Abstract
The influence of progesterone in the brain and on the behavior of females is fairly well understood. However, less is known about the effect of progesterone in the male system. In male rats, receptors for progesterone are present in virtually all vasopressin (AVP) immunoreactive cells in the bed nucleus of the stria terminalis (BST) and the medial amygdala (MeA). This colocalization functions to regulate AVP expression, as progesterone and/or progestin receptors (PR)s suppresses AVP expression in these same extrahypothalamic regions in the brain. These data suggest that progesterone may influence AVP-dependant behavior. While AVP is implicated in numerous behavioral and physiological functions in rodents, AVP appears essential for social recognition of conspecifics. Therefore, we examined the effects of progesterone on social recognition. We report that progesterone plays an important role in modulating social recognition in the male brain, as progesterone treatment lead to a significant impairment of social recognition in male rats. Moreover, progesterone appears to act on PRs to impair social recognition, as progesterone impairment of social recognition is blocked by a PR antagonist, RU-486. Social recognition is also impaired by a specific progestin agonist, R5020. Interestingly, we show that progesterone does not interfere with either general memory or olfactory processes, suggesting that progesterone seems critically important to social recognition memory. These data provide strong evidence that physiological levels of progesterone can have an important impact on social behavior in male rats.
Keywords: Vasopressin, Progestin Receptors, Progesterone, Social Recognition Behavior, Social memory
Introduction
There is a notable lack of knowledge about the role of the hormone progesterone in males. The vast body of our knowledge on progesterone and progestin receptor (PR) function comes from studies in females (Priest and Pfaff, 1995;Blaustein, 2008). Although progesterone has always been considered a “female hormone”, adult male rats have circulating levels of progesterone around 1.5 - 2 ng/ml (Auger and Vanzo, 2006;Andersen et al., 2004), compared to a range of 3 - 35 ng/ml in females that is seen throughout the rat estrus cycle (Weisz and Ward, 1980). Also, depending on the type of stressful event encountered, progesterone levels in males can approach 6 ng/ml (Andersen et al., 2004), suggesting a potential functional significance of this hormone in males. Recent studies demonstrate that progesterone and its receptor play an important, yet understudied, role in male behavior and physiology (Wagner, 2006). It is also important to note, that as males have higher levels of steroid receptor coactivators, which enhance steroid hormone action in many brain regions (Bian et al., 2011), it is likely that lower levels of progesterone are sufficient to elicit a physiological response within the male brain.
It has been shown that PRs are found in virtually every AVP-immunoreactive (AVP-ir) cell within the bed nucleus of the stria terminalis (BST) and medial amygdala (MeA) (Auger and De Vries, 2002). Indeed, this co-localization has functional implications for AVP regulation in the BST and MeA cells, as progesterone treatment results in a suppression of AVP-ir labeling within these cells, and two of the projection sites of these cells, the lateral septum (LS) and lateral habenula (LH) (Auger and Vanzo, 2006). Taken together, these data suggest an important role for progesterone regulation of AVP expression; however, it is unclear from these data if progesterone can regulate AVP-dependent behaviors.
One behavior that is linked to AVP in the LS, a site that receives AVP projections from the BST and MeA, is social recognition behavior. Social recognition paradigms capitalize on an animal's innate motivation to investigate unfamiliar conspecifics (Ferguson et al., 2002). An animal's ability to recognize a conspecific after an initial exposure typically lasts only 30 minutes; however, subcutaneous injections of AVP in rats and mice can facilitate social recognition by lengthening this social memory to 2 hours after initial exposure to a conspecific (Dantzer et al., 1987;Bielsky and Young, 2004). Social recognition is also enhanced by cite specific infusions of an AVP agonist or impaired by infusions of AVP antagonists directly into the lateral septum (Dantzer et al., 1988). Furthermore, castration, which depletes AVP in the BST and MeA, and in projection sites of these cells, also impairs social recognition (Bluthe et al., 1990). These studies demonstrate the importance of AVP in social recognition. As social recognition behavior is clearly AVP-dependent, and progesterone treatment functions to suppresses AVP-ir expression within the BST and MeA (Auger and Vanzo, 2006), we hypothesized that progesterone treatment would impair social recognition within the male brain.
Methods
Animals
Adult male Sprague-Dawley rats were bred in our animal facility from breeding stock obtained from Charles River (Charles River Laboratories, Inc., Wilmington, MA). Juvenile male stimulus animals (between 20-30 days old) were purchased directly from Charles River. All animals were group housed in our animal facility, unless otherwise noted, on a 12h light/12h dark cycle with lights off at 11:00 am, and had free access to food and water. This research was approved by the University of Wisconsin Animal Care and Use Committee. Different cohorts of animals were used in each experiment described below unless otherwise noted.
Drug treatments
All of the experiments described in this paper involved drug treatment administration via subcutaneous injection. Injections occurred during the “lights on” portion of the light cycle for three consecutive days. RU-486 (Steraloids, Newport R.I.; 5mg) was dissolved in 0.4 mL of vehicle (5% benzyl alcohol, 15% benzyl benzoate, sesame oil, all from Sigma-Aldrich Co., St Louis, MO, USA). Progesterone (Steraloids, Newport R.I.; 1mg) was dissolved in 0.1 mL of sesame oil. R5020 (Perkin Elmer, Boston MA; 20μg) was dissolved in 0.1 mL of sesame oil. 0.4 mL of vehicle was used as a control for RU-486 and 0.1 mL of sesame oil was used as a control for progesterone and R5020.
