Abstract
p53 levels are tightly regulated in normal cells, and thus the wild-type p53 protein is nearly undetectable until stimulated through a variety of stresses. In response to stress, p53 is released from its negative regulators, mainly Mdm2, allowing p53 to be stabilized to activate cell cycle arrest, senescence, and apoptosis programs. Many of the upstream signals that regulate wild type p53 are known; however, limited information for the regulation of mutant p53 exists. Previously, we demonstrated that wild-type and mutant p53R172H are regulated in a similar manner in the absence of Mdm2 or p16. Additionally, this stabilization of mutant p53 is responsible for the gain-of-function metastatic phenotype observed in the mouse. In this report, we examined the role of oncogenes, DNA damage, and reactive oxygen species, signals that stabilize wild type p53, on the stabilization of mutant p53 in vivo and the consequences of this expression on tumor formation and survival. These factors stabilized mutant p53 protein which often times contributed to exacerbated tumor phenotypes. These findings, coupled with the fact that patients carry p53 mutations without stabilization of p53, suggest that personalized therapeutic schemes may be needed for individual patients depending on their p53 status.
INTRODUCTION
The p53 pathway is impaired in most human cancers. More than 50% of human tumors carry p53 mutations, and most of these are missense mutations that result not only in loss of tumor-suppressing activities but also acquisition of oncogenic activities, defined as gain of function. Specifically, mutant p53 enhances proliferation and survival in cells, and tumorigenesis in mice when compared to cells or mice that are deficient for p53 (1).
Wild type p53 normally exists in a latent state, but becomes stabilized and activated in response to various genotoxic and cellular stress signals, allowing for transcriptional modulation of multiple genes that play important roles in controlling cell cycle progression, senescence, and apoptosis (2). The regulation of wild type p53 is mediated mainly by protein turnover. Primarily, its stability is regulated by murine double minute 2 (Mdm2), an E3 ubiquitin ligase that targets p53 for proteolytic degradation (3–7). Recently, several other ubiquitin ligases have been identified that also regulate p53 stability and include COP1 (8), Pirh2 (9), ARF-BP1/Mule (10), and Trim24 (11) although their roles in vivo are less clear.
The importance of DNA damage in p53 signaling has been extensively studied. In particular, exposure to chemotherapeutic drugs results in stabilization of wild type p53 through post-translational modifications in the amino terminus (2). These modifications disrupt the ability of Mdm2 to interact with p53 (12). The generation of reactive oxygen species (ROS), cytokines, and γ-radiation also play roles as a potent activators of p53 (13). Mechanistically, ROS activates p53 through direct damage to DNA. In addition, ROS contributes to crosstalk and activates other signaling pathways such as p38, JNK or NF-κB signaling resulting in synergy of p53 activation through phosphorylation, stabilization, and activation (14).
Two independent tumor suppressors, p16INK4a and p19ARF (p14ARF in humans) also impact the p53 signaling pathway through different mechanisms. p16INK4a loss, as occurs in some tumors, allows cyclinD/CDK4 kinase activity to phosphorylate Rb and results in its dissociation from E2F. Released E2F then transcriptionally activates p19ARF which binds Mdm2 and thereby affects p53 stabilization (15, 16). In cancers, p19ARF is also up regulated following oncogene activation (17–19). Mutant Ras or increased levels of c-Myc, for example, stimulate the ARF–Mdm2 complex formation (18, 20–22) which in turn, causes a sequestration of Mdm2 and subsequent stabilization of p53 (23).
Although the signaling pathways targeting wild type p53 are well documented, the regulatory signals that control mutant p53 levels are less well understood. Recently, we and others demonstrated that mutant p53 is inherently unstable in normal tissues and that some of the factors that regulate wild type p53 are also responsible for the stabilization of mutant p53 protein (24–26). Loss of Mdm2, for example, stabilizes mutant p53 in many normal tissues (26). In these experiments, stabilization of mutant p53 led to gain-of-function phenotypes manifested as increased tumor incidence and metastasis. Disruption of the Rb pathway via p16INK4a deletion also stabilized mutant p53 in vivo (26). We have now explored whether other signals that stabilize wild type p53 likewise affect mutant p53 stabilization. Since p53 is mutated in the majority of human tumors and expression of mutant p53 is often associated with poor outcomes, we examined a variety of cellular stimuli that may potentially stabilize mutant p53 in vivo and may thus lead to an enhanced the tumor phenotype. We found that activation of oncogenes stabilized mutant p53 resulting in more potent tumor phenotypes as compared to mice that only harbored the p53 mutation; however, these types of stimuli did not impact overall survival. On the other hand, when we examined the effect of doxorubicin, a chemotherapeutic DNA damaging agent, on mutant p53 stability, we observed stabilization of mutant p53 as well as decreased survival compared to the untreated p53 homozygous mutant mice. Furthermore, we analyzed the effect of a reactive oxygen species scavenger on mutant p53 protein stability and also observed a gain-of-function phenotype. In most cases, stabilization of mutant p53 led to worse phenotypes than the absence of p53. These results led us to propose that multiple cellular stress pathways that regulate wild type p53 also act to increase mutant p53 levels yielding gain-of-function phenotypes.
