Abstract
The ventral and dorsal medial geniculate (MGV and MGD) constitute the major auditory thalamic subdivisions providing thalamocortical inputs to layer IV and lower layer III of auditory cortex. No quantitative evaluation of this projection is available. Using biotinylated dextran amine (BDA)/biocytin injections, we describe the cortical projection patterns of MGV and MGD cells. In primary auditory cortex the bulk of MGV axon terminals are in layer IV/lower layer III with minor projections to supragranular layers and intermediate levels in infragranular layers. MGD axons project to cortical regions designated posterodorsal (PD) and ventral (VA) showing laminar terminal distributions that are quantitatively similar to the MGV-to-primary cortex terminal distribution. At the electron microscopic level MGV and MGD terminals are non-γ-aminobutyric acid (GABA)ergic with MGD terminals in PD and VA slightly but significantly larger than MGV terminals in primary cortex. MGV/MGD terminals synapse primarily onto non-GABAergic spines/dendrites. A small number synapse on GABAergic structures, contacting large dendrites or cell bodies primarily in the major thalamocortical recipient layers. For MGV projections to primary cortex or MGD projections to PD or VA, the non-GABAergic post-synaptic structures at each site were the same size regardless of whether they were in supragranular, granular, or infragranular layers. However, the population of MGD terminal-recipient structures in VA were significantly larger than the MGD terminal-recipient structures in PD or the MGV terminal-recipient structures in primary cortex. Thus, if terminal and postsynaptic structure size indicate strength of excitation then MGD to VA inputs are strongest, MGD to PD intermediate, and MGV to primary cortex the weakest.
INDEXING TERMS: electron microscopy, GABA, medial geniculate nucleus
The rat auditory thalamus or medial geniculate body (MGB) has two major subdivisions, ventral (MGV) and dorsal (MGD), whose cells project primarily to layers III and IV of auditory cortex (LeDoux et al., 1985; Romanski and LeDoux, 1993a, b; Kimura et al., 2003). MGV is the major thalamic component of the rat lemniscal pathway, with cells receiving their primary ascending input from the central nucleus of the inferior colliculus (IC; LeDoux et al., 1985) and projecting exclusively to primary auditory cortex, primarily to deep layer III and layer IV. Primary auditory cortex has been defined as any portion of auditory cortex that has a thick dense granular layer and receives its primary layer III/IV thalamocortical input from MGV. This includes the area called TE1 by Romanski and LeDoux (1993a,b), also designated areas Au1 and AuV by Paxinos and Watson (2007). Rat MGD also receives IC inputs, but these arise primarily from the dorsal cortex and external nucleus, extralemniscal components of the auditory system.
Many MGD cells also project to cortical layers III and IV, but these projections are described as terminating primarily in secondary/extralemniscal auditory cortex (areas 36 or TE2 and 20 or TE3). Kimura and colleagues (2003, 2004) have shown in the rat that the MGD layer III/IV projection primarily innervates two specific cortical areas they designated posterodorsal (PD) and ventral (VA). PD lies at the caudodorsal border of TE1, overlapping the boundary between TE1 and TE2, and may play a role in auditory spatial processing. VA lies in the ventral margin of TE1 extending ventrally into TE3 and may be involved in the emotional processing pathway (Donishi et al., 2006; Kimura et al., 2007, 2010).
Only one article has looked at the terminals of the auditory thalamocortical inputs in primary auditory cortex at the electron microscopic (EM) level. In that study Khazaria and Weinberg (1994) evaluated thalamocortical terminals arising from MGV cells that were confined to layer IV of primary cortex and showed that they make asymmetric synapses primarily on spines (32/34 synapses). No evaluation was made of any MGD terminals or MGV terminals in any other layers. Thus, although both the MGV and MGD projections have been shown qualitatively to terminate primarily in intermediate cortical layers and the MGV terminals in layer IV have been shown to terminate primarily on spines, many important questions remain to be answered in order to better understand the functions of these two thalamocortical pathways. These include 1) How do the laminar distributions of the MGV terminals in primary cortex and MGD terminals in PD and VA compare quantitatively? 2) What are the postsynaptic targets of MGV terminals outside of layer IV? 3) What are the post-synaptic targets of MGD terminals in areas PD and VA and how do these postsynaptic structures compare with the postsynaptic targets of MGV terminals? 4) Are the features (size, shape, etc.) of MGV and MGD terminals similar? To answer these questions we labeled projections from rat MGV and MGD and evaluated their anatomical properties at the light (LM) and electron microscopic levels. We also used antibody labeling to determine the γ-aminobutyric acid (GABA)ergic or non-GABAergic nature of the pre- and postsynaptic structures involved.
MATERIALS AND METHODS
All methods were approved by the University of Wisconsin School of Medicine and Public Health Animal Care and Use Committee. Animals were maintained in an Association for Assessment and Accreditation of Laboratory Animal Care-approved facility. Experiments were carried out in accordance with the guidelines published in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Adult male Long Evans rats (>300 g, Harlan, Houston, TX) were maintained on a 12-hour light/dark cycle in standard University of Wisconsin animal housing with food and water ad libitum. All necessary steps were used to minimize suffering and the number of animals used.
Extracellular injection experiments
Animals were anesthetized with sodium pentobarbital (35 mg/kg, I.P.) or with ketamine (70–90 mg/kg I.P.) plus xylazine (8–10 mg/kg I.P.), with supplemental doses of one-third the initial doses and xylazine supplements administered only one-third as often as ketamine supplements, as needed to maintain surgical level anesthesia. The deeply anesthetized rat was prepared for aseptic surgery and placed in the stereotaxic apparatus. Body temperature was maintained at 36°C by using a heating blanket with feedback control via a rectal thermometer.
MGV/MGD injections
To study thalamocortical terminals in auditory cortex, Neurobiotin (Vector, Burlingame, CA) or biotinylated dextran amine (BDA; Sigma, St. Louis, MO) microinjections were made into the MGV or MGD. Following anesthesia, the dorsal surface of the skull was exposed. A hole was drilled from 5.0 to 6.3 mm caudal to bregma and 3–4 mm lateral to the midline (the stereotaxic coordinates of MGV/MGD). A glass electrode (outer tip diameter of 20–30 μm, filled with 6% Neurobiotin in 0.9% NaCl or 8% BDA was guided to the exposed dorsal surface of the cortex and manually advanced 4 mm into the brain. According to the Paxinos and Watson (2007) brain atlas this would position the electrode a few hundred microns above the MGD. The electrode was then advanced in 5–10-μm steps while wide-band noise bursts from a free field speaker positioned 15 cm from the contralateral ear were presented (1 stimulus/sec). The electrode was advanced until an evoked auditory response was seen or before a depth of 6.5 mm was reached (the ventral edge of MGV). Neurobiotin or BDA was injected iontophoretically (Neurobiotin, 25–30 μA anodal current, 7 seconds on, 7 seconds off; BDA 5–0 μA anodal current, 7 seconds on, 7 seconds off) for either 20 (BDA) or 25 (Neurobiotin) minutes. The electrode was left in place for an additional 20 minutes and then withdrawn.
Postinjection processing
Section preparation
Tissue preparation has been described in detail elsewhere (Smith et al., 2010) and is summarized here. After the injections the electrode was removed, and the wound margins were closed and treated with a broad-spectrum antibiotic and lidocaine. Twenty-four hours (Neurobiotin injections) or 7 days (BDA injections) later the rat was given an overdose of sodium pentobarbital and perfused through the heart with 0.9% phosphate-buffered saline followed by 1,000 ml of a 3% paraformaldehyde/1% glutaraldehyde solution in 0.1 M sodium phosphate buffer (pH 7.4).
The tissue was refrigerated, and the brain was removed and placed in the same fixative overnight. Then 70–80-μm-thick sections were cut with a Vibratome, and the tracer was visualized by using the diaminobenzidine (DAB)-nickel/cobalt intensification method (Adams, 1981). Sections were rinsed in phosphate buffer, and these free-floating sections were inspected to determine whether the gross injection had successfully labeled axons and axon terminals in primary auditory cortex. Some of the sections containing the thalamocortical axon terminals were selected to be processed for EM. Sections not selected for EM were mounted on slides and dehydrated. Half of these sections were stained with cresyl violet and coverslipped whereas the other half were coverslipped unstained.
Those sections selected for EM analysis were fixed in 0.5% osmium tetroxide for 30 minutes, rinsed, and dehydrated through a series of graded alcohols and propylene oxide. Sections were then placed in unaccelerated Epon-Araldite resin and then transferred into a fresh batch of unaccelerated resin overnight. The sections were then embedded and flat-mounted in accelerated resin at 65°C. The portion of the embedded section containing the auditory cortex was isolated and mounted on a beam capsule. A camera lucida drawing of the flat-mounted tissue was made noting the location(s) of the labeled axons/terminals and nearby fiduciary structures. Then 70–80-nm thin sections were cut and mounted on coated nickel grids.
GABA postembedding immunogold labeling
An affinity-isolated anti-GABA antibody (Sigma-Aldrich, Catalog No. A2052, St. Louis, MO) at 1:250 dilution was used to evaluate the GABAergic nature of the terminals and postsynaptic structures (Table 1). The antibody was raised in rabbit against GABA conjugated to bovine serum albumin (BSA). The antibody was isolated from antiserum by immunospecific purification methods that remove proteins, including immunoglobulins, that do not bind specifically to GABA. Anti-GABA shows positive binding in a dot blot assay with GABA and with GABA-keyhole limpet hemocyaninin and negative binding with BSA (Sigma product information). Binding of the antibody to the tissue is not blocked by BSA.