Behavioral testing and Statistical analyses
Testing took place during the “lights off” portion of the light cycle under dim red light in our behavior room. All behavior was digitally recorded, unless otherwise noted, and then analyzed by a trained researcher blind to all treatments using The Observer (Noldus Information Technologies, Leesburg, VA) behavioral observation software. Sigma Stat 3.5 was used to conduct all statistical analyses. For the habituation-dishabituation study, a two-way repeated measures ANOVA was used to compare treatment by trial. A one-way ANOVA was run on habituation scores, which were calculated by subtracting the amount of investigation in trial 4 from the amount of investigation in trial 5. In the food-finding test, groups were compared using a Student's t test. Paired t tests were used to statistically analyze all other experiments.
Experiment 1 Habituation-Dishabituation, RU-486
Adapted from Winslow and Camacho (Winslow and Camacho, 1995). 4 month old male rats (n=23) were pretreated with RU-486 or vehicle, and then 2 hours later treated with progesterone or oil, for 3 days. During the 3 days of injections, the animals were separated from their cage mates and singly housed. On the third day of pretreatment and treatment, animals underwent behavioral testing 4 hours after the last round of injections. Testing occurred in the home cages of the adult male subjects. The test for each subject involved five, 1 minute trials. During the first four trials, the same juvenile male rat was placed in the subject's cage. On the fifth trial, a novel juvenile male rat was placed in the subject's cage. A 10 minute intertrial interval occurred between each of the five trials. Adult investigation of the juvenile was scored to include direct contact between the nose of the adult and the body of the juvenile and close (within 1 cm) following behavior. Cage directed behavior was also scored as a measure of basic locomotor and investigatory activity.
Experiments 2 and 3 Social Discrimination, RU486, R5020
The social discrimination paradigm was adapted from Engelmann et al. (Engelmann et al., 1995). In experiment 2, 3 month old male rats (n=40) received the same pretreatment with RU-486 or vehicle, and treatment with progesterone or oil as described above in the methods for experiment 1. In experiment 3, a different set of 3 month old male rats (n=40) received injections of progesterone, R5020 or oil for 3 days. Again, during the 3 days of injections, the animals were separated from their cage mates and singly housed. On the third day, injections of progesterone or oil were administered 4 hours before behavioral testing, while injections of R5020 were given 2 hours before behavioral testing, this time course for R5020 is when R5020 was empirically found to be the most behaviorally effective. Testing occurred in the home cages of the adult male rats. In trial 1, a male juvenile rat was placed in the home cage of the adult rat and the adult was allowed to freely investigate for 5 minutes. After 5 minutes, the juvenile was removed and the adult was alone in its cage for 30 minutes. After the 30 minute intertrial interval, the juvenile from trial 1 plus a novel juvenile were placed in the adult's cage, and the adult was again free to investigate for 5 minutes. The juvenile rats were distinguishable to the researcher scoring the video by unique tail marks drawn with permanent marker. Adult investigation of the juvenile(s) was scored to include direct contact between the nose of the adult and the body of the juvenile and close following behavior. Cage directed behavior was also scored as a measure of basic locomotor and investigatory activity.
Experiment 4 Olfactory tests, Preputial preference test
4 month old male rats (n=40) received the same pretreatment with RU-486 or vehicle, and treatment with progesterone or oil as described above in the habituationdishabituation paradigm. During the 3 days of injections, the animals were separated from their cage mates and singly housed. This test utilized preputial glands that were surgically removed from sacrificed male rats about 20-30 days old. The preputial glands were homogenized in ice cold tris buffered saline (TBS) and then centrifuged (Thompson et al., 2007). The supernatant (preputial extract) was then removed and stored at -80 degrees Celsius. On the day of behavior testing, the preputial extract was thawed and used in a preference test. While in its home cage, each adult male rat was exposed to two Nestlets (Ancare, Bellmore, N.Y.): one with 40 μL of preputial extract on it, the other with 40 μL of TBS. The subject rat was freely allowed to investigate both Nestlets for 5 minutes. Behavior was digitally recorded and scored for direct contact between the subject and the Nestlet. Animals used in this experiment were the same cohort as that used in experiment 2.
Experiment 4 Olfactory tests, Food-finding test
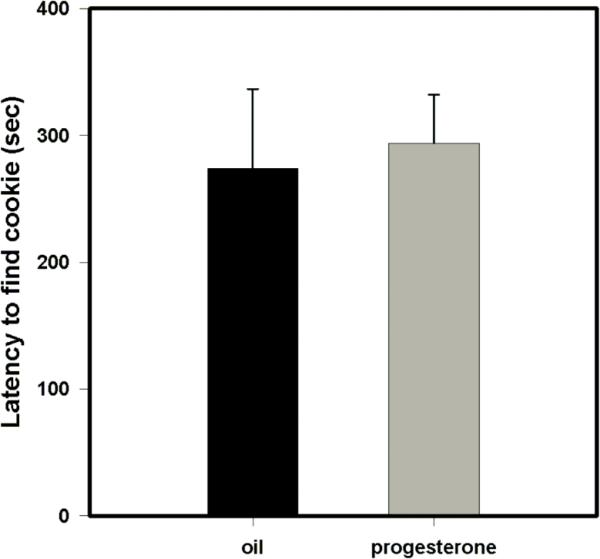
This paradigm was adapted from (Mencio-Wszalek et al., 1992). 6.5 month old rats (n=20) were treated with either progesterone or oil for three days. On the third day, injections occurred 4 hours before behavioral testing. A piece of chocolate chip cookie (average weight of 7.6 g) was buried at the center of a clean cage under a 1 cm layer of fresh bedding. Subjects were placed in the corner of the test cage and given a maximum of 10 minutes to uncover the cookie. The latency until the subject uncovered the buried cookie was measured in seconds with a stop watch.
