Abstract
Snake venoms consist of numerous molecules with diverse biological functions used for capturing prey. Each component of venom has a specific target, and alters the biological function of its target. Once these molecules are identified, characterized, and cloned; they could have medical applications. The activated clotting time (ACT) and clot rate were used for screening procoagulant and anticoagulant properties of 28 snake venoms. Crude venoms from Daboia russellii siamensis, Bothrops asper, Bothrops moojeni, and one Crotalus oreganus helleri from Wrightwood, CA, had procoagulant activity. These venoms induced a significant shortening of the ACT and showed a significant increase in the clot rate when compared to the negative control. Factor X activator activity was also measured in 28 venoms, and D. r. siamensis venom was 5–6 times higher than those of B. asper, B. moojeni, and C. o. helleri from Wrightwood County. Russell's viper venom-factor X activator (RVV-X) was purified from D. r. siamensis venom, and then procoagulant activity was evaluated by the ACT and clot rate. Other venoms, Crotalus atrox and two Naja pallida, had anticoagulant activity. A significant increase in the ACT and a significant decrease in the clot rate were observed after the addition of these venoms; therefore, the venoms were considered to have anticoagulant activity. Venoms from the same species did not always have the same ACT and clot rate profiles, but the profiles were an excellent way to identify procoagulant and anticoagulant activities in snake venoms.
Keywords: Russell's viper venom-factor X activator, Activated clotting time, Clot rate, Procoagulant, Anticoagulant
1. Introduction
There are more than 300 species of venomous snakes in the world, and their venoms have numerous molecules with diverse biomedical functions. Each component in the venom has different target and alters biological functions of the target's cells or molecules in various reactions. Disintegrins target platelets, hemorrhagic proteinases target basement membranes, fibrinogenases target fibrinogen, and procoagulants and anticoagulants target the clotting cascade. Snake venoms are excellent sources of molecules for drug discovery, and once purified, characterized, and cloned, could have potential applications in medicine.
Snake procoagulant molecules, especially from the Viperidae family, have been used in medical applications and as diagnostic tools. A procoagulant protein, Batroxobin from Bothrops atrox is useful for fibrinogen level assays (Reptilase™ time) and fibrinogen degradation products (FDPs) detection (Aronson, 1976; Stocker and Barlow, 1976; Johnson et al., 1977; Hutton and Warrell, 1993; Bell, 1997; Van Cott et al., 2002). Another known procoagulant from Russell's viper venom (RVV), RVV-factor X activator (RVV-X) is useful for measuring a lupus anticoagulant (Thiagarajan et al., 1986; Lo et al., 1989; Derksen and de Groot, 2004).
Procoagulant and anticoagulant properties are widely studied from the Viperidae family. Only a few anticoagulants have been isolated from snake venoms of the Elapidae family (Kini and Evans, 1991; Sundell et al., 2003; White, 2005; Gowda et al., 2006; Kumar et al., 2010).
In this report, we studied procoagulant and anticoagulants of 28 different snake venoms from nine species in two different families, Viperidae and Elapidae, using the Sonoclot assay and then compared them to purified RVV-X from crude Daboia russellii siamensis. Their activated clotting time (ACT) and clot rate signatures were determined.
2. Materials and methods
2.1. Venom
The National Natural Toxins Research Center (NNTRC) at Texas A&M University-Kingsville, Kingsville, TX, provided crude venoms from one Bothrops asper, one Bothrops moojeni, four Crotalus adamanteus, three Crotalus atrox, five Crotalus horridus, five Crotalus oreganus helleri, two Naja melanoleuca, and six Naja pallida. D. r. siamensis venom was obtained from the Queen Saovabha Memorial Institute (QSMI, Thai Red Cross Society, Bangkok) and was pooled venom from an underdetermined number of snakes. All snake venoms provided by the NNTRC were identified by their avid number and were never pooled. The avid number refers to individual snake venom. The geographical locations and avid numbers of snake venoms are listed in Table 2. The avid numbers are listed so that the same venoms could be used in future studies. Additional information about the snakes can be found on the NNTRC homepage (http://ntrc.tamuk.edu) by querying the snake venom by its avid number.
Table 2.
The ACT and clot rate of 28 snake venoms and purified RVV-X using the Sonoclot analyzer. Data was downloaded from Signature Viewer; software provided by Sienco, Inc. on an iMAC computer and analyzed by Microsoft Excel 2003.
| Venom (Avid #) | Geographical location | Procoagulant activity | Anticoagulant activity | Total protein (ng) | ACT (min)a | Clot rate (U)b | ||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD (n = 6) | P value | Mean ± SD (n = 6) | P value | |||||
| VIPERIDAE | ||||||||
| Bothrops asper | San Antonio Zoo, Texas | + | − | 10 | 2.18 ± 0.26 | 6.31 × 10−6 | 5.73 ± 0.82 | 2.80 × 10−4 |
| B. moojeni | Brownsville Zoo, Texas | + | − | 10 | 4.47 ± 0.27 | 4.67 × 10−5 | 6.41 ± 0.94 | 8.08 × 10−5 |
| Crotalus adamanteus | ||||||||
