Abstract
Accumulating evidence indicates that breast cancer is caused by cancer stem cells and cure of breast cancer requires eradication of breast cancer stem cells. Previous studies with leukemia stem cells have shown that NF-κB pathway is important for leukemia stem cell survival. In this study, by using MCF7 sphere cells as model of breast cancer stem-like cells, we evaluated the effect of NF-κB pathway specific inhibitors on human breast cancer MCF7 sphere cells. Three inhibitors including parthenolide (PTL), pyrrolidinedithiocarbamate (PDTC) and its analog diethyldithiocarbamate (DETC) were found to preferentially inhibit MCF7 sphere cell proliferation. These compounds also showed preferential inhibition in term of proliferation and colony formation on MCF7 side population (SP) cells, a small fraction of MCF7 cells known to enrich in breast cancer stem-like cells. The preferential inhibition effect of these compounds was due to inhibition of the NF-κB activity in both MCF7 sphere and MCF7 cells, with higher inhibition effect on MCF7 sphere cells than on MCF7 cells. PDTC was further evaluated in vivo and showed significant tumor growth inhibition alone but had better tumor growth inhibition in combination with paclitaxel in the mouse xenograft model than either PDTC or paclitaxel alone. This study suggests that breast cancer stem-like cells could be selectively inhibited by targeting signaling pathways important for breast cancer stem-like cells.
Keywords: Breast cancer stem-like cells, Side population cells, NF-κB, Sphere cells, Xenograft
Introduction
Breast cancer is the most frequent malignancy among women in Western countries, with an incidence in the U.S. of 111 cases per 100,000 woman-years (wy) and a mortality rate of 24 deaths per 100,000 wy [1]. Although the mortality of breast cancer has been decreasing [1, 2], which was believed to be the result of widespread mammography screening and the implementation of adjuvant therapy with tamoxifen and polychemotherapy [3, 4], breast cancer still is the most fatal disease for women in Western countries [1].
In 2003, Clarke and colleagues demonstrated that a highly tumorigenic subpopulation of breast cancer stem cells expressing CD44+CD24− surface marker in clinical specimen had the capacity to form tumors with as few as one hundred cells whereas tens of thousands of the bulk cells did not [5]. Recently, accumulating evidence indicates that breast cancer is originated from breast cancer stem cells, a rare population within breast tumor [5, 6]. Since the current cancer drugs, which are developed extensively based on their activity to inhibit bulk replicating cancer cells, may not be able to eliminate the cancer stem cells effectively, which have been demonstrated in a variety of tumors [7–13]. It is conceivable that improved breast cancer treatment requires eradication of cancer stem cells [6–8, 14, 15].
Although the breast cancer stem cells were initially identified in primary patient samples, cancer stem cells from patient samples are limited for breast cancer stem cell research because of the limited source. Meanwhile, some breast cancer cell lines were reported to harbor potential cancer stem-like cells. For instance, by using a sphere culture technique, MCF7 sphere cells were found to enrich breast cancer stem-like cells expressing CD44+CD24− [16]. Another approach to enrich breast cancer stem-like cells is to isolate the side population (SP) cells by flow cytometry [17, 18]. SP cells were first defined in hematopoietic system [19]. Although the detailed mechanism for the generation of the SP phenotype is still unknown, it is believed that some ATP-binding cassette (ABC) transporters including ABCG2/BCRP, ABCB1/MDR1 and ABCA3, might be involved in pumping out the fluorescent dye Hoechst 33342, causing the SP phenotype [20–22]. Based on the property to pump out Hoechst dye, SP cells could be isolated from different breast cancer cells lines, such as human breast cancer cell line MCF7 and SKBR3 [17, 18, 20]. Patrawala and colleagues reported that MCF7 SP cells had higher tumorigenicity than non-SP cells [18], which indicates that MCF7 SP cells enrich breast cancer stem-like cells.
In this study, based on the leukemia stem cell research, we intended to study the role of NF-κB pathway in breast cancer stem-like cell using MCF7 sphere cells and SP cells as models. Leukemia stem cells (LSC), the first cancer stem cell defined in the early 1990s [14, 23, 24], was the main cancer stem cell model for studying biology of cancer stem cells in the past decade. Leukemia stem cell study has benefited solid tumor stem cell study. For example, it was demonstrated the NF-κB pathway could be selectively targeted by pathway specific inhibitors including parthenolide (PTL) and pyrrolidine dithiocarbamate (PDTC) to preferentially inhibit LSC cells [25–27]. Here we first tested the sensitivity of breast cancer stem-like cells with known NF-κB pathway inhibitors. Our data indicated that PTL, PDTC and its analog DETC could preferentially inhibit both MCF7 sphere cells and SP cells, suggesting that these compounds were capable of preferentially inhibiting breast cancer stem-like cells. The mechanism was demonstrated to be mediated through inhibition of the NF-κB activity. In particular, these compounds could selectively inhibit the NF-κB activity better in MCF7 sphere cells than in MCF7 bulk cells. PDTC was further evaluated in the mouse xenograft model and found to be effective in inhibiting tumor growth alone and achieved a better tumor inhibition in combination with paclitaxel than PDTC or paclitael alone in vivo.
Materials and methods
Cell culture
Human breast cancer cell line MCF7 cells were obtained from ATCC (American Type Culture Collection). Cells were grown in DMEM medium (Invitrogen) supplemented with 10% fetal bovine serum (Invitrogen), 100 units/ml penicillin and 100 µg/ml streptomycin (Invitrogen), in a 37°C incubator containing 5% CO2. Sphere cell culture was performed according to the published protocol with some modifications [16, 28]. Briefly, single cells were plated in ultralow attachment plates (Corning, NY) at a density of 20,000 viable cells/ml in primary culture and 1,000 cells/ml in passages. Cells were grown in a serum-free mammary epithelial growth medium without bovine pituitary extract (MEGM, BioWhittaker), but supplemented with B27 (Invitrogen), 20 ng/ml and EGF and 20 ng/ml bFGF (BD Biosciences). In order to passage sphere cells, spheres were collected into 15 ml tube and allowed to settle for 15 min. Supernatant was removed. Sphere cells were dissociated enzymatically with 0.05% trypsin, 0.5 mM EDTA (Invitrogen) and mechanically by a glass Pasteur pipette. The cells obtained from dissociation were passed through a 40-µm sieve and analyzed microscopically for single cells and subjected to the following experiments.