Experiment 5 Object recognition test
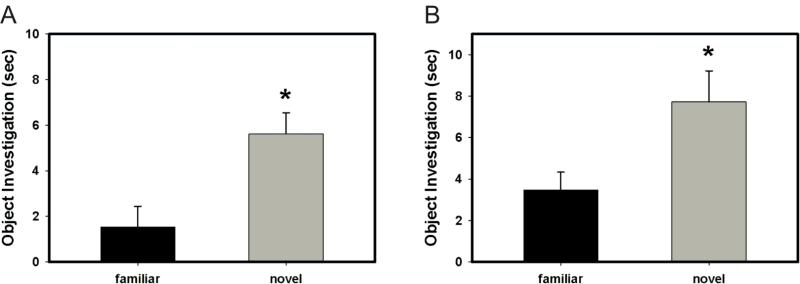
This paradigm was adapted from (Bevins and Besheer, 2006). 6.5 month old rats (n=20) were treated with either progesterone or oil for three days. On the third day, injections occurred 4 hours before behavioral testing. Identical objects (either large Lego blocks or metal tea balls) were placed in the back right and left corners of a Plexiglas chamber (60 × 38 × 39 cm). The subject was placed at the mid-point of the wall opposite the objects in a position facing away from the objects. For 5 minutes, the subject was freely allowed to investigate the objects. After a 1 hour intertrial interval, the subject was placed back in the same apparatus, which this time contained one of the objects from trial 1 and one novel object (if Lego blocks were used in trial 1, a tea ball was the novel object in trial 2 and vice versa). Again, the subject was allowed to freely investigate the objects for 5 minutes. Behavior was digitally recorded and scored for direct contact between the subject and the objects. Objects were counter balanced for location in chamber and for being the novel or familiar object across subjects.
Results
Experiment 1 Habituation-Dishabituation: RU-486
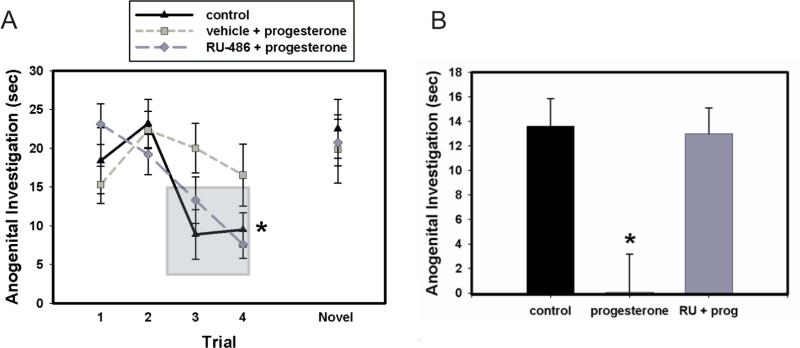
Since it has previously been shown that progesterone treatment decreases AVP immunoreactivity in the BST and the MeA, it was our goal to determine if progesterone would also affect social recognition, an AVP-dependent behavior. The group designated “control” consisted of the animals that were treated with RU-486 then oil (RU-486 + oil), and animals that were treated with vehicle then oil (vehicle + oil). As there were no significant difference between the two control groups (p > 0.05), these control groups were combined. In the habituation-dishabituation paradigm, we found a significant effect of exposure trial (F(4, 80) = 7.65, p< 0.001), as well as a significant interaction between treatment and trial (F(8, 80) = 2.4, p = 0.022). Post-hoc testing indicates a significant decrease in investigation between trial 1 and trials 3 and/or 4 in our control animals (p = 0.028 and p = 0.039 for trials 1 vs. trial 3 and trial 1 vs. 4, respectively). There was also a decrease in investigation between trial 1 and trials 3 and/or 4 in animals that were pretreated with RU-486 and then treated with progesterone (RU-486 + progesterone; p = 0.009 and p = <0.001 for trials 1 vs. trial 3 and trial 1 vs. 4, respectively). In contrast, animals treated with vehicle and then progesterone (vehicle + progesterone) displayed impaired social recognition, as they did not show significantly decreased investigation between trial 1 and trials 3 and/or 4 (p = 0.179 and p = 0.726 for trial 1 vs. trial 3 and trial 1 vs. 4, respectively). A habituation score (investigation in trial 5 minus investigation in trial 4) was also calculated to compare the treatment groups. Animals treated with vehicle and then progesterone (progesterone) were significantly different (F(2, 19) = 8.740, p = 0.002) from both the control group (control) and the animals treated with RU-486 and then progesterone (RU + prog). In summary, the control animals demonstrated normal recognition in the habituation-dishabituation paradigm, while the vehicle + progesterone group demonstrated impaired social recognition. Progesterone impairment of social recognition was blocked by pre-treatment with the progesterone antagonist, RU486 (Fig. 1a and 1b).
Figure 1.
