Abstract
Polylactide-glycolide (PLGA) nanoparticles have been developed as pulmonary drug delivery carriers. To investigate their behavior, small- (d50 = 74 nm) and large-sized (d50 = 250 nm) FITC-conjugated PLGA nanoparticles were intratracheally administered to rats and were traced for 5, 30 and 60 minutes and 24 hours after administration (HAT). Immunohistochemically, a, FITC-positive reaction was observed in type-I alveolar epithelial cells (type-I AEC), endothelial cells and alveolar macrophages in the lungs from 5 minutes after treatment (MAT) to 24 HAT in both nanoparticle groups. In the kidneys, a positive reaction was observed in proximal tubular epithelial cells at 30 MAT; the reaction peaked at 60 MAT and was reduced at 24 HAT, while no positive reaction was seen in other sites. Ultrascructurally, the number of membrane-bound vesicles, which were approximately 70 nm in size and hard to distinguish from pinocytic vesicles, apparently increased in type-I AEC and endothelial cells at 5 MAT in the small-sized group, in comparison with the control group receiving physiological saline. The number of vesicles in the large-sized group was almost same as that in the control group. On the other hand, in both nanoparticle groups, lysosomes filled with nanoparticles appeared in alveolar macrophages from 30 MAT to 24 HAT. These results indicate that PLGA nanoparticles might be quickly transferred from the alveolar space to the blood vessel via type-I alveolar epithelial cells and excreted into urine, and that there is a threshold for particle size, less than approximately 70 nm in diameter, with regard to absorption through the alveolar wall.
Keywords: drug delivery system, PLGA nanoparticles, pulmonary administration, ultrastructure, immunohistochemistry
Introduction
In preclinical studies, liposomes1–6, microparticles and nanoparticles using bioabsorbable polymers7–9 have been developed as drug delivery carriers for pulmonary administration of proteins, peptides and nucleic acids. The authors have developed composite particles containing an excipient and drug encapsulated in bioabsorbable polymeric nanoparticles for dry powder inhalation to treat lung and systemic diseases with improved pharmacological effects10. In in-vivo studies with beagles, it was found that the pharmacological effect of administering insulin encapsulated in PLGA nanoparticles after a single inhalation was 3.5 times higher than that of applying intravenous administration of insulin solution and that the blood glucose level was decreased after the single dosage within a matter of hours and returned to a normal value 24 hours later11. In a rat model of monocrotaline-induced pulmonary arterial hypertension (PAH), a single intratracheal instillation of NFκB decoy encapsulated in PLGA nanoparticles delivered the nanoparticles to lung, which mediated NFκB decoy and prevented monocrotaline-induced NFκB activation. It played a primary role in the pathogenesis of PAH and attenuated the development of PAH to improve the survival rates of rats12.
These pharmacological effects were the results of improved cell adherence, better cellular uptake, and sustained release of encapsulating drug due to the use of PLGA nanoparticles. Thus, the nanoparticles could be applied as drug delivery carriers for pulmonary administration. However, the behavior of PLGA nanoparticles after pulmonary drug administration has not been fully examined so far. Also, it is very important to evaluate the tissue pathology and histology to insure the safety of using PLGA nanoparticles in the early stages of studies before clinical trials. Our previous report applied the pathological and histological examinations of rat tissues to evaluate the dynamic state of a single dose of a drug encapsulated in PLGA nanoparticles within 24 hours of administration. After intratracheal instillation of FITC-encapsulated PLGA nanoparticles, FITC was found to be absorbed immediately through type-I alveolar epithelial cells13.
However, the dynamic state of the PLGA nanoparticles themselves could not be evaluated because the previous method could not determine whether the detected FITC was released from the encapsulated PLGA nanoparticles or in the encapsulated PLGA nanoparticles. Therefore, FITC-conjugated PLGA nanoparticles were used in this study. After they were administered intratracheally to rats, the FITC distributions in several rat tissues stained immunohistochemically were investigated by histological and pathological examinations because this method enables evaluation the distribution of PLGA nanoparticles simply and with high sensitivity, and the intracellular distribution of PLGA nanoparticles was evaluated with an electron microscope.
