Abstract
Cucurbits exude profusely when stems or petioles are cut. We conducted studies on pumpkin (Cucurbita maxima) and cucumber (Cucumis sativus) to determine the origin and composition of the exudate. Morphometric analysis indicated that the exudate is too voluminous to derive exclusively from the phloem. Cold, which inhibits phloem transport, did not interfere with exudation. However, ice water applied to the roots, which reduces root pressure, rapidly diminished exudation rate. Sap was seen by microscopic examination to flow primarily from the fascicular phloem in cucumber, and several other cucurbit species, but primarily from the extrafascicular phloem in pumpkin. Following exposure of leaves to 14CO2, radiolabeled stachyose and other sugars were detected in the exudate in proportions expected of authentic phloem sap. Most of this radiolabel was released during the first 20 s. Sugars in exudate were dilute. The sugar composition of exudate from extrafascicular phloem near the edge of the stem differed from that of other sources in that it was high in hexose and low in stachyose. We conclude that sap is released from cucurbit phloem upon wounding but contributes negligibly to total exudate volume. The sap is diluted by water from cut cells, the apoplast, and the xylem. Small amounts of dilute, mobile sap from sieve elements can be obtained, although there is evidence that it is contaminated by the contents of other cell types. The function of P-proteins may be to prevent water loss from the xylem as well as nutrient loss from the phloem.
The phloem is an extension of the symplast, carrying nutrients and signals, and often pathogens, from autotrophic to heterotrophic tissues. Because it plays a central role in plant function, researchers in many fields are interested in the analysis of mobile phloem sap. Unfortunately, the phloem is difficult to sample. Whereas the circulatory system of animals is easily accessed by venipuncture (the blood flows through extracellular space and is under modest pressure), sieve elements in the phloem are living cells under high turgor (Gould et al., 2004; Turgeon, 2010). Cutting sieve elements results in rapid sealing by several mechanisms: cell contents surge against the sieve plates, callose blocks the pores, phloem proteins coagulate, and, in legumes, forisomes disperse (Furch et al., 2010). This makes it difficult to obtain significant quantities of phloem sap from most plants.
One way to avoid these problems is to collect sap from the severed stylets of phloem-feeding insects, taking advantage of the insect’s ability to inhibit wound reactions with injected saliva (Will et al., 2007; Dinant et al., 2010). The stylet technique has been useful but is limited by the small volumes of sap obtained and also by the fact that exudation does not occur in all plant/insect combinations. EDTA can also be used to inhibit sieve-element sealing and thus facilitate exudation from cut phloem, although contamination is an issue if the EDTA induces leakage from cells (Turgeon and Wolf, 2009).
Considering these technical limitations, it is easy to see why investigators have been drawn to a limited number of plants, including cucurbits, which “bleed” freely from severed vascular tissue (Turgeon and Wolf, 2009). When the stems or petioles of cucurbits are cut, sap exudes from both cut surfaces for minutes or longer. Microliter to milliliter quantities of sap can be obtained by this method. The initial exudate is so copious that the site(s) of origin has not been resolved, but as exudation slows, sap can be seen emerging from the phloem.
Although this method has been in use at least from the time of Hartig in 1858 (Crafts, 1932), not all workers are convinced that cucurbit exudate represents pure, mobile phloem sap. For example, Cooil (1941) suggested that the sugar concentration in pumpkin (Cucurbita maxima) exudate is too low (0.2%–0.5%) to account for the rapid growth rate of the fruits. Cucurbit sap may also contain appreciable quantities of reducing sugars (Moose, 1938; Richardson et al., 1982), which are generally considered to be lacking in true phloem sap (Turgeon and Wolf, 2009).
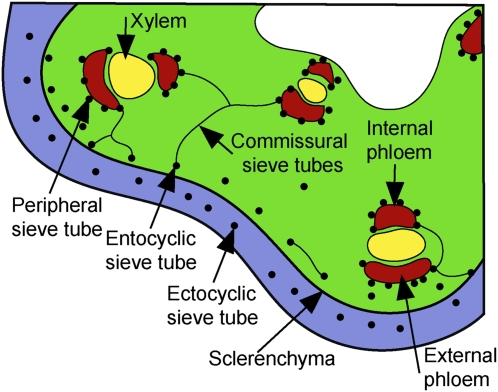
There is an additional problem with cucurbits in that the phloem is complex (Crafts, 1932). Sieve elements are present in the vascular bundles on both sides of the xylem (internal and external fascicular phloem) and also in an extensive system of sieve tubes outside the bundles (extrafascicular phloem), the role of which is not well understood (Fig. 1). The extrafascicular phloem of pumpkin consists of longitudinally oriented peripheral sieve tubes at the margins of the fascicular phloem and entocyclic sieve tubes just inside the sclerenchyma ring, connected by laterally oriented commissural sieve tubes. Extrafascicular phloem also includes the longitudinally oriented ectocyclic sieve tubes found outside the sclerenchyma ring (Fig. 1).
Figure 1.
Diagram of a transverse section of pumpkin stem adapted from Crafts (1932). The fascicular phloem (i.e. phloem in vascular bundles) is bicollateral, with both internal and external sieve tubes. The extrafascicular phloem inside the sclerenchyma ring is composed of peripheral sieve tubes closely adjacent to the vascular bundles, entocyclic sieve tubes just inside the sclerenchyma ring, and commissural sieve tubes that run transversely, linking these various elements to each other and to the fascicular phloem. Ectocyclic sieve tubes are found outside the sclerenchyma ring.
Zhang et al. (2010) recently provided evidence that sap exudes from the extrafascicular, rather than the fascicular, phloem of several cucurbit species. We find, in contrast, that both fascicular and extrafascicular phloem contribute to the exudate in different proportions, depending on the species. Zhang et al. (2010) also noted that the exudate contains significant amounts of hexose, again suggesting a source other than the fascicular phloem. In this study, we have analyzed sap from different types of phloem and find that the sugar profile depends on the site of origin of the sap. Our data also indicate that the phloem seals rapidly after a cut is made and that the xylem can contribute much of the fluid that exudes from the cut surface.
RESULTS
Fluid Exudes from Several Sources
The durations and volumes of exudation from the cut surfaces of cucumber (Cucumis sativus) and pumpkin stems or petioles are variable. In general, exudation periods are longer in pumpkin than in cucumber but differ from one plant to another and from one excision to another on the same plant. Cut stems tend to exude longer than cut petioles, and the proximal surface of a cut petiole exudes for a longer period than the distal surface. Plants brought into the dim light of the laboratory tend to exude longer than those sampled in the greenhouse.