Effect of treatment with progesterone or oil and pretreatment with RU-486 or vehicle on social recognition in the habituation-dishabituation paradigm. (A) Anogenital investigation of juvenile rat. Post-hoc analysis showed that animals in the control group (vehicle + oil and RU-486 + oil) exhibited normal social recognition (* p = 0.028 and p = 0.039 for trials 1 vs. trial 3 and trial 1 vs. 4, respectively), as did the animals in the RU-486 + progesterone group (* p = 0.009 and p < 0.001 for trials 1 vs. trial 3 and trial 1 vs. 4, respectively). Normal social recognition is identified when there is a significant decrease in investigation on trials 3 and 4 compared to trial 1, as indicated by the shaded box. Rats in the vehicle + progesterone group demonstrated impaired social recognition by not decreasing investigation in trials 3 and 4 (p = 0.179 and p = 0.726 for trial 1 vs. trial 3 and trial 1 vs. 4, respectively). (B) Habituation score, which was calculated by subtracting the amount of anogenital investigation in trial 4 from the amount of anogenital investigation in trial 5. Animals in the progesterone group were significantly different (* p = 0.002) from both the control group and the RU-prog group. Error bars represent SEM.
Experiment 2 Social discrimination: RU-486
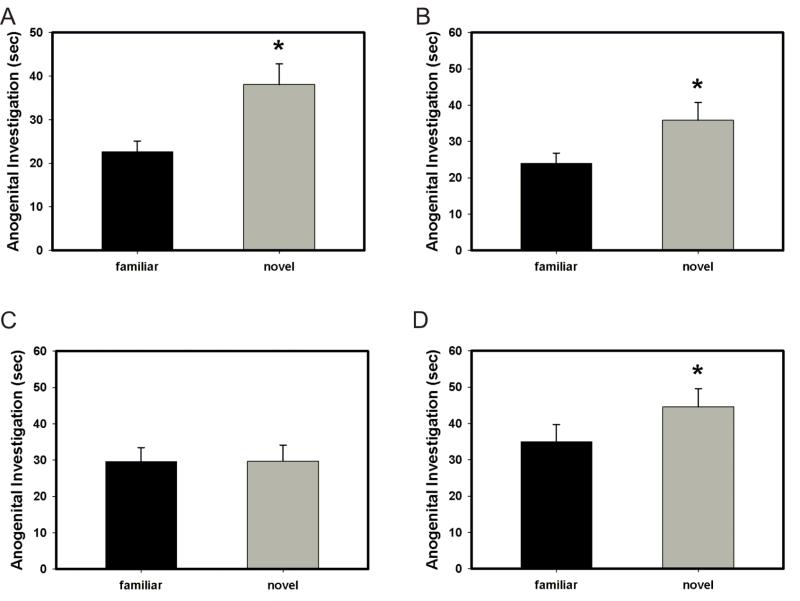
In order to confirm our results with a different social recognition paradigm, we tested social recognition using the social discrimination paradigm. In this paradigm, control animals (vehicle + oil, and RU486 + oil) discriminated normally (significantly more investigation of familiar juvenile than novel juvenile in trial 2 (p = 0.026, p = 0.021, respectively; Fig. 2a and 2b) while the animals in the vehicle + progesterone group failed to discriminate normally (p = 0.983, Fig. 2c). Importantly, the PR anatagonist, RU-486, blocked progesterone impairment of social recognition, as the amount of time an animal in this group (RU-486 + progesterone) spent investigating the novel juvenile as opposed to the familiar one was significantly different (p = 0.041 Fig. 2d).
Figure 2.
Effect of treatment with progesterone or oil and pretreatment with RU-486 or vehicle in the social discrimination paradigm. Anogenital investigation of juvenile rats in trial 2. (A) Animals in the vehicle + oil group discriminated normally (significantly more investigation of familiar juvenile than novel juvenile, * p = 0.026). (B) Animals in the RU-486 + oil group discriminated normally (* p = 0.021). (C) Animals in the vehicle + progesterone group failed to discriminate normally (p = 0.983). (D) Animals in the RU-486 + progesterone group discriminated normally (* p = 0.041), indicating that the PR antagonist RU-486 blocked the effect of progesterone on social discrimination. Error bars represent SEM.
Experiment 3 Social discrimination: R5020
In order to further demonstrate the specificity of progesterone induced impairment of social recognition through action on PR, we treated male rats with a synthetic progestin, R5020. Again, control animals treated with oil discriminated normally (p = 0.045, Fig. 3a); however, animals treated with either the non-metabolizable synthetic progestin, R5020, or progesterone both showed impaired social discrimination (p = 0.313 and p = 0.868, respectively; Fig. 3b and 3c).
Figure 3.
Effect of treatment with progesterone, oil, or synthetic progestin R5020 in the social discrimination paradigm. Investigation of juvenile rats in trial 2. (A) Animals in the oil group discriminated normally (* p = 0.045). (B) Animals treated with R5020 showed impaired social discrimination (p = 0.313). (C) Animals treated with progesterone showed impaired social discrimination (p = 0.868). Error bars represent SEM.
