Orchids comprise one of the largest and most diverse angiosperm families. Currently, about 24,500 orchid species have been reported, and there are many more to be discovered (Dressler, 2005). Due to their biological complexity, orchids have been proposed as an attractive system with which to address many fundamental biological questions (Tupac Otero et al., 2002; Cozzolino and Widmer, 2005; Dressler, 2005). In particular, orchid flowers are one of the best examples of coevolution between plants and pollinators and thus provide a unique opportunity to study development and evolution of flower forms and pollination biology. Highly modified flowers, selected for deceiving pollinators, are seen in approximately one-third of all orchid species (Cozzolino and Widmer, 2005; Tremblay et al., 2005), and the floral complexity of orchids has been proposed as one of the key factors behind their rapid species radiation (Gill, 1989). The orchid clade is also phylogenetically important, representing a petaloid monocot group that is distinct from other model species, e.g. maize (Zea mays), snapdragon (Antirrhinum majus), and Arabidopsis (Arabidopsis thaliana). However, despite their apparent importance, molecular and genetic approaches to orchid flower development and evolution are still in their infancy. To date, orchids remain underrepresented in studies at a molecular level (Dressler, 1981; McCook and Bateman, 1990; Peakall, 2007). One of the main obstacles is the availability of a suitable model species that is easy to maintain under laboratory conditions and has a wide range of mutants. Here, we report a mutant collection of the wind orchid (Neofinetia falcata) as a powerful tool to study orchid flower development. We also propose the wind orchid as an orchid model species with enormous advantages over other orchid species, which will answer biological questions unique to orchids as well as questions of plant evolution and development in broad terms.
AN OLD WIND ORCHID MUTANT COLLECTION
In far eastern Asian countries such as Korea, China, and Japan, growing orchids has a long history. Over the centuries, thousands of mutants and varieties of native orchids have been collected from their natural habitats. The wind orchid is one such orchid, the cultivation of which was recorded as early as 1665 (Reinikka, 1995). Wind orchids were particularly popular among warlords and samurai during the Edo period (1603–1868) in Japan and was thus often called the “Samurai orchid” or “Fuukiran,” meaning orchid of the rich and noble. In this period, owning the unusual mutant wind orchid was highly fashionable, and this motivated enthusiastic expeditions to hunt for valued wind orchid mutant plants from their natural habitats (Reinikka, 1995). Today, this passion for the wind orchid lives on, with orchid enthusiasts in Korea and Japan growing hundreds of different mutants of wind orchids. Recent advances in mass propagation of the wind orchid have made rare mutants widely available and continuously adds new mutants to the collection.
CHARACTERIZING THE MUTANT FLOWER MORPHOLOGIES OF THE WIND ORCHID
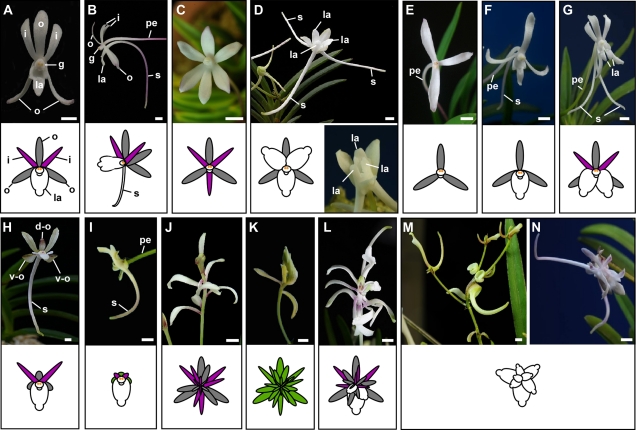
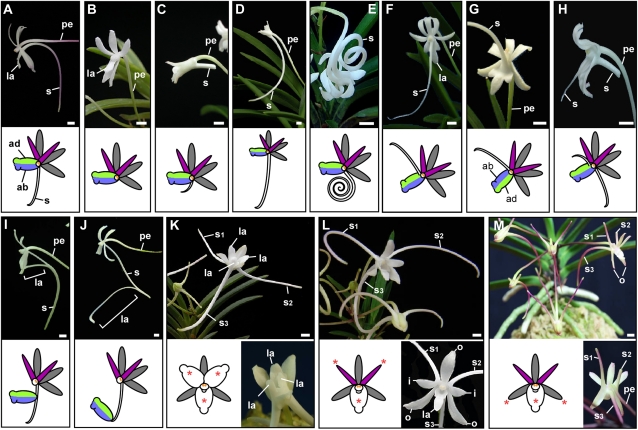
The wind orchid has a typical zygomorphic orchid flower form, with two whorls of tepals and a central gynostemium. The outer whorl consists of three similarly shaped outer tepals, whereas the inner whorl has two inner tepals and one highly modified labellum (lip tepal) with a long spur protruding from the abaxial side of the labellum (Fig. 1, A and B). The gynostemium (column), a reproductive organ with fused male and female organs, is positioned in the center and terminates the floral axis. The mutants reported here share peculiar flower forms and can be categorized into five major groups: 1) homeotic changes of floral organs, 2) increased or decreased organ number, 3) degenerated floral organ development, 4) changes in the determinacy of the flower, and 5) altered spur development. The spur mutants presented in Figure 2 can be further categorized into three characteristic groups: 1) altered spur length and curvature, 2) altered spur labellum patterning and positioning, and 3) absent or ectopic spurs.