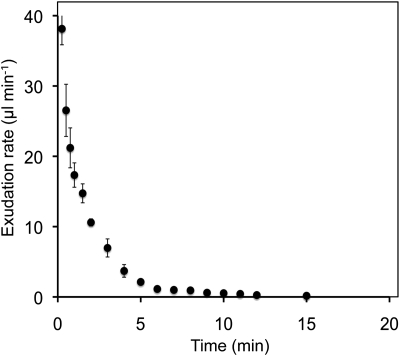
To measure exudation rates, cucumber plants were brought to the laboratory and the petioles of mature leaves were cut midway between the lamina and stem. The emerging fluid from the proximal surface was collected by blotting at intervals with preweighed filter paper and immediately weighing the paper again. In a representative experiment, the exudation rate, initially 38 μL min−1, declined rapidly (Fig. 2). A total of 59.4 μL exuded over a period of 15 min.
Figure 2.
Rate of exudation from the proximal cut surface of a pumpkin petiole (n = 3). The total volume exuded was 59.4 μL.
Could this amount of fluid exude entirely from the fascicular phloem? To answer this question, a morphometric analysis was conducted on the phloem of cucumber petioles by electron microscopy. The mean cross-sectional areas of individual sieve elements in the external and internal fascicular phloem were 71.0 ± 11.0 μm2 (se; n = 15) and 81.3 ± 13.9 μm2 (n = 13), respectively. Since there are 26.2 ± 1.4 (n = 4) sieve elements in the external phloem and 15.3 ± 0.8 (n = 4) sieve elements in the internal phloem, and six major vascular bundles, the combined cross-sectional areas of the fascicular sieve elements in the petiole was 1.86 × 104 μm2. In the case above, where 59.4 μL of sap exuded from a cut petiole, this would equal the volume of fascicular sieve elements in 3.18 m of phloem. Obviously, this value is unrealistic; since flow velocities in the cucurbits are in the range of 200 μm s−1 (Mullendore et al., 2010), it would take 4.4 h for 3.18 m of fluid to reach the cut surface, whereas in these experiments the time interval was only 15 min. Therefore, the fluid cannot represent only the sap inside fascicular sieve elements, especially when one considers that similar exudate volumes are often obtained from petioles of plants less than 1 m in height and that, once exudation stops, almost the same volume can be obtained by resevering the petiole at a point only several millimeters proximal to the original cut.
Due to the scattered and complex nature of the extrafascicular phloem, it is difficult to conduct a similar morphometric analysis on these cells. However, from the figures presented by Crafts (1932) and Kempers et al. (1993), it is reasonable to assume that no more than 200 longitudinally oriented extrafascicular sieve elements are severed when the petiole is cut. Since the diameter of a single extrafascicular sieve element is approximately 20 μm2 (measured in micrographs from Crafts [1932] and Cronshaw and Esau [1968]), the total cross-sectional area of extrafascicular sieve elements in the petiole is approximately 6.3 × 104 μm2, 3.4 times the area of the fascicular sieve elements. Exudation of 59.4 μL, therefore, would drain the volume of extrafascicular sieve elements in 0.9 m of phloem, again an excessive figure.
The cut cells at the surface of the stem or petiole also contribute fluid. Given that the cross-sectional area of a cucumber petiole is approximately 25 mm2 and the length of cortical cells is approximately 340 μm, cut cells contribute approximately 8.5 μL to the exudate volume. This is an appreciable portion of the fluid that erupts onto the surface immediately after a cut is made. However, when the initial fluid is blotted away, exudation continues, and it is unlikely to come from this source.
To test the source of the sap experimentally, we took advantage of the fact that long-distance phloem transport in cucurbits is severely inhibited by cold (Webb, 1967). To conduct these tests, an approximately 10-cm region near the middle of a cucumber petiole was packed in ice, kept in place with cheesecloth. Ten minutes later, the ice was pulled back from the distal end of the cold block, the petiole was quickly severed in the precooled region, and the exudate from the proximal cut surface was measured by blotting with preweighed filter paper, as described above. Cold did not inhibit exudation. Indeed, sap exuded more copiously from cold-treated (36.2 ± 3.2 μL [n = 5]) than control (22.8 ± 3.9 μL [n = 5]) petioles. This finding suggests either that that the major source of the fluid is not the phloem or that flow through the phloem is not subject to cold inhibition when the sieve tubes are cut.
Next, we conducted a series of experiments on cucumber and pumpkin measuring exudate volumes, as described above, in combination with a radiolabeling technique to determine if the exudate contains mobile sugars carried in the phloem. To apply the radiolabel, we exposed leaf blades to 14CO2. During the fixation period, the petioles were wrapped in aluminum foil to prevent inadvertent photosynthetic incorporation of leaked 14CO2. Stems or petioles were then cut after a 1-h chase period in room air. After a 1-h chase, radiolabeled sugars are in transit in the phloem, on their way to sinks (Webb and Gorham, 1964). Both exudate volume and 14C content were measured.
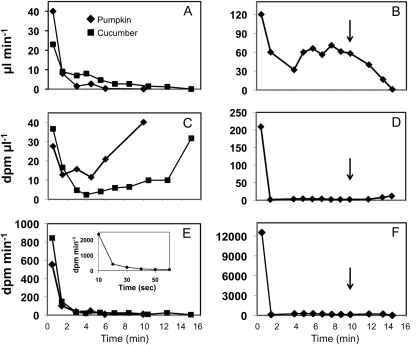
In cucumber plants, the volume of exudate declined rapidly, as described above (Fig. 3A). However, with pumpkin plants, two outcomes were apparent (Fig. 3, A and B). In some experiments, and in every case when cuts were made in the greenhouse, the exudate volume decreased rapidly, as in cucumber (Fig. 3A). However, in approximately 50% of the trials conducted on pumpkin in the laboratory, the exudate volume per unit of time at first decreased and then recovered to a moderate degree (Fig. 3B). In many cases, exudation persisted at that rate for an extended time, often for hours, yielding over 30 mL of fluid from proximal cut surfaces. Exudation sometimes persisted overnight. We also noted that the exudate from pumpkin was not as viscous as that from cucumber.
Figure 3.