Hoechst 33342 staining and flow cytometry analysis/sorting of SP cells
To identify and sort SP and non-SP fractions, cells were washed with PBS and detached from the culture dish with trypsin and EDTA, pelleted by centrifugation, and resuspended in 37°C DMEM containing 2% FBS at 1 × 106 cell/ml. Cell staining was performed according to the protocol [29] with slight modification. The cells were then incubated with Hoechst 33342 (Sigma) at 5 µg/ml either alone or in combination with 50 µM verapamil (Sigma) for 90 min at 37°C. Following staining, the cells were spun down and resuspended in HBSS (Invitrogen, Carlsbad, CA) containing 1 µg/ml propidium iodide and maintained at 4°C for flow cytometry analysis/sorting. Cell analysis and sorting were performed on a Moflo flow cytometer (Dako Cytomation, Fort Collins, CO. USA) equipped with a Coherent Enterprise II laser emitting MLUV at 351 nm and blue 488 nm lines. The Hoechst 33342 emission was first split using a 610dsp filter and then the red and the blue emissions were collected through a 670/30 nm and a 450/65 nm bandpass filters, respectively.
Cell proliferation assay
To test the sensitivity of MCF7 bulk cells and sphere cells to specific compounds, both MCF7 bulk cells and sphere single cells were seeded at concentration of 3 × 104 cells/ml in 96-well plates. After overnight incubation, serial concentrations of tested compounds were added. Each concentration was repeated three times. These cells were incubated in a humidified atmosphere with 5% CO2 for 3 days. Then, 20 µl MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) (Sigma) solution (4.14 mg/ml) was added to each well and incubated at 37°C for 4 h. The medium was removed and formazan was dissolved in DMSO and the optical density was measured at 590 nm using a Bio-assay reader (Bio-Rad, USA). The growth inhibition was determined using: Growth inhibition = (control’s O.D. − sample’s O.D.)/control’s OD.
To test the drug sensitivity of SP cells, both MCF7 SP and non-SP cells were sorted into 96-well culture plates at 500 cells per well and incubated in DMEM medium at 37°C in an incubator containing 5% CO2 for 24 h. Then cells were treated with compounds at concentration as indicated in the text. After 72 h exposure to the tested agents, proliferation of the cells was determined by using a fluorescence-based cell proliferation assay (CyQUANT Cell Proliferation Assay Kit, Molecular Probe). Fluorescence signal was detected by a Bio-assay reader (Bio-Rad, USA) according to the manufacturer’s instructions.
Colony formation assay
The colony formation assay was carried out in 35 mm dishes. Briefly, both SP and non-SP cells were plated in 35 mm dishes at 5,000 cells/well in 0.35% top agar in culture medium over a 0.5% agar layer. For compound testing group, compounds were added into the top agar at concentrations as indicated. Plates were further incubated in cell culture incubator for 12 days until colonies were large enough to be visualized. Colonies were stained with 0.01% Crystal Violet for 1 h and counted. Experiments were done in triplicate.
Nuclear extract preparation
Nuclear extracts were prepared according to the protocol by ActiveMotif Company (Active Motif, Carlsbad, CA). Cells were washed and collected in ice-cold PBS/PIB buffer and resuspended in 10 ml of ice-cold hypotonic buffer containing 20 mM HEPES (pH7.5), 5 mM NaF, 10 µM Na2MoO4, 0.1 mM EDTA. PBS/PIB buffer was prepared by adding 0.5 ml of PIB (125 mM NaF, 250 mM β-glycerophosphate, 250 mM para-nitrophenyl phosphate (PNPP) and 25 mM NaVO3) to 10 ml of 1× PBS prior to use. Following a 15 min incubation on ice, 50 µl of a 10% Nonidet P-40 solution was added and mixed by gentle pipetting. Nuclei were then pelleted by centrifugation at 14,000 × g for 30 s, washed with the hypotonic buffer and resuspended in 50 µl ice-cold complete lysis buffer (Active Motif, Carlsbad, CA). After the nuclear lysates were centrifuged, supernatants were collected for quantification of NF-κB activation. The protein concentrations in the supernatants were determined using the BCA protein assay kit (Pierce, Rockford, IL).
Quantification of NF-κB activation
Trans-AM NF-κB assay, an enzyme-linked immunosorbent assay (ELISA)—based method (Trans-AM NF-κB; Active Motif, Carlsbad, CA) was used for NF-κB activity quantification according to the manufacturer’s instruction. Briefly, cell nuclear extracts were placed in 96-well plates coated with an oligonucleotide containing the NF-κB consensus sequence, and the presence of active NF-κB was detected by using antibodies specific for p50 subunits that are not complexed to IκB and thus are able to bind the consensus sequence. A horseradish peroxidase (HRP)—conjugated secondary antibody is used to quantify NF-κB binding by conversion of an applied chromogenic substrate.
Antitumor activity of PDTC in tumor xenograft model
Female athymic nude mice (NCR-nu/nu, NCI) were housed under specific pathogen-free conditions. The in vivo experiments were performed in accordance with the guidelines of our institute. For mice in MCF7 xenograft study, the mice were given injections of β-estradiol (Sigma) dissolved in pure sesame oil (0.1 mg per 0.05 ml sesame oil per mouse, subcutaneously) 1 day before the injection of MCF7 cells and then at weekly intervals [30, 31]. Mice were inoculated subcutaneously with 2 × 107 MCF7 cells. When the tumor volumes reached 100–200 mm3, the mice were randomly divided into groups as indicated such that each group harbored tumors of a similar size. Each group included 5 mice. Stock solution of paclitaxel was prepared by dissolving the drug in a vehicle solution (EtOH:cremophor, 50:50 v/v). PDTC was dissolved in saline. Paclitaxel alone and paclitaxel in combination with PDTC stock solution were mixed with physiologic saline or saline containing PDTC (10:90 v/v). A dosing solution (200 µl) was intravenously injected, over 1 min via a tail vein. The PDTC dose was 60 mg/kg, and the paclitaxel dose was 10 mg/kg. Tumor measurements were done twice a week using traceable digital vernier calipers (Fisher). The tumor volumes were determined by measuring the length (l) and the width (w) and calculating the volume using the formula V = lw2/2.
Statistical analysis
The growth inhibition effect was compared by Student’s t test. P < 0.05 was considered statistically significant.