Materials and Methods
Materials
PLGA copolymer with a 75:25 ratio of lactic acid to glycolic acid and an average molecular weight of 20,000 (PLGA7520) from Wako Pure Chemical Industries (Osaka, Japan) and fluorescein-4-isothiocyanate (FITC) from Dojindo Laboratories (Kumamoto, Japan) were used in this study. FITC-conjugated PLGA (FITC-PLGA) was prepared by the method of Kim S.H. et al.14 using the above materials. Polyvinyl alcohol (PVA EG05, Nippon Synthetic Chemical Industry Co., Osaka, Japan) was used as the dispersant for the production of nanoparticles.
Preparation of FITC-PLGA nanoparticles
Three kinds of FITC-PLGA nanoparticles with different particles sizes were prepared by a previously reported emulsion solvent diffusion method in water15,16 respectively. First, 0.5 g of FITC-PLGA was dissolved in solution containing acetone and ethanol. Then, the organic solution was slowly poured into PVA aqueous solution stirred at 400 rpm and 40 °C to form a nanoparticle suspension. After vacuum evaporation of organic solvent, the suspension of FITC-PLGA nanoparticles was lyophilized to produce dry powder.
Physical evaluation of FITC-PLGA nanoparticles
The shape of freeze-dried nanoparticles was analyzed by field emission scanning electron microscopy (FE-SEM; JSM-7401F, JEOL Ltd., Tokyo, Japan). The number-equivalent mean particle diameter of the nanoparticles was determined from a cumulative distribution curve consisting 300 circle-equivalent diameters of particles obtained by FE-SEM image analysis with Image J (developed by the National Institutes of Health). The particle diameter is also known as the Heywood diameter. The surface charge of the nanoparticles dispersed in distilled water was measured by cataphoresis (Zetasizer Nano Z, Malvern, England).
Measurement of in vitro FITC release kinetics
The FITC elution ratio of FITC-PLGA nanoparticles was calculated by dividing the quantity of FITC released from the nanoparticles by the quantity of FITC included in the nanoparticles. The quantity of FITC released from the nanoparticles was obtained as follows: 100 mg of FITC-PLGA nanoparticles was dispersed in 10 mL of distilled water. Then, the solution was transferred to a dialysis membrane (inner phase) (Spectra/por®, 3.5 kDa molecular cutoff: 3.5 kDa, Spectrum Laboratories, Inc., Rancho Dominguez, CA, USA). After that, the membrane was dipped into 500 mL of distilled water (outer phase). After 24 hours, 1 mL of solution in the outer phase was sampled, and its FITC content was determined by fluorophotometer (FP-6500, JASCO Corporation, Tokyo, Japan) at the excitation and emission wavelengths of 490 and 520 nm, respectively.
The quantity of FITC included in the nanoparticles was measured as follows: 20 mg of FITC-PLGA nanoparticles 5 mL of acetonitrile. Then, 50 mL of distilled water was added to separate out the PLGA. After that, the suspension was filtrated through a hydrophilic PTFE membrane filter (pore size: 0.2 μm, Advantec Toyo Kaisha, Ltd., Tokyo, Japan), and the FITC content in the filtrate was determined by the fluorophotometer under the conditions described above.
Animals
A total of 36 male Sprague-Dawley rats aged 5 weeks were purchased from Charles River Laboratories Japan, Inc. (Kanagawa, Japan), and were housed individually in wire cages in an environmentally controlled room (temperature of 23 ± 3°C, relative humidity of 55 ± 20%, ventilation rate of 10–15 times per hour and a 12-h/12-h light/dark cycle), fed a commercial diet (CRF-1; Oriental Yeast Co., Ltd., Tokyo, Japan) and tap water ad libitum and used in the this experiment at 14 weeks of age. Animal experiments were conducted in accordance with the Guide for Animal Experimentation of Bozo Research Center Inc.
Experimental design
Animals were divided into the control group and small- and large-sized FITC-PLGA nanoparticle groups, which contained the same number of animals, and were intratracheally administered a single dose of 0.5 mL of saline suspension containing 40 mg of FITC-PLGA nanoparticles in the nanoparticle groups and physiological saline in the control group, according to the method described previously17. Three rats per group were sacrificed under ether anesthesia at 5, 30 and 60 min and 24 hours after treatment (HAT), and the lungs, livers, kidneys, brains, spleens and pancreases were removed for further analyses.