Figure 1.
Floral morphologies of wind orchid mutants and schematic diagrams of flowers (insets). A and B, A frontal (A) and side (B) view of the wild-type wind orchid flower. C and D, Mutants with a homeotic conversion of floral organs from a labellum to an inner tepal (C) and vice versa (D) with near-radial floral symmetry. E–G, Mutants with decreased (E and F) or increased (G) floral organ number. H and I, Mutants with degenerative tepal development. J to N, Flower determinacy mutants. These mutant flowers maintain meristematic activity and continue to produce tepals (J), leaf-like tepals (K), tepals and labella (L), or labella (M and N). i, Inner tepal (colored purple in the diagrams); o, outer tepal (gray in the diagrams); la, labellum (white in the diagrams); g, gynostemium; s, spur; pe, pedicel; d-o, dorsal outer tepal; v-o, ventral outer tepal. Bars = 3.00 mm.
Figure 2.
Spur morphologies of wind orchid mutants and schematic diagrams (insets). A, A wild-type flower with the spur protruding in the abaxial side of the labellum. B to E, Mutant flower with no (B), a short (C), a long (D), and a highly coiled spur (E). F to J, Spur patterning mutants. F, A mutant flower with the spur growing out from the adaxial (ad) side of the labellum. G, A flower with inverted ab-adaxiality of the labellum. H, A flower with an additional spur growing in the adaxial side of labellum as well as an abaxial spur. I and J, Mutant flowers with labellum positioned in the distal side of the spur. K to M, Mutants with three spurs. K, Three spurs due to the conversion of the inner tepal to labellum. L and M, A mutant flower with two ectopic spurs growing from the inner tepals (L) or two ventral outer tepals (M). Organs with spurs labeled with orange asterisks in the diagrams in K to M. I, Inner tepal; o, outer tepal; la, labellum; s, spur; pe, pedicel; ab (colored in lavender), abaxial side of labellum; ad (colored in green), adaxial side of labellum. Bars = 3.00 mm.
Several wind orchid mutants show homeotic changes in lateral organs. One of the most evident is a homeotic change between the labellum and an inner tepal seen in the Geum-sung (Golden star) mutant (Fig. 1C). In contrast to Geum-sung, Ok-hyang-ro (Jade incense burner) converts each inner tepal into a labellum (Fig. 1D). Notably, the conversion between a labellum and an inner tepal causes the disruption of floral zygomorphy, and these mutants form perfectly radially symmetrical flowers. Given that the spur is formed on the labellum, any substitution between a labellum and an inner tepal also affects the number of spurs in a flower. Thus, instead of the one spur seen in wild-type flowers (Fig. 1B), Ok-hyang-ro generates three spurs (Fig. 1D), while Geum-sung has no spur (Fig. 1C).
Additional or missing floral organs have been found in several mutants. Go-ya-ji-hwa, for example, has fewer tepals and no labellum compared to wild-type flowers (Fig. 1E). Most Go-ya-ji-hwa flowers have only three outer tepals, although occasionally flowers form between two and six tepals. Another mutant, Gui-gong-ja (Noble man), has three outer tepals and one labellum but lacks any inner tepals (Fig. 1F). Sang-a (Ivory tusk) has a normal number of tepals but forms an additional labellum. The name is derived due to the presence of an extra spur, causing the flower to resemble an elephant face with tusks (Fig. 1G).