TABLE 1.
Primary Antibody Used in This Study
| Antigen | Immunogen | Source | Dilution |
|---|---|---|---|
| γ-Aminobutyric acid (GABA) | Purified GABA conjugated to bovine serum albumin | Sigma-Aldrich (St. Louis, MO), rabbit polyclonal, #A2052 | 1:250 |
The mounted thin section was carefully immersed in solution A (0.745 g Tris buffer, 0.9 g NaCl, 0.1 ml Triton X in 100 ml dH2O, pH 7.6) and 5% BSA for 30 minutes, then in solution A and 1% BSA for 5 minutes, and then in solution A and 1% BSA containing the GABA antibody at 1:250 dilution at room temperature overnight. The following day the section was rinsed in solution A and 1% BSA twice for 5 minutes and once for 30 minutes and then rinsed in solution B (0.688 g Tris buffer, 0.9 g NaCl, 0.1 ml Triton X in 100 ml dH2O, pH 8.2) for 5 more minutes. The section was then immersed in the secondary antibody (goat anti-rabbit IgG with attached 15-nm gold particles) diluted 1:25 in solution B (pH 8.2) for 1 hour and rinsed twice for 5 minutes each in solution A and twice for 5 minutes in distilled water. After that they were fixed in 8% EM-grade glutaraldehyde, rinsed in distilled water, and then counterstained with uranyl acetate and lead citrate and rinsed again.
Sections were examined by using a Philips CM120 electron microscope. Control sections were prepared by the method described above, but the primary antibody step was omitted during the overnight immersion. When observed with the EM, control sections had a uniformly low background of around 0.5–0.6 gold particles/μm2. For the non-control sections, a “background” gold particle density was calculated for each thin section to account for possible processing differences between sections. Aggregates of two or more particles were counted as a single particle. Axon terminals or their postsynaptic targets were considered GABAergic if their particle density exceeded the mean gold particle density of the background by 5 times. Background density was measured over non-GABAergic structures such as blood vessel lumens, glial cells, etc.
In our rat auditory cortex tissue, cells in layer I immunostained for GABA, as did a subset of smaller neurons in layers II–VI. In contrast, larger somata with pyramidal morphology were GABA-negative. GABA-positive terminals typically contained flat or elliptical synaptic vesicles and, when viewed in appropriate plane of section, formed symmetrical contacts. These findings are consistent with those reported previously for GABA staining patterns in sensory cortex (e.g., Meinecke and Peters, 1987; Beaulieu et al., 1994). As an additional positive control, we looked at GABA antibody staining in the thalamic reticular nucleus (TRN) taken from the same tissue. Previous reports have indicated that virtually all cells in the rat TRN are GABAergic (Houser et al., 1980; de Biasi et al., 1986). The GABA antibody positively labeled cells in rat TRN with antibody levels that exceeded 5 times the background.
Analysis of axon terminals, light microscopy
To evaluate the distribution of labeled terminals, a high-magnification (1,000×) camera lucida drawing of a narrow strip of auditory cortex containing labeled terminals was made from the cortical surface to the white matter below layer VI. All en passant and en terminaux swellings (boutons) on labeled axons in this cortical strip were drawn. As they course through the neuropil, labeled thalamocortical axons of MGV and MGD cells typically maintain a fairly constant diameter that is interrupted at certain points by a noticeable increase in diameter after which the diameter rapidly returns to its original level. If the diameter change at one of these points was greater than 3 times the axon’s standard diameter as it coursed through the neuropil, it was considered a bouton. Figure 1 (arrows) illustrates some examples of what were deemed boutons.
Figure 1.

Boutons. A–D: Examples of swellings (arrows) on labeled MGV (A,B) and MGD (C,D) axons that were deemed boutons. Other swellings are present and unmarked. Scale bar = 10 μm in D (applies to A–D).
The terminology and atlas of Paxinos and Watson (2007) were used to identify auditory cortical regions. Two auditory areas, Au1 and AuV, receive their primary thalamic inputs from MGV (but see Discussion) and are characterized cytoarchitectonically in previous descriptions of rat primary auditory cortex (Zilles and Wree, 1985; Roger and Arnault, 1989). Au1 and AuV are distinguished from one another by anatomical and physiological features (Zilles et al., 1990; Romanski and LeDoux, 1993a,b; Polley et al., 2007; Storace et al., 2010, 2011). In our tissue, Au1 is characterized by its relatively wide overall cortical thickness, the relatively specific differentiation of its cortical layers, a comparatively wide layer IV with a high density of cells, a moderately low density of small and large pyramidal cells in layer V, and layers V and VI that together comprise half or slightly more than half the thickness of the cortical tissue. The dorsal border of Au1 is adjacent to a narrow region (AuD in Paxinos and Watson, 2007) where cortex often thins slightly in comparison, the cell density is lower in layer IV and sometimes layer III, and there appears to be a slight thinning of layer V and enlargement of layer VI. AuV is similar to Au1, except the differentiation of cortical layers is less clear in AuV than in Au1. The ventral border of AuV is adjacent to a region where cortex begins to thin considerably, the cell density in layer IV is lower, and the cortical layers are more poorly differentiated. Areas Au1 and AuV together may correspond to Area TE1, which is distinguished by its thick and dense granule cell layer (Roger and Arnault, 1989; Romanski and LeDoux, 1993a,b), and AuV may correspond to Area TE1v, which is positioned within the ventral portion of TE1 (Romanski and LeDoux, 1993a,b).
In our figures that contain photomicrographs or camera lucida representations of sections we first noted the locations of the cortical boundaries from Paxinos and Watson’s atlas (2007) at that particular rostrocaudal level and inserted our own boundary locations of auditory cortical subdivisions (Au1 and AuV) based on changes in the laminar features in our tissue. We have not labeled other, nonprimary cortical areas because of the present state of confusion in the literature concerning these areas. Our cortical boundary locations were in reasonable agreement with those shown by Paxinos and Watson at that rostrocaudal level.
We used the nomenclature of Kimura and colleagues, posterodorsal and ventral (PD and VA), to designate the cortical areas receiving MGD inputs. Several other studies have assigned different names to these areas. According to Kimura et al. (2004) PD lies immediately dorsal to the posterior end of TE1, running from about 4.8 to about 6.4 mm caudal to bregma and then bends “down” behind TE1 a location designated TE2d by Zilles et al. (1990). The portion of PD immediately above Au1 has also been called AuD by Paxinos and Watson (2007) whereas the portion of PD immediately caudal to Au1 is in the dorsal portion of an area designated P (Doron et al., 2002) or PAF (Pandya et al., 2008). VA lies primarily in ventral TE1 (AuV) but can extend ventrally into TE3V from around 3.6 to 5.6 or 6 mm caudal to bregma (Kimura et al., 2004; Donishi et al., 2006). This area ventral to primary auditory cortex called TE3V (Zilles et al., 1990; Romanski and LeDoux, 1993a,b) may also be analogous to the suprarhinal auditory field (SRAF) described as a tonotopically organized nonprimary auditory cortical area (Polley et al., 2007; Storace et al., 2010, 2011).
For figures containing sections through cortical tissue, cortical layer I–VI boundaries were added to the drawings based on the report of Zilles and Wree (1985), and the numbers of terminals in each cortical layer were counted. We defined supragranular layers as layers I, II, and the top half of layer III, the thalamocortical recipient layers as the bottom half of layer III, and all of layer IV and the infragranular layers as layers V and VI.
Analysis of axon terminals and their postsynaptic targets, electron microscopy
For analyzing neurobiotin/BDA-labeled axon terminals arising from MGV or MGD the following procedure was established. A given thin section was manually scanned at 10,000×. After identification of a labeled synaptic terminal, based on the presence of vesicles presynaptically and a postsynaptic thickening of the membrane apposed to the terminal, a photo of the terminal was taken at 29,000×, and its location was noted on the camera drawing that had been made of the block face prior to sectioning (see above). Using the 29,000× image, measurements of the labeled terminal and its postsynaptic target were made for each synapse by using NIH Image/J 1.60 software. Other terminal features were also noted.
Statistical tests
Statistical comparisons of EM measurements were made on profiles quantified via Minitab software. For all results, statistical analysis was done by using either Origin Pro7 or Microsoft Excel software. Data are presented as mean ± SD. A P value of 0.05 was considered to represent a significant difference.
Digital processing of images
Digitized light level photomicrographs were acquired in black and white with a Spot camera (Diagnostic Instruments, Sterling Heights, MI) mounted on a Nikon Eclipse E600 microscope using a 40× oil objective, brightfield illumination, and Nomarski optics. The final figures were prepared by using Adobe Photoshop (San Jose, CA), and tonal adjustments were applied across the entire image (levels and curves functions) with no further manipulations.
RESULTS
Light microscopy
We made nine injections that targeted the main subdivisions of the medial geniculate nucleus. Several were not confined to a particular subdivision, but two were confined to MGD and two were confined to MGV. The data presented here were collected from these experiments.