| 010-310-261 | Florida | N/A | N/A | 10 | 9.85 ± 1.83 | 0.27 | 3.09 ± 1.04 | 0.86 |
| 011-307-892 | Florida | N/A | N/A | 10 | 9.20 ± 1.91 | 0.24 | 3.25 ± 0.89 | 0.62 |
| 011-544-127 | Florida | N/A | N/A | 10 | 8.10 ± 1.32 | 0.68 | 3.70 ± 0.63 | 0.15 |
| 011-544-607 | Lady Co., Florida | N/A | N/A | 10 | 8.27 ± 0.90 | 0.70 | 3.32 ± 1.26 | 0.62 |
| C. atrox | ||||||||
| 001-098-257 | LaSalle & Dimmit Co., Texas | − | + | 10 | 13.92 ± 2.33 | 2.05 × 10−3 | 0.23 ± 0.23 | 5.32 × 10−4 |
| 010-359-784 | Kleberg, Texas | − | + | 10 | 12.98 ± 2.47 | 0.01 | 0.45 ± 0.21 | 8.53 × 10−4 |
| 010-525-522 | Reagan Co., Texas | − | + | 10 | 15.05 ± 2.95 | 1.67 × 10−3 | 0.47 ± 0.28 | 7.51 × 10−4 |
| C. horridus | ||||||||
| 010-327-277 | Garvin Co., Oklahoma | N/A | N/A | 10 | 8.46 ± 2.00 | 0.25 | 3.09 ± 0.77 | 0.83 |
| 010-519-540 | Payne Co., Oklahoma | N/A | N/A | 10 | 9.35 ± 1.33 | 0.75 | 2.17 ±0.19 | 0.08 |
| 010-595-578 | Payne Co., Oklahoma | N/A | N/A | 10 | 10.62 ± 1.65 | 0.23 | 2.53 ± 0.57 | 0.33 |
| 010-782-102 | Payne Co., Oklahoma | N/A | N/A | 10 | 9.77 ± 1.15 | 0.78 | 2.61 ± 0.65 | 0.43 |
| 010-825-782 | Jackson Co., Texas | N/A | N/A | 10 | 10.72 ± 0.79 | 0.06 | 2.75 ± 0.68 | 0.62 |
| C. o. helleri | ||||||||
| 010-328-029 | California | N/A | N/A | 10 | 9.22 ± 1.15 | 0.58 | 3.38 ± 0.60 | 0.40 |
| 010-367-284 | California | N/A | N/A | 10 | 8.88 ± 0.99 | 0.27 | 3.42 ± 0.83 | 0.42 |
| 011-084-009 | California | N/A | N/A | 10 | 9.70 ± 1.35 | 0.87 | 3.61 ± 0.83 | 0.25 |
| 011-085-061 | California | N/A | N/A | 10 | 9.08 ± 1.24 | 0.47 | 3.31 ±0.85 | 0.54 |
| 058-359-257 | Wrightwood, California | + | − | 10 | 3.73 ± 0.67 | 5.19 × 10−7 | 8.56 ± 1.13 | 2.91 × 10−6 |
| D. r. siamensis | QSMI, Thailand | + | − | 10 | 2.67 ± 0.23 | 1.11 × 10−5 | 8.85 ± 1.26 | 3.38 × 10−6 |
| ELAPIDAE | ||||||||
| Naja melanoleuca | ||||||||
| 455A475765 | Captive Born (Houston Zoo), Texas | N/A | N/A | 10 | 11.13 ± 1.52 | 0.07 | 2.90 ± 0.48 | 0.85 |
| 455A1E2864 | Captive Born (Houston Zoo), Texas | N/A | N/A | 10 | 10.07 ± 2.04 | 0.62 | 3.10 ± 0.80 | 0.83 |
| N. pallida | ||||||||
| 010-855-773 | San Antonio Zoo, Texas | N/A | N/A | 10 | 10.67 ± 1.09 | 0.11 | 1.26 ± 1.24 | 0.02 |
| 010-888-116 | San Antonio Zoo, Texas | − | + | 10 | 12.52 ± 2.60 | 0.03 | 1.01 ± 0.87 | 3.42 × 10−3 |
| 011-066-526 | San Antonio Zoo, Texas | N/A | N/A | 10 | 10.78 ± 1.26 | 0.11 | 1.32 ± 0.75 | 0.01 |
| 011-262-868 | San Antonio Zoo, Texas | N/A | N/A | 10 | 10.65 ± 0.77 | 0.08 | 2.44 ± 1.09 | 0.37 |
| 011-520-802 | San Antonio Zoo, Texas | − | + | 10 | 11.33 ± 1.16 | 0.02 | 1.79 ± 0.75 | 0.03 |
| 011-550-034 | San Antonio Zoo, Texas | N/A | N/A | 10 | 10.52 ± 1.85 | 0.31 | 1.03 ± 0.43 | 8.48 × 10−4 |
| Purified RVV-X | − | + | − | 1 | 2.57 ± 0.19 | 1.22 × 10−5 | 10.51 ± 1.69 | 2.40 × 10−6 |
| Negative control | − | N/A | N/A | − | 9.58 ±1.08 | − | 2.99 ± 0.92 | − |
| Positive control | − | + | − | − | 4.08 ± 0.63 | 4.62 × 10−6 | 8.81 ± 2.06 | 8.67 × 10−5 |
| Plasma control | − | −c | −c | − | −c | − | −c | − |
Bold letter indicates the significant shortening of the ACT by snake venoms when compared with the negative control at P < 0.05.
Italic letter indicates the significant prolonging of the ACT by snake venoms compared with the negative control at P < 0.05.
Grey-shaded letter indicates the significant increase in the clot rate by snake venoms compared with the negative control at P < 0.05.
Underlined letter indicates the significant reduction of the clot rate by snake venoms compared with the negative control at P < 0.05.
A “+” represents detectable activity.
A “−” indicates the absence of detectable activity.
N/A indicates no significant activity.
The time that plasma remains a liquid is reported as the ACT in minute.
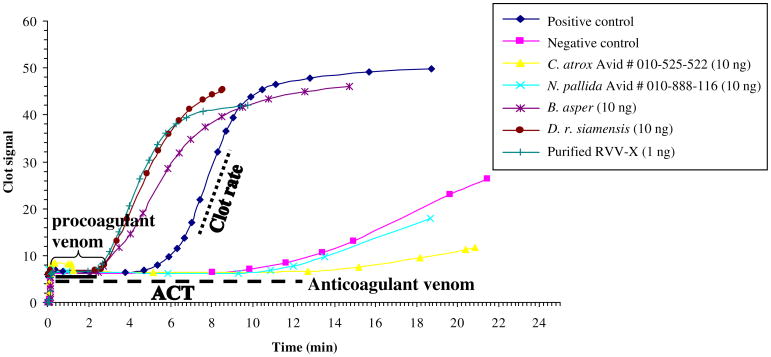
The clot rate is defined as the rate of fibrin polymerization, which is the slope in the linear part of the curves in Fig. 2 and is defined as the change clot signal with change in time (U = Δ signal/Δ time).
Pooled normal citrated plasma did not form clot.
2.2. Purification of RVV-X
RVV-X was purified from crude D. r. siamensis venom by a modification of the procedure of Kisiel et al. (1976). Briefly, samples of 125 mg of lyophilized crude D. r. siamensis venom were mixed with 5.0 mL of 0.1 M sodium phosphate buffer, pH 7.5 containing 1 mM benzamidine– HCl. Two hundred microliters of clear supernatant, at a concentration of 25 mg/mL, were applied into a Waters PROTEIN-PAK™ 300SW (7.5 × 300 mm) HPLC column. The column was previously equilibrated with the elution buffer (0.1 M sodium phosphate buffer, pH 7.5 containing 1 mM benzamidine). The collection process required 60 min, with a flow rate of 0.5 mL/min. A Waters 2487 Dual λ Absorbance Detector was used to monitor absorbencies at 280 nm. Each fraction was screened for factor X activator activity using chromogenic substrate S-2765. The molecular weight and protein patterns of each fraction were determined by SDS-PAGE. Fraction 3 samples had the highest molecular weight proteins with factor X activator activity (Fig. 1a) and had isoelectric point (pI) in the pH range 4–6 as determined by the Electrophoretic titration (ET) (Fig. 1b). The pooled fraction 3 peaks were dialyzed in 0.02 M Tris–HCl buffer, pH 8.0 and further purified by a DEAE anion exchange HPLC chromatography.