Results
PTL, PDTC and DETC preferentially inhibit MCF7 sphere cell proliferation
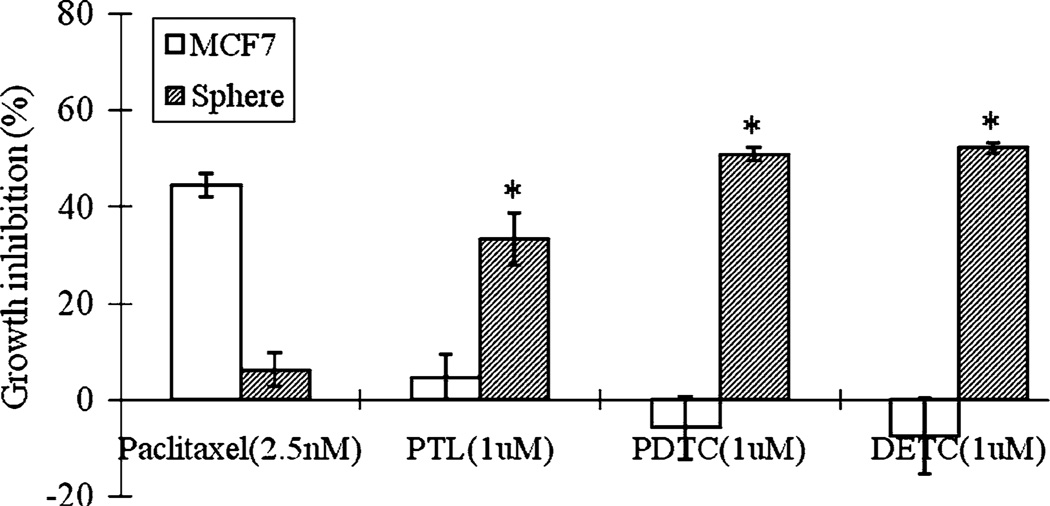
It has been shown that NF-κB pathway specific inhibitors, including MG-132, PTL and PDTC could selectively inhibit leukemia stem cell proliferation [25–27]. To study the sensitivity of breast cancer stem-like cell to NF-κB pathway inhibitors, 11 compounds targeting different steps of the NF-κB pathway, including antioxidants Curcumin [32]; PDTC [33]; DETC [34]; Quercetin [35], NF-κB phosphorylation inhibitors Sulfasalazine [36]; Sulindac [37]; Ibuprofen [38]; PTL [39] and NF-κB degradation inhibitors MG-132 [40]; Cyclosporin A [41]; Genistein [42], were tested in this study. MCF7 sphere cells were used as a model of breast cancer stem-like cells [16, 43]. Among all the inhibitors, antioxidants which inhibit NF-κB activation including PDTC and its analog DETC, and NF-κB phosphorylation inhibitors PTL were shown to preferentially inhibit sphere cell proliferation. As shown in Fig. 1, PTL, PDTC and DETC at 1 µM inhibited MCF7 sphere cell growth by 33.2, 50.8 and 52.2%, respectively, but did not show obvious growth inhibition effect on MCF7 bulk cells. In contrast, cancer drug paclitaxel gave better growth inhibition effect on bulk MCF7 cells by 44.5% at 2.5 nM but only inhibited sphere cells by 6.1%. These data indicate that, unlike paclitaxel that act primarily on replicating bulk MCF7 cells, PTL, PDTC and DETC selectively inhibited MCF7 sphere cell proliferation over MCF7 bulk cells.
Fig. 1.
Growth inhibition of PTL, PDTC, DETC and paclitaxel on MCF7 sphere cells compared with MCF7 bulk cells
PTL, PDTC and DETC could preferentially inhibit MCF7 SP cell proliferation and colony formation
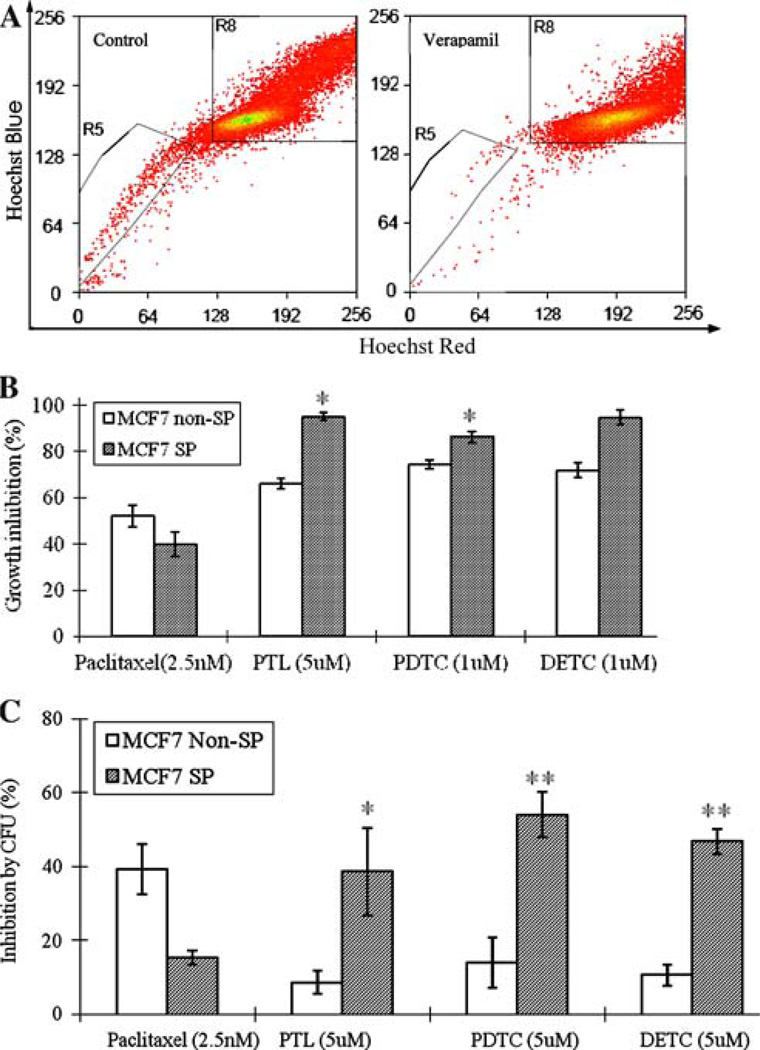
Apart from sphere cells cultured from MCF7 cells, side population (SP) cells isolated from MCF7 cells were also found to enrich breast cancer stem-like cells, which showed higher colony formation ability in vitro and in vivo tumorigenicity than non-SP fraction [18]. MCF7 cells were shown to contain 1.2% SP cells, which could significantly be blocked by ABC transporter inhibitor verapamil (0.12%) (Fig. 2a). It was of interest to test whether the various NF-κB inhibitors that are preferentially active against sphere cells as above have similar effect on MCF7 SP cells. For this purpose, both SP and non-SP cells were sorted out by flow cytometry as indicated by trapezoids on the left (R5) and right (R8), respectively (Fig. 2a), and seeded into 96 well plates at 500 cells/well. After overnight incubation, both SP and non-SP cells were treated with PDTC, DETC and PTL for 3 days. Cell proliferation was determined using a fluorescence-based cell proliferation assay as indicated above. Interestingly, unlike cancer drug paclitaxel (2.5 nM), which inhibited MCF7 SP cells by 39.3%, and non-SP cells by 52.2%, all the three compounds showed preferential inhibition for the MCF7 SP cells over the non-SP cells (Fig. 2b). PTL (5 µM) inhibited MCF7 SP cell growth by 95.2% and non-SP cells by 66.1% (Fig. 2b). Both PDTC and DETC also showed higher ability to inhibit both SP and non-SP proliferation. As shown in Fig. 2b, 1 µM of PDTC and DETC preferentially inhibited MCF7 SP cells by 86.3 and 94.5%, but inhibited non-SP cells by 74.4 and 71.9%, respectively.