MGD injections labeled axons that terminated primarily in layers III and IV of two regions that have been designated PD and VA by Kimura and colleagues (2004, 2006, 2007). Figure 2A shows the rostrocaudal extent of the two areas (black and gray enclosed areas) containing layer III/IV terminations in temporal cortex following a gross injection confined to MGD (Fig. 2B). The center of the MGD injection was located 5.3 mm caudal to bregma and a number of labeled MGD (very few MGV cells) were found in sections from 4.95 to 5.4 mm caudal to bregma. A few MGD cells could be seen in sections caudal to this. In auditory cortex, labeled terminals arising from these labeled MGD cells could be distinguished in all cortical layers in PD and VA but the bulk of them were confined to the lower half of layer III and all of layer IV (Fig. 2C). Figure 2C also illustrates that some layer VI pyramidal cells were backfilled as a result of the MGD injection. These cells were lightly labeled as were their axons as they traversed the neuropil and entered the white matter. To assure ourselves that the axon terminals we examined at the EM level did not arise from axon collaterals of backfilled layer VI cells we carefully followed the main axons of several of these cells as they ran through the neuropil. The axons were so light that it was difficult to distinguish any axon collaterals coming off the main axon and when we could the collateral rapidly faded before any boutons could be seen.
Figure 2.
MGD injection densely labels two discrete regions. A: Camera lucida drawings of five representative sections from one rat illustrating the two separate areas of layer III/IV label from caudal (right) to rostral (left). Enclosed light gray area in first three sections from left indicates the labeled area in layers III/IV that coincides with that designated ventral (VA) by Kimura et al. (2007). Dark gray areas in last three sections from right indicate the labeled area in layers III/IV that coincides with the area designated posterodorsal (PD) by Kimura et al., (2003). This labeling ends just dorsal to Au1 in adjacent region AuD. Numbers on each section represent approximate distance from Bregma in mm. Upper and middle dotted lines in each section enclose Au1 at this rostrocaudal level.. Middle and lower dotted lines enclose AuV. B: Photomicrograph of the thalamus at 5.3 mm posterior to Bregma at the center of the MGD injection site. C: Photomicrograph of the cortex at a level 4.3 mm posterior to Bregma illustrating thalamocortical terminal labeling as well as some backfilled cells in layer VI generated by the MGD injection seen in B. Scale bar = 1 mm in A; 0.5 mm in B; 200 μm in C. Abbreviations: Au1 = primary auditory cortex; AuV = auditory cortex, ventral; CA3 = field CA3 of hippocampus; DLG = dorsal lateral geniculate nucleus; MGD = medial geniculate nucleus, dorsal; MGV = medial geniculate nucleus, ventral; wm = white matter.
All of the labeled terminals we examined at the LM and EM levels were well labeled, so we are confident that they were all terminals arising from thalamocortical axons and not from backfilled cortical cells. Backfilled layer VI cells were also seen following MGV injections (Fig. 3C) but their axons were very light and collaterals were difficult to distinguish even at their source and quickly faded. Our MGV and MGD injections also backfilled some cells in those areas that have previously been reported to project to MGV/MGD including the inferior and superior colliculi, dorsal nucleus of the lateral lemniscus, nucleus of the brachium of the inferior colliculus (NBIC), cuneiform nucleus, and the reticular formation immediately below the IC. Only one of these areas, namely, the NBIC, has been reported to also project to the auditory cortex as well (Budinger et al., 2006) so it is feasible that some of the terminals in the cortex, after injection of MGB, could theoretically have arisen from backfilled cells in NBIC that project to both MGB and auditory cortex.
Figure 3.
MGV injection labels layer III/IV in primary cortex. A: Camera lucida drawings of five representative sections from one rat illustrating the area of layer III/IV label (enclosed area) from caudal (right) to rostral (left). Darker gray in enclosed areas represents denser label, and lighter gray in enclosed area represents moderate label. Numbers on each section represent approximate distance from Bregma in mm. Upper and middle dotted lines in each section enclose Au1 at this rostrocaudal level. Middle and lower dotted lines enclose AuV. B: Photomicrograph of the thalamus at 5.5 mm posterior to Bregma at the center of the MGV injection site. C: Photomicrograph of the cortex at a level 5 mm posterior to Bregma illustrating the thalamocortical terminal labeling as well as some backfilled cells in layer VI generated by the MGV injection. Scale bar = 1 mm in A; 0.5 mm in B; 200 μm in C. Abbreviations: Au1 = primary auditory cortex; AuV = auditory cortex, ventral; CA3 = field CA3 of hippocampus; DLG = dorsal lateral geniculate nucleus; MGD = medial geniculate nucleus, dorsal; MGV = medial geniculate nucleus, ventral; wm = white matter.
As with the MGD injections, an injection into MGV (Fig. 3B) labeled terminals in all cortical layers as well but the majority were confined to the lower half of layer III and all of layer IV (Fig. 3C). Although varying in the amount of temporal cortex in the dorsoventral plane occupied in each section, these MGV thalamocortical terminals in layers III and IV ran continuously for a considerable distance rostrocaudally from around 3.5 to 6.4 mm posterior to bregma (Fig. 3A). Our MGV injection seen in Figure 3B was located at around 5.5 mm caudal to bregma and labeled a large number of MGV cells that were concentrated from around 5.1 to 5.7 mm. However, labeled MGV cells could be seen from the caudalmost extent of the nucleus (6.6 mm caudal to Bregma) to as far rostral as 4.9 mm.
Figures 4 and 5 illustrate our method for generating a more quantitative measure of the layer distribution of terminals in order to determine if there were differences between MGD terminal distributions in PD and VA and MGV terminals in primary cortex. Figure 4A and D shows the axonal boutons found in the cortical layers in a strip of tissue in regions VA (Fig. 4A,B) and PD (Fig. 4C,D). Each strip is centered in the region of label and within anatomically defined area Au1. A comparison of the plots and percentages shown in A and D indicates that the terminal distributions are essentially the same in both regions. Superficial layers are sparsely innervated whereas the terminals in layers III and IV account for 55–60% of the total number. Terminal concentrations drop sharply as one moves from layer IV into layer V but then increase slightly in superficial layer VI only to drop off again in deep layer VI.
Figure 4.
Distribution of MGD boutons in areas PD and VA. A: Distribution of boutons on BDA-labeled MGD axons in a strip of auditory cortex, shown in B, running from the cortical surface to the white matter through the area designated VA taken 3.5 mm caudal to Bregma. Each dot represents a bouton. The larger dark spheres in layer VI represent the location of retrogradely labeled cell bodies. Dotted lines divide the strip into 50-μm parcels. Location of layer boundaries is indicated to the left. Plot of the number of boutons in each 50-μm parcel and percentage of the total boutons in each layer is indicated to the right. B: Camera lucida drawing of a section 3.5 mm posterior to Bregma. Location of the layer III/IV labeling in this section is highlighted and the gray bar through it indicates the location of the strip of tissue seen in A. Boundaries of primary cortical areas Au1 and AuV are indicated by dotted lines. The boundary between AuV and Au1 is at the bottom edge of the gray bar. C: Camera lucida drawing of a section approximately 5.8 mm posterior to Bregma. Location of the layer III/IV labeling in this section highlighted and the gray bar through it indicates the location of the strip of tissue seen in D. The dorsal border of Au1 is at dorsal edge of the shaded bar. D: Distribution of boutons on BDA-labeled MGD axons in a strip of auditory cortex, shown in C, within the region designated PD. The larger dark spheres in layer VI represent the location of retrogradely labeled cell bodies. Dotted lines divide the strip into 50-μm parcels. Location of layer boundaries is indicated to the left. Plot of the number of boutons in each 50-μm parcel and percentage of the total boutons in each layer is indicated to the right. Scale bar = 1 mm in B,C. Abbreviations: Au1 = primary auditory cortex; AuV = auditory cortex, ventral; LP = lateral posterior nucleus; MGD = medial geniculate nucleus, dorsal; MGV = medial geniculate nucleus, ventral; Po = posterior thalamic nucleus; VPM = ventral posteromedial thalamic nucleus.
Figure 5.
Distribution of MGV boutons in the cortical layers of primary cortex. A: Camera lucida drawing of a section 5.9 mm posterior to Bregma. Location of the layer III/IV labeling in this section is highlighted and the gray bar through it indicates the location of the strip of tissue seen in B. Dotted lines indicate the boundaries of Au1 and AuV. B: Distribution of boutons on BDA-labeled MGV axons in the strip of auditory cortex shown in A. Each dot represents a bouton. The larger dark spheres in layer VI represent the location of retrogradely labeled cell bodies. Dotted lines divide the strip into 50-μm parcels. Location of layer boundaries is indicated to the left. Plot of the number of boutons in each 50-μm parcel and percentage of the total boutons in each layer is indicated to the right. Scale bar = 1 mm in A. Abbreviations: Au1 = primary auditory cortex; AuV = auditory cortex, ventral; MGB = medial geniculate body; SC = superior colliculus.
Figure 5 shows the MGV axonal boutons found in the cortical layers in a strip of tissue in an area of primary cortex. The layer distribution in this strip (Fig. 5B) is very similar to that described above for MGD terminal distribution in PD and VA. Superficial layers were sparsely innervated whereas the terminals in layers III and IV accounted for around 60% of the total. Deep to layer IV the terminal density dropped in layer V and then increased slightly again in superficial layer VI only to drop off again in deep layer VI. Thus it would appear that, in terms of terminal distributions in individual layers, the MGD innervation of PD and VA and the MGV innervation of primary cortex are quite similar.