Immunohistochemical evaluation
Each organ was preserved in neutral buffered formalin fixative, embedded in paraffin and sectioned. After deparaffinization, the sections were reacted immunohistochemically with anti-FITC monoclonal antibody (1:1000, American Research Products, Waltham, MA, USA) using Envision polymer reagent (DakoCytomation, Carpinteria, CA, USA) and visualized with 3,3′-diaminobenzidine tetrahydrochloride and counterstained with hematoxylin.
Ultrastructural evaluation of the lungs
Tissue samples were taken from three different areas in the left lobe that appeared yellow in color in the FITC-PLGA nanoparticle groups and three randomly selected areas in the control group. The samples were fixed with a mixture of 0.5% glutaraldehyde and 1.5% paraformaldehyde under deaeration by a vacuum pump (RP 75, JEOL Ltd., Tokyo, Japan) at 100 L/min for 5 min, postfixed with 1% osmium tetroxide and embedded in epoxy resin. Ultrathin sections were double-stained with uranyl acetate and lead citrate and observed under a transmission electron microscope (JEM-100CX II, Nippon Denshi, Tokyo, Japan).
Results and Discussion
Characterization of FITC-PLGA nanoparticles
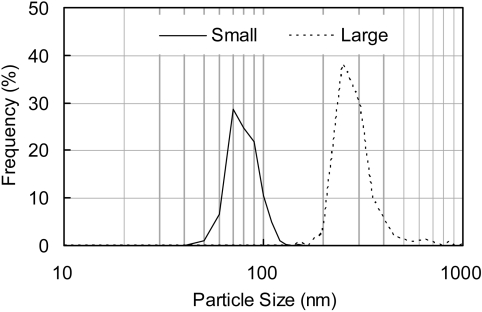
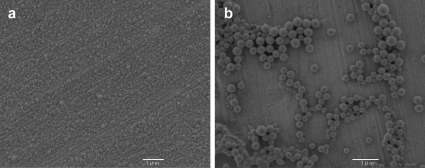
Two kinds of FITC-PLGA nanoparticles were prepared, smaller particles (mean diameter 74 nm) and larger particles (mean diameter 250 nm). The particle size distribution is shown in Fig. 1, and an FE-SEM photograph of each particle, which exhibited the same features as the FITC-loaded PLGA nanoparticles in our previous report13, is shown in Fig. 2. The surface charge of these particles was about −17 mV. The FITC elution ratio for the small-sized FITC-PLGA nanoparticles was found to be 1.9%. Therefore, FITC detection could be used as the marker to trace the dynamic state of PLGA nanoparticles.
Fig. 1.
Particle size distribution of FITC-conjugated PLGA nanoparticles in water.
Fig. 2.
Scanning electron microscope photograph of the small-sized (a) and large-sized FITC-PLGA nanoparticles (b).
Necropsy findings
No abnormal findings were observed in the control group. In both the small- and large-sized FITC-PLGA nanoparticle groups, the whole lungs turned yellow in color, from the main bronchi to the periphery, and the color remained until 24 HAT.
Immunohistochemical evaluation of FITC-PLGA nanoparticles
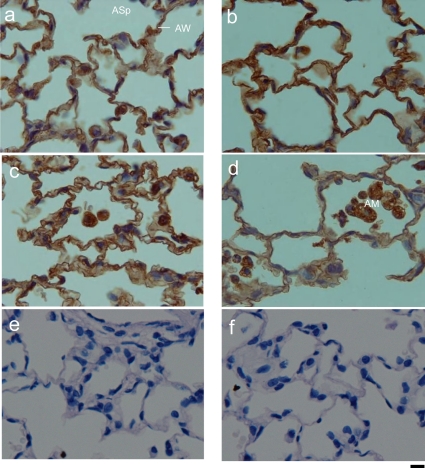
In the lungs, no immunopositive reaction for FITC was observed in the control group. As shown in the Fig. 3, a FITC-positive reaction was found in the alveolar wall at 5 minutes after treatment (MAT) in the small-sized nanoparticle group. The positive reaction was also detected at 24 HAT (Fig. 3d). This result suggests that uptake of PLGA nanoparticles might be rapid and that the nanoparticles might remain at least until 24 HAT. In addition, a FITC-positive reaction was observed in the alveolar macrophages from 5 MAT to 24 HAT. This indicates that the PLGA nanoparticles might be recognized as foreign substances by alveolar macrophages.