Other mutants show flowers with degenerated floral organ development. Some of the best examples are Hong-bi-jeob (Red flying butterfly; Fig. 1H) and Ho-jeob-ji-mu (Butterfly dance; Fig. 1I). In these mutants the tepal development is defective. In Hong-bi-jeob, the labellum and two inner tepals develop normally, but the three outer tepals show arrested growth. In particular, the growth arrest can be seen in the dorsal half of two ventral outer tepals (Fig. 1H, v-o) and in the distal part of the dorsal outer tepal (Fig. 1H, d-o). In Hong-bi-jeob, all tepals acquire a leaf-like identity, with the growth of each tepal arresting at a scale-like structure, whereas the labellum develops normally (Fig. 1I).
In the wild-type wind orchid, formation of the gynostemium terminates the floral axis (Fig. 1A). This floral determinacy is lost in some mutants (Fig. 1, J–N), where the continued meristematic activity in the center of the flower produces additional lateral organs. The type of lateral organs produced varies between the mutants. The indeterminate meristematic center of the Nam-geuk-ji-mu (Fig. 1J) and the San-chui-jun (Fig. 1K) flowers continuously generate tepals. Although Nam-geuk-ji-mu produces normal-looking tepals, San-chui-jun generates leaf-like tepals showing chimerical features between the leaf and the tepal. In Choon-geub-jeon, the flower meristem keeps generating tepals and labella (Fig. 1L). It appears that each gynostemium is substituted by an individual flower, creating a Russian doll-like phenotype. Sa-man-sib-ja-hwa (Fig. 1M) and O-de-mo-yang (Fig. 1N) mainly generate labella but occasionally produce scale-like tepals. All indeterminate flower mutants can be further categorized into two groups: one group with the elongated floral axis between lateral organs (Fig. 1, J, L, M) and a second group without the elongated floral axis between lateral organs (Fig. 1, K and N).
The wind orchid mutant collection also includes a wide variety of spur mutants. The wild-type spur is about 5 cm long and slightly curved (Fig. 2A). Choo-dong exhibits normal labellum and tepal morphology but fails to develop a spur (Fig. 2B). This is distinct from the Geum-sung flower (Fig. 1C), which also lacks a spur but due to a substitution from the labellum to a tepal. Other mutants exhibit variations in spur length. The No-gye flower has a severely reduced (about 1 cm long) spur (Fig. 2C), whereas the spur of Baek-ryung reaches a length of 7 to 10 cm (Fig. 2D). The curvature of the spur varies among mutants from straight to highly coiled as seen in Hwang-joa-ji-mu (Fig. 2E).
Patterning or positioning of the labellum and spur is altered in a number of mutants. In the wild-type flower, the spur develops from the abaxial side of the labellum (Fig. 2A). In contrast, in Chun-shim, the spur develops from the adaxial side of the labellum (Fig. 2F). The flower of Byen-gyung-ji-hwa (Fig. 2G) is superficially similar to that of Chun-shim (Fig. 2F). However, the adaxial and the abaxial sides of the labellum are inverted in Byen-gyung-ji-hwa (Fig. 2G). In the Yong-seol-jo flower, two spurs develop from both adaxial and abaxial sides of the labellum (Fig. 2H). The position of the labellum is altered in Mo-jung and Sul-ki-yi-jak, where the labellum is positioned in the middle (Fig. 2I) or the distal end of the spur (Fig. 2J).
Several mutants have flowers with three spurs. A conversion from the inner tepals to labella generates three labella and three spurs (Fig. 2K, Ok-hyang-ro). Another route to creating three spurs without extra labella is through ectopic spur formation on tepals. Two mutants possess flowers with one labellum and three spurs. A recently reported Korean mutant, Sam-kac-san (Mt. Triangle), has three spurs, with two ectopic spurs growing from the abaxial side of the inner tepals (Fig. 2L). In Hwa-guye, two ectopic spurs grow from the two ventral outer tepals (Fig. 2M).