Radiolabeled exudate from cut stems of pumpkin and cucumber. Leaf blades were exposed to 14CO2 for 15 min, and 45 min later, stems were cut. Three representative experiments are shown. Charts on the left record exudation rate (A), 14C concentration (C), and 14C exudation rate (E) in cucumber and pumpkin. The inset in E records 14C exudation in a separate pumpkin experiment over a shorter time period. The three panels on the right record an experiment in which exudation rate (B) in a pumpkin plant declined, then rose again, then declined when ice water was applied to the soil (arrows).
In the experiment recorded in Figure 3B, we waited until exudation stabilized and at the 10-min time point poured an excess of ice water on the surface of the soil to decrease root pressure (see below). The rate of exudation declined rapidly. This experiment was repeated over 10 times with consistent results.
In these experiments on both pumpkin and cucumber, 14C was detected in the initial flush of exudate (Fig. 3, C and D), as described by Shalitin and Wolf (2000). The concentration of radiolabel (dpm mL−1) in the sap declined rapidly over time. In the majority of cases, where flux of exudate remained low (Fig. 3C), 14C content increased again, sometimes reaching concentrations approaching those of the initial exudate. Although 14C concentrations increased over time, the volumes of exudate at the longer time points had greatly declined, with the result that the total 14C exuded per unit of time continued to be very low after the first minute (Fig. 3E).
To examine the time course of 14C exudation at shorter intervals, we repeated the experiment with pumpkin plants, collecting stem exudate by blotting every 10 s (Fig. 3E, inset). The concentration of 14C in the exudate declined rapidly over the first 30 s. Over half of the 14C had already exuded within 10 s, and the amount exuded in the first 20 s equaled 65% of the total released when exudation stopped (8.5 min). To determine the amount of 14C in the exudate compared with that in the petiole, we conducted additional experiments in which we counted total 14C in the exuded fluid and also extracted and counted the 14C in an adjacent 1-cm segment of petiole. The amount of 14C in the petiole segments was 8.9 ± 1.6 (n = 4) times that in the exudate. In other words, the 14C in the exudate equaled that in 1.1 mm of adjacent tissue. In the case of persistent exudation in pumpkin plants (Fig. 3, B, D, and F), the 14C concentration remained very low until ice water was applied, at which point it also rose, although slightly (Fig. 3D), and the total 14C exuded per unit of time after ice-water treatment was negligible (Fig. 3F).
Water from the Xylem
The large volumes of exudate obtained in many experiments suggested that much of the fluid originated from the xylem, forced out of the cut surface by root pressure. Lee et al. (2004) measured positive root pressure in cucumber plants. To test this possibility, we relied on the fact that root pressure rapidly declines when roots are chilled (Lee et al., 2004), whereas cold inhibition of phloem transport is a localized phenomenon (Webb, 1967). Therefore, root chilling should inhibit xylem water flux, but not sap movement through sieve tubes, in the aerial portion of the plant. Consistent with this reasoning, and with the hypothesis that phloem sap is diluted by xylem water, when ice water was poured on the soil of pumpkin plants exuding persistently in the laboratory, the exudation rate declined rapidly (Fig. 3B) while the concentration of 14C in the exudate rose (Fig. 3D).
We next considered the possibility that the xylem contributes to exudation in all cases, not just those involving pumpkin plants in the laboratory, in which flow is persistent. As another test for xylem transport, silicon was used as a tracer. Silicon is transported in the xylem but not in the phloem (Epstein and Bloom, 2005). The experiments were conducted on cucumber plants growing in the sun in the greenhouse, because previous experience had shown that the smallest volumes of exudate, generally less than 20 μL, were obtained from such plants and exudation commonly stopped within 10 min. In these experiments, 200 mL of potassium silicate solution (56 μL L−1) was applied to the soil and exudates were collected at different time points from severed petioles. At zero time, the concentration of silicate in exudate was 4.5 ± 0.21 μL L−1 (n = 3). Four hours later, the concentration had risen to 6.3 ± 0.57 μL L−1 (n = 3), a statistically significant increase (t test), and 24 h later, the concentration was 11.0 ± 1.4 μL L−1.
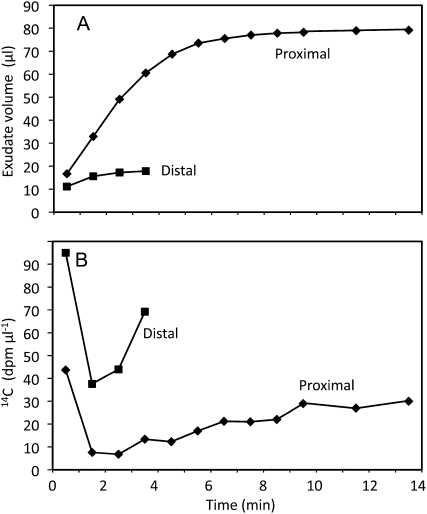
As another test, we reasoned that if exudate contains water from the xylem, phloem-mobile constituents should be more dilute in the fluid issuing from the proximal side of a cut, since this tissue is in continuity with the vascular system of the roots. To test this hypothesis, we labeled mature leaves with 14CO2, as described above. After a 1-h chase period, the petioles were cut and both 14C and sugars exuding from the two surfaces were analyzed. By the time exudation ceased (15 min), the total amount of 14C released from the two sides was the same; the ratio of 14C in the proximal and distal sides was 1.08 ± 0.17 (n = 4). However, the proximal side exuded approximately four times more fluid than the distal side (Fig. 4A). Therefore, the concentration of exuded 14C was considerably lower in exudate from the proximal side (Fig. 4B), as predicted. Over time, the 14C concentration on both sides fell and rose again, as in previous experiments (compare Figs. 3C and 4B).
Figure 4.
Volume (A) and 14C concentration (B) in exudate from the proximal and distal surfaces of a cut cucumber petiole. The leaf blade was exposed to 14CO2 for 15 min, and the petiole was severed 45 min later.
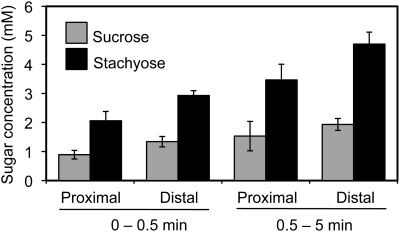
These experiments also provided an opportunity to test the phloem mobility of unlabeled sugars exuding from the two surfaces. If the aggregate sugars in the exudate, measured by HPLC, derive from the sieve tubes with the 14C-labeled sugars (in other words, if they are in the same pool), they should be diluted to the same extent as they collect on the two surfaces of the cut. This proved not to be true. The sugar concentrations, both stachyose and Suc, were much more similar on the two sides of the cut than were the concentrations of 14C (Fig. 5). This suggests that at least some of the exuded stachyose and Suc does not originate from the sieve tubes.