Fig. 3.
Immunohistochemistry for FITC in the lungs. Sections from the small-sized FITC-PLGA nanoparticle group at 5 minutes (a), 30 minutes (b), 60 minutes (c) and 24 hours (d) and from the control group at 5 minutes (e) and 24 hours (f). AW: alveolar wall, ASp: alveolar space, AM: alveolar macrophage. Bars = 10 μm.
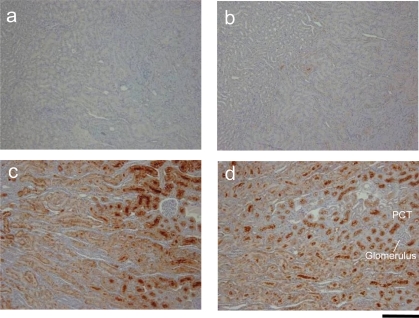
In the kidneys, no immunopositive reaction for FITC was observed in the control group. As shown in the Fig. 4, a FITC-positive reaction firstly appeared in proximal tubules at 30 MAT and was detected in proximal tubules until 24 HAT, whereas no distinct positive reaction for FITC was observed in the glomeruli. Taken together with the minimal elution of FITC from the PLGA-nanoparticles as mentioned above, it is considered that the FITC-PLGA nanoparticles are absorbed in the lungs, transferred to the blood circulation and finally excreted into urine; the detailed excretion pathway will be determined by further studies. It may also be suggested that intractable glomerulonephritis is unlikely to be contracted by administering FITC-PLGA nanoparticles.
Fig. 4.
Immunohistochemistry for FITC in the kidneys. Sections from the small-sized FITC-PLGA nanoparticle group at 5 minutes (a), 30 minutes (b), 60 minutes (c) and 24 hours (d). PCT: proximal convoluted tubule. Bars = 50 μm.
As for the liver, which plays an important role in metabolism of foreign substances, little FITC was found in the portal vein area, central vein area or Kupffer cells at 24 HAT. Also, little FITC was found in the spleen, pancreas or brain.
Ultrastructural evaluation of FITC-PLGA nanoparticles in the lungs
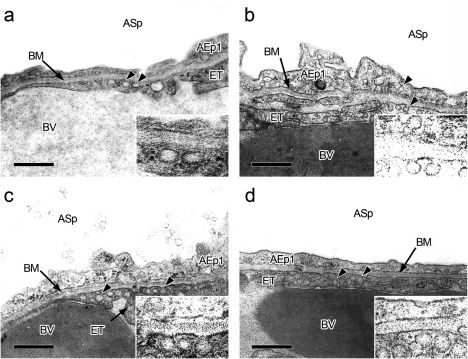
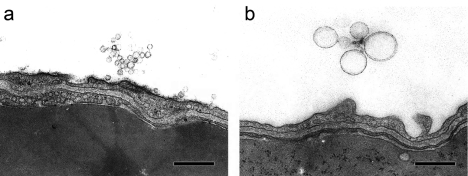
In the control group, pinocytic vesicles, which were approximately 70 nm in diameter, were observed in type-I alveolar epithelial cells and endothelial cells in the alveolar wall (Fig. 5a). Although similar vesicles were observed in the nanoparticle groups, the number, particular in type-I alveolar epithelial cells, was greatly increased in the small-sized nanoparticle group at 5 MAT to 24 HAT (Fig. 5b, c, d), while the number in the large-sized group was comparable to that in the control group. The nanoparticles present in alveolar spaces were characterized by an electron lucent (like they were empty), round body bounded by a single thin membranous substance (Fig. 6). This feature was quite similar to that of pinocytic vesicles in type-I alveolar epithelial cells and endothelial cells when the diameter was around 70 nm. Therefore, nanoparticles could not be definitively distinguished from pinocytic vesicles when they were present in cytoplasm; however, it may be reasonable to consider that the nanoparticles approximately 70 nm in size coexisted with pinocytic vesicles. Similar vesicles could not be detected in type-II alveolar epithelial cells and the interstitium of the alveolar wall. These results indicate that uptake of the nanoparticles injected into the lungs by type-I alveolar epithelial cells and transfer to the blood stream via endothelial cells might occur quickly and that there is a threshold for particle size, less than 70 nm in diameter, with regard to absorption through the alveolar wall.