EXPLOITING THE WIND ORCHID MUTANT COLLECTION
To understand orchid flower evolution and development, it is necessary to determine the underlying molecular mechanisms. The rich and constantly expanding collection of wind orchid flower mutants provides a unique and useful tool for dissecting the manifold aspects of orchid flower development as well as flower evolution in other angiosperms. These include floral zygomorphy, floral organ pattern formation, and spur development. Recruitment of floral zygomorphy is one of the key steps of orchid evolution (Rudall and Bateman, 2002). Basal orchid genera, such as Apostasia and Neuwiedia, with hexamerous radial flowers (three inner tepals), have acquired floral zygomorphy by altering the ventral inner tepal into a labellum along with stamen suppression (Bateman and Rudall, 2006). Geum-sung, an atavistic mutant (Fig. 1C), and Ok-hyang-ro, a mutant with three labella (Fig. 1D), will provide key information for understanding the radial-to-zygomorphic transition in orchid flowers. Several studies report that the TCP transcription factor CYCLOIDEA, a key regulator in establishing floral zygomorphy in several dicot species (Cubas et al., 1999; Hileman et al., 2003; Citerne et al., 2006; Feng et al., 2006; Busch and Zachgo, 2007; Broholm et al., 2008; Kim et al., 2008; Zhang et al., 2010), may also be involved in orchid flower development (Rudall and Bateman, 2002; Rajkumari and Longjam, 2005). Currently, however, there is no direct evidence indicating that TCP genes are involved in orchid floral zygomorphy. It has been suggested that combinations of different B function MADS-box paralogs (APETALA3-LIKE and PISTILLATA-LIKE genes) determine tepal and labellum identities, termed “orchid code” (Tsai et al., 2004; Mondragón-Palomino and Theissen, 2008, 2011; Tsai et al., 2008). The role of MADS-box genes and the orchid code hypothesis can be rigorously tested utilizing the wind orchid mutants with altered tepal identities and patterning. In addition, studying A and C MADS-box genes (APETALA1 and APETALTA2, AGAMOUS), LEAFY, SUPERMAN, and WUSCHEL-AGAMOUS pathways in mutant flowers with altered floral patterning and determinacy (Fig. 1, E–G, J–N) will contribute to our understanding of pattern formation and organogenesis in petaloid monocots. Furthermore, this will also help to resolve the homology among orchid tepals, grass floral organs, and dicot petals/sepals, which will shed new light on flower development and evolution in angiosperms as a whole.
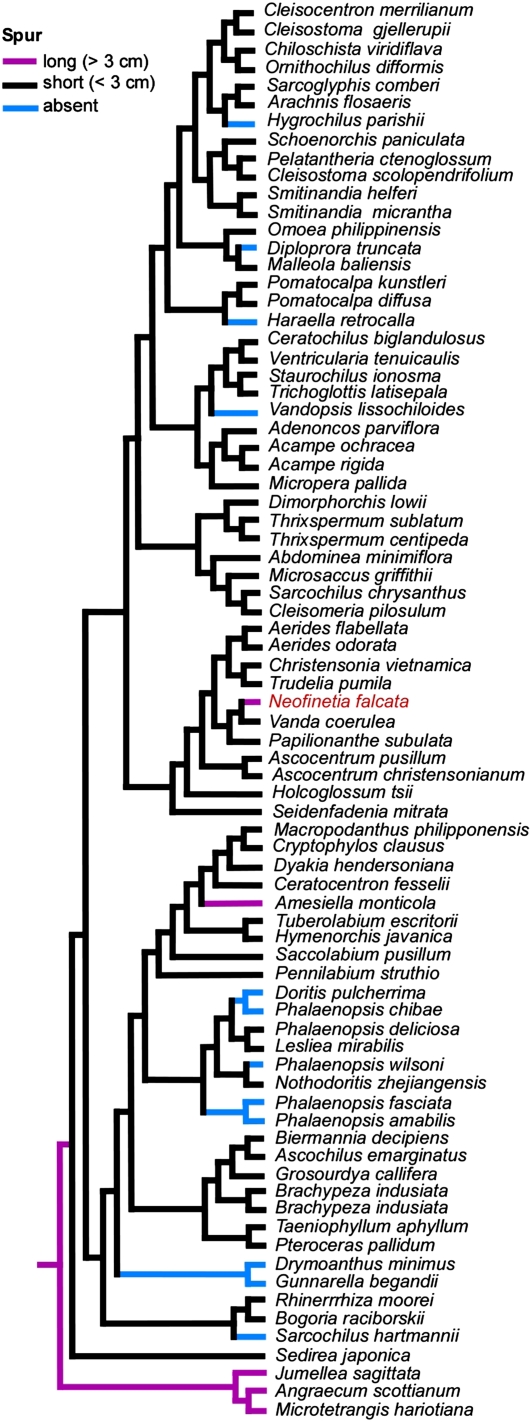
The spur is suggested to be one of the key innovations that led to rapid species diversification in many plant lineages (Hodges, 1997; Bell et al., 2009). Darwin hypothesized that the long spur of an orchid, Angraecum sesquipedale, was an outcome of an elaborate coevolution with the proboscis length of its insect pollinator (Darwin, 1877). The pollinator involved was found in Madagascar about 40 years later (Rothschild and Jordan, 1903). Despite its long history of interest, to date, very little is known about the molecular mechanisms underlying orchid spur development and evolution. Analysis of the phylogenetic relationship of the wind orchid and closely related orchid species showed that the prevailing short spur (<3 cm) status (Fig. 3, clades in black) evolved from the ancestral long spur (>3 cm) status (Fig. 3, clades in purple). Subsequent recruitments of a long spur (occurring twice, including in the wind orchid, clades in purple) and/or loss of the spur (Fig. 3, clades in blue) occurred independently (Topik et al., 2005). The roles of the KNOX genes, HIRZINA and INVAGINATA, in spur development have been reported in snapdragon (Antirrhinum majus) and Linaria vulgaris, respectively (Golz et al., 2002; Box et al., 2011). Studying these KNOX genes as well as lateral organ adaxial-abaxial polarity genes such as YABBY, KANADI, REVOLUTA, and PHABULOSA in the wind orchid spur mutants (Fig. 2) will shed light on our understanding of orchid spur development such as positioning and patterning.