A similarity in the MGV and MGD terminal distribution is also seen if the layers are subdivided into supragranular (all of layers I and II and upper half of III), thalamocortical (TC) recipient (lower half of layer III and all of layer IV), and infragranular (all of layers V and VI) regions. Around half of the terminals were found in TC-recipient layers (MGD to VA = 51.5%, MGD to PD = 48.8%, MGV to Au1 = 55.1%), a rather high concentration considering that lower layer III and layer IV make up only around 16–17% of the total thickness of cortex. A much lower concentration was seen in supraganular layers (MGD to VA = 15.3%, MGD to PD = 15.6%, MGV to Au1 = 15.4%) and an intermediate level in infragranular layers (MGD to VA = 33.2%, MGD to PD = 35.7%, MGV to primary cortex = 29.4%).
Electron microscopy
We evaluated more than 200 labeled MGD terminals in all layers in both area PD (218 terminals) and area VA (210 terminals) following MGD injections and in primary auditory cortex (215 terminals) following MGV injections. In a previous study (Smith et al., 2010) we evaluated thalamocortical projections to auditory cortex from the suprageniculate nucleus (SG) and corticocortical projections from visual cortex to auditory cortex and showed that the number of boutons seen in the light microscopic tissue are a reasonably accurate reflection of the synaptic terminals in a given layer seen at the EM level. A similar evaluation here on MGD and MGV projections confirmed this. The laminar distribution of boutons observed at the LM level was similar to the distribution of terminals positively identified as terminals the EM level (Fig. 6A).
Figure 6.
A comparison of labeled thalamocortical terminals at the LM and EM levels. A: Terminal/bouton distribution across supragranular (supra. = layers I, II, upper half of III), thalamocortical recipient (TC rec. = layer IV, lower half of III), and infragranular (infra. = layers V, VI) layers of cortex for MGV terminals in primary cortex and MGD terminals in VA and PD. Dark bars for each area represent the percentage of synaptic terminals found in that area during the EM analysis for MGV terminals (top dark bar) and MGD terminals in VA (middle dark bar) and PD (lower dark bar). White bars in each area represent the percentage of boutons in that area seen on labeled MGV axons (top white bar) and MGD axons in VA (middle dark bar) and PD (lower dark bar) during the LM analysis. B: Cumulative histograms of areas of MGV terminals in primary cortex and MGD terminals in PD and VA. Each point on a curve represents the percentage of all terminals smaller and equal to that size. The MGV curve shifted to the left indicates a distribution of smaller terminal areas. C: Evaluation of terminal areas for MGV terminals in primary cortex, MGD terminals in PD and VA and SG terminals in primary cortex. Box represents the 25–75th percentile, bar represents the 5–95th percentile, and dot and horizontal bar in box represent mean and median values, respectively. Abbreviations: E.M. = electron microscope; L.M. = light microscope; MGD = medial geniculate nucleus, dorsal; MGV = medial geniculate nucleus, ventral; PD = posterodorsal; SG = suprageniculate thalamic nucleus; VA = ventral.
Comparison of MGV terminals with previous data
The only previous quantitative descriptions of rat thalamocortical terminals in primary auditory cortex focused exclusively on MGV terminals in layer IV (Khazaria and Weinberg, 1994) or on terminals from SG (Smith et al., 2010), which synapse primarily in superficial and deep layers of auditory cortex. Khazaria and Weinberg (1994) measured 34 labeled rat MGV terminals in layer IV of primary cortex. Our measurements (Fig. 6B,C) of the areas of MGV terminals in this region (lower layer III/layer IV, n = 107, 0.45 ± 0.22 μm2) and the terminals we measured in all layers (n = 208, 0.42 ± 0.2 μm2) are in good agreement with theirs (n = 34, 0.43 ± 0.03 μm2). A comparison of our MGV terminals with SG terminals in Au1 that we reported on previously (Smith et al., 2010) indicates that MGV terminals in primary cortex are significantly larger (P = 0.000035) than SG thalamocortical terminals (n = 152, 0.29 ± 0.17μm2), which were found primarily in supragranular and infragranular layers (Fig. 6C).
Comparison of MGV and MGD terminals
Virtually all of the MGV and MGD terminals were not labeled with the GABA antibody; in only 5 of >600 terminals evaluated was the GABA-positive criterion reached. The areas of the large majority of both MGD and MGV terminals ranged from 0.1 to 1 μm2 (Fig. 6B). A one-way ANOVA on the size of the terminals arising from MGD and terminating in VA or PD and arising from MGV and terminating in primary cortex showed significant differences in the means [F(2,625) = 4.56, P < 0.011). Post hoc t-tests for the differences in the means showed that the size of thalamocortical terminals arising from MGD axons found in PD (n = 218, area = 0.486 ± 0.24 μm2) and VA (n = 210, area = 0.474 ± 0.22 μm2) were not significantly different from each other (P = 0.6); however, the MGD terminals in either PD or VA were significantly larger than the MGV thalamocortical terminals in primary cortex (0.42 ± 0.2 μm2, PD vs MGV P = 0.0045, VA vs MGV P = 0.017) (Fig. 6C).
Figures 7 and 8 illustrate typical MGD terminals in both PD (Fig. 7A–E) and VA (Fig. 7F–H) and typical MGV terminals (Fig. 8). The reaction product within the labeled terminal was usually sufficiently dark to make it difficult to distinguish features usually described for presynaptic profiles. However, it was possible to distinguish round vesicles and either one (Figs. 7C,H, 8B–F, arrows) or two (Figs. 7B,D–G, 8A, arrows) asymmetric synaptic densities for both MGD and MGV terminals. Both MGD and MGV terminals could occasionally be seen surrounding (Fig. 7D, black asterisk) or virtually engulfing (Fig. 7E, black asterisk) postsynaptic processes.
Figure 7.
MGD terminals. A–E: Electron micrographs showing examples of MGD terminals (white asterisks) in area designated PD. F–H: Electron micrographs showing examples of MGD terminals (white asterisks) in area designated VA. Terminals made asymmetric contacts with either single (A,C,H) or multiple (B,D,E–G) active zones (black arrows). In some instances the postsynaptic spinous process would indent the terminal (D, black asterisk) and could sometimes be surrounded by the terminal (E, black asterisk). Scale bar = 1 μm in C (applies to A–H).
Figure 8.

MGV terminals. A–F: Examples of MGV terminals (white asterisks) synapsing on small dendrites and spines in primary cortex. Terminals made asymmetric synaptic contacts with either single (black arrows in BF) or multiple (black arrows in A) active zones. Star in D illustrates a GABA-labeled terminal. Scale bar = 1 μm in F (applies to A–F).
Postsynaptic targets
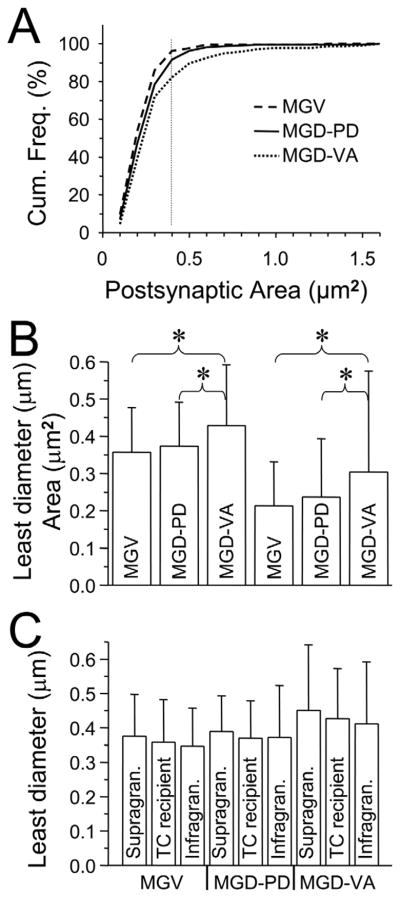
We also evaluated the postsynaptic targets of these MGD and MGV terminals. The large majority of the labeled terminals arising from MGV (208/215) and MGD (PD 214/218, VA 206/210) synapsed on small postsynaptic structures that were GABA immunonegative (Figs. 7, 8). In the rat visual cortex, measurements of the areas of spines on pyramidal cells (Suzuki et al., 2007) indicated that the majority of spines ranged from 0.1 to 0.4 μm2 (mean = 0.29 μm2), with a few as large as 0.6 μm2. In the rat somatosensory cortex, the mean spine area was reported to be 0.435 μm2 (Vees et al., 1998). Figure 9A is a cumulative frequency plot that illustrates the areas of the structures postsynaptic to our MGV terminals in primary cortex and to MGD terminals in PD and VA. If we conservatively use the range of most spine areas in rat visual cortex (0.1–0.4 μm2) as indicative of a postsynaptic spine in auditory cortex (Fig. 9A, vertical dotted line), then 96% of MGV terminals, 92% of MGD terminals in PD, and 82% of MGD terminals in VA are synapsing on spines. The larger postsynaptic structures could potentially be spines or small dendrites. Thus in referring to postsynaptic structures, for the remainder of the article we will use the term “spines/dendrites” even though the large majority of these are spines. One-way ANOVA showed a significant difference in both the means of the areas and least diameters of the spines/dendrites post-synaptic to these MGD and MGV GABA-immunonegative terminals [areas, F(2,632) =12.48, P < 4.80 × 10−6: least diameters, F(2,631) = 16.03, P < 1.62 × 10−7). Post hoc analysis of these means indicated that the MGD terminals in VA synapsed on spines/dendrites that were significantly larger (least diameter = 0.43 ± 0.16 μm, area = 0.30 ± 0.27 μm) than the spines/dendrites post-synaptic to MGD terminals in PD (least diameter = 0.37 ± 0.12 μm, area = 0.24 ± 0.16 μm, P = 0.0008) and MGV terminals in primary cortex (least diameter = 0.36 ± 0.12 μm, area = 0.21 ± 0.12 μm, P = 0.00056) (Fig. 9B).