Fig. 1.
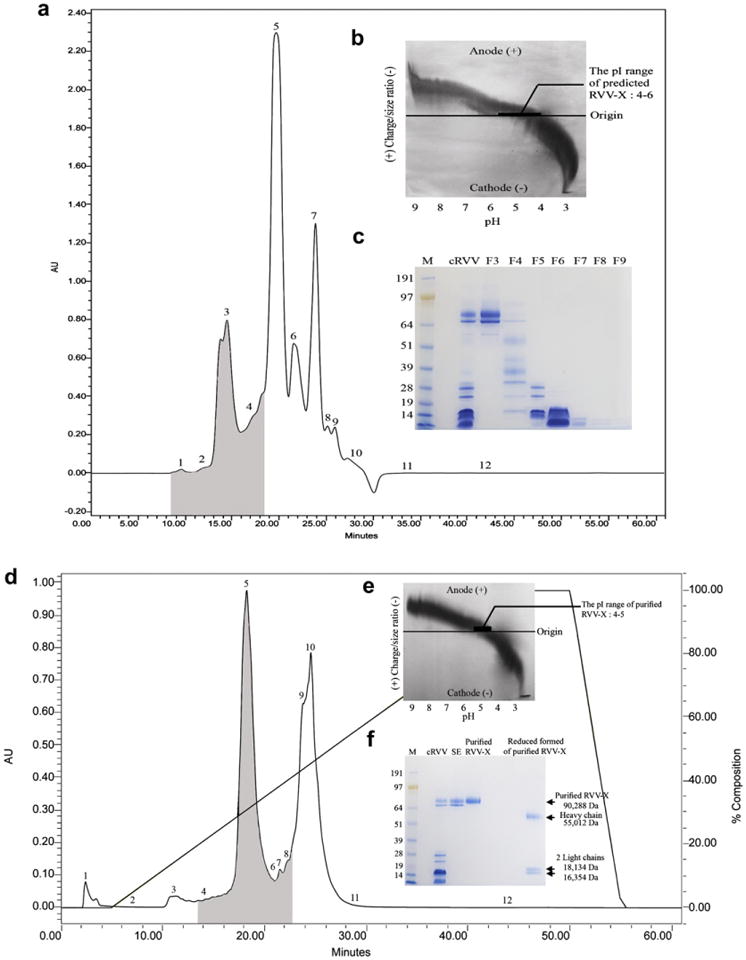
(a) Size exclusion (SE) chromatographic profile of lyophilized crude D. r. siamensis venom (cRVV). The grey-shaded areas indicate the location of factor X activator activity using chromogenic substrate S-2765. (b) The ET profile of the pooled fraction 3 with factor X activator activity from the 300SW SE HPLC column is shown in the upper right corner. The ET was performed by using a Pharmacia Biotech PhastSystem. A polyacrylamide isoelectric focusing (IEF) PhastGel with pH gradient 3–9 was generated in the first dimension. The gel was then rotated 90°, and a volume of 3 μL (2 mg/mL) of pooled fraction 3 was applied to the pH gradient gel. The gel was stained with silver nitrate. (c) SDS-PAGE analysis of venom fractions from SE HPLC column. Crude and fractionated cRVV were run on 4–12% Bis–Tris Gel under non-reducing conditions at 200 V for 50 min. The gel was stained with RapidStain. Lane 1: SeeBlue Plus2 Markers (Invitrogen™); lane 2: cRVV (20 μg); lane 3: fraction 3 (F3; 7 μg); lane 4: fraction 4 (F4; 7 μg); lane 5: fraction 5 (F5; 7 μg); lane 6: fraction 6 (F6; 7 μg); lane 7: fraction 7 (F7; 7 μg); lane 8: fraction 8 (F8; 7 μg); lane 9: fraction 9 (F9; 7 μg). (d) DEAE anion exchange HPLC profile from fraction 3 from the 300SW SE HPLC column. The grey-shaded areas indicate the location of factor X activator activity using chromogenic substrate S-2765. (e)The ET profile of purified RVV-X. A volume of 3 μL(0.5 mg/mL) of purified RVV-X (fraction 5) was applied to the pH gradient gel. The gel was stained with silver nitrate. (f) SDS-PAGE analysis of purified RVV-X from purification step. Samples from each purification step were run on 4–12% Bis–Tris Gel under non-reducing and reducing conditions at 200 V for 50 min. Gel was stained with RapidStain. Lane 1: SeeBlue Plus2 Markers (Invitrogen™); lane 2: cRVV (20 μg); lane 3: fraction 3 from SE (6 μg); lane 4: fraction 5 from DEAE anion exchange (purified RVV-X; 6 μg); lane 5: reduced form of purified RVV-X (7 μg).
The optimal conditions for the second step of purification were determined by the ET curve using a Pharmacia Biotech PhastSystem (Perez et al., 2001). Two hundred microliters of the pooled venom fraction 3 at the concentration of 31.93 mg/mL were applied into a Waters PROTEIN-PAK™ 5PW (7.5 × 75 mm) HPLC column, which was previously equilibrated with 0.02 M Tris–HCl buffer, pH 8.0. The fractions were eluted using 0.02 M Tris–HCl buffer, pH 8.0 with a 0–0.5 M NaCl salt gradient. The collection required 60 min with a flow rate of 1 mL/min. A Waters 2487 Dual λ absorbance detector was used to monitor absorbencies at 280 nm. Breeze software was used to control the pumps and store data. Fractions of various volumes were collected and retention times of the fractions were recorded. Each fraction was screened for factor X activator activity using chromogenic substrate S-2765. Two anion exchange runs were made and fraction 5 samples with factor X activator activity were pooled. The molecular weight and purity of purified RVV-X were determined by SDS-PAGE and verified by mass spectrometry, which was carried out by the laboratory for Biological Mass Spectrometry, Texas A&M University, College Station, Texas. The ET curve was used to predict pI of purified RVV-X.