Figure 3.
Phylogenetic relationship and spur morphology of the wind orchid and closely related orchid species (adapted from Topik et al., 2005). The short spur status (clades in black) evolved from the ancestral long spur status (clades in purple). Later, several recruitments of long spur (clades in purple) and no spur (clades in blue) occurred independently.
WIND ORCHID AS A PROMISING ORCHID MODEL SPECIES
With the long-standing historical interest and recent development of orchid research, a need for an orchid model species is pressing. An orchid model species facilitates integrated research and enables us to focus common resources, which will accelerate orchid research. The wind orchid has an immense potential as an orchid model species. The wind orchid mutant collection has been established over several centuries and covers a wide range of mutant phenotypes, all of which are readily available. These include mutants with defects in flower lateral organ patterning, organogenesis, floral determinacy, floral zygomorphy, and spur development. In particular, the range of spur mutants exceeds any other plant species. Moreover, the wind orchid offers even greater collections of flower color, leaf shape, and leaf color (variegated pattern) mutants. The mutant collection often contains several independently occurring mutants exhibiting identical or similar phenotypes, providing an invaluable resource that no other orchid species can offer. Along with this collection, the wind orchid has several other advantages over other orchid species. The wind orchid is easy to grow and is amenable to producing flowers under laboratory conditions. It is of small stature (5–10 cm), allowing up to 100 plants to be grown within a square meter. Tissue culture methods for clonal propagation and optimized germination systems are well established in the wind orchid (Chung, 1979; Ichihashi and Islam, 1999; Islam and Ichihashi, 1999). Agrobacterium-mediated transformation of the wind orchid further enables functional analyses utilizing transgenic plants (Niimi et al., 2001). The wind orchid is diploid (2n = 38) and has a relatively small genome size (1C = 2.35 pg, about 6 times bigger than Arabidopsis) among orchids (Leitch et al., 2009). Both selfing and outcrossing are possible and a crossed flower yields thousands of seeds, allowing genetic analyses in different mutant backgrounds (Chung, 1979; Arditti, 1992). Currently, the most promising way to utilize the wind orchid mutant collection is a candidate gene approach. This will be accelerated by the ongoing development of deep sequencing and transcriptome analyses. With decreasing costs of whole-genome sequencing (Drmanac, 2011), even forward genetic approaches may be possible in the long term. Subsequently, findings from the wind orchid can be translated to closely and distantly related orchid species, providing genetic toolkits with which to dissect the morphological diversity among orchid species. Exploiting this extensive mutant collection with combined molecular and ecological approaches will advance our understanding of development and evolution of the extraordinary morphologies, as well as the unique plant-animal and plant-fungus interactions, found in the orchid family. Furthermore, orchids represent an important petaloid monocot group, distinct from the majority of wind-pollinated monocot flowers with sepal-like floral organs such as lemma and lodicules. Studies on the wind orchid will thus advance our understanding on angiosperm flower development and evolution in broad terms.