MATERIALS AND METHODS
Generation of Eµ-myc and K-rasLA1 cohorts
p53515A /+, p53−/−, K-rasLA1 and p53515A/515A mice were maintained on more than 95% of C57BL/6 background (24, 27, 28). Eµ-myc mice (29) were crossed to p53515A/515A to generate p53515A/+ Eµ-myc. The background of Eµ-myc, Eµ-myc p53515A /+, or Eµ-myc p53+/− mice were 75% C57BL/6 and 25% 129Sv. Tails from Eµ-myc, Eµ-myc p53515A/+, or Eµ-myc p53+/− mice were genotyped using primers previously described (30). K-rasLA1 mice (27) were crossed to p53515A/515A to generate p53515A/+ K-rasLA1 mice. p53515A/+ K-rasLA1 mice were further crossed with p53515A/515A to generate p53515A/515A K-rasLA1 mice. To determine mouse genotypes, PCR analysis was performed on tail DNA using published primer sets for the p53−, p53515A, and K-rasLA1 alleles (24, 27, 28, 31). The animal studies were performed according to the MD Anderson and IACUC guidelines.
Statistics
Survival curves were plotted by the Kaplan-Meier method using GraphPad Prism to assay statistical differences. A factor was considered statistically significant if it had a two-sided P value of < 0.05.
Immunohistochemistry (IHC) analysis
IHC analysis was performed as previously described (32). Protein expression was analyzed using rabbit α-p53 (CM5) antibodies (Vector Laboratories) at 1:200 a dilution for 2 hours at 37°C, and visualized by ABC and DAB kits from (Vector Laboratories). Slides were counterstained with Nuclear Fast Red. Hematoxylin and eosin (H&E) staining was used for pathological analysis of tumors.
Western blotting
Protein lysates were prepared from either the tissues or tumors of mice. 50 µg of total proteins were resolved on an SDS-PAGE and transferred to nitrocellulose membranes (GE Bioscience). After blocking with 5% skim milk in PBS-0.1% Tween 20 (PBS-T) for 1 h at room temperature, membranes were incubated with α-p53 (CM5, Vector Laboratories, 1:1000 dilution) antibodies at 4°C overnight. Membranes were then washed with PBS-T and incubated with anti-rabbit horseradish peroxidase-conjugated secondary antibody and visualized with ECL plus (GE Bioscience). Anti-β-actin or Vinculin (Sigma, 1:5000) antibodies were used as a loading control. All antibodies were diluted in blocking buffer.
Treatment of mice
Two day-old homozygous pups were treated with 2 Gray (Gy) of γ-radiation. For doxorubicin treatment, pups were injected with either PBS or 2µg of doxorubicin suspended in PBS per gram of body weight at P5 and P6. Five hours after the last injection, pups were sacrificed. Protein expression in tissue was measured using western blotting analysis as described above. For reactive oxygen species (ROS) experiments, parent mice were treated with the ROS scavenger N-acetyl-cystein (NAC) for two weeks prior to mating through drinking water and continued while pups were nursing. At day five, homozygous mutant pups were irradiated with 2 Gy. Four hours after irradiation, mice were injected with either PBS or 2µg of NAC in PBS per gram of body weight. One hour later, pups were sacrificed and the protein expression was examined.
Measurement of intracellular ROS
The dye 5(and 6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (CM-H2DCFDA, Molecular Probes, Eugene, OR, USA) was used to detect intracellular ROS. The fluorescence intensity of intracellular DCFDA is a linear indicator of ROS in the stained cells (33). To compare intracellular ROS in thymocytes from p53515A/515A mice, freshly isolated thymocytes were incubated with 10 µM DCFDA in the culture medium (RPMI 1640) for 30 min at 37°C. The cells were harvested and the DCFDA fluorescence profiles determined by flow cytometry analysis with a Coulter Epics software program, version 4.02.
Loss of heterozygosity (LOH)
LOH at the p53 locus in Eµ-myc p53515A /+ and Eµ-myc p53+/− lymphomas was determined by PCR amplification using primers 5’-TACTCTCCTCCCCTCAATAAGCTATTC-3’ (exon 5) and 5’-AGTCTAGGCTGGAGTCAACTGTCT-3’ (intron 6). PCR amplicons were separated by gel electrophoresis followed by DNA purification of the correct products as previously described ((34)). Sequencing was performed using the exon 5 primers and analyzed using Chromas software.
RESULTS
K-ras activation stabilized the p53R172H mutant and exacerbated tumor progression
Wild type p53 is stabilized and activated in response to hyper-proliferative signals, and thus protects cells from aberrant growth signals. Tissue culture stabilizes wild type and mutant p53 and is thus not an optimal system to study these effects (24). Therefore, we examined the effect of oncogenes on mutant p53 protein stabilization in vivo by taking advantage of two tumor prone mouse models that are driven by hyper-proliferative signals. The first is the mouse carrying an oncogenic allele of K-rasLA1 (27). Following a spontaneous recombination event in the Ras allele, the recombined cells express activated Ras and thus drive tumor progression. The second is the Eµ-myc transgenic mouse model that expresses high levels of c-Myc in B-cells and rapidly develops B-cell lymphomas (29). Myc overexpression elevates p53 activity (30, 35). These were crossed to a p53 mutant mouse model inheriting the p53515A missense mutation, encoding the mutant p53R172H protein, and to p53-null mice for comparison of stabilization of mutant protein and tumor phenotypes. We generated homozygous p53 mutant mice that carry the K-rasLA1 allele to avoid wild-type p53 activation by K-ras. The latent K-rasLA1 allele is spontaneously recombined and expresses an active ras protein harboring a glycine to aspartic acid alteration at amino acid 12 (27). We examined whether activated K-ras expression in the lungs affected p53 stability in 4 week-old mice, prior to overt tumor development. By immunohistochemistry (IHC), we observed that mutant p53 is visible only in hyperplastic lesions in lungs from young K-rasLA1 p53515A/515A but not in the lungs from young K-rasLA1 mice with wild type p53 (Figure 1A). The level of p53 protein expression in different genotypes was also analyzed by western blotting. p53 levels were higher in the lungs from K-rasLA1 p53515A/515A mice as compared to wild type, K-rasLA1, or p53515A/515A mice at four weeks of age (Figure 1B). The absence of wild type p53 staining may be due to the fact that cells activating wild type p53 initiate senescence or apoptotic programs, quickly rendering p53 undetectable. Stabilization of mutant p53 was also examined in tumors from K-rasLA1 p53515A/515A mice. IHC revealed that mutant p53 was expressed in all tumors (Figure 1C), which contrasts to the 75% detection rate in tumors that spontaneously arise in p53515A/515A mice ((26), and this study). Thus, activation of K-ras stabilizes mutant p53 in vivo.