2.3. Human normal citrated plasma
Pooled normal citrated plasma was prepared by taking samples from five healthy human donors. From each donor, thirty-six milliliters of blood was drawn with a butterfly needle attached to 19 gauge 3/4″ long. Blood was collected by gravity into a 50 mL plastic test tube containing 4 mL of 3.2% sodium citrate. After the blood was drawn, the tube was inverted gently twice to ensure full citration of blood. The citrated blood was centrifuged at 2000 g for 20 min at 25°C. The plasma was standardized by pooling the plasma from different individuals and 0.7 mL was aliquoted into 1.5 mL plastic tubes, and frozen immediately at −80°C.
2.4. Procoagulant and anticoagulant activities
The procoagulant and anticoagulant activities of B. asper, B. moojeni, C. adamanteus, C. atrox, C. horridus, C. o. helleri, D. r. siamensis, N. melanoleuca, N. pallida, and purified RVV-X from D. r. siamensis were measured using the Sonoclot analyzer, which measures the activated clotting time (ACT) and clot rate by measuring viscosity changes of a whole blood or plasma sample (von Kaulla et al., 1975). The time that plasma remains a liquid is reported as the ACT. The clot rate is defined as the rate of fibrin polymerization, which is the slope in the linear part of the curves in Fig. 2 and is defined as the change clot signal with change in time (U = Δsignal/Δtime). A cuvette was placed into the cuvette holder, which maintains the temperature at 37°C A pre-warmed 10 μL sample (37°C) of a 0.3 M CaCl2 was added to one side of the cuvette. A 10 μL sample of each venom at a concentration of 1 μg/mL or a 10 μL of purified RVV-X at a concentration of 0.1 μg/mL was added to the other side of the cuvette. A constant volume of 330 μL of citrated plasma (50 mg/mL) was added to the cuvette. The ACT and clot rate were measured and the data was downloaded from Signature Viewer, software provided by Sienco, Inc. on an iMAC computer and analyzed by Microsoft Excel 2003. The negative control was pooled normal citrated human plasma that was reconstituted with only CaCl2. The positive control was pooled normal citrated plasma that was activated with glass beads contained in the cuvette and 0.3 M CaCl2. The plasma control contained no CaCl2.
Fig. 2.
Data from Sonoclot signature further analyzed by Microsoft Excel 2003 obtained when pooled human citrated plasmas were activated by four representative snake venoms and purified RVV-X. Other venom samples were tested with the Sonoclot analyzer (data not shown). The time that plasma remains a liquid is reported as the ACT in minute. The clot rate is defined as the rate of fibrin polymerization, which is the slope in the linear part of the curves and is defined as the change clot signal with change in time (U = Δsignal/Δtime). The solid line indicates the shorten ACT activated with the procoagulant venoms. Dashed line indicates the prolonged ACT induced by the anticoagulant venoms.
2.5. Factor X activator activity
Factor X activator activities in venoms from B. asper, B. moojeni, C. adamanteus, C. atrox, C. horridus, C. o. helleri, D. r. siamensis, N. melanoleuca, N. pallida, and purified RVV-X from D. r. siamensis were tested using a factor Xa-specific chromogenic substrate S-2765. Twenty-five microliters of pooled normal human citrated plasma (50 mg/mL) was dissolved in Tris–EDTA buffer was incubated at 37°C for 3 min. Then, 25 μL of chromogenic substrate S-2765 was added and mixed within 30 s. Twenty-five microliters of each crude venom, or purified RVV-X from crude D. r. siamensis venom at a concentration of 10 μg/mL was premixed with 0.1 M CaCl2 in equal quantities and incubated at 37°C for 3 min. The reaction was stopped by 20% acetic acid. The yellow color was monitored at 405 nm. The negative control was prepared by mixing the plasma, chromogenic substrate, 20% of acetic acid, and water instead of the venom. The plasma control was prepared by mixing the chromogenic substrate, crude venom, 20% of acetic acid, and water instead of the plasma. Each test was performed in duplicate.
2.6. Statistical analysis
The results were expressed as the mean ± standard deviation (SD). Their significance was analyzed by the student's t-test. The level of significance was at P < 0.05. P value was compared with the negative control.
3. Results
3.1. Purification of RVV-X
RVV-X was purified from crude D. r. siamensis venom by a two-step procedure with approximately 2% yield, and had a fourfold increase in specific activity (Table 1). The molecular weight of purified RVV-X was verified by Mass spectrometry (90,288 Da). The heavy chain and two light chains had a molecular weight of 55,012, 18,134, and 16,354 Da, respectively (Fig. 1f). The molecular weight of purified RVV-X is similar to the RVV-X that was reported by Gowda et al. (1994). In addition, we demonstrated that the pI of purified RVV-X was 4–5 and revealed that RVV-X migrated differently at all pHs, which means that RVV-X factor has many different carboxyl and amine side chains (Fig. 1e). The electrophoretic titration band was very broad, which could mean that there were many different proteins with slightly different pI. RVV-X is known to be a glycoprotein with varying sialic acid molecules, which causes variation in the pI (Amphlett et al., 1982; Gowda et al., 1994). The appearance of a very broad band on the ET gel is probably the result of glycosylation. Purified RVV-X was used as the positive control and used to compare other venoms.
Table 1.
Purification of RVV-X from crude D. r. siamensis venom.
| Purification step | Volume (mL) | Protein concentration (mg/mL)a | Total protein (mg)b | Specific activity (nkat/ng)c | Recovery of protein (%)d | Purification factor (fold)e |
|---|---|---|---|---|---|---|
| Crude D. r. siamensis venom | 5 | 25.00 | 125.00 | 0.31 | 100 | 1.00 |
| 300SW size exclusion | 36.83 | 0.234 | 8.63 | 0.71 | 6.90 | 2.29 |
| 5PW DEAE | 6.04 | 0.407 | 2.46 | 1.24 | 1.97 | 4.00 |
Protein concentration was determined by spectrophotometer at 280 nm.
The total protein (mg) was calculated by multiplying (total volume; mL) × (venom protein concentration; mg/mL).
One katal (kat) was the amount of enzyme that converts one mole of chromogenic substrate S-2765 per second. One nanokatal (nkat) was 1 × 10−9 mole of product released per second. Specific RVV-X activity was calculated by 1 nkat by total protein (ng) in each sample. One nkat was calculated by the following equation: [OD 405 nm/(ε × T × 10−9)], where the enzyme activity is measured at 405 nm, ε is the extinction coefficient of chromogenic substrate S-2765 (1.27 × 104 mol−1 Lcm−1 at Emax = 405 nm), T is reaction time in second.
Recovery of protein was defined as the total protein recovered of at each step of purification.