Acknowledgments
We thank the Korean Neofinetia Club “Pungppamo” (www.pungnan.com) and Mr. Kook Hyung Han for providing the photos of the mutants and Dr. Liz Sheffield, Dr. Patrick Gallois, Dr. Giles Johnson, Dr. Thomas Nuhse, and Dr. Rebecca Wright for critical discussion of the manuscript.
References
- Arditti J. (1992) Fundamentals of Orchid Biology. John Wiley & Sons, New York [Google Scholar]
- Bateman RM, Rudall PJ. (2006) The good, the bad, and the ugly: using naturally occurring terata to distinguish the possible from the impossible in orchid floral evolution. Aliso 22: 481–496 [Google Scholar]
- Bell AK, Roberts DL, Hawkins JA, Rudall PJ, Box MS, Bateman RM. (2009) Comparative micromorphology of nectariferous and nectarless labellar spurs in selected clades of subtribe Orchidinae (Orchidaceae). Bot J Linn Soc 160: 369–387 [Google Scholar]
- Box MS, Dodsworth S, Rudall PJ, Bateman RM, Glover BJ. (2011) Characterization of Linaria KNOX genes suggests a role in petal-spur development. Plant J 68: 703–714 [DOI] [PubMed] [Google Scholar]
- Broholm SK, Tähtiharju S, Laitinen RAE, Albert VA, Teeri TH, Elomaa P. (2008) A TCP domain transcription factor controls flower type specification along the radial axis of the Gerbera (Asteraceae) inflorescence. Proc Natl Acad Sci USA 105: 9117–9122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch A, Zachgo S. (2007) Control of corolla monosymmetry in the Brassicaceae Iberis amara. Proc Natl Acad Sci USA 104: 16714–16719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JD. (1979) Seed culture of Neofinetia falcata in vitro: basic study on asymbiotic germination of seeds and growth of seedlings. Plant Tissue Cult 6: 49–65 [Google Scholar]
- Citerne HL, Pennington RT, Cronk QCB. (2006) An apparent reversal in floral symmetry in the legume Cadia is a homeotic transformation. Proc Natl Acad Sci USA 103: 12017–12020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzolino S, Widmer A. (2005) Orchid diversity: an evolutionary consequence of deception? Trends Ecol Evol 20: 487–494 [DOI] [PubMed] [Google Scholar]
- Cubas P, Vincent C, Coen E. (1999) An epigenetic mutation responsible for natural variation in floral symmetry. Nature 401: 157–161 [DOI] [PubMed] [Google Scholar]
- Darwin C. (1877) On the Various Contrivances by Which British and Foreign Orchids Are Fertilised by Insects, Ed 2. Murray, London [Google Scholar]
- Dressler RL. (1981) Classification of the Orchidaceae and their probable origin. Proc Int Bot Congr 13: 127 [Google Scholar]
- Dressler RL. (2005) How many orchid species? Selbyana 26: 155–158 [Google Scholar]
- Drmanac R. (2011) The advent of personal genome sequencing. Genet Med 13: 188–190 [DOI] [PubMed] [Google Scholar]
- Feng XZ, Zhao Z, Tian ZX, Xu SL, Luo YH, Cai ZG, Wang YM, Yang J, Wang Z, Weng L, et al. (2006) Control of petal shape and floral zygomorphy in Lotus japonicus. Proc Natl Acad Sci USA 103: 4970–4975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill DE. (1989) Fruiting Failure Pollinator Inefficiency and Speciation in Orchids. Academy of Natural Sciences Publications, Philadelphia [Google Scholar]
- Golz JF, Keck EJ, Hudson A. (2002) Spontaneous mutations in KNOX genes give rise to a novel floral structure in Antirrhinum. Curr Biol 12: 515–522 [DOI] [PubMed] [Google Scholar]
- Hileman LC, Kramer EM, Baum DA. (2003) Differential regulation of symmetry genes and the evolution of floral morphologies. Proc Natl Acad Sci USA 100: 12814–12819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges SA. (1997) Floral nectar spurs and diversification. Int J Plant Sci 158: S81–S88 [Google Scholar]