Figure 1.
K-rasLA1 stabilizes mutant p53R172H and results in a more aggressive tumor phenotype. (A) p53 levels were examined using Immunohistochemistry (IHC). Paraffin embedded lungs from 4-week old K-rasLA1 or K-rasLA1 p53515A/515A mice were stained with a p53 antibody. (B) Western blot analysis of p53 in lungs from 4-week old wild-type (Wt), K-rasLA1 (K-ras), p53515A/515A or K-rasLA1 p53515A/515A mice. Vinculin serves as a loading control. (C) IHC analysis was performed to analyze p53 expression in paraffin embedded lung adenocarcinomas from K-rasLA1 p53515A/515A mice. (D) Kaplan-Meier curves indicate survival of K-rasLA1 (K-ras), p53515A/515A, p53−/− K-rasLA1 p53−/−, and K-rasLA1 p53515A/515A mice.
Despite stabilization of p53R172H, the survival of K-rasLA1 p53515A/515A and K-rasLA1 p53−/− was similar (Figure 1D). However, the K-rasLA1 p53515A/515A mice developed more advanced and metastatic lung adenocarcinomas as compared to K-rasLA1 p53−/− mice (Table 1). This fact is highlighted by the observation that 55.4% of the tumors from K-rasLA1 p53515A/515A mice were adenocarcinomas, while only 27.8% of K-rasLA1 p53−/− mice developed adenocarcinomas. Taken altogether, these data suggest the shift to a more aggressive tumor phenotype is the result of stabilized p53R172H gain-of-function activities following oncogenic K-ras stimulation.
Table 1.
Tumor spectrum of K-ras LA1 p53515A/515A and K-ras LA1 p53−/−mice.
| Tumor type | K-ras LA1 p53515A/515A (%) | K-ras LA1 p53−/− (%) |
|---|---|---|
|
Adenocarcinoma (Multifocal) Adenocarcinoma (Multifocal) early Adenocarcinoma (Multifocal) early Adenocarcinoma + lymphoma Metastatic adenocarcinoma Poorly differentiated adenocarcinoma Sarcoma Osteosarcoma (Mutifoocal) Angiosarcoma (High grade) Spindle cell sarcoma (High grade) Sarcoma in soft tissue Metastatic sarcoma in lung Lymphoma Carcinoma (Teratocarcinoma) Others (Hemorrhage liver or arm) |
31 (55.4) 18 (32.1) 7 (12.5) 2 (3.6) 2 (3.6) 2 (3.6) 14 (25) 2 (3.6) 2 (3.6) 8 (3.6) 2 (3.6) 0 8 (14.3) 1 (1.8) 2 (3.6) |
5 (27.8) 4 (22.2) 0 1 (5.6) 0 0 8 (44.4) 0 2 (11.1) 4 (22.2) 1 (5.6) 1 (5.6) 5 (27.8) 0 0 |
| Total tumor #; Total mice # (# of tumors per mouse) | 56 : 26 (2.15) | 18 : 8 (2.25) |
c-Myc stabilized p53R172H mutant protein but did not accelerate tumor progression
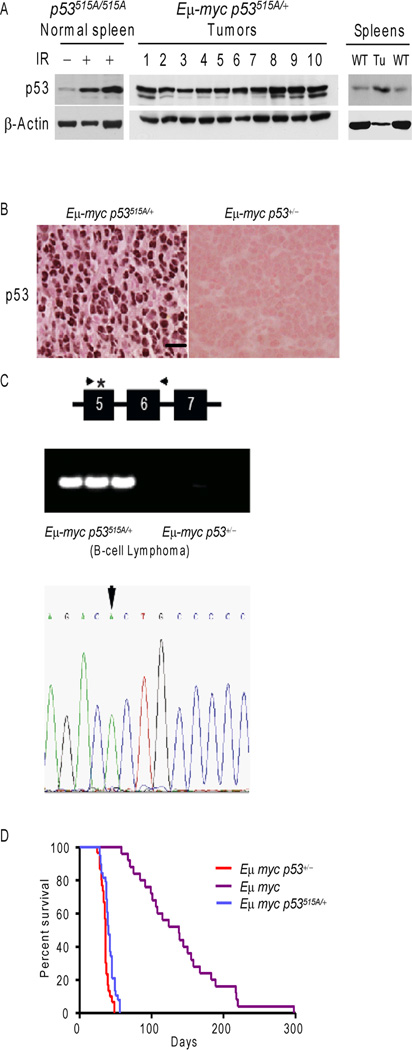
To expand the generality of these findings to other oncogenes, we next assayed how overexpression of c-Myc influenced the stability of p53R172H in vivo. We generated heterozygous p53 mutant mice harboring the Eµ-myc transgene due to pre-natal lethality of homozygous mutant p53 mice with Eµ-myc (30). Western blot and IHC analysis of Eµ-myc p53515A/+ lymphomas showed that all Eµ-myc p53515A/+ splenic lymphomas overexpressed p53 as compared to wild type spleens (Figure 2A and B). On the other hand, Eµ-myc p53+/− lymphomas, which delete the one wild type p53 allele, failed to express p53 (Figure 2B, and (35)). To explore whether the expressed p53 in lymphomas from Eµ-myc p53515A/+ is wild type or mutant protein, we analyzed the p53 loci for loss of heterozygosity (LOH). Lymphomas from the Eµ-myc p53515A/+ mice retained a p53 allele, while Eµ-myc p53+/− mice lost their single wild type p53 allele (Figure 2C). Sequencing revealed that 100% (15/15) Eµ-myc p53515A /+ lymphomas lost the wild type p53 allele (Figure 2C). Thus, increased expression of c-Myc stabilized p53R172H in vivo. We also monitored the survival of Eµ-myc p53515A/+ and Eµ-myc p53+/− mice. The Eµ-myc p53515A/+ mice succumbed to lymphomas at a similar rate as the Eµ-myc p53+/− mice showing mean survival of 40 days and 38 days, respectively (Figure 2D). Thus, while the mutant p53 allele was retained and the protein was stable, no alteration in tumor development was observed.