Fig. 5.
Representative transmission electron microphotographs of vesicles (arrowheads) in the lungs from the control (a) and the small-sized FITC-PLGA nanoparticle groups at 5 minutes (b), 30 minutes (c), and 60 minutes (d) after treatment (inserts: higher magnification of the vesicles). AEp1: type-I alveolar epithelial cell; ASp: alveolar space; BM: basement membrane; BV: blood vessel; ET: endothelial cell. Bars = 500 nm.
Fig. 6.
Representative transmission electron microphotographs of intra-alveolar nanoparticles from the small-sized (a) and large-sized FITC-PLGA nanoparticle groups (b) at 30 minutes after treatment. Bars = 500 nm.
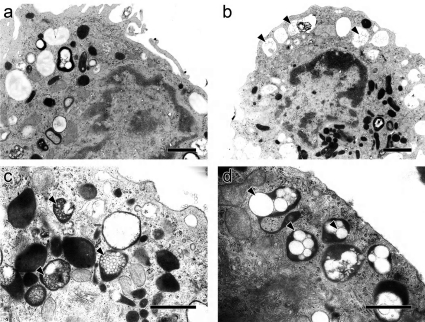
On the other hand, alveolar macrophages that contained secondary lysosomes with many nanoparticles, ranging from 50 to 500 nm, were observed in the nanoparticle groups (Fig. 7) at 5 MAT and later. This finding suggests that uptake of nanoparticles by not only type-I alveolar epithelial cells but also alveolar macrophages was quick and that larger-sized and agglomerating nanoparticles are likely to be captured by phagocytosis by alveolar macrophages.
Fig. 7.
Representative transmission electron microphotographs of alveolar macrophages from the control (a) and small-sized FITC-PLGA nanoparticle groups (b) at 30 minutes after treatment. Higher magnification of the nanoparticles (arrowhead) in lysosomes from the small-sized (c) and large-sized FITC-PLGA nanoparticle groups (d) at 30 minutes. Bars =1 μm.
In the present experiment, we provide not only fundamental information but also a clue for elucidating the absorption pathway of nanoparticles in lung tissue. However, to clarify the uptake mechanism, absorption rate and quantity rate of these particles, sufficient data need to be collected in further experiments.
Acknowledgments
This work was supported in part by a Grant-in-Aid for Cancer Research from the Ministry of Health, Labour and Welfare of Japan. The authors would like to thank Ms. Sachiko Nakai and Ms. Megumi Inagaki (Saiseikai Chuwa Hospital) for their technical assistance.
References
- 1.Wong JP, Yang H, Blasetti KL, Schnell G, Conley J, Schofield LN. Liposome delivery of ciprofloxacin against intracellular Francisella tularensis infection. J Control Release. 92: 265–273 2003. [DOI] [PubMed] [Google Scholar]
- 2.Sweeney LG, Wang Z, Loebenberg R, Wong JP, Lange CF, Finlay WH. Spray-freeze-dried liposomal ciprofloxacin powder for inhaled aerosol drug delivery. Int J Pharm. 305: 180–185 2005. [DOI] [PubMed] [Google Scholar]
- 3.Saari M, Vidgren MT, Koskinen MO, Turjanmaa VM, Nieminen MM. Pulmonary distribution and clearance of two beclomethasone liposome formulations in healthy volunteers. Int J Pharm. 181: 1–9 1999. [DOI] [PubMed] [Google Scholar]
- 4.Skubitz KM, Anderson PM. Inhalational interleukin-2 liposomes for pulmonary metastases: a phase I clinical trial. Anticancer Drugs. 11: 555–563 2000. [DOI] [PubMed] [Google Scholar]
- 5.Rijnders BJ, Cornelissen JJ, Slobbe L, Becker MJ, Doorduijn JK, Hop WC, Ruijgrok EJ, Löwenberg B, Vulto A, Lugtenburg PJ, de Marie S. Aerosolized liposomal amphotericin B for the prevention of invasive pulmonary aspergillosis during prolonged neutropenia: a randomized, placebo-controlled trial. Clin Infect Dis. 46: 1401–1408 2008. [DOI] [PubMed] [Google Scholar]