Fig. 2.
(a) A representative MCF7 SP profile for sorting. MCF7 cells were stained as described in Methods. The MCF7 SP and non-SP regions are indicated by trapezoinds on the left (R5) and right (R8), respectively. (b) Growth inhibition of PTL, PDTC, DETC and paclitaxel on MCF7 SP cells, compared with MCF7 non-SP cells. (c) Inhibition of colony formation by PTL, PDTC, DETC and paclitaxel on MCF7 SP and non-SP cells
We further evaluated the effects of these compounds on MCF7 SP cells using colony formation assay. Interestingly, all the three compounds showed higher inhibition of colony formation for MCF7 SP cells than for non-SP cells. As shown in Fig. 2c, PDTC and DETC (5 µM) inhibited colony formation ability of MCF7 SP cells by 54.1 and 46.8% but the same treatment only gave inhibition on non-SP cells by 14.0 and 10.7%, respectively. Similarly, PTL (5 µM) inhibited colony formation of MCF7 SP cells by 38.7% but 8.7% for non-SP cells (Fig. 2c). In contrast to the above NF-κB inhibitors, the control cancer drug paclitaxel showed the reverse inhibition effect, that is, paclitaxel inhibited MCF7 SP and non-SP colony formation by 15.4 and 39.2%, respectively.
Taken together, these data indicate that, unlike cancer drug paclitaxel, PTL, PDTC and DETC could preferentially inhibit MCF7 SP cell proliferation and colony formation over non-SP cells.
PTL, PDTC and DETC preferentially inhibit NF-κB activity in MCF7 sphere cells
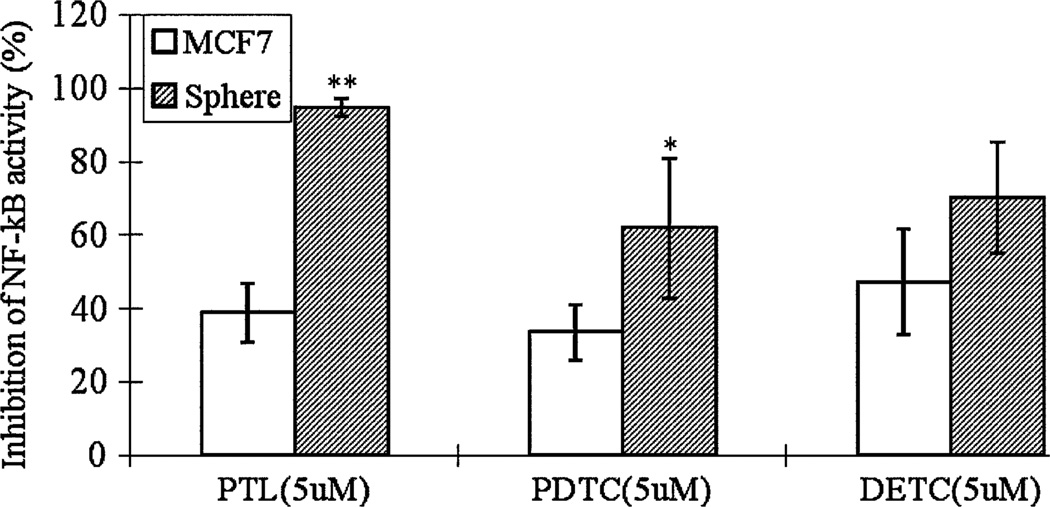
To investigate the mechanism by which breast cancer stem-like cells are more sensitive to these inhibitors, we compared the NF-κB activity in sphere cells to the bulk MCF7 cells under the same treatment. An ELISA-based method (Trans-AM NF-κB; ActiveMotif, Carlsbad, CA) was used to quantify the NF-κB activation because of its high sensitivity to detect and quantify NF-κB activation compared to the more traditional method EMSA [44] or Western blot or gel shift assay. Although there is no significant difference in the basal level of NF-κB activity in MCF7 bulk cells and MCF7 sphere cells (data not shown), all the three compounds, PTL, PDTC and DETC, reduced the NF-κB activity within MCF7 sphere cells but had less effect for MCF7 bulk cells (Fig. 3). As shown in Fig. 3, when treated with 5 µM PTL, PDTC and DETC for 24 h, NF-κB activity in MCF7 sphere cells was decreased by 94.6, 61.8 and 70.1%, respectively. However, the treatment only inhibited the NF-κB activity within MCF7 cells by 38.8, 33.6 and 47.3%, respectively (Fig. 3). These data suggest that the preferential inhibition effects of PTL, PDTC and DETC were caused by inhibition of the NF-κB activity.
Fig. 3.