Figure 2.
c-Myc expression in B-cells harboring one p53515A allele results in loss of wild type p53 and stabilization of mutant p53. (A) Western blot analysis of p53 in lymphomas from Eµ-myc p53515A/+ mice. Wild type (WT) and irradiated (IR) spleens were used for comparison of c-Myc levels. All spleens were lysed by tissue homogenization in NP-40 lysis buffer, followed by centrifugation to remove insoluble proteins and debris. β-actin serves as a loading control. (B) IHC of Eµ-myc p53515A/+ and Eµ-myc p53+/− lymphomas using a p53 antibody. (C) PCR analysis of the p53 locus in lymphomas from Eµ-myc p53515A/+ and Eµ-myc p53+/− mice. The PCR primers were designed to amplify p53 from exon 5 to intron 6. The asterisk denoted the p53515A mutation in Exon 5. Loss of heterozygosity at the p53 locus in Em-myc p53515A/+ lymphomas was analyzed by sequencing of PCR product. The tumors showed LOH as the 515 nucleotide (arrow) had only one mutant peak and lost the wild type peak. (D) Kaplan-Meier curves indicating survival of Eµ-myc p53515A/+, Eµ-myc p53+/−, and Eµ-myc mice.
Mutant p53 stabilized by p16INK4a loss does not affect overall survival
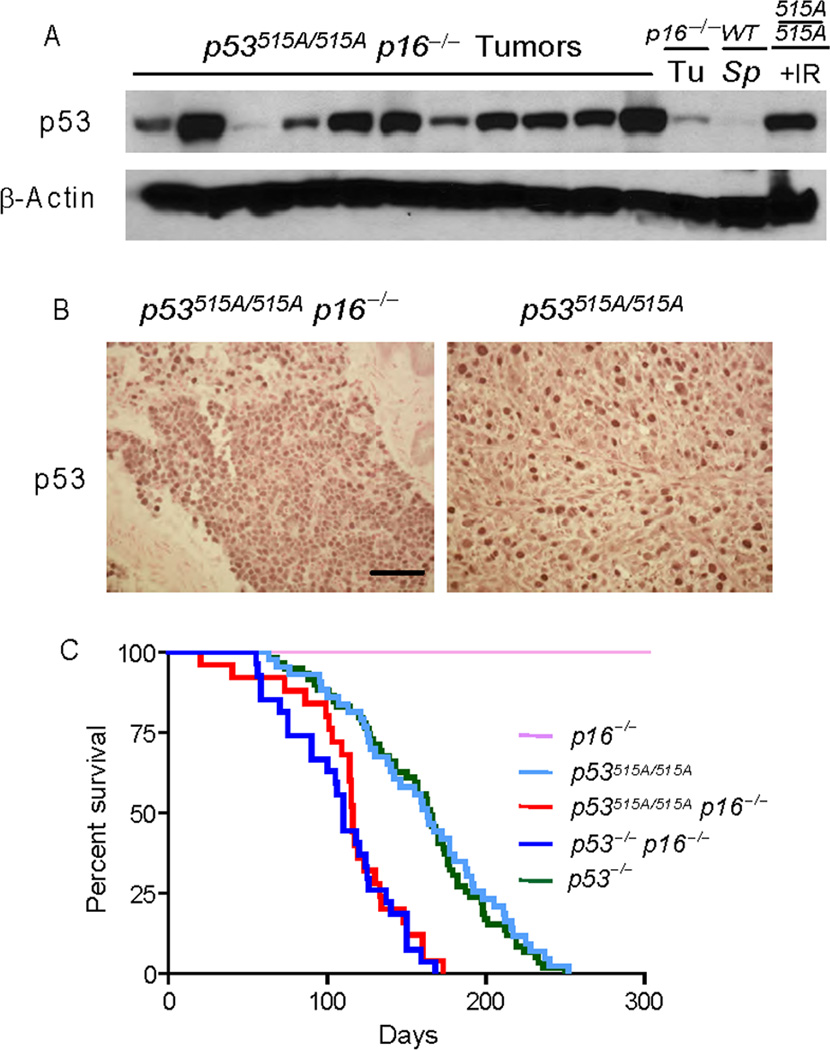
Given the fact that oncogenic activation resulted in stabilization of mutant p53, we next examined how loss of a second tumor suppressor, p16Ink4a, affected mutant p53 stability and tumor progression. Previously, we showed that mutant protein is stabilized in a variety of tissues from young mice in the absence of p16INK4a (26). To this end, we analyzed the effect of stabilized mutant protein in a cohort of p16INK4a−/− p53515A/515A mice with stable p53R172H. Western blot and IHC analysis revealed that mutant p53 protein was detectible in all (11 of 11) p16INK4a−/− p53515A/515A tumors at varying levels (Figure 3A and B). Thus, p16INK4a loss also resulted in stabilization of p53R172H in all tumors. While the survival of p53 homozygous mutant and p53−/− mice in a p16INK4a null background was not statistically significant (Figure 3C), loss of p16 enhanced the mutant p53 gain-of-function phenotype. We observed that 5 of 17 (29.4%) of the p16INK4a−/− p53515A/515A mice developed metastatic sarcomas, in contrast to the lack of metastatic disease in the p16INK4a−/− and p53515A/515A mice (26).