- 6.Wittgen BP, Kunst PW, van der Born K, van Wijk AW, Perkins W, Pilkiewicz FG, Perez-Soler R, Nicholson S, Peters GJ, Postmus PE. Phase I study of aerosolized SLIT cisplatin in the treatment of patients with carcinoma of the lung. Clin Cancer Res. 13: 2414–2421 2007. [DOI] [PubMed] [Google Scholar]
- 7.Kawashima Y, Yamamoto H, Takeuchi H, Fujioka S, Hino T. Pulmonary delivery of insulin with nebulized DL-lactide/glycolide copolymer (PLGA) nanospheres to prolong hypoglycemic effect. J Control Release. 62: 279–287 1999. [DOI] [PubMed] [Google Scholar]
- 8.Ohashi K, Kabasawa T, Ozeki T, Okada H. One-step preparation of rifampicin/poly (lactic-co-glycolic acid) nanoparticle-containing mannitol microspheres using a four-fluid nozzle spray drier for inhalation therapy of tuberculosis. J Control Release. 135: 19–24 2009. [DOI] [PubMed] [Google Scholar]
- 9.Tomoda K, Ohkoshi T, Hirota K, Sonavane GS, Nakajima T, Terada H, Komuro M, Kitazato K, Makino K. Preparation and properties of inhalable nanocomposite particles for treatment of lung cancer. Colloids Surf B Biointerfaces. 71: 177–182 2009. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto H, Hoshina W, Kurashima H, Takeuchi H, Kawashima Y, Yokoyama T, Tsujimoto H. Engineering of poly (lactic-co-glycolic acid) nano-composite particle for dry powder inhalation dosage forms of insulin with spray fluidized bed granulating system. J Soc Powder Technol Jpn. 41: 514–521 2004. [Google Scholar]
- 11.Tsujimoto H, Hara K, Kawashima Y. Evaluation of glycaemia control in beagle dogs by the administration of insulin encapsulated PLGA nano-composite preparations. J Soc Powder Technol Jpn. 42: 765–772 2005. [Google Scholar]
- 12.Kimura S, Egashira K, Ling C, Nakano K, Iwata E, Miyagawa M, Tsujimoto H, Hara K, Morishita R, Sueishi K, Tominaga R, Sunagawa K. Nanoparticle-mediated delivery of nuclear factor κB decoy into lungs ameliorates monocrotaline-induced pulmonary arterial hypertension. Hypertension. 53: 877–883 2009. [DOI] [PubMed] [Google Scholar]
- 13.Hara K, Tsujimoto H, Tsukada Y, Huang CC, Kawashima Y, Tsutsumi M. Histological examination of PLGA nanospheres for intratracheal drug administration. Int J Pharm. 356: 267–273 2008. [DOI] [PubMed] [Google Scholar]
- 14.Kim SH, Jeong JH, Chun KW, Park T. Target-specific cellular uptake of PLGA nanoparticles coated with poly(L-lysine)-poly(ethylene glycol)-folate. Langmuir. 21: 8852–8857 2005. [DOI] [PubMed] [Google Scholar]
- 15.Kawashima Y, Okumura M, Takenaka H. Spherical crystallization: direct spherical agglomeration of salicylic acid crystals during crystallization. Science. 216: 1127–1128 1982. [DOI] [PubMed] [Google Scholar]
- 16.Kawashima Y, Yamamoto H, Takeuchi H, Hino T, Niwa T. Properties of a peptide containing DL-lactide/glycolide copolymer nanospheres prepared by novel emulsion diffusion methods. Eur J Pharm Biopharm. 45: 41–48 1998. [DOI] [PubMed] [Google Scholar]
- 17.Oka Y, Mitsui M, Kitahashi T, Sakamoto A, Kusuoka O, Tsunoda T, Mori T, Tsutsumi M. A reliable method for intratracheal instillation of materials to the entire lung in rats. J Toxicol Pathol. 19: 107–109 2006. [Google Scholar]