Experiment 4 Olfactory tests
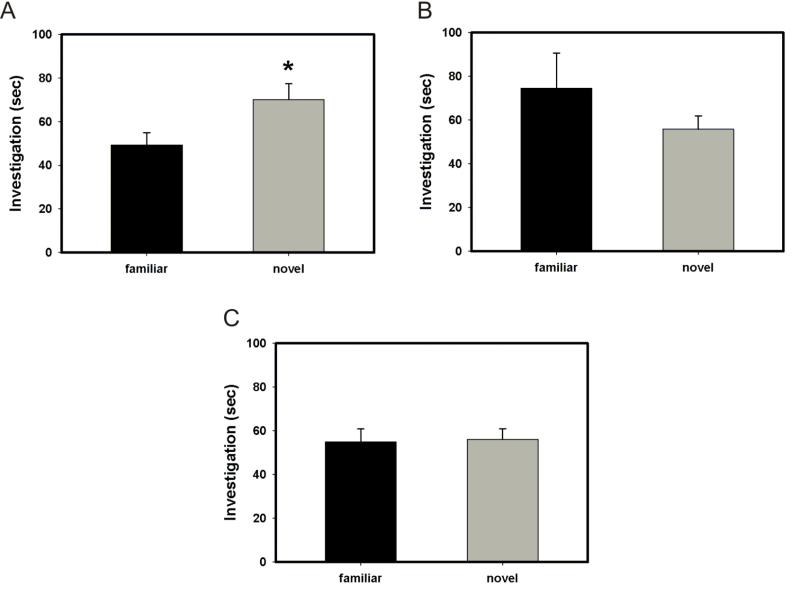
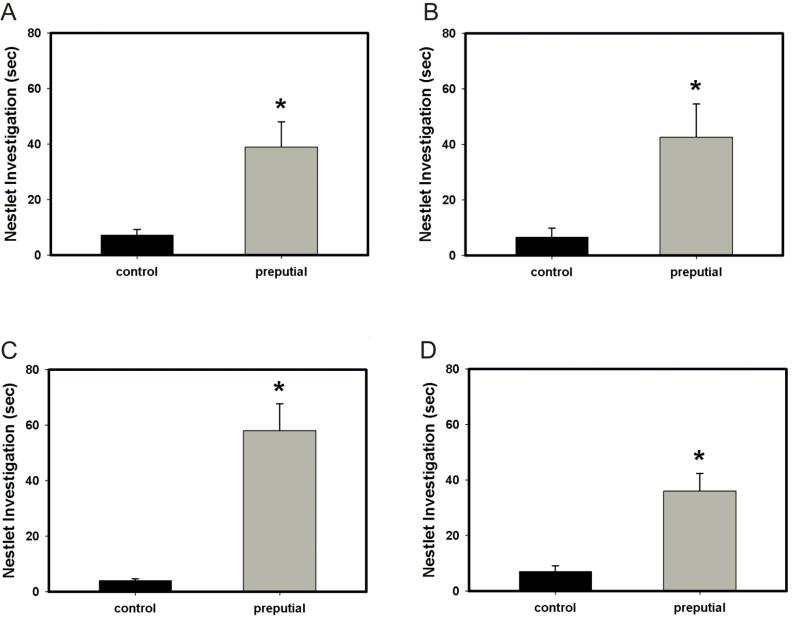
In the preputial preference test, a test of preference for social vs. non-social odors, all treatment groups (vehicle + oil, vehicle + progesterone, RU-486 + progesterone and RU-486 + oil) preferred the preputial-treated Nestlet (preputial) compared to the TBS-treated Nestlet (control) (vehicle + oil, p = 0.008; vehicle + progesterone, p < 0.001; RU-486 + progesterone, p = 0.004; RU-486 + oil, p = 0.007; Fig. 4a – 4d). Similarly, in a test of food-finding ability, animals treated with either oil or progesterone did not statistically differ from each other in latency to uncover a hidden piece of cookie (p = 0.783; Fig. 5). These data suggest that progesterone did not interfere with general olfaction.
Figure 4.
Effect of treatment with progesterone or oil and pretreatment with RU-486 or vehicle on preference for preputial-treated Nestlet vs. control Nestlet. (A-D) All treatment groups (vehicle + oil, RU-486 + oil, vehicle + progesterone and RU-486 + progesterone) preferred the preputial-treated Nestlet (preputial) compared to the TBS-treated Nestlet (control) (vehicle + oil, * p = 0.008; RU-486 + oil, * p = 0.007; vehicle + progesterone, * p < 0.001; RU-486 + progesterone, * p = 0.004; Fig. 4a – 4d). Error bars represent SEM.
Figure 5.
Effect of treatment with progesterone or oil on food-finding ability. There was no statistical difference between treatment groups in latency to uncover a hidden piece of cookie (p = 0.783). Error bars represent SEM.
Experiment 5 Object recognition: Progesterone vs. oil
In the object recognition paradigm, oil and progesterone treated animals significantly preferred to investigate a novel object compared to a familiar object (p < 0.001 and p = 0.04, respectively; Fig. 6a and 6b). These data suggest that progesterone does not impair memory in general.
Figure 6.
Effect of treatment with progesterone or oil on object recognition. (A) Animals treated with oil significantly preferred investigating the novel object over the familiar object (* p < 0.001). (B) Animals treated with progesterone significantly preferred investigating the novel object over the familiar object (* p = 0.04).
Discussion
Our data support the notion that physiological levels of progesterone, by acting on progestin receptors (PRs), can have a significant modulatory action on social recognition memory within the male brain. We also report that progesterone impairment of social recognition is not a result of impaired olfactory ability or general impairment of memory systems. Taken together, these data provide strong evidence that progesterone interferes with social recognition memory in adult male rats.
Although the effect of progesterone via PRs appears to be specific to social recognition memory, the presence of PRs in the olfactory bulb suggested the possibility that the impairment in social recognition following progesterone treatment could be caused by a general impairment of olfactory ability. We controlled for this possibility, in two different tests of olfactory functioning. In both paradigms, progesterone did not impair olfactory performance. This suggests that the impairment in social recognition following progesterone treatment is more likely due to interference with social memory, rather than an impairment of olfactory ability. These results are supported by a 1978 study by Soares and Kalberer, where progesterone treatment prevented a typical social odor preference in male mice (Soares and Kalberer, 1978), but it did not alter latency scores in a food-finding task. It appears that in male mice, as in our male rats, progesterone seems to only impair memory for social olfactory cues and not general olfactory processing (Soares and Kalberer, 1978).