Figure 3.
p16Ink4a loss stabilizes mutant p53R172H but does not affect survival. (A) Stabilized p53 protein in tumors from p16−/− p53515A/515A mice. Western blot analysis of p16−/− p53515A/515A tumors blotted with a p53 antibody. β-actin serves as a loading control. (B) Stabilization of mutant p53 protein in tumors from p16−/− p53515A/515A by IHC. (C) Survival of p16−/− p53515A/515A was compared with p16−/−, p53515A/515A, p53−/− and p16−/− p53−/− using Kaplan-Meier curves.
Reactive oxygen species (ROS) stabilized mutant p53 protein in vivo
It is widely appreciated that the p53 pathway is activated in response to gains (oncogenes) or losses (tumor suppressors) of genetic material. However, exogenous factors have also been implicated in activation of wild-type p53. One factor, oxidative stress, is caused by reactive oxygen species (ROS) and is known to stabilize and activate wild-type p53. High levels of ROS are often observed in solid tumors and are elevated following radiation treatment in cancer patients (36). To test the role of ROS in stabilization of mutant p53 in vivo, we treated pups with γ–radiation to first induce ROS and then treated with the ROS scavenger N-acetyl-cystein (NAC). We first measured ROS induction in the thymus of p53515A/515A pups following irradiation. As shown in Figure 4A, thymi from p53515A/515A mice had three fold higher levels of ROS after irradiation. We next irradiated additional pups in order to examine the levels of p53R172H. Four hours after treatment with ionizing radiation, the pups were then intraperitoneally injected with NAC or phosphate buffered saline (PBS). Protein expression was analyzed one hour after NAC or PBS treatment. For this experiment, we treated two mice for each condition. Mutant p53 protein was increased in the spleen and thymus after irradiation; however, mutant p53 stabilization was dampened following treatment with NAC in both the thymus and spleen (Figure 4B). This result indicates that increased ROS levels resulting from γ-radiation also stabilize mutant p53.
Figure 4.
Reactive oxygen species (ROS) stabilizes p53R172H. (A) ROS were measured in thymocytes from p53515A/515A mice after 2 Gy γ-irradiation. Thymocytes were prepared from three individual mice with or without radiation. Two hours post treatment, cells were incubated with 10µM 5 (and 6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (DCFDA) and their fluorescence was measured by FACS. (B) Four pups were treated with radiation. After four hours, two were then treated with N-acetyl-cystein (NAC), a reactive oxygen species (ROS) scavenger, intraperitoneally. Western blot analysis was carried out using thymus and spleen samples from p53515A/515A mice with (+) or without (−) NAC one hour later. Spleen and thymus samples from untreated mice were used as control.
Doxorubicin stabilized mutant p53 protein in vivo
While we have shown that both ionizing radiation and ROS result in stabilization of mutant p53 in vivo, other DNA damaging agents are also often employed in clinical settings. The impact of these chemotherapeutic agents on the stabilization of mutant p53 is currently unknown. Doxorubicin is used as a treatment strategy for multiple tumor types. It induces DNA damage resulting in wild type p53 activation. To determine whether doxorubicin treatment resulted in the stabilization of mutant p53 in vivo, we injected doxorubicin into 5 and 6 day-old pups. Western blot analysis revealed that p53 levels increased after doxorubicin treatment not only in the thymus of homozygous mutant mice but also in wild type and p53 heterozygous mice (Figure 5A). Homozygous p53 mutant pups treated with doxorubicin once a day for two consecutive days starting at P5 were monitored for tumor formation and survival. Doxorubicin treated mutant mice showed no difference in survival as compared to doxorubicin treated p53-null mice (Figure 5B). Interestingly, treated p53 homozygous mutant mice died significant earlier due to increased tumor burden than untreated p53 mutant mice, suggesting that timing or mechanisms by which the mutant p53 protein is stabilized may impact tumor onset.
Figure 5.
Doxorubicin or radiation stabilizes mutant p53. (A) p53 levels were analyzed by western blot analysis of thymus from wild-type, p53515A /+ or p53515A/515A pups 24 hours after the last injection of doxorubicin. (B&C) Kaplan-Meier curves indicating survival of doxorubicin treated (B) or γ-irradiated mice of different genotypes (C). (D) p53 levels in tumors from γ-irradiated p53515A/515A mice.
