha M. Global Health Risks: Mortality and burden of disease attributable to selected major risks. World Health Organization; 2009. pp. 1–70. [Google Scholar]
- 10.Martinez AW, Phillips ST, Carrilho E, Whitesides GM. Anal Chem. 2010;82:3–10. doi: 10.1021/ac9013989. [DOI] [PubMed] [Google Scholar]
- 11.Reches M, Mirica KA, Dasgupta R, Dickey MD, Butte MJ, Whitesides GM. ACS Appl Mater Interfaces. 2010;2:1722–1728. doi: 10.1021/am1002266. [DOI] [PubMed] [Google Scholar]
- 12.Mirica KA, Phillips ST, Mace CR, Whitesides GM. J Agric Food Chem. 2010;58:6565–6569. doi: 10.1021/jf100377n. [DOI] [PubMed] [Google Scholar]
- 13.Martinez AW, Phillips ST, Butte MJ, Whitesides GM. Angew Chem, Int Ed. 2007;46:1318–1320. doi: 10.1002/anie.200603817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nie Z, Deiss F, Liu X, Akbulut O, Whitesides GM. Lab Chip. 2010;10:3163–3169. doi: 10.1039/c0lc00237b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao W, Ali MM, Aguirre SD, Brook MA, Li Y. Anal Chem. 2008;80:8431–8437. doi: 10.1021/ac801008q. [DOI] [PubMed] [Google Scholar]
- 16.Dungchaia W, Chailapakula O, Henry CS. Anal Chim Acta. 2010;674:227–233. doi: 10.1016/j.aca.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 17.Li X, Tian J, Shen W. Anal Bioanal Chem. 2010;396:495–501. doi: 10.1007/s00216-009-3195-9. [DOI] [PubMed] [Google Scholar]
- 18.Fu E, Liang T, Houghtaling J, Ramachandran S, Ramsey SA, Lutz B, Yager P. Anal Chem. 2011;83:7941–7946. doi: 10.1021/ac201950g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng CM, Martinez AW, Gong J, Mace CR, Phillips ST, Carrilho E, Mirica KA, Whitesides GM. Angew Chem, Int Ed. 2010;122:4881–4884. doi: 10.1002/anie.201001005. [DOI] [PubMed] [Google Scholar]
- 20.Chin CD, Laksanasopin T, Cheung YK, Steinmiller D, Linder V, Parsa H, Wang J, Moore H, Rouse R, Umviligihozo G, Karita E, Mwambarangwe L, Braunstein SL, van de Wijgert J, Sahabo R, Justman JE, El-Sadr W, Sia SK. Nat Med. 2011;17:1015–1020. doi: 10.1038/nm.2408. [DOI] [PubMed] [Google Scholar]
- 21.LaBarre P, Hawkins KR, Gerlach J, Wilmoth J, Beddoe A, Singleton J, Boyle D, Wiegl B. PLoS One. 2011;6:e19738. doi: 10.1371/journal.pone.0019738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maupin PH. One-step test for Aspartate Aminotransferase. 5,834,226. US. 1998 November 10;
- 23.Bowers GN, McComb RB. Clin Chem. 1966;12:70–89. [PubMed] [Google Scholar]
- 24.Wilkinson JH, Boutwell JH, Winsten S. Clin Chem. 1969;15:487–495. [PubMed] [Google Scholar]
- 25.Keiding R, MHörder M, Gerhardt Denmark W, Pitkänen E, Tenhunen R, Strömme JH, Theodorsen L, Waldenström J, Tryding NLW. Scand J Clin Lab Invest. 1974;33:291–306. [Google Scholar]
- 26.Bradford MM. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 27.Matsuzawa T, Katunuma N. Anal Biochem. 1966;17:143–153. doi: 10.1016/0003-2697(66)90017-0. [DOI] [PubMed] [Google Scholar]
- 28.Del Corso A, Cappiello M, Buono F, Moschini R, Paolicchi A, Mura U. J Biochem Bioph Methods. 2006;67:123–130. doi: 10.1016/j.jbbm.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 29.Capps RDI, Batsakis JG, Briere RO, Calam RRL. Clin Chem. 1966;12:406–413. [PubMed] [Google Scholar]
- 30.Allain CC, Henson CP, Nadel MK, Knoblesdorff AJ. Clin Chem. 1973;19:223–227. [PubMed] [Google Scholar]
- 31.Wu T-W, Dappen GM. Colorimetric Assay for Bilirubin. 4,069,017. US. 1978 January 17;