Purification factor was the number of times that specific factor X activator activity increased over D. r. siamensis venom.
3.2. Procoagulant and anticoagulant activities
Venom from viperid and elapid snakes from various geographical locations were examined for procoagulant or anticoagulant activities using the ACT and clot rate (Table 2). Purified RVV-X and crude venoms of B. asper, B. moojeni, C. o. helleri (Wrightwood), and D. r. siamensis had the shortest ACT (2.18–4.47 min) and highest clot rates (5.73– 8.85 U), suggesting the presence of predominant procoagulants in these venoms (Table 2). Prolonged ACT (11.33–15.05 min) and a low clot rate (0.23–2.44 U) was found in crude venom of C. atrox (Reagan) and N. pallida snakes suggesting predominant anticoagulants are present in these venoms.
The Sonoclot signatures of purified RVV-X and four representative snake venoms with procoagulant and anticoagulant properties are shown in Fig. 2. Two of the venoms have the signature of procoagulant venoms (D. r. siamensis and B. asper) with a shortened ACTs and increased clot rates. The other two venoms (C. atrox and N. pallida) have a signature of anticoagulant venoms with extended ACTs and slower clot rates. The signatures of all 28 venoms were determined, but not shown; however, all the ACT and clot rates are found in Table 2.
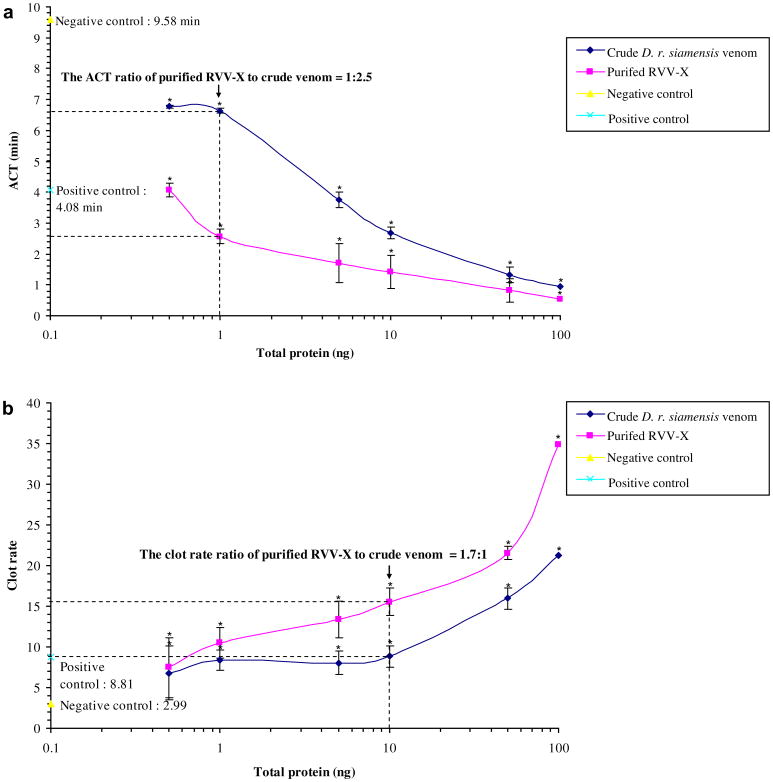
RVV-X was purified from crude D. r. siamensis and the procoagulant activity was evaluated by the ACT and clot rate. The procoagulant activity of purified RVV-X was compared to crude venom. Crude D. r. siamensis and purified RVV-X significantly shortened the ACT (Fig. 3a) and enhanced the clot rate in a dose-dependent manner (Fig. 3b). The ACT with 1 ng of purified RVV-X was approximately 2.5 times shorter than the ACT with crude D. r. siamensis venom. The clot rate at 10 ng was 1.7 times higher with purified RVV-X than crude D. r. siamensis venom.
Fig. 3.
Effect of crude D. r. siamensis venom and purified RVV-X at various concentrations on (a) ACT and (b) clot rate of pooled normal citrated plasma using the Sonoclot analyzer. A 10 μL of a 0.3 M CaCl2 was added to one side of the cuvette. A 10 μL of D. r. siamensis venom and purified RVV-X at various concentrations was added to the other side of the cuvette. The pooled normal citrated plasma concentration was 50 mg/mL and 330 μL of pre-warmed (37°C) pooled normal citrated plasma was added to the cuvette. Data represent the mean ± SD (n = 6). An asterisk (*) indicates statistic significance compared with the negative control at P < 0.05. Their significance was analyzed by the student's t-test.
3.3. Factor X activator activity
The factor X activator activity of snake venoms was examined by assessing the hydrolysis of chromogenic substrate S-2765. Significant factor X activator activity was observed after activating the substrate with D. r. siamensis, B. asper, B. moojeni, C. o. helleri from Wrightwood County, and purified RVV-X as shown in Table 3. The specific factor X activator activity of crude D. r. siamensis venom was 5–6 times higher than crude B. asper, B. moojeni, and C. o. helleri from Wrightwood County. However, purified RVV-X from D. r. siamensis had a 4-fold higher specific factor X activator activity than crude venom (Table 3). The other venoms, negative control, and plasma control had no factor X activator activity (data not shown).
Table 3.
Factor X activator activity of various snake venoms using factor Xa-specific chromogenic substrate S-2765.
| Venom (Avid #) | Geographical location | Total protein (ng) | Specific activity (nkat/ng)a | P valueb |
|---|---|---|---|---|
| B. asper | San Antonio Zoo, TX | 125 | 0.051 | 1.51 × 10−2 |
| B. moojeni | Brownsville Zoo, TX | 125 | 0.068 | 5.84 × 10−3 |
| C. o. helleri 058-359-257 | Wrightwood, CA | 125 | 0.059 | 8.54 × 10−3 |
| D. r. siamensis | QSMI, Thailand | 125 | 0.310 | 3.23 × 10−5 |
| Purified RVV-X | 125 | 1.240 | 2.41 × 10−5 |
One katal (kat) was the amount of enzyme that converts one mole of chromogenic substrate S-2765 per second. One nanokatal (nkat) was 1 × 10−9 mole of product released per second. Specific activity was calculated by 1 nkat by total protein (ng) in each sample. One nkat was calculated by the following equation: [OD 405 nm/(ε × T × 10−9)], where the enzyme activity is measured at 405 nm, ε is the extinction coefficient of chromogenic substrate S-2765 (1.27 × 104mol−1Lcm−1 at Emax = 405 nm), T is reaction time in second.
Their significance was analyzed by the student's t-test. The level of significance was at P < 0.05. P value compared with the negative control.