We report that progesterone impaired social recognition memory; however, novel object memory was not impaired by progesterone treatment. In agreement with our data, another study examined the effect of progesterone on working memory. It was found that progesterone did not impair performance in a novel object task, but it did impair performance on a spatial task (Sun et al., 2010). These data suggest that the mechanism by which progesterone influences memory systems may be task-specific. As we have previously reported that progesterone treatment in adult male rats decreases AVP expression within the BST and MeA (Auger and Vanzo, 2006), it is possible that progesterone impairs social recognition via suppression of AVP expression. This potential pathway would be consistent with the well known role that AVP in the BST and MeA plays in social recognition.
Data from numerous labs have indicated the importance of AVP in social recognition. When AVP antagonists are infused into the LS, one of the projection sites of the AVP cells in the BST and MeA), social recognition is impaired; conversely, LS infusions of AVP agonists enhance social recognition (Dantzer et al., 1988). Also, castration, which depletes AVP in the cells of the BST and MeA and decreases the density of fibers projecting from these cells to the LS (De Vries et al., 1985) blocks social recognition (Bluthe et al., 1990). Additionally, while one study showed that animals lacking the AVP V1A receptor show deficits in social recognition (Bielsky et al., 2004), another lab showed that mice lacking the V1A receptor did not have impairments in this behavior (Wersinger et al., 2007). However, this same lab showed that animals lacking AVP V1b receptors show deficits in social recognition (Wersinger et al., 2002), taken together these data suggest that mechanisms altering AVP levels or AVP signaling have a strong potential to influence this AVP-dependant behavior. We hypothesize that the impairment in social recognition memory that we observed is mediated through the suppression in AVP levels that are seen following progesterone treatment in the BST and MeA (Auger and Vanzo, 2006). This hypothesis is consistent with data from a study that examined the role of AVP in both social and non-social behavioral paradigms. Everts and Koolhaas, 1997, have shown that infusions of a V1A receptor antagonist into the LS are able to block social recognition in rats, but not novel object recognition (Everts and Koolhaas, 1997). These data suggest that these two different forms of memory occur via different pathways within the brain. While social recognition memory is dependent upon AVP transmission in the LS (Everts and Koolhaas, 1997), perhaps non-social memory is dependent upon other, non-AVP dependent, mechanisms.
The notion that progesterone can influence memory or structures associated with memory is not new, as it has been shown that spine density in the hippocampus is regulated over the estrus cycle in female rats (Woolley and McEwen, 1993). Specifically, dendritic spine density declines as estrogen levels fall, but they decline even faster if progesterone is administered during the decline in estrogen levels (Woolley and McEwen, 1993). On a behavioral level, changes in progesterone levels are associated with memory impairments in rodents (Vallee et al., 2001). Also, progesterone induced impairment of social memories has been demonstrated in humans. Exogenous progesterone exposure impairs women's ability to remember socially relevant stimuli (i.e., human faces) (van Wingen et al., 2007). In this study, progesterone treatment is associated with decreased activity in the amygdala and in the fusiform face area as measured by event-related fMRI (van Wingen et al., 2007). The authors suggest that the “social recognition” memory tested is impaired by elevated allopregnanolone, a neuroactive metabolite of progesterone, levels in subjects treated with progesterone (van Wingen et al., 2007). Although this study cannot rule out the possibility that progesterone itself is influencing social memories, it has been suggested that progesterone-induced memory impairments result from the activity of allopregnanolone in the brain.
Allopregnanolone potentiates the effects of GABA at the GABAA receptor, thereby increasing its inhibitory effect, and it is through this effect that allopregnanolone influences memory as well as a number of other behaviors (Dubrovsky, 2005). Although the mechanism of progesterone/allopregnanolone action on memories is not clear, allopregnanolone does appear to impair non-social learning and memory tasks in rodents. Previous data indicate allopregnanolone can impair non-social memory in male mice (Ladurelle et al., 2000;Johansson et al., 2002). In a Y-maze novelty discrimination task, control animals preferentially explored a novel arm; however, mice receiving infusions of allopregnanolone into the lateral ventricles for several days did not investigate a novel, previously unexplored, arm in the maze, suggesting a form of spatial memory impairment (Ladurelle et al., 2000). Allopregnanolone also impairs learning in the Morris water maze following i.v. injection for several days. Animals treated with allopregnanolone show an increased latency to find the platform in the maze compared to controls (Johansson et al., 2002). In both of these experiments, motor behavior was not impaired by allopregnanolone treatment, only the learning and/or memory performance. These data, along with our data, suggest that progesterone, or its metabolites, may differentially affect social and non-social memories. The mechanism by which this may occur is unclear, but our data concerning the effects of PR modulators on social recognition memory argue that progesterone is functioning to impair social recognition memory through classical interaction with PRs, rather than through action on other receptors after its conversion to neuroactive metabolites.
Our current data also appear to extend our previous findings regarding progesterone suppression of AVP (Auger and Vanzo, 2006). That is, progesterone suppression of AVP may underlie progesterone impairment of social recognition. The mechanism by which progesterone influences AVP is likely through PRs, and our RU-486 and R5020 results presented here support that notion. Interestingly, PRs are found in discrete areas of the male brain and many of these areas play a large role in social behavior. Not surprisingly, PRs within the male brain have been reported to be expressed in areas classically associated with reproduction (Guerra-Araiza et al., 2001). However, PRs have also been found to be expressed within the olfactory bulb as well as the hippocampus (Guerra-Araiza et al., 2001). PRs are also found in areas implicated in fear, stress, and anxiety (Walker et al., 2003;Brinton et al., 2008;Auger and De Vries, 2002). The distribution of PRs in the male brain suggests that they can have a number of functional implications in the male system; however, few studies have addressed the functional role of PRs within these brain regions. We demonstrate a functional role for progesterone, as well as PRs, in regulating social recognition memory in male rats. The specific distribution of PRs in the male brain, along with our current data, indicate that progesterone action in the male brain may regulate a small, targeted, number of social and emotional processes.