Stabilization of p53R172H by γ-radiation alters survival
Previously, we observed that γ-irradiation of four week-old p53 homozygous mutant mice resulted in a longer half-life of the mutant protein over a 15 hour time period as compared to the wild type protein (26). To further explore the effects of mutant p53 stabilization, we treated pups with a low dose of radiation as p53−/− pups treated with γ-radiation show significant differences in survival as compared to untreated mice (37). Irradiated p53515A/515A pups dies significantly sooner than irradiated p53−/− mice and non-treated control mice (Figure 5C, p=0.036, p<0.0001; respectively). Importantly, 100% of the tumors from irradiated mice expressed stable mutant p53 (Figure 5D). This result is contrasted by the fact that only 75% of spontaneous tumors from p53515A/515A mice expressed stable mutant p53 (26), and this study). Together, these results suggest that radiation-induced stability of mutant p53 results in a deleterious gain-of-function phenotype manifested by development of multiple tumors and decreased survival as compared to irradiated p53−/− mice.
DISCUSSION
Mutant p53, like wild type p53, is inherently unstable (26). However, p53R172H becomes stabilized in the absence of Mdm2 and p16Ink4a, and also has an extended half-life as compared to wild type p53 in response to DNA damage signals (26). These data indicate that mutant p53 may be stabilized by mechanisms that also stabilize wild-type p53, implying that chemotherapeutic strategies that aim to activate wild-type p53 will also stabilize mutant p53. Since p53 is mutated in more than 50% of tumors and expression of mutant p53 is associated with poor outcomes, we asked whether other factors; such as those that contribute to human tumor formation (oncogenic activation) and clinical therapeutics (IR and doxorubicin), stabilize mutant p53.
Two potent oncogenes, K-ras and c-Myc, were used to determine their impact on mutant p53 stability in in vivo mouse models. Expression of each oncogene resulted in the stabilization of mutant p53 but did not dramatically shorten life span compared to p53-null mice. Significantly, the K-rasLA1 p53515A/515A displayed a more aggressive tumor phenotype compared to the K-rasLA1 p53−/− mice; 55% adenocarcinoms and 28% adenocarcinoms, respectively. These results confirm that the gain-of-function ability of p53R172H is associated with stabilized mutant protein and indicate that p53 mutations are more detrimental than p53 loss. However, this does not occur in Eµ-Myc p53515A/+ mice suggesting either the timing of stabilization or tissue specific differences impact manifestation of the gain-of-function phenotypes.
Many chemotherapeutic strategies aim to activate the p53 tumor suppressor signaling pathway. Here, we tested whether different DNA damaging agents such as doxorubicin, γ–radiation, and reactive oxygen species affect mutant p53 protein levels in vivo. Doxorubicin and γ–radiation are standard therapeutic agents used to treat various cancers as they induce a powerful DNA damaging response which elicits a p53 response. Our findings demonstrate that these therapeutic strategies stabilize p53 expression regardless of mutation status. As a result, γ-radiation yielded a gain-of-function phenotype by decreasing survival of mutant mice. Given that irradiated p53515A/515A mice die sooner than irradiated p53−/− mice, it is tempting to speculate that harmful outcomes may occur in human patients that harbor mutated p53 following therapeutic treatment regimens. Furthermore, the median survival for doxorubicin treated p53515A/515A and p53−/− mice was 99 and 126 days, respectively. Given the potential clinical importance of these preliminary findings, it will be imperative going forward to compare the outcome and survival of patients harboring wild type or mutant p53.
In addition to chemotherapeutic treatment, oxidative stress, mainly caused by reactive oxygen species, is an important factor leading to the activation of the p53 pathway (38, 39). Accumulating data suggests that tumors treated with radiation contain high amount of ROS (40). Studies in p53−/− mice have demonstrated that the antioxidant function of p53 may directly contribute to the prevention of tumor development (41). This may have important implications for p53 in the regulation of redox-sensitive survival pathways. Our results showed that decreasing the level of ROS resulted in decreased mutant p53 protein expression. This result indicates the gain-of-function phenotype resulting from stabilized mutant p53 may be overcome by inhibiting DNA damage caused by ROS and suggests that management of ROS levels in patients with mutant p53 may be warranted.
The survival of mutant mice was clearly affected in response to certain stresses, but not others. Irradiated mutant mice showed a significant decreased latency in tumor formation as compared to p53−/− mice. Interestingly, however, the survival of p53 mutant mice that are also p16−/− or have activated Ras was not different from p53−/− mice in these respective backgrounds, even though there were changes in tumor spectrum and metastatic potential. Therefore, other cooperating events, timing of insult, or tissue specificity may all contribute to outcome.
In conclusion, our data indicate that p53R172H is regulated by many of the same signals that regulate wild-type p53. The importance of this study cannot be overemphasized, especially with regards to tumor treatment. The molecular mechanisms of mutant p53 stabilization present a fundamental conundrum in therapeutic intervention, not only Li-Fraumeni syndrome patients but also for cancer patients with spontaneous p53 mutations. These data suggest that direct knowledge of a patient’s p53 status may be critical in preventing unintended consequences when determining therapeutic strategies. This study also emphasizes the need for individually tailored treatment for cancer patients depending upon their p53 mutation status.
ACKNOWLEDGMENTS
We thank members of the Lozano lab for helpful discussion and technical advice. This study was supported by NIH Grants CA46392 and CA34936 (to GL). DNA sequencing and veterinary core facilities were supported by an NCI Cancer Center Support Grant CA16672.