Figure 5.
Suc and stachyose concentrations in exudate taken from the proximal and distal sides of cut cucumber petioles. Exudates were collected at 0.5 and 5 min after the petiole was severed. Samples from 10 to 15 petioles per plant were combined (n = 3 plants).
What causes exudation to cease? Perhaps the xylem and the rest of the apoplast becomes plugged with phloem exudate that rapidly becomes viscous upon exposure to air. Pumpkin exudate is usually less viscous than that from cucumber, which could explain why exudation often lasts longer in pumpkin than in cucumber. To test this hypothesis, the surface of cut cucumber stems was treated with the thiol reagent 2-mercaptoethanol (1% [v/v]; approximately 50 μL) after exudation had ceased. This treatment caused flow to resume for up to 5 min, but with reduced volume flow, in approximately 90% of the experiments.
Droplets Exude from the Fascicular and Extrafascicular Phloem
Identifying the site of phloem exudation soon after a cut has been made has previously not been possible, due to the rapid efflux of copious quantities of fluid over the cut surface. We took advantage of the fact that ice water applied to the roots diminishes the flux of water through the xylem and thus allows individual drops of phloem sap to be visualized.
To conduct these experiments, ice water was poured onto the surface of the soil of pumpkin or cucumber plants. Approximately 10 min later, the stem, or one of the petioles, was severed. Fluid rapidly flooded the proximal cut surface, but this excessive exudation ceased within 10 s. The proximal surface was then rapidly blotted dry, and after 2 to 3 s, when renewed exudation was visible as localized droplets, a second cut was made approximately 2 mm proximal to the original cut. This second cut stopped further exudation and allowed individual spots of fluid to be visualized on the surface of the 2-mm slice of tissue with a stereomicroscope.
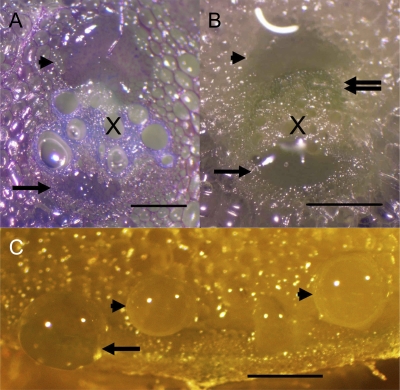
In cucumber, the origin of the droplets was particularly easy to see, because the fluid rapidly became rubbery on exposure to air and remained in place when the tissue was stained with toluidine blue (0.01% [v/v]; Fig. 6A). The rubbery consistency was noted in sap emanating from all types of phloem. Most of the exudate droplets issued from the fascicular phloem and only occasionally from the extrafascicular phloem. Approximately twice as many droplets were counted over the internal fascicular phloem as over the external fascicular phloem. By making repeated cuts over a 30-cm-long segment of cucumber stem and measuring exudates in capillary tubes, it was determined that the volume issuing from the internal fascicular phloem was 3.7 times that issuing from the external fascicular phloem. Droplets from the entocyclic sieve elements were more common than those from the ectocyclic phloem. Droplets from the commissural extrafascicular and peripheral extrafascicular phloem were rare.
Figure 6.
Micrographs of exuding droplets. A, Cucumber vascular bundle stained with toluidine blue. The xylem (X) is stained blue, and other cells are stained purple. A small exuding droplet is seen over the external fascicular phloem (arrow). A larger droplet over the internal phloem (arrowhead) originated in the fascicular phloem but has grown to cover the extrafascicular phloem as well. B, Unstained pumpkin vascular bundle. An exuding droplet has emerged from the external fascicular phloem (arrow), which is uncommon in pumpkin. Another droplet is visible over the peripheral, internal extrafascicular phloem (arrowhead). The green region (double arrow) is the internal fascicular phloem. The white, crescent-shaped line is a diffraction image. C, Unstained pumpkin petiole section. Drops of exudate are visible over the ectocyclic phloem (arrow) and entocyclic phloem (arrowheads). Bars = 0.5 mm.
In pumpkin, the sap from all types of phloem was less sticky than exudate from cucumber, making staining impractical. Nonetheless, the position of the droplets could be determined because the phloem was evident from its green color on either side of the colorless xylem (Zhang et al., 2010). Almost all of the exuding droplets associated with the vascular bundles (more than 90%) emanated from the peripheral, extrafascicular sieve tubes, as described by Zhang et al. (2010; Fig. 6, B and C). Exudate from the commissural sieve tubes between the bundles was also noted, but it was not as abundant as that from the peripheral sieve tubes. On occasion, but only rarely, droplets exuded from the fascicular phloem, as indicated by their position immediately adjacent to the xylem (Fig. 6B). Exudate from the edge of the stem, from both the ectocyclic and entocyclic sieve tubes, was much more abundant than in cucumber (Fig. 6C).
We also conducted less extensive trials on other species in the Cucurbitaceae. Zucchini (Cucurbita pepo) exuded primarily from the extrafascicular phloem in a pattern much like pumpkin, whereas watermelon (Citrullus lanatus), bitter apple (Citrullus colocynthis), luffa (Luffa acutangula), calabash (Lagenaria siceraria), and winter melon (Benincasa hispida) exuded primarily from the fascicular phloem, in a pattern much like cucumber.
The Sugar Composition of Exudate Depends on Its Origin
To determine the composition of exudate from different types of phloem, we followed the procedure described above for applying ice water to the soil and viewing droplets. Exudate was collected with capillary tubes and analyzed by HPLC. Due to insufficient volumes, not all exudate types in the two species were analyzed.