Figure 9.
Structures postsynaptic to labeled terminals. A: Cumulative histograms (cf. Fig. 6B) of the area of structures postsynaptic to MGV terminals in primary cortex (n = 206) and MGD terminals in PD (n = 214) and VA (n = 214). Curves shifted to the right indicate a distribution of larger postsynaptic structures. Vertical dotted line indicates the largest size of most pyramidal cell spines in rat visual cortex (Suzuki et al., 2007). B: Plot of the least diameter (left three bars, mean and SD) and area (right three bars, mean and SD) of all structures in all layers that were postsynaptic to labeled MGV and MGD terminals. Asterisks indicate significant differences between the two bars at the end of the parenthesis. C: Least diameter (mean and SD) of structures postsynaptic to labeled MGV and MGD terminals in supragranular (layers I, II, upper III), TC recipient (layer IV, lower layer III), and TC infragranular (layers V,VI) regions. Structures postsynaptic to MGV terminals in supragranular layers (n = 31, least diameter = 0.38 ± 0.12 μm, area = 0.22 μm2), postsynaptic to MGV terminals in TC-recipient layers (n = 106, least diameter = 0.36 ± 0.12 μm, area = 0.21 μm2), postsynaptic to MGV terminals in infragranular layers (n = 70, least diameter = 0.35 ± 0.11 μm, area = 0.21 μm2). Structures postsynaptic to MGD terminals in supragranular layers of PD (n = 34, least diameter = 0.39 ± 0.10 μm) TC-recipient layers of PD (n = 128, least diameter = 0.37 ± 0.11 μm) infragranular layers of PD (n = 50, least diameter = 0.37 ± 0.15 μm). Structures postsynaptic to MGD terminals in supragranular layers of VA (n = 43, least diameter = 0.45 ± 0.19 μm), TC recipient layers of VA (n = 118, least diameter = 0.43 ± 0.15 μm), infragranular layers of VA (n = 51, least diameter = 0.41 ± 0.18 μm). No significant differences were seen between layers in a given area. Abbreviations: MGD = medial geniculate nucleus, dorsal; MGV = medial geniculate nucleus, ventral; PD = posterodorsal; TC = thalamocortical; VA = ventral.
We were also interested in knowing if the spines/dendrites postsynaptic to a given input were the same regardless of whether they were located in supragranular, TC recipient, or infragranular layers. Differences might imply that the influence of these inputs were potentially stronger/weaker in a layer-dependent manner. The results of one-way ANOVA evaluations showed that the mean areas and least diameters of the structures postsynaptic to MGD terminals in PD were not significantly different regardless of their location in supragranular, thalamocortical recipient or infragranular layers (Fig. 9C) [area, F(2,209) = 0.11, P = 0.90: diameter, F(2,209) = 0.36, P = 0.70]. The same was true for MGD terminals in VA [area, F(2,209) = 0.675, P = 0.51: diameter, F(2,201)= 0.48, P = 0.62) and MGV terminals in Au1 [area, F(2,204) = 0.11, P = 0.89: length, F(2,204) = 0.62, P = 0.54).
We next asked how the size of the spines/dendrites onto which labeled terminals were synapsing compared with the “typical postsynaptic structure” in each layer. To do this we measured a population of 300 randomly selected postsynaptic structures in all layers of primary cortex that were contacted by terminals that were not labeled with BDA (least diameter = 0.46 ± 0.21 μm, area = 0.48 ± 0.6 μm2). Compared with this population, dendrites postsynaptic to our labeled MGD and MGV thalamocortical terminals were significantly smaller. Thus, although spines/dendrites postsynaptic to MGD terminals in VA are significantly larger than those postsynaptic to MGD terminals in PD and MGV terminals in primary cortex, they are still not as large as the general population of postsynaptic structures.
A small population of both MGV (n = 8) and MGD (n = 7) terminals synapsed on GABAergic postsynaptic structures, typically very large dendrites or cell bodies (Fig. 10). Of these synapses on GABAergic structures, none were found in supragranular layers (I, II, superficial III), 11/15 were found in TC recipient layers (deep III, IV), and 4/15 were found in infragranular layers (V, VI).
Figure 10.
MGD terminals synapsing on a GABAergic cell. A: Electron micrograph of two labeled MGD terminals (white asterisks) synapsing on a GABAergic cell (cb) and its dendrite (d) in layer IV of the region designated PD. Cell body and dendrite are outlined in black for clarity. B: Enlarged view of the terminal on the dendrite (d). C: Enlarged view of the terminal synapsing on the cell body (cb). In addition, this terminal is also synapsing on a nearby process (s). Scale bar = 2 μm in A; 1 μm in B (applies to B,C).
DISCUSSION
General comments
We have quantitatively compared features of the projection patterns of thalamocortical cells in the lemniscal MGV pathway and the extralemniscal MGD pathway. Our results are in broad agreement with previously described features of this system. In primary auditory cortex MGV axon termination is meager in supragranular layers, somewhat higher in infragranular layers, and dense in layer IV and upper layer III. MGD axons project to areas designated PD and VA. Quantitatively the laminar distribution of these terminals in both PD and VA is very similar to the MGV terminal distribution in primary cortex. The terminals from MGV and MGD are non-GABAergic. MGD terminals in both PD and VA are slightly but significantly larger than MGV terminals. Postsynaptically, MGV and MGD terminals synapse primarily onto non-GABAergic spines/dendrites. For a given thalamocortical projection (MGV to primary cortex or MGD to PD or VA), the postsynaptic structures are the same size regardless of whether they are in supragranular, TC-recipient, or infragranular layers, but as a population the VA terminal recipient structures are significantly larger. Those few terminals synapsing on GABAergic structures do so primarily deep to supragranular layers and are onto large dendrites or cell bodies.
As with any thalamocortical system, several features of the circuitry will determine how auditory thalamic inputs from MGD and MGV inputs will influence the auditory cortex. These include features such as 1) differences in the inputs to MGD versus MGV cells, 2) differences in MGD versus MGV cell features including intrinsic membrane properties, 3) differences in the information carried by MGV versus MGD cells, and 4) differences in the synaptic features of the thalamocortical terminals including differences in their targets. These features are discussed below including how the data presented here might be incorporated.
MGV versus MGD cells: anatomy
What are the anatomical features of the thalamocortical cells giving rise to the projections evaluated here? MGV cells are primarily tufted cells (e.g., Winer, 1986; Clerici and Coleman, 1990) that are virtually all thalamocortical and receive both excitatory and inhibitory inputs (Saint Marie et al., 1997; Peruzzi et al., 1997) from the central nucleus of the IC (Oliver, 1984). Dorsal division cells can be either tufted or stellate, both of which can be thalamocortical, but a considerable number project instead to the amygdala (Doron and LeDoux, 2000). Both cell types receive excitatory and inhibitory IC inputs arising primarily from the dorsal cortex. Excitatory IC terminals in MGV are larger than those in MGD (Bartlett et al., 2000). Corticothalamic terminals in MGV and MGD are primarily the small variety from layer VI cells, but MGD receives an additional large terminal input from cortical layer V cells (Bartlett et al., 2000; Rouiller and Welker, 2000). We have noted in this paper that ascending thalamocortical output terminals of MGD cells are slightly but significantly larger than those from MGV cells. Thus the major inputs from the IC and the cortex to MGV and MGD as well as the outputs of these two areas differ in their size and, as a result, presumably their influence.
MGV versus MGD cells: physiology
Intracellular recordings in brain slices from rat MGV and MGD cells (Bartlett and Smith, 1999) showed that MGV and MGD cells have similar intrinsic membrane properties. Like thalamocortical cells in other areas of sensory thalamus, tufted MGV TC cells and tufted or stellate MGD TC cells respond to depolarization with either single spikes (tonic response) or a three to five high-frequency (>200 Hz) spikes (burst response) depending on the membrane potential (Hu, 1995; Peruzzi et al., 1997; Tennigkeit et al., 1997; Bartlett and Smith, 1999). Thus the temporal information being sent to the cortex is intimately related to membrane potential, as exemplified by the finding for thalamocortical synapses in visual cortex that individual spikes in a burst are twice as likely to elicit a postsynaptic spike than a single spike (Swadlow and Gusev, 2001). Such a result indicates that bursting provides a more reliable, powerful way of getting information into the cortex. Ramcharan et al. (2005) noted that in awake monkeys MGD cells show more bursting and lower spontaneous activity than their MGV counterparts. This may be a result of neuromodulators that can alter the resting potential and thus the firing properties of MGV and MGD cells in different ways.
In response to cholinergic and serotonergic inputs, virtually all MGV cells and some MGD cells depolarized; however, there was a population of MGD cells that hyperpolarized (Varela and Sherman 2007, 2009). Brainstem centers that send neuromodulators to MGB are more active during waking, leading Varela and Sherman to speculate that in the awake condition almost all MGV cells and some MGD cells are depolarized and respond in tonic mode whereas the MGD cells that hyperpolarized to these neuromodulators would be in burst mode. MGD cell bursting combined with our observation that MGD axons have larger terminals would presumably mean that a more secure spike output would be elicited from cortical cells by bursting MGD cells with terminals in PD and VA than from nonbursting MGV cells with terminals in primary cortex.