4. Discussion
The measurement of plasma ACT and clot rate after activation with snake venoms and purified RVV-X from crude D. r.siamensis venom was a fast and reproducible way to screen and classify venoms as procoagulant or anticoagulant (Table 2). Snake venoms from the same species generally had the similar signatures as determined by the ACT and clot rate, but in some cases the signatures were different. Snake venoms are complex mixtures of many molecules, and since venoms of the same species can be different, this is a good reason to only pool venoms of the same species if they have the same activities. If venoms are pooled, you may be diluting the protein of interest and make the purification process more difficult.
Purified RVV-X and venoms in Table 2 defined as procoagulants had a shortened ACTs and extended clot rates (Table 2 and Fig. 2). Crude D. r. siamensis venom had the second highest ACT and the highest clot rate of all crude venoms tested (Table 2). With ten times less purified RVV-X, the ACT was slightly shorter (2.57 min) and clot rate was higher (10.51 U) than crude D. r. siamensis venom. The procoagulant activity of crude D. r. siamensis venom was the result of factor X activator in crude D. r. siamensis venom. RVV-X converts FX to the active form of factor X (FXa), with its co-factor in plasma forming the prothrombinase complex. Then, the prothrombinase complex activates prothrombin to thrombin, which shortened the time of coagulation and increased the clot rate (Furie and Furie, 1976; Kisiel et al., 1976; Jackson and Nemerson, 1980).
Factor X activators are common in viperid and crotalid snake venoms, and this study, as well as others, suggest that snake venoms with procoagulant activity could have factor X activators (Franssen et al., 1983; Hemker et al., 1984; el-Asmar et al., 1986; Hofmann and Bon, 1987; Komori et al., 1990; Maruyama et al., 1992; Farid et al., 1993; Samel and Siigur, 1995; Siigur et al., 2001; Tans and Rosing, 2001). The chromogenic substrate S-2765 assay was used to measure factor X activator activity in purified RVV-X and 28 other snake venoms. D. r. siamensis venom had the highest factor X activity, and the activity increased by a factor of 4 when purified (Table 1). Factor X activator was also found in venoms from B. asper, B. moojeni, and C. o. helleri from Wrightwood County and also had a shortened ACT, an increased clot rate, and caused hydrolysis of a chromogenic substrate S-2765. Purification of factor X activators from of B. asper, B. moojeni, and C. o. helleri venoms should be determined to further substantiate the presence of factor X activator in these venoms.
Previous studies have indicated that D. r. siamensis contains RVV-X and Russell's viper venom-factor V activator (RVV-V), both of which accelerate the clotting of plasma (Hanahan et al., 1972; Kisiel et al., 1976; Takeya et al., 1992; Gowda et al., 1994). Factor X activator-like proteins were also found in B. asper (Nahas et al., 1979) and B. moojeni (Furtado et al., 1991). Not all venoms from the same species had the same procoagulant or anticoagulant activities. In this study, only C. o. helleri venom from Wrightwood County had procoagulant activity, and this is the first report of the factor X activator activity in C. o. helleri venom. The other four C. o. helleri venoms had neither procoagulant nor anticoagulant activities, which may to be due to low concentrations of these venoms. However, the variations of clotting parameters of the C. o. helleri venoms were supported by factor X activator activity using chromogenic substrate S-2765 (Table 3). It would be a mistake to pool the five C. o. helleri venom from California snakes if the goal was to isolate a procoagulant, since four of the venoms had no procoagulant activity. Initial screening is necessary before pooling venoms for purification of biological important molecules. Similar results were reported by Salazar et al. (2009), which demonstrated the hemostatic variations in these snake venoms. The same species from different geographical locations possess procoagulant activity in contrast to the other venoms in nearby counties having fibrinolytic activity with low coagulant activity.
Snake venoms, particularly from the families Crotalidae and Viperidae, are complex mixtures of numerous molecules that can possess both procoagulant and anticoagulant properties (Kornalík, 1985; Markland, 1998; Braud et al., 2000; Matsui et al., 2000; White, 2005). Dambisya et al. (1994) have reported that the different concentrations of Calloselasma rhodostoma venom observed the dual effect. In this study, based on the ACT and clot rate data, both crude D. r. siamensis venom and purified RVV-X (0.5-100 ng) only had procoagulant activity (Fig. 3). However, the dual effect of other venoms should be further investigated and may be a useful tool for diagnosis and predicting coagulopathy in snakebites.
This study provided no insights to the mechanism of an extended ACT and slower clot rate since the anticoagulant molecules were not purified or characterized. Crude venom samples of C. atrox venom and two different crude venom samples of N. pallida had anticoagulant activities. Generally, a significantly prolonged ACT was consistent with a significantly reduced clot rate, which was found in human plasma induced with 10 ng of C. atrox and two different crude venom samples of N. pallida. Three other different crude venom samples of N. pallida significantly decreased the clot rate without a significant increase in the ACT. This may be due to low concentrations of these molecules in these snake venoms. However, non-clotting plasma was observed when with activated pooled normal citrated plasma with 100 μg of N. melanoleuca and N. pallida venoms was used (data not shown). This is the first study to report that N. melanoleuca and N. pallida venoms were able to prevent the coagulation process. Further studies are needed to determine the anticoagulant molecules in these venoms. In addition, the anticoagulant effects of C. atrox venoms were consistent with the findings of other studies, which reported that C. atrox venom contains many fibrinolytic components such as plasminogen activator, atroxase-α, β-chain fibrinogenase (Bajwa et al., 1980, 1981a,b; Willis and Tu, 1988; Markland, 1998). These toxins act on fibrin or fibrinogen, or act as a plasminogen activator, causing blood clot lyses.
The ACT and clot rate profiles were used to rapidly screen venoms and identify procoagulant or anticoagulant activities in 28 snake venoms. This preliminary data could be the foundation for future research. The procoagulant molecules could be used to develop clot-promoting drugs for external uses in patients with hemophilia or other bleeding conditions. Anticoagulant molecules in these venoms should be further investigated for drug discovery in stroke patients or patients with thrombotic disorders.
Acknowledgments
This work was funded by the Royal Golden Jubilee (RGJ) Ph.D. scholarship, the National Research Council of Thailand and the NNTRC, Texas A&M University-Kingsville: NIH/Viper Resource Center #5 P40 RR018300-07. We thank Bill Russell and David Russell for determining the molecular weight by mass spectrometry. We would like to thank Tracey Alvarado and Danielle Calhoun, students in the Summer Research Program at Texas A&M University-Kingsville, USA, for their assistance. We are grateful to Nora Diaz De Leon, the NNTRC administrative officer for technical assistance, and we gratefully acknowledge all of the NNTRC staff.