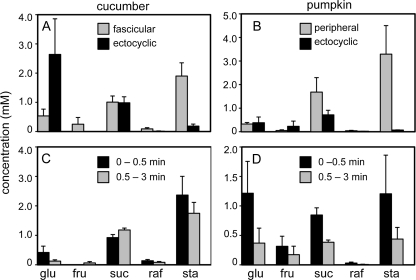
In cucumber, exudates from the internal and external fascicular phloem could not be isolated separately and therefore were combined for analysis (Fig. 7A). The concentrations of transport sugars (stachyose, raffinose, and Suc) were very low (less than 5 mm). Stachyose was the most abundant sugar, followed by Suc. Small amounts of Glc and Fru and traces of raffinose were also detected. The exudate from the ectocyclic and entocyclic phloem also blended together. This sap contained abundant Glc, a small amount of Suc, very little stachyose, and minor traces of raffinose and Fru (Fig. 7A).
Figure 7.
Exudate sugar concentrations. Sugar concentrations are shown in cucumber (A and C) and pumpkin (B and D). In A and B, ice water was applied to the soil, and 10 min later, the stem was cut. After rapid blotting, exudate was collected from different phloem types. Data are from approximately 100 cuts per plant (n = 3 plants). In C and D, ice water was not applied to the soil. The stems were cut, and exudate from all phloem types was sampled together. Data are from three to five cuts per plant (n = 3 plants).
In pumpkin, there was not enough fascicular phloem exudate for analysis. However, the sugar profile of the peripheral extrafascicular phloem was similar to that of the fascicular phloem of cucumber. Stachyose was the most abundant sugar (Fig. 7B), but as in cucumber the concentrations of all sugars were less than 5 mm. The exudate from mixed ectocyclic and entocyclic phloem contained mostly Suc, with smaller amounts of hexose and stachyose (Fig. 7B). In additional experiments, leaves were labeled with 14CO2, and 2 h later, droplets were collected from different phloem sources and analyzed by scintillation counting. Radiolabel was consistently present in all types of phloem exudate with the exception of the entocyclic/ectocyclic phloem, which remained unlabeled.
Since cucurbit exudate is collected in most studies from the entire cut surface of the stem or petiole, we also analyzed fluid acquired by this method, without ice-water treatment. Stems were cut and, without blotting, collections were made of the copious fluid exuded in the first 0.5 min (early collection) and the smaller amount of fluid that exuded over the next 2.5 min (late collection), combining droplets from all sources.
In cucumber, the early and late collections were similar in both the absolute and relative concentrations of sugars (Fig. 7C). Stachyose dominated, followed by Suc, with lesser amounts of monosaccharide and raffinose. In pumpkin, sugars were more concentrated in the early collection, although the profiles at early and late times were similar, with substantial proportions of Glc, Suc, and stachyose (Fig. 7D).
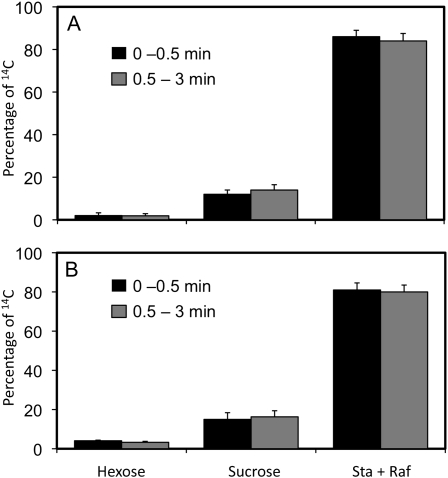
As another test of the composition of mobile material in the exudate, mature leaf blades were exposed to 14CO2 for 15 min, and 45 min later, exudate was collected from cut stem surfaces. Radiolabel in the exudate was analyzed by thin-layer chromatography. Similar patterns were found for both cucumber (Fig. 8A) and pumpkin (Fig. 8B), with 80% to 85% of the labeled sugar as stachyose, 10% as Suc, 2% to 4% as hexoses, and trace amounts as raffinose (Fig. 8).
Figure 8.
14C in photosynthetically labeled sugars. Leaves of pumpkin (A) and cucumber (B) were exposed to 14CO2 for 15 min. Forty-five minutes later, stems were cut and exudates were collected over two time periods. Three to five sampling cuts were made per plant. Radiolabeled sugars were analyzed by thin-layer chromatography (n = 3 plants).
DISCUSSION
Origin of the Exudate
Exudate from cut stems or petioles of cucurbits is often used as a source of phloem sap. The results presented here indicate that this sap has several origins, that it contains both mobile phloem constituents and other compounds, and that it is highly dilute. The contribution from different types of phloem depends on the species.
Exudation occurs in successive, overlapping stages. In the first stage, immediately after a cut is made, fluid rapidly floods the surface of the wound. Cut parenchyma cells release a considerable amount of sap, and since these cells are highly vacuolated, it is dilute. The phloem also contributes to the initial flood of exuded liquid. Since the phloem is under pressure, it is reasonable to assume that sap bursts from both fascicular and extrafascicular sieve tubes before they have a chance to seal. Indeed, as reported previously by Shalitin and Wolf (2000), when leaf blades are provided with 14CO2, radiolabeled sugars exude from cut petioles and stems, primarily as [14C]stachyose, with smaller amounts of [14C]raffinose and [14C]Suc. These are the sugars reported to be mobile in the cucurbits by Webb and Gorham, 1964. There is also a small quantity of [14C]hexose, but the absence of hexose in phloem sap obtained by aphid stylectomy in other families (Turgeon and Wolf, 2009) suggests that these 14C-labeled monosaccharides are not mobile but derive instead from the hydrolysis of other sugars in companion cells, and perhaps other neighboring cell types, and are pulled into the phloem when the pressure is released.
Although radiolabeling studies confirm the presence of phloem contents in exudate, two independent lines of evidence suggest that it is a minor constituent. First, sugar concentrations are very low, less than 5 mm, indicating considerable dilution. Aphid stylectomy studies have not been conducted on cucurbits, but other evidence suggests that the sugar concentration in the phloem is as high as in other plants. Pristupa (1983) demonstrated histochemically that the sugar content in the phloem of pumpkin leaves is much greater than in surrounding mesophyll cells. Haritatos et al. (1996) measured the concentrations of Suc, raffinose, and stachyose in microdissected minor veins of melon (Cucumis melo) and found the total to be 534 mm. Zhang et al. (2010) estimated the sum concentration of these sugars in pumpkin vascular bundles, again by microdissection, to be approximately 1 m. Finally, Voitsekhovskaja et al. (2006) measured, by aphid stylectomy, a total of 820 mm sugar in the phloem sap of Alonsoa meridionalis, a species in the Scrophulariaceae that loads raffinose and stachyose by polymer trapping, as do the cucurbits. Therefore, assuming the true sieve element concentration to be approximately 800 mm, true phloem sap contributes less than 1% of the exudate volume.