Other in vivo recordings (e.g., Aitkin and Webster, 1971, 1972; Whitfield and Purser, 1972; Calford and Webster, 1981; Calford, 1983; Allon and Yeshurun, 1985; Imig and Morel, 1985; Morel et al., 1987; Edeline, et al., 1999) primarily from species other than rat and primarily under anesthetized conditions show that MGV cells have narrow frequency tuning and short latencies and are tonotopically arranged. MGD cells are not tonotopically organized. Like MGV cells, some MGD cells are sharply tuned and have short response latencies, but many show broad frequency tuning with longer and more variable latencies and a more rapid habituation to repetitive stimuli. There is no evidence to indicate if the MGD cells with MGV-like response patterns or those with the more broad frequency tuning or members of both populations project to auditory cortex.
Influence of MGV/MGD thalamocortical terminals on pyramidal cells
Our data indicate that the majority of MGV or MGD terminals are primarily on non-GABAergic spines and small dendrites. Studies in several species including rat and human (Meyer et al., 1989; Fitzpatrick and Henson, 1994; Smith and Populin, 2000; Barbour and Callaway, 2008; Richardson et al., 2009) have shown that spiny stellate neurons are rare in auditory cortex, indicating that most of our terminals are synapsing on the spines of pyramidal cells. Stimulation of MGV or MGD in a thalamocortical slice preparation (Cruikshan et al., 2002; Rose and Metherate, 2005; Lee and Sherman, 2008) generated glutamatergic excitatory postsynaptic potentials (epsps) in layer III and IV pyramidal cells (in an area designated “AII” for MGD stimulation, in “AI” for MGV stimulation) with both AMPA and NMDA components but no metabotropic component. The spiny location of many of our synapses might lead to the conclusion that they are at some distance from pyramidal cell bodies and thus might have only a minor effect on cell activity. However, both MGV and MGD inputs were described as generating “large” epsps (Lee and Sherman, 2008). In addition, Richardson et al. (2009) showed that MGV synapses in layer III/IV terminate on spines within 100 μm of the pyramidal cell body and generate larger epsps than cortico-cortical inputs that were more widely distributed on the dendritic tree. All of this would indicate that, at least in layer III/IV, both MGV and MGD terminals on spines are close enough to the cell body that they can have a strong influence on the cell’s level of excitation.
Lee and Sherman (2008) also indicated that additional synaptic features such as jitter, rise time, and paired pulse ratio (a measure of synaptic depression) at MGV and MGD synapses were indistinguishable and speculated that their functional role may be “identical.” Unfortunately, although mean and standard deviation values were given for all of these features including amplitude, any significant differences between MGD versus MGV-generated epsps were not described. As a result we cannot speculate as to whether our observation that MGD terminals in PD and VA were larger than MGV terminals and synapsed on larger postsynaptic structures in PD could explain any potential differences in these features.
Influence of MGV/MGD thalamocortical terminals on interneurons
Our data indicate that only a small percentage of thalamocortical inputs from both MGV and MGD terminated on GABAergic structures, suggesting that thalamic input to interneurons is weak, i.e., that feedforward inhibition in auditory cortex is weak. In vitro physiological data from our and other labs lend strength to this argument and suggest an important difference between auditory cortex and the cortices of other sensory modalities. Three measures of the strength of feedforward inhibition are the number of interneurons, the amount/strength of ascending input to these interneurons, and the amount/strength of disynaptic inhibition seen in pyramidal cells that would be generated by these feedforward interneurons. Our lab has reported on a type of interneuron in mouse auditory cortex that is distributed through layers I–V and has been genetically engineered to express green fluorescent protein (Verbny et al., 2006). These interneurons were shown to receive an MGV input but it was weak and rarely suprathreshold, whereas inputs to pyramidal cells were stronger and frequently suprathreshold. In addition, disynaptic inhibition in these interneurons and pyramidal cells in layers II–IV was not observed. In rat auditory cortex, Rose and Metherate (2005) noted that another category of interneurons designated FS are relatively rare in layers III/IV. They also noted that, although these rare FS cells display epsps in response to MGV stimulation, these epsps are no larger than those seen in pyramidal cells in the same layers.
Finally, recordings from these FS interneurons or layer II–IV pyramidal cells during MGV stimulation rarely showed disynaptic inhibitory postsynaptic potentials (ipsps), and if they did they were weak. In contrast to these findings in auditory cortex, recordings from somatosensory thalamocortical slices (Agmon and Connors, 1992) indicate a different circuitry. Here FS interneurons in layers II–VI receive strong and usually suprathreshold excitatory inputs from thalamocortical inputs. In addition, pyramidal cells in the same layers receive strong disynaptic inhibition to activation of the same TC input. Thus our anatomical data together with the slice physiology provide a strong argument that auditory cortical feedforward inhibition plays a lesser role in controlling cell activity than in somatosensory cortex.
Comparisons of the effects MGD and MGV inputs
As detailed above, Lee and Sherman (2008), using a thalamocortical slice preparation, have described the synaptic responses of some cells in secondary auditory cortex to MGD stimulation and compared these with responses of primary auditory cortical cells to MGV stimulation. Unfortunately, this is the only report of the action of MGD inputs on cortical cells and it is unclear whether these recordings were from cells in VA or PD or both. There are some evoked response data that describe the effects of these CT inputs from MGD on their two cortical targets and compare them with MGV activated responses. Epicortical recordings using 64-electrode arrays draped over auditory cortex showed that the two evoked responses arising from the approximate termination sites of MGV and MGD have different configurations (Di and Barth, 1992; Brett et al., 1994). Click responses evoked a positive-negative biphasic waveform in the MGV-recipient primary auditory cortex thought to be generated by the rapid sequential activation of supragranular and then infragranular pyramidal cells (Barth and Di, 1990). In contrast, identical click stimuli generated a waveform with a positive peak but little or no negative peak in areas corresponding to the MGD-recipient PD and VA regions (Brett et al., 1994). These findings would indicate 1) that the similar evoked response from PD and VA are because these MGD inputs are activating similar cell populations (our data showing that the laminar distribution of MGD terminal in these two areas is very similar, which might help to strengthen this possibility); and 2) that cortical cell populations receiving MGV inputs in primary cortex and MGD inputs in PD and VA may be influenced differently by their respective inputs. The similar laminar distributions of MGV inputs to primary cortex and MGD inputs to PD and VA that we have documented here would seem to weaken this argument. Alternately, the absence of the later peak might reflect the strong MGV-driven input of primary cortex to PD (Romanski and LeDoux, 1993a) that could potentially alter/eliminate this later component.
Functions of PD and VA
What might be the function of these two separate areas to which MGD is projecting? Recordings from area PD (Horikowa et al., 1988; Doron et al., 2002) indicated that there is a reversal of the frequency map from low-frequency CFs (<10 kHz) at the posterior end of the primary auditory field to higher frequencies CFs > 10 kHz in PD. Pandya and colleagues (2008) showed that cells in this area posterior to primary cortex (their area PAF) have longer latencies, adapt more readily to repetitive stimuli, respond to wider frequency ranges, and might therefore be involved in processing auditory information on “larger spectral and longer temporal scales.” Whether the area designated PD is physically located within the area designated PAF remains to be determined. Based on the connections of PD with posterior parietal cortex, which in turn connects with prefrontal cortex and the high best frequencies (>15 kHz) recorded from this region, Kimura et al. (2004, 2010) have postulated that area PD is involved in auditory spatial processing.
The second region, VA, elicited evoked responses, indicating that cells here may respond to low frequencies (below 15 kHz). According to Kimura and colleagues (2007), VA does not project to the amygdala but does project to the caudal part of insula, an area thought to be pivotal for fear conditioning. VA also projects corticocortically to all layers of nonprimary cortex including PD and elsewhere. Its projection to PD is heavier than the reciprocal projection from PD, which is to layer I. Kimura and colleagues (Kimura et al., 2007; Donishi et al., 2006) think that VA gives rise to a stream of auditory information out of the temporal cortex that may be related to emotion or may play a role in recognizing novel sounds for effective behavior and memory formation.
Some unresolved confusion has arisen as to the nature of the area VA and whether it should be considered a primary or secondary cortical area. Initially, based on cytoarchitectonic features and the presence or absence of a thalamic input from MGV, temporal cortex was subdivided into regions designated TE1, TE2, and TE3, with TE1 designated the core or primary auditory cortex (Zilles and Wree, 1995). Romanski and LeDoux (1993a) noted that, although the ventral one-third of TE1 (which they called TE1v) resembled the dorsal two-thirds cytoarchitecturally and received inputs from MGV (the hallmarks of a primary auditory cortical region), the MGV input was not as strong as the MGV projection to the dorsal two-thirds of TE1. This, coupled with their report (Romanski and LeDoux, 1993b) of a projection of some TE1v cells to the amygdala (one of the hallmarks of a secondary cortex area), led them to speculate that TE1v might constitute a secondary cortical area. Subsequently, descriptions and figures from Kimura showing the location of MGD terminals (Kimura et al., 2006, their Figs. 1, 2E,F, 3, 4A–C) indicated that a considerable portion of the labeled MGD terminals in “VA” are located in this ventral aspect of TE1.