REFERENCES
- 1.Brosh R, Rotter V. When mutants gain new powers: news from the mutant p53 field. Nat Rev Cancer. 2009;9:701–713. doi: 10.1038/nrc2693. [DOI] [PubMed] [Google Scholar]
- 2.Appella E, Anderson CW. Post-translational modifications and activation of p53 by genotoxic stresses. Eur J Biochem. 2001;268:2764–2772. doi: 10.1046/j.1432-1327.2001.02225.x. [DOI] [PubMed] [Google Scholar]
- 3.Freedman DA, Levine AJ. Nuclear export is required for degradation of endogenous p53 by MDM2 and human papillomavirus E6. Mol Cell Biol. 1998;18:7288–7293. doi: 10.1128/mcb.18.12.7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387:296–299. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 5.Honda R, Tanaka H, Yasuda H. Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53. FEBS Lett. 1997;420:25–27. doi: 10.1016/s0014-5793(97)01480-4. [DOI] [PubMed] [Google Scholar]
- 6.Kubbutat MH, Jones SN, Vousden KH. Regulation of p53 stability by Mdm2. Nature. 1997;387:299–303. doi: 10.1038/387299a0. [DOI] [PubMed] [Google Scholar]
- 7.Roth J, Dobbelstein M, Freedman DA, Shenk T, Levine AJ. Nucleo-cytoplasmic shuttling of the hdm2 oncoprotein regulates the levels of the p53 protein via a pathway used by the human immunodeficiency virus rev protein. Embo J. 1998;17:554–564. doi: 10.1093/emboj/17.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dornan D, Wertz I, Shimizu H, Arnott D, Frantz GD, Dowd P, O'Rourke K, Koeppen H, Dixit VM. The ubiquitin ligase COP1 is a critical negative regulator of p53. Nature. 2004;429:86–92. doi: 10.1038/nature02514. [DOI] [PubMed] [Google Scholar]
- 9.Leng RP, Lin Y, Ma W, Wu H, Lemmers B, Chung S, Parant JM, Lozano G, Hakem R, Benchimol S. Pirh2, a p53-induced ubiquitin-protein ligase, promotes p53 degradation. Cell. 2003;112:779–791. doi: 10.1016/s0092-8674(03)00193-4. [DOI] [PubMed] [Google Scholar]
- 10.Chen D, Kon N, Li M, Zhang W, Qin J, Gu W. ARF-BP1/Mule is a critical mediator of the ARF tumor suppressor. Cell. 2005;121:1071–1083. doi: 10.1016/j.cell.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 11.Allton K, Jain AK, Herz HM, Tsai WW, Jung SY, Qin J, Bergmann A, Johnson RL, Barton MC. Trim24 targets endogenous p53 for degradation. Proc Natl Acad Sci U S A. 2009;106:11612–11616. doi: 10.1073/pnas.0813177106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ho JW, Song JZ, Leung YK. Activation of p53 by specific agents in potential cancer therapy. Curr Med Chem Anticancer Agents. 2005;5:131–135. doi: 10.2174/1568011053174819. [DOI] [PubMed] [Google Scholar]
- 13.Soussi T, Lozano G. p53 mutation heterogeneity in cancer. Biochem Biophys Res Commun. 2005;331:834–842. doi: 10.1016/j.bbrc.2005.03.190. [DOI] [PubMed] [Google Scholar]
- 14.Temkin V, Karin M. From death receptor to reactive oxygen species and c-Jun N-terminal protein kinase: the receptor-interacting protein 1 odyssey. Immunol Rev. 2007;220:8–21. doi: 10.1111/j.1600-065X.2007.00560.x. [DOI] [PubMed] [Google Scholar]
- 15.Hahn WC, Weinberg RA. Modelling the molecular circuitry of cancer. Nat Rev Cancer. 2002;2:331–341. doi: 10.1038/nrc795. [DOI] [PubMed] [Google Scholar]
- 16.Sherr CJ. The INK4a/ARF network in tumour suppression. Nat Rev Mol Cell Biol. 2001;2:731–737. doi: 10.1038/35096061. [DOI] [PubMed] [Google Scholar]
- 17.de Stanchina E, McCurrach ME, Zindy F, Shieh SY, Ferbeyre G, Samuelson AV, Prives C, Roussel MF, Sherr CJ, Lowe SW. E1A signaling to p53 involves the p19(ARF) tumor suppressor. Genes Dev. 1998;12:2434–2442. doi: 10.1101/gad.12.15.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pomerantz J, Schreiber-Agus N, Liégeois NJ, Silverman A, Alland L, Chin L, Potes J, Chen K, Orlow I, Lee H-W, Cordon-Cardo C, DePinho RA. The Ink4a Tumor Suppressor Gene Product, p19Arf, Interacts with MDM2 and Neutralizes MDM2's Inhibition of p53. Cell. 1998;92:713–723. doi: 10.1016/s0092-8674(00)81400-2. [DOI] [PubMed] [Google Scholar]
- 19.Zindy F, Eischen CM, Randle DH, Kamijo T, Cleveland JL, Sherr CJ, Roussel MF. Myc signaling via the ARF tumor suppressor regulates p53-dependent apoptosis and immortalization. Genes Dev. 1998;12:2424–2433. doi: 10.1101/gad.12.15.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamijo T, Weber JD, Zambetti G, Zindy F, Roussel MF, Sherr CJ. Functional and physical interactions of the ARF tumor suppressor with p53 and Mdm2. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:8292–8297. doi: 10.1073/pnas.95.14.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stott FJ, Bates S, James MC, McConnell BB, Starborg M, Brookes S, Palmero I, Ryan K, Hara E, Vousden KH, Peters G. The alternative product from the human CDKN2A locus, p14(ARF), participates in a regulatory feedback loop with p53 and MDM2. Embo J. 1998;17:5001–5014. doi: 10.1093/emboj/17.17.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Xiong Y, Yarbrough WG. ARF promotes MDM2 degradation and stabilizes p53: ARF-INK4a locus deletion impairs both the Rb and p53 tumor suppression pathways. Cell. 1998;92:725–734. doi: 10.1016/s0092-8674(00)81401-4. [DOI] [PubMed] [Google Scholar]