Inhibition of NF-κB activity of MCF7 cells and MCF7 sphere cells after treatment with PTL, PDTC and DETC. Cells were treated with PTL, PDTC and DETC at 5 µM for 24 h. Nuclear extract was prepared from control and treated cells. The quantification of NF-κB activation was performed using Trans-AM NF-κB kit from ActiveMotif
Effects of PDTC on tumor growth in nude mice
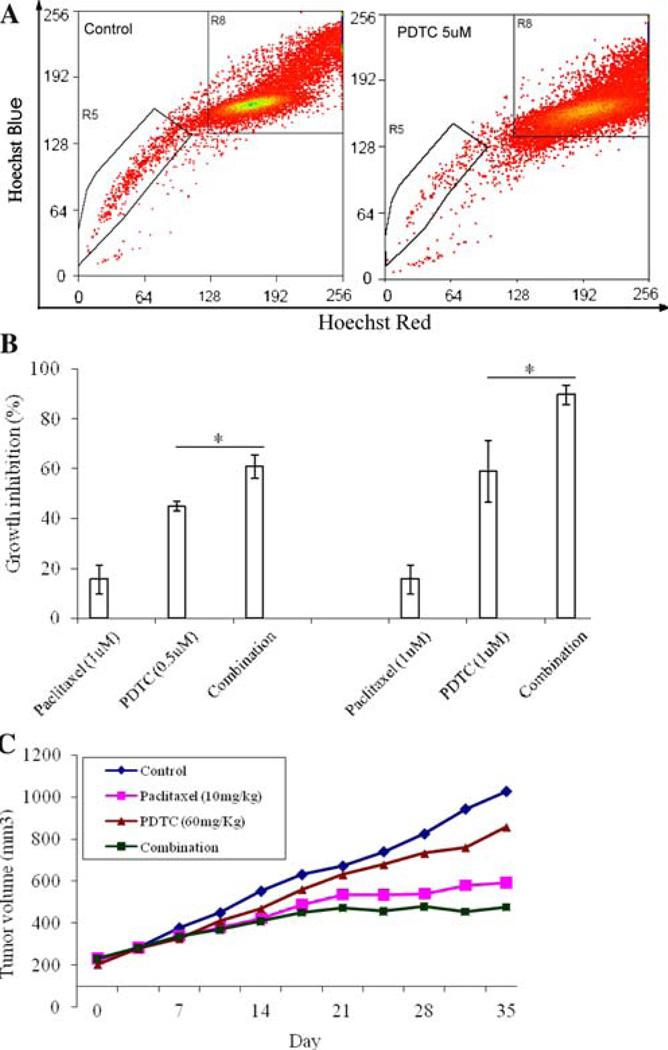
PDTC was chosen for further investigation in this study. To provide evidence that PDTC specifically inhibited cancer stem-like cells within MCF7 cells, we treated MCF7 cells with PDTC (5 µM) for 24 h and examined the SP fraction within MCF7 cells by flow cytometry. Interestingly, PDTC significantly decreased the SP fraction within the MCF7 cells. As shown in Fig. 4a, the SP fraction within MCF7 cells was only 0.27% after treatment, compared with 1.20% in control MCF7 cells (Fig. 4a). Since PDTC specifically inhibited breast cancer stem-like cells, it is possible that combination of PDTC with clinical drug like paclitaxel could have enhanced effects on treatment of breast cancer. To test this, we performed both in vitro and in vivo experiments to compare the combination effect of PDTC with clinical drug paclitaxel with either drug alone. Indeed, combination of PDTC with paclitaxel showed synergistic effect on MCF7 sphere cells in vitro. PDTC alone at the concentrations of 0.5 and 1 µM inhibited MCF7 sphere cell growth by 44.8 and 58.8% whereas paclitaxel alone inhibited MCF7 sphere cell growth by 15.6% (Fig. 4b). In contrast, the combination of PDTC with paclitaxel showed better growth inhibition effects than either PDTC or paclitaxel alone. As shown in Fig. 4b, combinations of paclitaxel (1 µM) with PDTC (0.5 µM) and with PDTC (1 µM) inhibited MCF7 sphere cells by 60.7 and 89.6%, respectively. We further evaluated the antitumor effect of PDTC in MCF7 nude mouse xenograft model. As shown in Fig. 4c, PDTC alone showed certain antitumor effect in vivo. When combined with paclitaxel, PDTC produced a better inhibitory effect on tumor growth than either PDTC or paclitaxel alone (Fig. 4c). DETC, which is an analog of PDTC, was also tested in the xenograft model and found to have similar growth inhibitory effects as PDTC (data not shown).
Fig. 4.
Antitumor effect of PDTC in vitro and in vivo. (a) Decreasing SP fraction within MCF7 cells by PDTC. (b) Growth inhibition effect of PDTC, paclitaxel and combination on MCF7 sphere cells. (c) In vivo anti-tumor growth effect of PDTC (60 mg/kg) paclitaxel (10 mg/kg) and combination on MCF7 xenograft. Treatment was given twice a week for four weeks. Tumor sizes were measured twice weekly
Discussion
Although the breast cancer stem cells are the first solid tumor stem cells identified in 2003 [5], breast cancer stem cell research is still in its early stage and a feasible model is needed for breast cancer stem cell research. Currently, most cancer stem cells were identified in primary patient samples, which is limited for cancer stem cell research because of their limited supply. In contrast, cancer stem cells from cell lines could be a promising model for cancer stem cell research because of its unlimited availability and easy handling. Very recently, sphere cells and side population cells isolated or cultured from human breast cancer cell line MCF7 were reported to enrich breast cancer stem-like cells [16, 18], which have higher tumorigenicity and can be used as a model for breast cancer stem cell study [43]. Besides the model, new approaches are also needed to target breast cancer stem cells. Leukemia stem cell research established the original paradigm of cancer stem cells and has shed light on the study to identify and characterize solid tumor stem cells [45–47]. Importantly, it may also suggest the direction for elimination of solid tumor stem cells. For instance, one promising approach to eliminate leukemia stem cell is to target signaling pathways important for leukemia stem cell survival or self renewal [48–50]. Some signaling pathways, including the PI3K pathway, NF-κB pathway, mTOR signaling and PTEN signaling, were found to be preferentially important for leukemia stem cell survival or/and self-renewal, which could be selectively targeted for elimination of leukemia stem cells [25–27, 51, 52].