Our data (see Fig. 2) also indicate that region VA (which appears to correspond to TE1v or AuV) receives a considerable input from MGD (a hallmark of secondary cortex). Our data would also indicate that this area receives an MGV input as well (see Fig. 3A). In another report, Polley et al. (2007) recorded from a tonotopically organized region they designated ventral auditory field (VAF) located immediately ventral to primary auditory cortex (A1). Based on the cortical location and the fact that cells retrogradely labeled from this area were located in both MGV and MGD, they felt that this region corresponds to VA. The similar tonotopic organization compared with A1 (Au1) would lead to speculation that this area might be considered primary. However, a comparison of responses of cells in A1 and VAF indicated that there were significant differences in response area shape, threshold, onset delays, and rate intensity functions. Subsequent studies from this group (Storace et al., 2010, 2011) indicated that the MGV cell population projecting to VAF is different from the MGV cells projecting to A1. Thus, this area, called VA by Kimura, appears to be synonymous with areas or portions of areas designated TE1v, AuV, or VAF by others. The cytoarchitecture of this region, projections from auditory thalamus to this region, outputs from this region, and responses of cells in this region give mixed messages as to how it should be classified in the cortical hierarchy.
Final comments
Our study has focused on the anatomical aspects of MGV inputs to primary auditory cortex and MGD inputs to PD and VA. Some features are very similar in that both are sending a quantitatively similar feedforward-type projection pattern to their respective cortical areas with terminals most dense in layers III and IV and that both projections appear to be non-GABAergic and are terminating primarily on cortical spines, all of which would support the notion that they are both acting as cortical drivers (Lee and Sherman, 2010). We have also uncovered some differences in that MGD terminals are larger and in PD they synapse on larger postsynaptic structures. Although both these differences are statistically significant, they are not large. Further studies will be required to determine if these anatomical variations lead to significantly different cortical response features.
Acknowledgments
We thank Anna Kowalkowski for technical assistance on this project. We dedicate this paper to Jeff Winer, a pioneer in thalamocortical structure and function.
Grant sponsor: National Institute on Deafness and Other Communication Disorders; National Institutes of Health; Grant numbers: R01 DC006212 (to P.H.S.) and R01 DC006013 (to M.I.B. and P.H.S.); Grant sponsor: The University of Wisconsin School of Medicine and Public Health; Grant number: PRJ39MN (to D.J.U.).
LITERATURE CITED
- Adams JC. Heavy metal intensification of DAB-based HRP reaction product. J Histochem Cytochem. 1981;29:775. doi: 10.1177/29.6.7252134. [DOI] [PubMed] [Google Scholar]
- Agmon A, Connors BW. Correlation between intrinsic firing patterns and thalamocortical synaptic responses of neurons in mouse barrel cortex. J Neurosci. 1992;12:319–329. doi: 10.1523/JNEUROSCI.12-01-00319.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitkin LM, Webster WR. Tonotopic organization of the medial geniculate body of the cat. Brain Res. 1971;26:402–405. [PubMed] [Google Scholar]
- Aitkin LM, Webster WR. Medial geniculate body of the cat: organization and responses to tonal stimuli of neurons in ventral division. J Neurophysiol. 1972;35:365–380. doi: 10.1152/jn.1972.35.3.365. [DOI] [PubMed] [Google Scholar]
- Allon N, Yeshurun Y. Functional organization of the medial geniculate body’s subdivisions of the awake squirrel monkey. Brain Res. 1985;360:75–82. doi: 10.1016/0006-8993(85)91222-3. [DOI] [PubMed] [Google Scholar]
- Barbour DL, Callaway EM. Excitatory local connections of superficial neurons in rat auditory cortex. J Neurosci. 2008;28:11174–11185. doi: 10.1523/JNEUROSCI.2093-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett EL, Smith PH. Anatomic, intrinsic, and synaptic properties of dorsal and ventral division neurons in rat medial geniculate body. J Neurophysiol. 1999;81:1999–2016. doi: 10.1152/jn.1999.81.5.1999. [DOI] [PubMed] [Google Scholar]
- Bartlett EL, Stark JM, Guillery RW, Smith PH. Comparison of the fine structure of cortical and collicular terminals in the rat medial geniculate body. Neuroscience. 2000;100:811–828. doi: 10.1016/s0306-4522(00)00340-7. [DOI] [PubMed] [Google Scholar]
- Barth DS, Di S. Three-dimensional analysis of auditory evoked potentials in rat neocortex. J Neurophysiol. 1990;64:1527–1536. doi: 10.1152/jn.1990.64.5.1527. [DOI] [PubMed] [Google Scholar]
- Beaulieu C, Campistron G, Crevier C. Quantitative aspects of the GABA circuitry in the primary visual cortex of the adult rat. J Comp Neurol. 1994;339:559–572. doi: 10.1002/cne.903390407. [DOI] [PubMed] [Google Scholar]
- Brett B, Di S, Watkins L, Barth DS. A horseradish peroxidase study of parallel thalamocortical projections responsible for the generation of mid-latency auditory-evoked potentials. Brain Res. 1994;647:65–95. doi: 10.1016/0006-8993(94)91399-4. [DOI] [PubMed] [Google Scholar]
- Budinger E, Heil P, Hess A, Scheich H. Multisensory processing via early cortical stages: connections of the primary cortical auditory field with other sensory systems. Neuroscience. 2006;143:1065–1083. doi: 10.1016/j.neuroscience.2006.08.035. [DOI] [PubMed] [Google Scholar]
- Calford MB. The parcellation of the medial geniculate body of the cat defined by the auditory response properties of single units. J Neurosci. 1983;3:2350–2364. doi: 10.1523/JNEUROSCI.03-11-02350.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calford MB, Webster WR. Auditory representation within principal division of cat medial geniculate body: an electrophysiology study. J Neurophysiol. 1981;45:1013–1028. doi: 10.1152/jn.1981.45.6.1013. [DOI] [PubMed] [Google Scholar]
- Clerici WJ, Coleman JR. Anatomy of the rat medial geniculate body: I. Cytoarchitecture, myeloarchitecture, and neocortical connectivity. J Comp Neurol. 1990;297:14–31. doi: 10.1002/cne.902970103. [DOI] [PubMed] [Google Scholar]
- Cruikshank SJ, Rose HJ, Metherate R. Auditory thalamocortical synaptic transmission in vitro. J Neurophysiol. 2002;87:361–384. doi: 10.1152/jn.00549.2001. [DOI] [PubMed] [Google Scholar]
- de Biasi S, Frassoni C, Spreafico R. GABA immunoreactivity in the thalamic reticular nucleus of the rat. A light and electron microscopic study. Brain Res. 1986;399:143–147. doi: 10.1016/0006-8993(86)90608-6. [DOI] [PubMed] [Google Scholar]
- Di S, Barth DS. The functional anatomy of middle-latency auditory evoked potentials: thalamocortical connections. J Neurophysiol. 1992;68:425–431. doi: 10.1152/jn.1992.68.2.425. [DOI] [PubMed] [Google Scholar]
- Donishi D, Kimura A, Okamoto K, Tamai Y. “Ventral” area in the rat auditory cortex: a major auditory field connected with the dorsal division of the medial geniculate body. Neuroscience. 2006;141:1553–1567. doi: 10.1016/j.neuroscience.2006.04.037. [DOI] [PubMed] [Google Scholar]
- Doron NN, LeDoux JE. Cells in the posterior thalamus project to both amygdala and temporal cortex: a quantitative retrograde double-labeling study in the rat. J Comp Neurol. 2000;425:257–274. [PubMed] [Google Scholar]
- Doron NN, LeDoux JE, Semple MN. Redefining the tonotopic core of rat auditory cortex; physiological evidence for a posterior field. J Comp Neurol. 2002;453:345–360. doi: 10.1002/cne.10412. [DOI] [PubMed] [Google Scholar]
- Edeline JM, Manunta Y, Nodal FR, Bajo VM. Do auditory responses recorded from awake animals reflect the anatomical parcellation of the auditory thalamus? Hear Res. 1999;131:135–152. doi: 10.1016/s0378-5955(99)00026-x. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick DC, Henson OW., Jr Cell types in the mustached bat auditory cortex. Brain Behav Evol. 1994;43:79–91. doi: 10.1159/000113626. [DOI] [PubMed] [Google Scholar]
- Horikawa J, Ito S, Hosokawa Y, Homma T, Murata K. Tonotopic representation in the rat auditory cortex. Proc Jpn Acad. 1988;64:260–263. [Google Scholar]
- Houser DR, Vaughn JE, Barber RP, Roberts E. GABA neurons are the major cell type of the nucleus reticular thalami. Brain Res. 1980;200:341–354. doi: 10.1016/0006-8993(80)90925-7. [DOI] [PubMed] [Google Scholar]
- Hu B. Cellular basis of temporal synaptic signaling: an in vitro electrophysiological study in rat auditory thalamus. J Physiol (Lond) 1995;483:167–182. doi: 10.1113/jphysiol.1995.sp020576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imig TJ, Morel A. Tonotopic organization in ventral nucleus of medial geniculate body in the cat. J Neurophysiol. 1985;53:309–340. doi: 10.1152/jn.1985.53.1.309. [DOI] [PubMed] [Google Scholar]