The second line of evidence that sieve tube sap is a minor constituent of exudate comes from a comparison of morphometric data and 14C content in the exuded fluid. Morphometry indicates that sap would have to flow out of meters of fascicular sieve tube length to account for the volumes that issue from a cucumber petiole. In striking contrast, comparisons of phloem-mobile 14C in exudate and in the petiole itself demonstrate that the 14C actually comes from only 1.1 mm of adjacent tissue, a difference of several orders of magnitude. Put another way, assuming that the radiolabel comes from the fascicular sieve tubes alone, 1.1 mm of total sieve tube length in the cucumber petiole contains only 0.02 μL of fluid. Therefore, while the fascicular sieve elements release mobile sugars and probably other cell contents, they contribute negligibly to overall exudate volume.
The contribution that the ectocyclic/entocyclic sieve tubes make to the initial outpouring of fluid is more difficult to ascertain, because the compounds in these cells did not become radiolabeled in the course of our 14C-transport studies. However, sugar composition of the exudate provides a clue. In cucumber, the sap that floods the entire cut surface contains only a modest proportion of Glc, even though Glc is the primary sugar released by the ectocyclic/entocyclic phloem. This suggests that ectocyclic/entocyclic sieve elements contribute little to the overall sap volume in this species. In contrast, the proportion of Glc in pumpkin exudate is high. This may be due to a larger contribution from ectocyclic/entocyclic sieve tubes, which would be consistent with studies indicating that, in pumpkin, the P-protein bodies of extrafascicular phloem tend not to disperse as readily as those from the fascicular phloem (Cronshaw and Esau, 1968; Evert et al., 1973) and thus do not as readily plug the phloem.
The xylem also contributes to exudate volume if plants are kept in dim light long enough for the stomata to close, reducing transpiration and allowing the buildup of root pressure. Even if the xylem is under tension, cutting the petiole or stem eliminates transpirational pull and presumably allows root pressure to force water back onto the surface. This explains why silicon, which is mobile in the xylem, but not the phloem (Epstein and Bloom, 2005) is detected in exudate obtained from greenhouse plants. The contribution of xylem water to the sap explains why plants in the relative darkness of the laboratory exude more than those sampled in the greenhouse.
Plants also exude a greater volume, over a longer period of time, from proximal cut surfaces in comparison with the distal surface. Again, this suggests a contribution of water from the xylem, and this hypothesis is consistent with radiolabeling data, which indicate that mobile 14C is much more dilute on the proximal than on the distal side of a cut.
Once the initial burst of exudation occurs, sap continues to flow from the cut surface. The 14C content of the exudate drops within seconds, suggesting that the phloem involved in long-distance transport of these compounds seals more quickly than the xylem. Initial sealing may be due to the surge of P-protein and ruptured cell contents onto the sieve plates to form slime plugs (Evert et al., 1973; Furch et al., 2010). However, plugging is not immediate. If the flow of water from the xylem is inhibited by pouring ice water on the soil, drops of sap can be seen emerging from the phloem. This is consistent with the observations of Kempers et al. (1993) that dye solution is able to pass through apparently plugged sieve plates, indicating that, at least for some time, they do not act as complete barriers to fluid movement. Not all of the vascular bundles exude at this stage, indicating that the sealing mechanism is irregular and sporadic, blocking some sieve tubes more readily than others.
In cucumber, most phloem exudate comes from fascicular sieve tubes, and the unlabeled sugar profile is almost identical to that of radiolabeled sugars: predominantly stachyose with small amounts of raffinose and Suc. In pumpkin, droplets exude primarily from the extrafascicular phloem, as reported by Zhang et al. (2010). Indeed, we were not able to obtain fascicular phloem exudate in sufficient quantity for analysis from pumpkin. However, sugars that issue from the peripheral extrafascicular phloem of pumpkin, at the edges of the vascular bundles, and from commissural sieve elements in the cortex are very similar to those that exude from fascicular cucumber phloem. While it is perilous to compare data from different species, the combined evidence from cucumber and pumpkin suggests a free exchange of the contents of fascicular and extrafascicular sieve tubes, with the exception of the ectocyclic/entocyclic sieve tubes at the edge of the stem. In support of this conclusion, data from autoradiography (Webb and Gorham, 1964) and dye-tracer studies (Zhang et al., 2010) demonstrate that the fascicular and commissural sieve tubes are in symplastic continuity. In contrast to the data of Zhang et al. (2010), we found that cucumber and watermelon exude primarily from the fascicular, rather than the extrafascicular, phloem, as do C. colocynthis, L. acutangula, L. siceraria, and B. hispida.
The Copious Nature of the Exudate, and the Sealing Mechanisms
It is likely that the exudate from cucurbits is copious because they are vines. Vines are “structural parasites.” They take advantage of the architecture of other plants to support large leaf areas with minimal investment in stem structure. To compensate for the narrowness of the stems, vines have wider xylem vessels than freestanding species (Ewers and Fisher, 1991; Ewers et al., 1991), and hydraulic conductivity (flow rate per unit of pressure gradient per unit of stem transverse area) is higher in vines than in trees (Gartner et al., 1990). The phloem of cucurbits also appears to be adapted to the vine habit (Ewers and Fisher, 1991). The sieve tubes are especially wide, as are the sieve pores. Mullendore et al. (2010) determined that sieve tube-specific conductivity in pumpkin is four to 14 times higher than in species from other families studied. The dimensions of the sieve tubes in the cucurbits presumably make them difficult to seal.
What eventually stops fluid loss from the xylem and phloem? From experiments in which exudation resumed after 2-mercaptoethanol treatment, it appears that phloem proteins that spill onto the cut surface form a waterproof layer over the entire surface as they oxidize. Since there is much less pressure in the xylem than in the phloem, the xylem plugs first. These results suggest that the role of P-protein in the cucurbits may be to prevent excessive water loss from wounded xylem as much as it is to seal wounded phloem. From this vantage point, P-protein can be viewed as an adaptation to the vine growth form, a caulking material that coats and waterproofs wounded tissue.
In the case of pumpkin, the xylem appears not to plug adequately if the plants are first transferred to dim light, allowing pressure to build in the vessels. In these instances, the rate of exudation briefly declines but then rises again, perhaps because phloem exudate in pumpkin is not as viscous as that of cucumber and is unable to fully inhibit water flow. Root pressure then forces water through the ends of the xylem vessels, cleaning away the exuded phloem sap and allowing the flow of essentially unadulterated water over an extended period.