In this study, by using MCF7 sphere cells as a model, we investigated the effect of NF-κB pathway inhibitors on breast cancer stem-like cells. Interestingly, NF-κB pathway inhibitors, including PTL, PDTC and DETC, were found to preferentially inhibit MCF7 sphere cell proliferation over parental MCF7 cells. PTL is a compound extracted from Tanacetum parthenium [53] and is known to inhibit the NF-κB pathway by preventing IkBa degration [54], inhibiting IkB kinase b [55] and alkylating of p65 [56]. PDTC and DETC are known to be antioxidants which inhibit the NF-κB pathway through blocking activation of nuclear factor kappa B (NF-kappa B) [33] and also inhibiting the IKK activity [57] and IκB-κ [58]. Like their effect on breast cancer stem-like cells revealed in this study, both PTL and PDTC could preferentially inhibit leukemia stem cell [26, 27]. Cancer drug paclitaxel is different from PTL, PDTC and DETC, in that it had better activity for bulk MCF7 cells than for MCF7 sphere cells. In addition, all the three compounds, unlike paclitaxel, also showed a preferential inhibitory effect on colony formation of MCF7 SP cells. Since both MCF7 sphere cells and SP cells are known to enrich in breast cancer stem-like cells [16, 18], these data indicate that the three compounds, PTL, PDTC and DETC, which are different from common cancer drug paclitaxel, could preferentially inhibit breast cancer stem-like cells. It is interesting to note that while there is no apparent difference in the basal level of NF-κB activity of the MCF7 sphere cells and bulk cells, treatment with PTL. PDTC and DETC preferentially inhibited the NF-κB activity in the sphere cells over bulk cells (Fig. 3). NF-κB is known to be a suppressor of apoptosis, and it is conceivable that its role in the more quiescent cancer stem-like sphere cells is more important than in the bulk cells. The mechanism of action of PTL, PDTC and DETC for MCF7 sphere cells is related to the inhibition of NF-κB activity. These compounds caused greater inhibition of the NF-κB activity in MCF7 sphere cells than in MCF7 bulk cells, suggesting that the NF-κB pathway might be preferentially vulnerable in MCF7 sphere cells than the MCF7 bulk cells. Further studies are needed to determine what step in the NF-κB pathway is more important in sphere cell survival.
To substantiate the in vitro activity of PDTC on breast cancer stem-like cells, we evaluated PDTC alone and in combination with paclitaxel in the mouse xenograft model. PDTC alone showed significant inhibition effect on tumor growth. Interestingly, when combined with paclitaxel, PDTC had a higher inhibition on tumor growth than paclitaxel or PDTC alone (Fig. 4c). DETC, an analog of PDTC, also showed similar tumor growth inhibition effect in vivo as PDTC (data not shown). Our results are consistent with the previous finding that PTL in combination with docetaxel could reduce metastasis and improve survival in a xenograft model of breast cancer [59]. Taken together, this study indicates that it is possible to inhibit breast cancer stem cells by targeting the NF-κB pathway. Future studies are needed to investigate other signaling pathways for breast cancer stem cells that may be exploited for development of new drugs that target cancer stem cells for improved treatment of breast cancer.
Acknowledgements
Support from NIH grant AI44063, Ho Ching Yang Fellowship of Johns Hopkins Bloomberg School of Public Health, and the Johns Hopkins Center for AIDS Research, is gratefully acknowledged.
Abbreviations
- PTL
Parthenolide
- PDTC
Pyrrolidinedithiocarbamate
- DETC
Diethyldithiocarbamate
- SP
Side population
- ABC
ATP-binding cassette
References
- 1.Howe HL, Wingo PA, Thun MJ, Ries LA, Rosenberg HM, Feigal EG, Edwards BK. Annual report to the nation on the status of cancer (1973 through 1998), featuring cancers with recent increasing trends. J Natl Cancer Inst. 2001;93:824–842. doi: 10.1093/jnci/93.11.824. [DOI] [PubMed] [Google Scholar]
- 2.Peto R, Boreham J, Clarke M, Davies C, Beral V. UK and USA breast cancer deaths down 25% in year 2000 at ages 20–69 years. Lancet. 2000;355:1822. doi: 10.1016/S0140-6736(00)02277-7. [DOI] [PubMed] [Google Scholar]
- 3.EBCT. Polychemotherapy for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 1998;352:930–942. [PubMed] [Google Scholar]
- 4.EBCT. Tamoxifen for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 1998;351:1451–1467. [PubMed] [Google Scholar]
- 5.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Hajj M, Becker MW, Wicha M, Weissman I, Clarke MF. Therapeutic implications of cancer stem cells. Curr Opin Genet Dev. 2004;14:43–47. doi: 10.1016/j.gde.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Jones RJ, Matsui WH, Smith BD. Cancer stem cells: are we missing the target? J Natl Cancer Inst. 2004;96:583–585. doi: 10.1093/jnci/djh095. [DOI] [PubMed] [Google Scholar]
- 8.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 9.Donnenberg VS, Donnenberg AD. Multiple drug resistance in cancer revisited: the cancer stem cell hypothesis. J Clin Pharmacol. 2005;45:872–877. doi: 10.1177/0091270005276905. [DOI] [PubMed] [Google Scholar]
- 10.Guzman ML, Jordan CT. Considerations for targeting malignant stem cells in leukemia. Cancer Control. 2004;11:97–104. doi: 10.1177/107327480401100216. [DOI] [PubMed] [Google Scholar]
- 11.Costello RT, Mallet F, Gaugler B, Sainty D, Arnoulet C, Gastaut JA, Olive D. Human acute myeloid leukemia CD34+/CD38− progenitor cells have decreased sensitivity to chemotherapy and Fas-induced apoptosis, reduced immunogenicity, and impaired dendritic cell transformation capacities. Cancer Res. 2000;60:4403–4411. [PubMed] [Google Scholar]