As numerous factors regulate social recognition memory, such as vasopressin, oxytocin and dopamine (Keverne and Curley, 2004) it is not clear if progesterone action on one or a number of these systems influences social recognition. It is also important to note that progesterone might be acting to alter the expression of other steroid receptors that are important for regulating AVP expression and social recognition memory (De Vries et al., 1985) (Imwalle et al., 2002). For example, progesterone reduces estrogen receptor expression in the BST (DonCarlos et al., 1995), which could reduce AVP expression, and this may be the mechanism by which social recognition memory is impaired in progesterone treated animals. Future research will be necessary to elucidate the molecular pathways involved in the progesterone impairment of social recognition memory.
In conclusion, our data suggest that progesterone is an important molecule in regulating social recognition in the male brain. Progesterone appears to interfere with the formation of social recognition memory without interfering with general non-spatial memory or olfactory functioning. Nonetheless, the data presented here indicate that progesterone has an important function in the male system that may be instrumental in mediating social interactions.
Highlights.
-We report that progesterone is important in modulating social recognition in the male brain.
-Progesterone treatment leads to a significant impairment in social recognition in male rats.
-Progesterone impairment of social recognition is blocked by a PR antagonist, RU-486.
-Social recognition is also impaired by the synthetic progestin, R5020.
-Progesterone did not interfere with either general memory or olfactory processes.
Acknowledgements
This work was supported by National Institute of Mental Health – National Research Service Award (NRSA) T32 Gm007507, a National Science Foundation (NSF) Grant IOS-1122074 to CJA as well as the Psychology Department, the Graduate School and the College of Letters and Sciences at the University of Wisconsin-Madison.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Andersen ML, Bignotto M, Machado RB, Tufik S. Different stress modalities result in distinct steroid hormone responses by male rats. Braz J Med Biol Res. 2004;37:791–797. doi: 10.1590/s0100-879x2004000600003. [DOI] [PubMed] [Google Scholar]
- Auger CJ, De Vries GJ. Progestin receptor immunoreactivity within steroid-responsive vasopressinimmunoreactive cells in the male and female rat brain. J Neuroendocrinol. 2002;14:561–567. doi: 10.1046/j.1365-2826.2002.00809.x. [DOI] [PubMed] [Google Scholar]
- Auger CJ, Vanzo RJ. Progesterone treatment of adult male rats suppresses arginine vasopressin expression in the bed nucleus of the stria terminalis and the centromedial amygdala. J Neuroendocrinol. 2006;18:187–194. doi: 10.1111/j.1365-2826.2005.01400.x. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Besheer J. Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study ‘recognition memory’. Nat Protoc. 2006;1:1306–1311. doi: 10.1038/nprot.2006.205. [DOI] [PubMed] [Google Scholar]
- Bian C, Zhang D, Guo Q, Cai W, Zhang J. Localization and sex-difference of steroid receptor coactivator-1 immunoreactivities in the brain of adult female and male mice. Steroids. 2011;76:269–279. doi: 10.1016/j.steroids.2010.11.009. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Hu SB, Szegda KL, Westphal H, Young LJ. Profound impairment in social recognition and reduction in anxiety-like behavior in vasopressin V1a receptor knockout mice. Neuropsychopharmacology. 2004;29:483–493. doi: 10.1038/sj.npp.1300360. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Young LJ. Oxytocin, vasopressin, and social recognition in mammals. Peptides. 2004;25:1565–1574. doi: 10.1016/j.peptides.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Blaustein JD. Neuroendocrine regulation of feminine sexual behavior: lessons from rodent models and thoughts about humans. Annu Rev Psychol. 2008;59:93–118. doi: 10.1146/annurev.psych.59.103006.093556. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Schoenen J, Dantzer R. Androgen-dependent vasopressinergic neurons are involved in social recognition in rats. Brain Res. 1990;519:150–157. doi: 10.1016/0006-8993(90)90073-k. [DOI] [PubMed] [Google Scholar]
- Brinton RD, Thompson RF, Foy MR, Baudry M, Wang J, Finch CE, Morgan TE, Pike CJ, Mack WJ, Stanczyk FZ, Nilsen J. Progesterone receptors: form and function in brain. Front Neuroendocrinol. 2008;29:313–339. doi: 10.1016/j.yfrne.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Bluthe RM, Koob GF, Le MM. Modulation of social memory in male rats by neurohypophyseal peptides. Psychopharmacology (Berl) 1987;91:363–368. doi: 10.1007/BF00518192. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Koob GF, Bluthe RM, Le MM. Septal vasopressin modulates social memory in male rats. Brain Res. 1988;457:143–147. doi: 10.1016/0006-8993(88)90066-2. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Buijs RM, Van Leeuwen FW, Caffe AR, Swaab DF. The vasopressinergic innervation of the brain in normal and castrated rats. J Comp Neurol. 1985;233:236–254. doi: 10.1002/cne.902330206. [DOI] [PubMed] [Google Scholar]