Ethical statement: This research was approved by Texas A&M University-Kingsville Human Subjects Committee.
Abbreviations
- ACT
activated clotting time
- ET
electrophoretic titration
- pI
isoelectric point
- RVV
Russell's viper venom
- RVV-X
Russell's viper venom-factor X activator
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
Footnotes
Conflict of interest: The authors declare that there are no conflicts of interest.
References
- Amphlett GW, Byrne R, Castellino FJ. Cation binding properties of the multiple subforms of RVV-X, the coagulant protein from Vipera russelli. Biochemistry. 1982;21:125–132. doi: 10.1021/bi00530a022. [DOI] [PubMed] [Google Scholar]
- Aronson DL. Comparison of the actions of thrombin and the thrombin-like venom enzymes ancrod and batroxobin. Thromb Haemost. 1976;36:9–13. [PubMed] [Google Scholar]
- Bajwa SS, Markland FS, Russell FE. Fibrinolytic enzyme(s) in western diamondback rattlesnake (Crotalus atrox) venom. Toxicon. 1980;18:285–290. doi: 10.1016/0041-0101(80)90007-0. [DOI] [PubMed] [Google Scholar]
- Bajwa SS, Markland FS, Russell FE. Fibrinolytic and fibrinogen clotting enzymes present in the venoms of western diamondback rattlesnake, Crotalus atrox, eastern diamondback rattlesnake, Crotalus adamanteus, and southern Pacific rattlesnake, Crotalus viridis helleri. Toxicon. 1981a;19:53–59. doi: 10.1016/0041-0101(81)90117-3. [DOI] [PubMed] [Google Scholar]
- Bajwa SS, Patkos GB, Markland FS. Clinical potential of fibrinolytic enzyme(s) of western diamondback rattlesnake (Crotalus atrox) venom. Proc West Pharmacol Soc. 1981b;24:165–168. [PubMed] [Google Scholar]
- Bell WR., Jr Defibrinogenating enzymes. Drugs. 1997;54(Suppl. 3):18–30. doi: 10.2165/00003495-199700543-00005. [DOI] [PubMed] [Google Scholar]
- Braud S, Bon C, Wisner A. Snake venom proteins acting on hemostasis. Biochimie. 2000;82:851–859. doi: 10.1016/s0300-9084(00)01178-0. [DOI] [PubMed] [Google Scholar]
- Dambisya YM, Lee TL, Gopalakrishnakone P. Action of Calloselasma rhodostoma (Malayan pit viper) venom on human blood coagulation and fibrinolysis using computerized thromboelastography (CTEG) Toxicon. 1994;32:1619–1626. doi: 10.1016/0041-0101(94)90320-4. [DOI] [PubMed] [Google Scholar]
- Derksen RH, de Groot PG. Tests for lupus anticoagulant revisited. Thromb Res. 2004;114:521–526. doi: 10.1016/j.thromres.2004.06.009. [DOI] [PubMed] [Google Scholar]
- el-Asmar MF, Shaban E, Hagag M, Swelam N, Tu A. Coagulant component in Cerastes cerastes (Egyptian sand viper) venom. Toxicon. 1986;24:1037–1044. doi: 10.1016/0041-0101(86)90130-3. [DOI] [PubMed] [Google Scholar]
- Farid T, Nasser H, Zaki K, el-Asmar MF. Low molecular weight factor X activator from Cerastes vipera (Sahara sand viper) venom. Toxicon. 1993;31:1007–1017. doi: 10.1016/0041-0101(93)90260-p. [DOI] [PubMed] [Google Scholar]
- Franssen JH, Janssen-Claessen T, Van Dieijen G. Purification and properties of an activating enzyme of blood clotting factor X from the venom of Cerastes cerastes. Biochim Biophys Acta. 1983;747:186–190. doi: 10.1016/0167-4838(83)90139-5. [DOI] [PubMed] [Google Scholar]
- Furie BC, Furie B. Coagulant protein of Russell's viper venom. Methods Enzymol. 1976;45:191–205. doi: 10.1016/s0076-6879(76)45019-x. [DOI] [PubMed] [Google Scholar]
- Furtado MF, Maruyama M, Kamiguti AS, Antonio LC. Comparative study of nine Bothrops snake venoms from adult female snakes and their offspring. Toxicon. 1991;29:219–226. doi: 10.1016/0041-0101(91)90106-2. [DOI] [PubMed] [Google Scholar]
- Gowda DC, Jackson CM, Hensley P, Davidson EA. Factor X-activating glycoprotein of Russell's viper venom. Polypeptide composition and characterization of the carbohydrate moieties. J Biol Chem. 1994;269:10644–10650. [PubMed] [Google Scholar]
- Gowda CD, Nataraju A, Rajesh R, Dhananjaya BL, Sharath BK, Vishwanath BS. Differential action of proteases from Trimeresurus malabaricus, Naja naja and Daboia russellii venoms on hemostasis. Comp Biochem Physiol C Toxicol Pharmacol. 2006;143:295–302. doi: 10.1016/j.cbpc.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Hanahan DJ, Rolfs MR, Day WC. Observations on the Factor V activator present in Russell's viper venom and its action on Factor V. Biochim Biophys Acta. 1972;86:205–211. doi: 10.1016/0304-4165(72)90107-9. [DOI] [PubMed] [Google Scholar]
- Hemker HC, van Dam-Mieras MC, Devilée PP. The action of Echis carinatus venom on the blood coagulation system. Demonstration of an activator of factor X. Thromb Res. 1984;35:1–9. doi: 10.1016/0049-3848(84)90307-4. [DOI] [PubMed] [Google Scholar]
- Hofmann H, Bon C. Blood coagulation induced by the venom of Bothrops atrox. 2. Identification, purification, and properties of two factor X activators. Biochemistry. 1987;26:780–787. doi: 10.1021/bi00377a019. [DOI] [PubMed] [Google Scholar]
- Hutton RA, Warrell DA. Action of snake venom components on the haemostatic system. Blood Rev. 1993;7:176–189. doi: 10.1016/0268-960x(93)90004-n. [DOI] [PubMed] [Google Scholar]
- Jackson CM, Nemerson Y. Blood coagulation. Annu Rev Biochem. 1980;49:765–811. doi: 10.1146/annurev.bi.49.070180.004001. [DOI] [PubMed] [Google Scholar]