In the later phases of exudation, once the xylem has sealed, discrete droplets of fluid continue to exude from the phloem. The concentration of 14C in these droplets increases (Fig. 3C), as expected if it mixes with progressively less xylem water. While the 14C-labeling experiments indicate that this sap emanates from the phloem, the sugar concentration data indicate that it is nonetheless dilute. Where does the rest of the fluid come from? It seems reasonable to suggest that when the sieve tubes are cut and opened to atmospheric pressure, they act as osmometers. In other words, the high solute content of the sieve tubes pulls water in from the apoplast and drives the diluted sap onto the cut surface. Theoretically, this osmotically driven process would continue indefinitely in the absence of sieve tube sealing, since sieve tubes will always contain more solute than apoplastic water. However, the sieve tubes do progressively seal. This process is initiated by the formation of slime plugs and callose (Furch et al., 2010). Finally, oxidized P-proteins create an impenetrable barrier over the cut surface, and exudation stops.
Can Cucurbit Exudate Be Used as a Source of Phloem Sap?
Cucurbit exudate, either from cucumber or pumpkin, contains phloem-mobile material. This is clear from experiments in which radiolabeled compounds issue from cut petioles and stems after the leaf blades are exposed to 14CO2. However, the presence of hexose indicates that nonmobile material is also present. Hexoses, and potentially other components of the sap, including ions, small molecules, and macromolecules, could have their origin in companion cells, phloem parenchyma cells, or cell walls. It is also possible that nonmobile, structural components of the sieve tubes are displaced by pressure release and exude onto the surface. It is common practice to blot the surface immediately after a cut is made to remove contaminants from cut cells. Unfortunately, from the kinetics of 14C discharge, it is evident that blotting also removes much of the material that will exude from the sieve tubes.
Once the wound is blotted, fluid may continue to collect over the entire surface, providing an opportunity to collect many microliters of sap. In cucumber, the sap obtained between the 0.5- and 3-min time points contains a small proportion of hexose, but in pumpkin, hexose contamination is more severe, apparently due to a large contribution from entocyclic/ectocyclic phloem. The small proportion of hexose in cucumber exudate, in comparison with stachyose, suggests that it may be relatively pure, mobile sap. However, this conclusion is based on the assumption that stachyose is a reliable marker for sieve tube content, an assumption that may not be warranted, since the exudation patterns of 14C-labeled compounds do not fully match those of unlabeled stachyose. It is likely that at least some of the stachyose comes from other cell types, which is not surprising, since it is a storage, as well as a transport, compound (Sprenger and Keller, 2000). Therefore, a high stachyose-hexose ratio is not necessarily convincing proof that exudate is authentic phloem sap.
Compounds other than hexoses may also be used to assess contamination, although the complex compartmentation of the phloem and surrounding cells makes interpretation difficult. For example, Rubisco is a useful marker, since chloroplasts are present in the cells surrounding the sieve tubes but not the sieve tubes themselves. However, unless the chloroplasts are ruptured, Rubisco will not be released; therefore, its measurement could underestimate contamination from cytosolic compounds.
Contamination from entocyclic/ectocyclic phloem can be avoided by waiting until exudation is limited to small droplets associated with the vascular bundles. In cucumber, the droplets issue from the fascicular phloem. However, even these droplets cannot be assumed to be pure phloem sap; they are dilute, indicating that most of the fluid comes from outside the phloem, and they potentially contain contaminants from the sources listed above.
In short, there does not yet seem to be a foolproof method of assessing the degree of contamination, or determining the origin of specific molecular species, in the fluid that emerges from wounded cucumber or pumpkin tissue. Cucurbit exudate contains ions and molecules in transit in the sieve tubes, but unequivocal identification of these materials in the face of potential contamination from other sources remains a challenge.
MATERIALS AND METHODS
Plant Material and Exudate Collection
Cucumber (Cucumis sativus) and pumpkin (Cucurbita maxima) plants were grown for 3 to 4 weeks in Metromix 360 in a greenhouse (400–1,400 μmol photons m−2 s−1) or in a growth chamber (600–700 μmol photons m−2 s−1), as indicated in “Results.” Exudates were collected in pipette tips or, for smaller volumes, in capillaries, 0.5 to 8 μL in volume. The volume of fluid in partially filled capillaries was determined by measuring the length of the filled volume with a stereomicroscope. The capillaries were immediately placed in a 1.5-mL Eppendorf tube containing 20 μL of 1% (v/v) 2-mercaptoethanol, and the exudate was expelled by centrifugation at 8,000 rpm for 1 min. The 2-mercaptoethonol prevented exudate gelling. In some cases, the radiolabel in exudate was counted by expelling sap in capillaries onto filter paper and placing the paper directly in a scintillation vial.
Radiolabeling
Attached leaf blades of cucumber or pumpkin were sealed in a plastic cuvette and exposed under light from a 1,000-W incandescent metal halide lamp (300 μmol photons m−2 s−1) for 15 min to 14CO2 (1.0 MBq) generated from Na214CO3 (6.6 × 105 MBq mmol−1) by the addition of excess 80% (v/v) lactic acid. The petioles were wrapped in aluminum foil to prevent the inadvertent photosynthetic fixation of 14CO2 by the petiole itself. Radiolabel was counted in Ecoscint scintillation solution (National Diagnostics).
Identification of Sugars
Soluble carbohydrates were extracted by mixing exudate with approximately 6 volumes of methanol, chloroform, and water (12:5:3, v/v/v). The aqueous phase was separated by the addition of water (0.6 volumes). The neutral fraction was obtained by passing the aqueous phase through anion- and cation-exchange resins (Haritatos et al., 2000). The neutral fraction was then freeze dried, resuspended in water, and analyzed by thin-layer chromatography and autoradiography (Haritatos et al., 2000) or by HPLC, as described (McCaskill and Turgeon, 2007; Srivastava et al., 2008).
Silicon Analysis
A potassium silicate solution (200 mL, 56 μL L−1; adjusted to pH 6.5 with NaOH) was applied to the surface of well-watered soil. Exudates were collected from severed petioles at different time points after application, and 2-mercaptoethanol was added to the exudates to a final concentration of 1% (v/v), as described above. Silicon was analyzed with an iCAP 6000 emission spectrometer (Thermo Electron).