- DonCarlos LL, Malik K, Morrell JI. Region-specific effects of ovarian hormones on estrogen receptor immunoreactivity. Neuroreport. 1995;6:2054–2058. doi: 10.1097/00001756-199510010-00024. [DOI] [PubMed] [Google Scholar]
- Dubrovsky BO. Steroids, neuroactive steroids and neurosteroids in psychopathology. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:169–192. doi: 10.1016/j.pnpbp.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Wotjak CT, Landgraf R. Social discrimination procedure: an alternative method to investigate juvenile recognition abilities in rats. Physiol Behav. 1995;58:315–321. doi: 10.1016/0031-9384(95)00053-l. [DOI] [PubMed] [Google Scholar]
- Everts HG, Koolhaas JM. Lateral septal vasopressin in rats: role in social and object recognition? Brain Res. 1997;760:1–7. doi: 10.1016/s0006-8993(97)00269-2. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Young LJ, Insel TR. The neuroendocrine basis of social recognition. Front Neuroendocrinol. 2002;23:200–224. doi: 10.1006/frne.2002.0229. [DOI] [PubMed] [Google Scholar]
- Guerra-Araiza C, Reyna-Neyra A, Salazar AM, Cerbon MA, Morimoto S, Camacho-Arroyo I. Progesterone receptor isoforms expression in the prepuberal and adult male rat brain. Brain Res Bull. 2001;54:13–17. doi: 10.1016/s0361-9230(00)00410-x. [DOI] [PubMed] [Google Scholar]
- Imwalle DB, Scordalakes EM, Rissman EF. Estrogen receptor alpha influences socially motivated behaviors. Horm Behav. 2002;42:484–491. doi: 10.1006/hbeh.2002.1837. [DOI] [PubMed] [Google Scholar]
- Johansson IM, Birzniece V, Lindblad C, Olsson T, Backstrom T. Allopregnanolone inhibits learning in the Morris water maze. Brain Res. 2002;934:125–131. doi: 10.1016/s0006-8993(02)02414-9. [DOI] [PubMed] [Google Scholar]
- Keverne EB, Curley JP. Vasopressin, oxytocin and social behaviour. Curr Opin Neurobiol. 2004;14:777–783. doi: 10.1016/j.conb.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Ladurelle N, Eychenne B, Denton D, Blair-West J, Schumacher M, Robel P, Baulieu E. Prolonged intracerebroventricular infusion of neurosteroids affects cognitive performances in the mouse. Brain Res. 2000;858:371–379. doi: 10.1016/s0006-8993(00)01953-3. [DOI] [PubMed] [Google Scholar]
- Mencio-Wszalek T, Ramirez VD, Dluzen DE. Age-dependent changes in olfactory-mediated behavioral investigations in the male rat. Behav Neural Biol. 1992;57:205–212. doi: 10.1016/0163-1047(92)90164-y. [DOI] [PubMed] [Google Scholar]
- Priest CA, Pfaff DW. Actions of sex steroids on behaviours beyond reproductive reflexes. Ciba Found Symp. 1995;191:74–84. doi: 10.1002/9780470514757.ch5. [DOI] [PubMed] [Google Scholar]
- Soares MJ, Kalberer WD. Progesterone: effects on investigatory preferences, aggression, and olfaction inorchidectomized, testosterone-treated mice. Behav Biol. 1978;23:260–266. doi: 10.1016/s0091-6773(78)91942-9. [DOI] [PubMed] [Google Scholar]
- Sun WL, Luine VN, Zhou L, Wu HB, Weierstall KM, Jenab S, Quiniones-Jenab V. Acute progesterone treatment impairs spatial working memory in intact male and female rats. Ethn Dis. 2010;20:S1–S7. [PubMed] [Google Scholar]
- Thompson RN, Napier A, Wekesa KS. Chemosensory cues from the lacrimal and preputial glands stimulate production of IP3 in the vomeronasal organ and aggression in male mice. Physiol Behav. 2007;90:797–802. doi: 10.1016/j.physbeh.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee M, Mayo W, Koob GF, Le MM. Neurosteroids in learning and memory processes. Int Rev Neurobiol. 2001;46:273–320. doi: 10.1016/s0074-7742(01)46066-1. [DOI] [PubMed] [Google Scholar]
- van Wingen G, van Broekhoven F, Verkes RJ, Petersson KM, Backstrom T, Buitelaar J, Fernandez G. How progesterone impairs memory for biologically salient stimuli in healthy young women. J Neurosci. 2007;27:11416–11423. doi: 10.1523/JNEUROSCI.1715-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner CK. The many faces of progesterone: a role in adult and developing male brain. Front Neuroendocrinol. 2006;27:340–359. doi: 10.1016/j.yfrne.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Weisz J, Ward IL. Plasma testosterone and progesterone titers of pregnant rats, their male and female fetuses, and neonatal offspring. Endocrinology. 1980;106:306–316. doi: 10.1210/endo-106-1-306. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Caldwell HK, Martinez L, Gold P, Hu SB, Young WS., III Vasopressin 1a receptor knockout mice have a subtle olfactory deficit but normal aggression. Genes Brain Behav. 2007;6:540–551. doi: 10.1111/j.1601-183X.2006.00281.x. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Ginns EI, O'Carroll AM, Lolait SJ, Young WS., III Vasopressin V1b receptor knockout reduces aggressive behavior in male mice. Mol Psychiatry. 2002;7:975–984. doi: 10.1038/sj.mp.4001195. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Camacho F. Cholinergic modulation of a decrement in social investigation following repeated contacts between mice. Psychopharmacology (Berl) 1995;121:164–172. doi: 10.1007/BF02245626. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol. 1993;336:293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]