- 12.Graham SM, Jorgensen HG, Allan E, Pearson C, Alcorn MJ, Richmond L, Holyoake TL. Primitive, quiescent, Philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro. Blood. 2002;99:319–325. doi: 10.1182/blood.v99.1.319. [DOI] [PubMed] [Google Scholar]
- 13.Angstreich GR, Matsui W, Huff CA, Vala MS, Barber J, Hawkins AL, Griffin CA, Smith BD, Jones RJ. Effects of imatinib and interferon on primitive chronic myeloid leukaemia progenitors. Br J Haematol. 2005;130:373–381. doi: 10.1111/j.1365-2141.2005.05606.x. [DOI] [PubMed] [Google Scholar]
- 14.Behbod F, Rosen JM. Will cancer stem cells provide new therapeutic targets? Carcinogenesis. 2005;26:703–711. doi: 10.1093/carcin/bgh293. [DOI] [PubMed] [Google Scholar]
- 15.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 16.Ponti D, Costa A, Zaffaroni N, Pratesi G, Petrangolini G, Coradini D, Pilotti S, Pierotti MA, Daidone MG. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65:5506–5511. doi: 10.1158/0008-5472.CAN-05-0626. [DOI] [PubMed] [Google Scholar]
- 17.Kondo T, Setoguchi T, Taga T. Persistence of a small subpopulation of cancer stem-like cells in the C6 glioma cell line. Proc Natl Acad Sci USA. 2004;101:781–786. doi: 10.1073/pnas.0307618100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patrawala L, Calhoun T, Schneider-Broussard R, Zhou J, Claypool K, Tang DG. Side population is enriched in tumorigenic, stem-like cancer cells, whereas ABCG2+ and ABCG2− cancer cells are similarly tumorigenic. Cancer Res. 2005;65:6207–6219. doi: 10.1158/0008-5472.CAN-05-0592. [DOI] [PubMed] [Google Scholar]
- 19.Goodell MA, Rosenzweig M, Kim H, Marks DF, DeMaria M, Paradis G, Grupp SA, Sieff CA, Mulligan RC, Johnson RP. Dye efflux studies suggest that hematopoietic stem cells expressing low or undetectable levels of CD34 antigen exist in multiple species. Nat Med. 1997;3:1337–1345. doi: 10.1038/nm1297-1337. [DOI] [PubMed] [Google Scholar]
- 20.Hirschmann-Jax C, Foster AE, Wulf GG, Goodell MA, Brenner MK. A distinct “side population” of cells in human tumor cells: implications for tumor biology and therapy. Cell Cycle. 2005;4:203–205. [PubMed] [Google Scholar]
- 21.Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M, Nakauchi H, Sorrentino BP. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med. 2001;7:1028–1034. doi: 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]
- 22.Jonker JW, Freeman J, Bolscher E, Musters S, Alvi AJ, Titley I, Schinkel AH, Dale TC. Contribution of the ABC-transporters Bcrp1 and Mdr1a/1b to the side population phenotype in mammary gland and bone marrow of mice. Stem Cells. 2005;23:1059–1065. doi: 10.1634/stemcells.2005-0150. [DOI] [PubMed] [Google Scholar]
- 23.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 24.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 25.Guzman ML, Neering SJ, Upchurch D, Grimes B, Howard DS, Rizzieri DA, Luger SM, Jordan CT. Nuclear factor-kappaB is constitutively activated in primitive human acute myelogenous leukemia cells. Blood. 2001;98:2301–2307. doi: 10.1182/blood.v98.8.2301. [DOI] [PubMed] [Google Scholar]
- 26.Guzman ML, Rossi RM, Karnischky L, Li X, Peterson DR, Howard DS, Jordan CT, Liesveld JL, Phillips GL, Swiderski CF, Grimes BA, Szilvassy SJ, Neering SJ, Upchurch D, Grimes B, Rizzieri DA, Luger SM, Lemischka IR, Pettigrew AL, Meyerrose T, Rossi R, Phillips GL. The sesquiterpene lactone parthenolide induces apoptosis of human acute myelogenous leukemia stem and progenitor cells. Blood. 2005;16:708–712. doi: 10.1182/blood-2004-10-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malaguarnera L, Pilastro MR, DiMarco R, Scifo C, Renis M, Mazzarino MC, Messina A. Cell death in human acute myelogenous leukemic cells induced by pyrrolidinedithiocarbamate. Apoptosis. 2003;8:539–545. doi: 10.1023/a:1025550726803. [DOI] [PubMed] [Google Scholar]
- 28.Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, Wicha MS. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253–1270. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kasukabe T, Okabe-Kado J, Kato N, Sassa T, Honma Y. Effects of combined treatment with rapamycin and cotylenin A, a novel differentiation-inducing agent, on human breast carcinoma MCF-7 cells and xenografts. Breast Cancer Res. 2005;7:R1097–R1110. doi: 10.1186/bcr1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hardman WE, Moyer MP, Cameron IL. Fish oil supplementation enhanced CPT-11 (irinotecan) efficacy against MCF7 breast carcinoma xenografts and ameliorated intestinal side-effects. Br J Cancer. 1999;81:440–448. doi: 10.1038/sj.bjc.6690713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh S, Aggarwal BB. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) [corrected] J Biol Chem. 1995;270:24995–25000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- 33.Schreck R, Meier B, Mannel DN, Droge W, Baeuerle PA. Dithiocarbamates as potent inhibitors of nuclear factor kappa B activation in intact cells. J Exp Med. 1992;175:1181–1194. doi: 10.1084/jem.175.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hill WD, Hess DC, Carroll JE, Wakade CG, Howard EF, Chen Q, Cheng C, Martin-Studdard A, Waller JL, Beswick RA. The NF-kappaB inhibitor diethyldithiocarbamate (DDTC) increases brain cell death in a transient middle cerebral artery occlusion model of ischemia. Brain Res Bull. 2001;55:375–386. doi: 10.1016/s0361-9230(01)00503-2. [DOI] [PubMed] [Google Scholar]
- 35.Musonda CA, Chipman JK. Quercetin inhibits hydrogen peroxide (H2O2)-induced NF-kappaB DNA binding activity and DNA damage in HepG2 cells. Carcinogenesis. 1998;19:1583–1589. doi: 10.1093/carcin/19.9.1583. [DOI] [PubMed] [Google Scholar]