Acknowledgments
We thank Andre Jagendorf and Tom Slewinski for discussions and helpful readings of the manuscript and Andre Jagendorf for technical assistance.
References
- Cooil BJ. (1941) Significance of phloem exudate of Cucurbita pepo with reference to translocation of organic materials. Plant Physiol 16: 61–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crafts AS. (1932) Phloem anatomy, exudation, and transport of organic nutrients in cucurbits. Plant Physiol 7: 183–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronshaw J, Esau K. (1968) P protein in the phloem of Cucurbita. II. The P protein of mature sieve elements. J Cell Biol 38: 292–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinant S, Bonnemain JL, Girousse C, Kehr J. (2010) Phloem sap intricacy and interplay with aphid feeding. C R Biol 333: 504–515 [DOI] [PubMed] [Google Scholar]
- Epstein E, Bloom AJ. (2005) Mineral Nutrition of Plants: Principles and Perspectives, Ed 2. Sinauer Associates, Sunderland, MA [Google Scholar]
- Evert RF, Eschrich W, Eichhorn SE. (1973) P-protein distribution in mature sieve elements of Cucurbita maxima. Planta 109: 193–201 [DOI] [PubMed] [Google Scholar]
- Ewers FW, Fisher JB. (1991) Why vines have narrow stems: histological trends in Bauinia (Fabaceae). Oecologia 88: 233–237 [DOI] [PubMed] [Google Scholar]
- Ewers FW, Fisher JB, Fichtner K. (1991) Water flux and xylem structure in vines. In FE Putz, HA Mooney, eds, The Biology of Vines. Cambridge University Press, Cambridge, UK, pp 127–160 [Google Scholar]
- Furch ACU, Zimmermann MR, Will T, Hafke JB, van Bel AJE. (2010) Remote-controlled stop of phloem mass flow by biphasic occlusion in Cucurbita maxima. J Exp Bot 61: 3697–3708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartner BL, Bullock SH, Mooney HA, By Brown V, Whitbeck JL. (1990) Water transport properties of vine and tree stems in a tropical deciduous forest. Am J Bot 77: 742–749 [Google Scholar]
- Gould N, Minchin PEH, Thorpe MR. (2004) Direct measurements of sieve element hydrostatic pressure reveal strong regulation after pathway blockage. Funct Plant Biol 31: 987–993 [DOI] [PubMed] [Google Scholar]
- Haritatos E, Keller F, Turgeon R. (1996) Raffinose oligosaccharide concentrations measured in individual cell and tissue types in Cucumis melo L. leaves: implications for phloem loading. Planta 198: 614–622 [DOI] [PubMed] [Google Scholar]
- Haritatos E, Medville R, Turgeon R. (2000) Minor vein structure and sugar transport in Arabidopsis thaliana. Planta 211: 105–111 [DOI] [PubMed] [Google Scholar]
- Kempers R, Prior DAM, Bel AJE, Oparka KJ. (1993) Plasmodesmata between sieve element and companion cell of extrafascicular stem phloem of Cucurbita maxima permit passage of 3 kDa fluorescent probes. Plant J 4: 567–575 [Google Scholar]
- Lee SH, Singh AP, Chung GC, Ahn SJ, Noh EK, Steudle E. (2004) Exposure of roots of cucumber (Cucumis sativus) to low temperature severely reduces root pressure, hydraulic conductivity and active transport of nutrients. Physiol Plant 120: 413–420 [DOI] [PubMed] [Google Scholar]
- McCaskill A, Turgeon R. (2007) Phloem loading in Verbascum phoeniceum L. depends on the synthesis of raffinose-family oligosaccharides. Proc Natl Acad Sci USA 104: 19619–19624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moose CA. (1938) Chemical and spectroscopic analysis of phloem exudate and parenchyma sap from several species of plants. Plant Physiol 13: 365–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullendore DL, Windt CW, Van As H, Knoblauch M. (2010) Sieve tube geometry in relation to phloem flow. Plant Cell 22: 579–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pristupa NA. (1983) Distribution of ketosugars among cells of conducting bundles of the Cucurbita pepo leaf. Sov Plant Physiol. 30: 372–378 [Google Scholar]
- Richardson PT, Baker DA, Ho LC. (1982) The chemical composition of cucurbit vascular exudates. J Exp Bot 33: 1239–1247 [Google Scholar]
- Shalitin D, Wolf S. (2000) Cucumber mosaic virus infection affects sugar transport in melon plants. Plant Physiol 123: 597–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprenger N, Keller F. (2000) Allocation of raffinose family oligosaccharides to transport and storage pools in Ajuga reptans: the roles of two distinct galactinol synthases. Plant J 21: 249–258 [DOI] [PubMed] [Google Scholar]
- Srivastava AC, Ganesan S, Ismail IO, Ayre BG. (2008) Functional characterization of the Arabidopsis AtSUC2 sucrose/H+ symporter by tissue-specific complementation reveals an essential role in phloem loading but not in long-distance transport. Plant Physiol 148: 200–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeon R. (2010) The puzzle of phloem pressure. Plant Physiol 154: 578–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeon R, Wolf S. (2009) Phloem transport: cellular pathways and molecular trafficking. Annu Rev Plant Biol 60: 207–221 [DOI] [PubMed] [Google Scholar]
- Voitsekhovskaja OV, Koroleva OA, Batashev DR, Knop C, Tomos AD, Gamalei YV, Heldt HW, Lohaus G. (2006) Phloem loading in two Scrophulariaceae species: what can drive symplastic flow via plasmodesmata? Plant Physiol 140: 383–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb JA. (1967) Translocation of sugars in Cucurbita melopepo. IV. Effects of temperature change. Plant Physiol 42: 881–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb JA, Gorham PR. (1964) Translocation of photosynthetically assimilated C14 in straight-necked squash. Plant Physiol 39: 663–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will T, Tjallingii WF, Thönnessen A, van Bel AJE. (2007) Molecular sabotage of plant defense by aphid saliva. Proc Natl Acad Sci USA 104: 10536–10541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Tolstikov V, Turnbull C, Hicks LM, Fiehn O. (2010) Divergent metabolome and proteome suggest functional independence of dual phloem transport systems in cucurbits. Proc Natl Acad Sci USA 107: 13532–13537 [DOI] [PMC free article] [PubMed] [Google Scholar]