- Johnson PJ, White Y, Woolf IL, Williams R. Reptilase time in cirrhosis and hepatocellular carcinoma. Br Med J. 1977;2:869–870. doi: 10.1136/bmj.2.6091.869-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kini RM, Evans HJ. Inhibition of platelet aggregation by a fibrinogenase from Naja nigricollis venom is independent of fibrinogen degradation. Biochim Biophys Acta. 1991;1095:117–121. doi: 10.1016/0167-4889(91)90073-7. [DOI] [PubMed] [Google Scholar]
- Kisiel W, Hermodson MA, Davie EW. Factor X activating enzyme from Russell's viper venom: isolation and characterization. Biochemistry. 1976;15:4901–4906. doi: 10.1021/bi00667a023. [DOI] [PubMed] [Google Scholar]
- Komori Y, Nikai T, Sugihara H. Isolation and characterization of factor X activator from the venom of Vipera aspis aspis. Int J Biochem. 1990;22:1053–1060. doi: 10.1016/0020-711x(90)90213-m. [DOI] [PubMed] [Google Scholar]
- Kornalík F. The influence of snake venom enzymes on blood coagulation. Pharmacol Ther. 1985;29:353–405. doi: 10.1016/0163-7258(85)90008-7. [DOI] [PubMed] [Google Scholar]
- Kumar MS, Devaraj VR, Vishwanath BS, Kemparaju K. Anticoagulant activity of a metalloprotease: further characterization from the Indian cobra (Naja naja) venom. J Thromb Thrombolysis. 2010;29:340–348. doi: 10.1007/s11239-009-0379-2. [DOI] [PubMed] [Google Scholar]
- Lo SC, Oldmeadow MJ, Howard MA, Firkin BG. Comparison of laboratory tests used for identification of the lupus anticoagulant. Am J Hematol. 1989;30:213–220. doi: 10.1002/ajh.2830300405. [DOI] [PubMed] [Google Scholar]
- Markland FS. Snake venoms and the hemostatic system. Toxicon. 1998;36:1749–1800. doi: 10.1016/s0041-0101(98)00126-3. [DOI] [PubMed] [Google Scholar]
- Maruyama M, Kamiguti AS, Tomy SC, Antonio LC, Sugiki M, Mihara H. Prothrombin and factor X activating properties of Bothrops erythromelas venom. Ann Trop Med Parasitol. 1992;86:549–556. doi: 10.1080/00034983.1992.11812706. [DOI] [PubMed] [Google Scholar]
- Matsui T, Fujimura Y, Titani K. Snake venom proteases affecting hemostasis and thrombosis. Biochim Biophys Acta. 2000;1477:146–156. doi: 10.1016/s0167-4838(99)00268-x. [DOI] [PubMed] [Google Scholar]
- Nahas L, Kamiguti AS, Barros MA. Thrombin-like and factor X-activator components of Bothrops snake venoms. Thromb Haemost. 1979;41:314–328. [PubMed] [Google Scholar]
- Perez JC, McKeller MR, Pérez JC, Sánchez EE, Ramírez MS. An internet database of crotaline venom found in the United States. Toxicon. 2001;39:621–632. doi: 10.1016/s0041-0101(00)00186-0. [DOI] [PubMed] [Google Scholar]
- Salazar AM, Guerrero B, Cantu B, Cantu E, Rodríguez-Acosta A, Pérez JC, Galán JA, Tao A, Sánchez EE. Venom variation in hemostasis of the southern Pacific rattlesnake (Crotalus oreganus helleri): isolation of hellerase. Comp Biochem Physiol C Toxicol Pharmacol. 2009;149:307–316. doi: 10.1016/j.cbpc.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samel M, Siigur J. Medium molecular weight factor X activating enzyme from Vipera berus berus venom. Toxicon. 1995;33:41–52. doi: 10.1016/0041-0101(94)00143-v. [DOI] [PubMed] [Google Scholar]
- Siigur E, Tõnismägi K, Trummal K, Samel M, Vija H, Subbi J, Siigur J. Factor X activator from Vipera lebetina snake venom, molecular characterization and substrate specificity. Biochim Biophys Acta. 2001;1568:90–98. doi: 10.1016/s0304-4165(01)00206-9. [DOI] [PubMed] [Google Scholar]
- Stocker K, Barlow GH. The coagulant enzyme from Bothrops atrox venom (batroxobin) Methods Enzymol. 1976;45:214–223. doi: 10.1016/s0076-6879(76)45021-8. [DOI] [PubMed] [Google Scholar]
- Sundell IB, Ranby M, Zuzel M, Robinson KA, Theakston RD. In vitro procoagulant and anticoagulant properties of Naja naja naja venom. Toxicon. 2003;42:239–247. doi: 10.1016/s0041-0101(03)00137-5. [DOI] [PubMed] [Google Scholar]
- Takeya H, Nishida S, Miyata T, Kawada S, Saisaka Y, Morita T, Iwanaga S. Coagulation factor X-activating enzyme from Russell's viper venom, (RVV-X). A novel metalloproteinase with disintegrin (platelet aggregation inhibitor)-like and C-type lectin-like domains. J Biol Chem. 1992;267:14109–14117. [PubMed] [Google Scholar]
- Tans G, Rosing J. Snake venom activators of factor X: an overview. Haemostasis. 2001;31:225–233. doi: 10.1159/000048067. [DOI] [PubMed] [Google Scholar]
- Thiagarajan P, Pengo V, Shapiro SS. The use of the dilute Russell viper venom time for the diagnosis of lupus anticoagulants. Blood. 1986;68:869–874. [PubMed] [Google Scholar]
- Van Cott EM, Smith EY, Galanakis DK. Elevated fibrinogen in an acute phase reaction prolongs the reptilase time but typically not the thrombin time. Am J Clin Pathol. 2002;118:263–268. doi: 10.1309/WUB3-72JT-E50M-EU8J. [DOI] [PubMed] [Google Scholar]
- von Kaulla KN, Ostendorf P, von Kaulla E. The impedance machine: a new bedside coagulation recording device. J Med. 1975;6:73–88. [PubMed] [Google Scholar]
- White J. Snake venoms and coagulopathy. Toxicon. 2005;45:951–967. doi: 10.1016/j.toxicon.2005.02.030. [DOI] [PubMed] [Google Scholar]
- Willis TW, Tu AT. Purification and biochemical characterization of atroxase, a nonhemorrhagic fibrinolytic protease from western diamondback rattlesnake venom. Biochemistry. 1988;27:4769–4777. doi: 10.1021/bi00413a028. [DOI] [PubMed] [Google Scholar]