- 36.Weber CK, Liptay S, Wirth T, Adler G, Schmid RM. Suppression of NF-kappaB activity by sulfasalazine is mediated by direct inhibition of IkappaB kinases alpha and beta. Gastroenterology. 2000;119:1209–1218. doi: 10.1053/gast.2000.19458. [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto Y, Yin MJ, Lin KM, Gaynor RB. Sulindac inhibits activation of the NF-kappaB pathway. J Biol Chem. 1999;274:27307–27314. doi: 10.1074/jbc.274.38.27307. [DOI] [PubMed] [Google Scholar]
- 38.Palayoor ST, Youmell MY, Calderwood SK, Coleman CN, Price BD, Rosenzweig KE, Youmell MB. Constitutive activation of IkappaB kinase alpha and NF-kappaB in prostate cancer cells is inhibited by ibuprofen radiosensitization of human tumor cells by the phosphatidylinositol 3-kinase inhibitors wortmannin and LY294002 correlates with inhibition of DNA-dependent protein kinase and prolonged G2-M delay. Oncogene. 1999;18:7389–7394. doi: 10.1038/sj.onc.1203160. [DOI] [PubMed] [Google Scholar]
- 39.Hehner SP, Heinrich M, Bork PM, Vogt M, Ratter F, Lehmann V, Schulze-Osthoff K, Droge W, Schmitz ML. Sesquiterpene lactones specifically inhibit activation of NF-kappa B by preventing the degradation of I kappa B-alpha and I kappa B-beta. J Biol Chem. 1998;273:1288–1297. doi: 10.1074/jbc.273.3.1288. [DOI] [PubMed] [Google Scholar]
- 40.Meriin AB, Gabai VL, Yaglom J, Shifrin VI, Sherman MY. Proteasome inhibitors activate stress kinases and induce Hsp72. Diverse effects on apoptosis. J Biol Chem. 1998;273:6373–6379. doi: 10.1074/jbc.273.11.6373. [DOI] [PubMed] [Google Scholar]
- 41.Meyer S, Kohler NG, Joly A. Cyclosporine A is an uncompetitive inhibitor of proteasome activity and prevents NF-kappaB activation. FEBS Lett. 1997;413:354–358. doi: 10.1016/s0014-5793(97)00930-7. [DOI] [PubMed] [Google Scholar]
- 42.Natarajan K, Manna SK, Chaturvedi MM, Aggarwal BB. Protein tyrosine kinase inhibitors block tumor necrosis factor-induced activation of nuclear factor-kappaB, degradation of I-kappaBalpha, nuclear translocation of p65, and subsequent gene expression. Arch Biochem Biophys. 1998;352:59–70. doi: 10.1006/abbi.1998.0576. [DOI] [PubMed] [Google Scholar]
- 43.Phillips TM, McBride WH, Pajonk F. The response of CD24(−/low)/CD44+ breast cancer-initiating cells to radiation. J Natl Cancer Inst. 2006;98:1777–1785. doi: 10.1093/jnci/djj495. [DOI] [PubMed] [Google Scholar]
- 44.Renard P, Ernest I, Houbion A, Art M, Le Calvez H, Raes M, Remacle J. Development of a sensitive multi-well colorimetric assay for active NFkappaB. Nucleic Acids Res. 2001;29:E21. doi: 10.1093/nar/29.4.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weissman I. Stem cell research: paths to cancer therapies and regenerative medicine. Jama. 2005;294:1359–1366. doi: 10.1001/jama.294.11.1359. [DOI] [PubMed] [Google Scholar]
- 46.Bonnet D. Cancer stem cells: AMLs show the way. Biochem Soc Trans. 2005;33:1531–1533. doi: 10.1042/BST0331531. [DOI] [PubMed] [Google Scholar]
- 47.Jordan CT. Cancer stem cell biology: from leukemia to solid tumors. Curr Opin Cell Biol. 2004;16:708–712. doi: 10.1016/j.ceb.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 48.Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med. 2006;355:1253–1261. doi: 10.1056/NEJMra061808. [DOI] [PubMed] [Google Scholar]
- 49.Polyak K, Hahn WC. Roots and stems: stem cells in cancer. Nat Med. 2006;12:296–300. doi: 10.1038/nm1379. [DOI] [PubMed] [Google Scholar]
- 50.Zhang M, Rosen JM. Stem cells in the etiology and treatment of cancer. Curr Opin Genet Dev. 2006;16:60–64. doi: 10.1016/j.gde.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 51.Xu Q, Simpson SE, Scialla TJ, Bagg A, Carroll M. Survival of acute myeloid leukemia cells requires PI3 kinase activation. Blood. 2003;102:972–980. doi: 10.1182/blood-2002-11-3429. [DOI] [PubMed] [Google Scholar]
- 52.Yilmaz OH, Valdez R, Theisen BK, Guo W, Ferguson DO, Wu H, Morrison SJ. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441:475–482. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- 53.Guzman ML, Jordan CT. Feverfew: weeding out the root of leukaemia. Expert Opin Biol Ther. 2005;5:1147–1152. doi: 10.1517/14712598.5.9.1147. [DOI] [PubMed] [Google Scholar]
- 54.Hehner SP, Hofmann TG, Droge W, Schmitz ML. The antiinflammatory sesquiterpene lactone parthenolide inhibits NF-kappa B by targeting the I kappa B kinase complex. J Immunol. 1999;163:5617–5623. [PubMed] [Google Scholar]
- 55.Kwok BH, Koh B, Ndubuisi MI, Elofsson M, Crews CM. The anti-inflammatory natural product parthenolide from the medicinal herb Feverfew directly binds to and inhibits IkappaB kinase. Chem Biol. 2001;8:759–766. doi: 10.1016/s1074-5521(01)00049-7. [DOI] [PubMed] [Google Scholar]
- 56.Garcia-Pineres AJ, Castro V, Mora G, Schmidt TJ, Strunck E, Pahl HL, Merfort I. Cysteine 38 in p65/NF-kappaB plays a crucial role in DNA binding inhibition by sesquiterpene lactones. J Biol Chem. 2001;276:39713–39720. doi: 10.1074/jbc.M101985200. [DOI] [PubMed] [Google Scholar]
- 57.MacKenzie CJ, Paul A, Wilson S, de Martin R, Baker AH, Plevin R. Enhancement of lipopolysaccharide-stimulated JNK activity in rat aortic smooth muscle cells by pharmacological and adenovirus-mediated inhibition of inhibitory kappa B kinase signalling. Br J Pharmacol. 2003;139:1041–1049. doi: 10.1038/sj.bjp.0705330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peng Y, Power MR, Li B, Lin TJ. Inhibition of IKK down-regulates antigen + IgE-induced TNF production by mast cells: a role for the IKK-IkappaB-NF-kappaB pathway in IgE-dependent mast cell activation. J Leukoc Biol. 2005;77:975–983. doi: 10.1189/jlb.0204115. [DOI] [PubMed] [Google Scholar]
- 59.Sweeney CJ, Mehrotra S, Sadaria MR, Kumar S, Shortle NH, Roman Y, Sheridan C, Campbell RA, Murry DJ, Badve S, Nakshatri H. The sesquiterpene lactone parthenolide in combination with docetaxel reduces metastasis and improves survival in a xenograft model of breast cancer. Mol Cancer Ther. 2005;4:1004–1012. doi: 10.1158/1535-7163.MCT-05-0030. [DOI] [PubMed] [Google Scholar]