- Ichihashi S, Islam MO. (1999) Effects of complex organic additives on callus growth in three orchid genera, Phalaenopsis, Doritaenopsis, and Neofinetia. J Jpn Soc Hortic Sci 68: 269–274 [Google Scholar]
- Islam MO, Ichihashi S. (1999) Effects of sucrose, maltose and sorbitol on callus growth and plantlet regeneration in Phalaenopsis, Doritaenopsis and Neofinetia. J Jpn Soc Hortic Sci 68: 1124–1131 [Google Scholar]
- Kim M, Cui ML, Cubas P, Gillies A, Lee K, Chapman MA, Abbott RJ, Coen E. (2008) Regulatory genes control a key morphological and ecological trait transferred between species. Science 322: 1116–1119 [DOI] [PubMed] [Google Scholar]
- Leitch IJ, Kahandawala I, Suda J, Hanson L, Ingrouille MJ, Chase MW, Fay MF. (2009) Genome size diversity in orchids: consequences and evolution. Ann Bot (Lond) 104: 469–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCook LM, Bateman RM. (1990) Homeosis in orchid flowers a potential mechanism for saltational evolution. Am J Bot 77: 145 [Google Scholar]
- Mondragón-Palomino M, Theissen G. (2008) MADS about the evolution of orchid flowers. Trends Plant Sci 13: 51–59 [DOI] [PubMed] [Google Scholar]
- Mondragón-Palomino M, Theissen G. (2011) Conserved differential expression of paralogous DEFICIENS- and GLOBOSA-like MADS-box genes in the flowers of Orchidaceae: refining the 'orchid code'. Plant J 66: 1008–1019 [DOI] [PubMed] [Google Scholar]
- Niimi Y, Chen L, Hatano T. (2001) Gene transformation by using Agrobacterium in some orchidaceous plants. In Proceedings of the 7th Asia Pacific Orchid Conference. Nagoya, Japan, pp 95–98 [Google Scholar]
- Peakall R. (2007) Speciation in the Orchidaceae: confronting the challenges. Mol Ecol 16: 2834–2837 [DOI] [PubMed] [Google Scholar]
- Rajkumari JD, Longjam RS. (2005) Orchid flower evolution. J Genet 84: 81–84 [DOI] [PubMed] [Google Scholar]
- Reinikka M. (1995) A History of the Orchid, Ed New. Timber Press, Portland, OR [Google Scholar]
- Rothschild W, Jordan K. (1903) A revision of the lepidopterous family Sphingidae. In Novitates Zoologicae, Vol IX, Supplement, Version 2. Hazell Watson & Viney, Ltd., Aylesbury, UK [Google Scholar]
- Rudall PJ, Bateman RM. (2002) Roles of synorganisation, zygomorphy and heterotopy in floral evolution: the gynostemium and labellum of orchids and other lilioid monocots. Biol Rev Camb Philos Soc 77: 403–441 [DOI] [PubMed] [Google Scholar]
- Topik H, Yukawa T, Ito M. (2005) Molecular phylogenetics of subtribe Aeridinae (Orchidaceae): insights from plastid matK and nuclear ribosomal ITS sequences. J Plant Res 118: 271–284 [DOI] [PubMed] [Google Scholar]
- Tremblay RL, Ackerman JD, Zimmerman JK, Calvo RN. (2005) Variation in sexual reproduction in orchids and its evolutionary consequences: a spasmodic journey to diversification. Biol J Linn Soc Lond 84: 1–54 [Google Scholar]
- Tsai WC, Kuoh CS, Chuang MH, Chen WH, Chen HH. (2004) Four DEF-like MADS box genes displayed distinct floral morphogenetic roles in Phalaenopsis orchid. Plant Cell Physiol 45: 831–844 [DOI] [PubMed] [Google Scholar]
- Tsai WC, Pan ZJ, Hsiao YY, Jeng MF, Wu TF, Chen WH, Chen HH. (2008) Interactions of B-class complex proteins involved in tepal development in Phalaenopsis orchid. Plant Cell Physiol 49: 814–824 [DOI] [PubMed] [Google Scholar]
- Tupac Otero J, Ackerman JD, Bayman P. (2002) Diversity and host specificity of endophytic Rhizoctonia-like fungi from tropical orchids. Am J Bot 89: 1852–1858 [DOI] [PubMed] [Google Scholar]
- Zhang WH, Kramer EM, Davis CC. (2010) Floral symmetry genes and the origin and maintenance of zygomorphy in a plant-pollinator mutualism. Proc Natl Acad Sci USA 107: 6388–6393 [DOI] [PMC free article] [PubMed] [Google Scholar]