- 23.Korgaonkar C, Zhao L, Modestou M, Quelle DE. ARF function does not require p53 stabilization or Mdm2 relocalization. Mol Cell Biol. 2002;22:196–206. doi: 10.1128/MCB.22.1.196-206.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lang GA, Iwakuma T, Suh YA, Liu G, Rao VA, Parant JM, Valentin-Vega YA, Terzian T, Caldwell LC, Strong LC, El-Naggar AK, Lozano G. Gain of function of a p53 hot spot mutation in a mouse model of Li-Fraumeni syndrome. Cell. 2004;119:861–872. doi: 10.1016/j.cell.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 25.Olive KP, Tuveson DA, Ruhe ZC, Yin B, Willis NA, Bronson RT, Crowley D, Jacks T. Mutant p53 gain of function in two mouse models of Li-Fraumeni syndrome. Cell. 2004;119:847–860. doi: 10.1016/j.cell.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Terzian T, Suh YA, Iwakuma T, Post SM, Neumann M, Lang GA, Van Pelt CS, Lozano G. The inherent instability of mutant p53 is alleviated by Mdm2 or p16INK4a loss. Genes Dev. 2008;22:1337–1344. doi: 10.1101/gad.1662908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson L, Mercer K, Greenbaum D, Bronson RT, Crowley D, Tuveson DA, Jacks T. Somatic activation of the K-ras oncogene causes early onset lung cancer in mice. Nature. 2001;410:1111–1116. doi: 10.1038/35074129. [DOI] [PubMed] [Google Scholar]
- 28.Jacks T, Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT, Weinberg RA. Tumor spectrum analysis in p53-mutant mice. Curr Biol. 1994;4:1–7. doi: 10.1016/s0960-9822(00)00002-6. [DOI] [PubMed] [Google Scholar]
- 29.Adams JM, Harris AW, Pinkert CA, Corcoran LM, Alexander WS, Cory S, Palmiter RD, Brinster RL. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature. 1985;318:533–538. doi: 10.1038/318533a0. [DOI] [PubMed] [Google Scholar]
- 30.Post SM, Quintas-Cardama A, Terzian T, Smith C, Eischen CM, Lozano G. p53-dependent senescence delays Emu-myc-induced B-cell lymphomagenesis. Oncogene. 2010;29:1260–1269. doi: 10.1038/onc.2009.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng S, El-Naggar AK, Kim ES, Kurie JM, Lozano G. A genetic mouse model for metastatic lung cancer with gender differences in survival. Oncogene. 2007;26:6896–6904. doi: 10.1038/sj.onc.1210493. [DOI] [PubMed] [Google Scholar]
- 32.Evans SC, Viswanathan M, Grier JD, Narayana M, El-Naggar AK, Lozano G. An alternatively spliced HDM2 product increases p53 activity by inhibiting HDM2. Oncogene. 2001;20:4041–4049. doi: 10.1038/sj.onc.1204533. [DOI] [PubMed] [Google Scholar]
- 33.Qiang W, Cahill JM, Liu J, Kuang X, Liu N, Scofield VL, Voorhees JR, Reid AJ, Yan M, Lynn WS, Wong PK. Activation of transcription factor Nrf-2 and its downstream targets in response to moloney murine leukemia virus ts1-induced thiol depletion and oxidative stress in astrocytes. J Virol. 2004;78:11926–11938. doi: 10.1128/JVI.78.21.11926-11938.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Post SM, Quintas-Cardama A, Pant V, Iwakuma T, Hamir A, Jackson JG, Maccio DR, Bond GL, Johnson DG, Levine AJ, Lozano G. A high-frequency regulatory polymorphism in the p53 pathway accelerates tumor development. Cancer Cell. 2010;18:220–230. doi: 10.1016/j.ccr.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eischen CM, Weber JD, Roussel MF, Sherr CJ, Cleveland JL. Disruption of the ARF-Mdm2-p53 tumor suppressor pathway in Myc-induced lymphomagenesis. Genes Dev. 1999;13:2658–2669. doi: 10.1101/gad.13.20.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martindale JL, Holbrook NJ. Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol. 2002;192:1–15. doi: 10.1002/jcp.10119. [DOI] [PubMed] [Google Scholar]
- 37.Kemp CJ, Wheldon T, Balmain A. p53-deficient mice are extremely susceptible to radiation-induced tumorigenesis. Nat Genet. 1994;8:66–69. doi: 10.1038/ng0994-66. [DOI] [PubMed] [Google Scholar]
- 38.Kastan MB, Onyekwere O, Sidransky D, Vogelstein B, Craig RW. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991;51:6304–6311. [PubMed] [Google Scholar]
- 39.Li JM, Fan LM, George VT, Brooks G. Nox2 regulates endothelial cell cycle arrest and apoptosis via p21cip1 and p53. Free Radic Biol Med. 2007;43:976–986. doi: 10.1016/j.freeradbiomed.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shackelford RE, Kaufmann WK, Paules RS. Oxidative stress and cell cycle checkpoint function. Free Radic Biol Med. 2000;28:1387–1404. doi: 10.1016/s0891-5849(00)00224-0. [DOI] [PubMed] [Google Scholar]
- 41.Sablina AA, Budanov AV, Ilyinskaya GV, Agapova LS, Kravchenko JE, Chumakov PM. The antioxidant function of the p53 tumor suppressor. Nat Med. 2005;11:1306–1313. doi: 10.1038/nm1320. [DOI] [PMC free article] [PubMed] [Google Scholar]