- 32.Tostmann A, Boeree MJ, Aarnoutse RE, de Lange WCM, van der Ven AJAM, Dekhuijzen R. J Gastroen Hepatol. 2008;23:192–202. doi: 10.1111/j.1440-1746.2007.05207.x. [DOI] [PubMed] [Google Scholar]
- 33.González de Requena D, Núñez M, Jiménez-Nácher I, Soriano V. AIDS. 2002;16:290–291. doi: 10.1097/00002030-200201250-00020. [DOI] [PubMed] [Google Scholar]
- 34.Christian B, Okuma J, Hawkins C, Chalamilla G, Spiegelman D, Nagu T, Kanyangarara M, Mugusi F, Fawzi W. Prevalence of Hepatitis B and C Co-infection and Response to Antiretroviral Therapy among HIV-infected Patients in an Urban Setting in Tanzania. 17th Conference on Retroviruses & Opportunistic Infections; San Francisco, CA. 2010. [Google Scholar]
- 35.den Brinker M, Wit FWNM, Wertheim-van Dillen PME, Jurriaans S, Weel J, van Leeuwen R, Pakker NG, Reissa P, Danner SA, Weverling GJ, Lange JMA. AIDS. 2000;14:2895–2902. doi: 10.1097/00002030-200012220-00011. [DOI] [PubMed] [Google Scholar]
- 36.Hossain PMD, Kawar BMD, El Nahas MMD., PhD N Engl J Med. 2007;356:213–215. doi: 10.1056/NEJMp068177. [DOI] [PubMed] [Google Scholar]
- 37.Wild D. The Immunoassay Handbook. Elsevier, Inc; San Diego, CA: 2005. [Google Scholar]
- 38.Carrilho E, Martinez AW, Whitesides GM. Anal Chem. 2009;81:7091–7095. doi: 10.1021/ac901071p. [DOI] [PubMed] [Google Scholar]
- 39.Burtis CA, Ashwood ER, Bruns DE. Teitz Textbook of Clinical Chemistry and Molecular Diagnostics. Elsevier Inc; St. Louis: 2006. [Google Scholar]
- 40.Martinez AW, Phillips ST, Wiley BJ, Gupta M, Whitesides GM. Lab Chip. 2008;8:2146–2150. doi: 10.1039/b811135a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.GX and GR filter membranes are both fabricated from an asymmetric polysulfone material, which minimizes cell lysis during separation and maximizes isolated plasma.
- 42.Liu L, Qiu CL, Chen Q, Zhang SM. J Alloys Compd. 2006;425:268–273. [Google Scholar]
- 43.Premarketing Clinical Evaluation. Food and Drug Administration; 2009. FDA Guidance for Industry Drug-Induced Liver Injury; pp. 1–28. [Google Scholar]
- 44.Statland BE. Clinical Decision Levels for Lab Tests. Medical Economics Books; Ann Arbor, MI: 2008. [Google Scholar]
- 45.The overall color of a reaction zone is determined by the average of all the colors in that zone. If there is incomplete wetting of a zone, part of the zone may remain undeveloped or underdeveloped, which would result in a skewed intensity value for that reaction zone.
- 46.Goutelle S, Maurin M, Rougier F, Barbaur X, Bourguignon L, Ducher M, Maire P. Fundam Clin Pharm. 2008;22:633–648. doi: 10.1111/j.1472-8206.2008.00633.x. [DOI] [PubMed] [Google Scholar]
- 47.Arakawa T, Prestrelski SJ, Kenney WC, Carpenter JF. Adv Drug Delivery Rev. 2001;46:307–326. doi: 10.1016/s0169-409x(00)00144-7. [DOI] [PubMed] [Google Scholar]
- 48.RGB space is an additive color space, where white has the maximum intensity (255), and the appearance of a colored spot on a white background will show a decrease in intensity. For the ALP assay, the presence of ALP resulted in a decrease in grayscale intensity (White = 255, Black = 0). For the AST assay, the presence of AST resulted in an increase in total color intensity. For the Protein assay, the presence of BSA resulted in a decrease in the red intensity.
- 49.Diagnostics For All. 2011 November 12; http://dfa.org/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.