- Kharazia VN, Weinberg RJ. Glutamate in thalamic fibers terminating in layer IV of primary sensory cortex. J Neurosci. 1994;14:6021–6032. doi: 10.1523/JNEUROSCI.14-10-06021.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A, Donishi T, Sakoda T, Hazama M, Tamai Y. Auditory thalamic nuclei projections to the temporal cortex in the rat. Neuroscience. 2003;117:1003–1016. doi: 10.1016/s0306-4522(02)00949-1. [DOI] [PubMed] [Google Scholar]
- Kimura A, Donishi T, Okamoto K, Tamai Y. Efferent connections of “posterodorsal” auditory area in the rat cortex: implications for auditory spatial processing. Neuroscience. 2004;128:399–419. doi: 10.1016/j.neuroscience.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Kimura A, Donishi T, Okamoto K, Imbe H, Tamai Y. Efferent connections of the ventral auditory area in the cortex: implications for auditory processing related to emotion. Eur J Neurosci. 2007;25:2819–2834. doi: 10.1111/j.1460-9568.2007.05519.x. [DOI] [PubMed] [Google Scholar]
- Kimura A, Imbe H, Donishi T. Efferent connections of an auditory area in the caudal insular cortex of the rat: anatomical nodes for cortical streams of auditory processing and cross-modal sensory interactions. Neuroscience. 2010;166:1140–1157. doi: 10.1016/j.neuroscience.2010.01.032. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Ruggiero DA, Reis DJ. Projections to the subcortical forebrain from anatomically defined regions of the medial geniculate body in the rat. J Comp Neurol. 1985;242:182–213. doi: 10.1002/cne.902420204. [DOI] [PubMed] [Google Scholar]
- Lee CC, Sherman SM. Synaptic properties of thalamic and intracortical inputs to the first- and higher-order cortical areas in the auditory and somatosensory systems. J Neurophysiol. 2008:317–326. doi: 10.1152/jn.90391.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Sherman SM. Drivers and modulators in the central auditory pathways. Front Neurosci. 2010;4:79–86. doi: 10.3389/neuro.01.014.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinecke DL, Peters A. GABA immunoreactive neurons in rat visual cortex. J Comp Neurol. 1987;261:388–404. doi: 10.1002/cne.902610305. [DOI] [PubMed] [Google Scholar]
- Meyer G, Gonzalez-Hernandez TH, Ferres-Torres R. The spiny stellate neurons in layer IV of human auditory cortex. A Golgi study. Neuroscience. 1989;33:489–498. doi: 10.1016/0306-4522(89)90401-6. [DOI] [PubMed] [Google Scholar]
- Morel A, Rouiller E, de Ribaupierre Y, de Ribaupierre F. Tonotopic organization of the medial geniculate body (MGB) of lightly anesthetized cats. Exp Brain Res. 1987;69:24–42. doi: 10.1007/BF00247026. [DOI] [PubMed] [Google Scholar]
- Oliver DL. Neuron types in the central nucleus of the inferior colliculus that project to the medial geniculate body. Neuroscience. 1984;11:409–424. doi: 10.1016/0306-4522(84)90033-2. [DOI] [PubMed] [Google Scholar]
- Pandya PK, Rathbun DL, Moucha R, Navzer DE, Kilgard MP. Spectral and temporal processing in rat posterior auditory cortex. Cereb Cortex. 2008;18:301–314. doi: 10.1093/cercor/bhm055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6. New York: Academic Press; 2007. [DOI] [PubMed] [Google Scholar]
- Peruzzi D, Bartlett E, Smith PH, Oliver DL. A monosynaptic GABAergic input from the inferior colliculus to the medial geniculate body in rat. J Neurosci. 1997;17:3766–3777. doi: 10.1523/JNEUROSCI.17-10-03766.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polley DB, Read HL, Storace DA, Merzenich MM. Multiparametric auditory receptive field organization across five cortical fields in albino rat. J Neurophysiol. 2007;97:3621–3638. doi: 10.1152/jn.01298.2006. [DOI] [PubMed] [Google Scholar]
- Ramcharan EJ, Gnadt JW, Sherman SM. Higher-order thalamic relays burst more than first-order relays. Proc Natl Acad Sci U S A. 2005;102:12236–12241. doi: 10.1073/pnas.0502843102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson RJ, Blundon JA, Bayazitov IT, Zakharenko SS. Connectivity patterns revealed by mapping of active inputs on dendrites of thalamorecipient neurons in the auditory cortex. J Neurosci. 2009;29:6406–6417. doi: 10.1523/JNEUROSCI.0258-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger M, Arnault P. Anatomical study of the connections of the primary auditory area in the rat. J Comp Neurol. 1989;287:339–356. doi: 10.1002/cne.902870306. [DOI] [PubMed] [Google Scholar]
- Romanski LM, LeDoux JE. Organization of rodent auditory cortex: anterograde transport of PHA-L from MGV to temporal cortex. Cereb Cortex. 1993a;3:499–514. doi: 10.1093/cercor/3.6.499. [DOI] [PubMed] [Google Scholar]
- Romanski LM, LeDoux JE. Information cascade from primary auditory cortex to the amygdala: corticocortical and corticoamygdaloid projections of temporal cortex in the rat. Cereb Cortex. 1993b;3:515–532. doi: 10.1093/cercor/3.6.515. [DOI] [PubMed] [Google Scholar]
- Rose HJ, Metherate R. Auditory thalamocortical transmission is reliable and temporally precise. J Neurophysiol. 2005;94:2019–2030. doi: 10.1152/jn.00860.2004. [DOI] [PubMed] [Google Scholar]
- Rouiller EM, Welker E. A comparative analysis of the morphology of corticothalamic projections in mammals. Brain Res Bull. 2000;53:727–741. doi: 10.1016/s0361-9230(00)00364-6. [DOI] [PubMed] [Google Scholar]
- Saint Marie RL, Stanforth DA, Jubelier EM. Substrate for rapid feedforward inhibition of the auditory forebrain. Brain Res. 1997;765:173–176. doi: 10.1016/s0006-8993(97)00654-9. [DOI] [PubMed] [Google Scholar]
- Smith PH, Populin LC. Fundamental differences between the thalamocortical recipient layers of the cat auditory and visual cortices. J Comp Neurol. 2001;436:508–519. doi: 10.1002/cne.1084. [DOI] [PubMed] [Google Scholar]
- Smith PH, Manning KA, Uhlrich DJ. Evaluation of inputs to rat primary auditory cortex from the suprageniculate nucleus and extrastriate visual cortex. J Comp Neurol. 2010;518:3679–3700. doi: 10.1002/cne.22411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storace DA, Higgins NC, Read HL. Thalamic label patterns suggest primary and ventral auditory fields are distinct core regions. J Comp Neurol. 2010;518:1630–1646. doi: 10.1002/cne.22345. [DOI] [PubMed] [Google Scholar]
- Storace DA, Higgins NC, Read HL. Thalamocortical pathway specialization for sound frequency resolution. J Comp Neurol. 2011;519:177–193. doi: 10.1002/cne.22501. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Zhou H, Neumaier JF, Pham TA. Opposing functions of CREB and MKK1 synergistically regulate the geometry of dendritic spines in visual cortex. J Comp Neurol. 2007;503:605–617. doi: 10.1002/cne.21424. [DOI] [PubMed] [Google Scholar]
- Swadlow HA, Gusav AG. The impact of “bursting” thalamic impulses at a neocortical synapse. Nat Neurosci. 2001;4:402–408. doi: 10.1038/86054. [DOI] [PubMed] [Google Scholar]
- Tennigkeit F, Puil E, Schwartz DW. Firing modes and membrane properties in lemniscal auditory thalamus. Acta Otolaryngol. 1997;117:254–257. doi: 10.3109/00016489709117782. [DOI] [PubMed] [Google Scholar]
- Varela C, Sherman SM. Differences in response to muscarinic activation between first order and higher order thalamic relays. J Neurophysiol. 2007;98:3538–3547. doi: 10.1152/jn.00578.2007. [DOI] [PubMed] [Google Scholar]
- Varela C, Sherman SM. Differences in responses to serotonergic activation between first and higher order thalamic nuclei. Cereb Cortes. 2009;19:1776–1786. doi: 10.1093/cercor/bhn208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vees AM, Micheve KD, Beaulieu C, Descarries L. Increased number and size of dendritic spines in ipsilateral barrel field cortex following unilateral whisker trimming in postnatal rats. J Comp Neurol. 1998;400:110–124. [PubMed] [Google Scholar]
- Verbny YI, Erdelyi F, Szabo G, Banks MI. Properties of a population of GABAergic cells in murine auditory cortex weakly excited by thalamic stimulation. J Neurophysiol. 2006;96:3194–3208. doi: 10.1152/jn.00484.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield IC, Purser D. Microelectrode study of the medial geniculate body in unanesthetized free-moving cats. Brain Behav Evol. 1972;6:311–328. doi: 10.1159/000123718. [DOI] [PubMed] [Google Scholar]
- Winer JA. The medial geniculate body of the cat. Adv Anat Embryol Cell Biol. 1985;86:1–97. [PubMed] [Google Scholar]
- Zilles K, Wree A. Cortex: areal and laminar structure. In: Paxino G, editor. The rat nervous system. Vol. 1. Sydney: Academic Press; 1985. pp. 375–415. [Google Scholar]
- Zilles K, Wree A, Dausch N. Anatomy of the neocortex; neurochemical organization. In: Kolb B, Tees RC, editors. The cerebral cortex of the rat. Cambridge, MA: MIT Press; 1990. pp. 113–150. [Google Scholar]