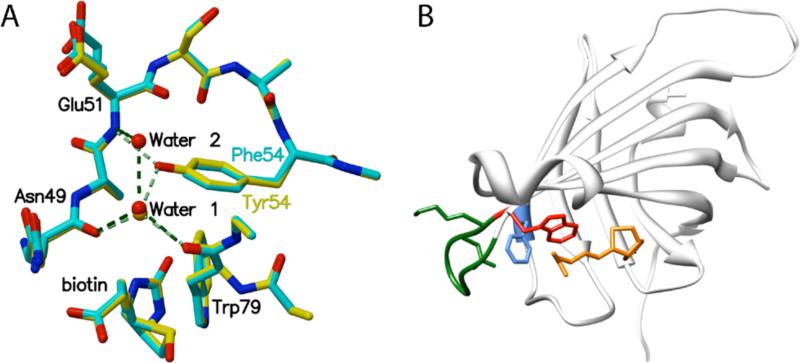
Figure 2.
(A) Superposition of details from biotin bound Y54F (blue) and wild type streptavidin (yellow) structures. Removal of the Y54 hydroxyl group disrupts a hydrogen bonding network involving Trp79, Glu51, Asn49, and a bound water molecule (Water 1). The mutation results in a small cavity filled by a new bound water molecule (Water 2) in one of two subunits in the bound crystal structure. (B) Disruption of the hydrogen bond network involving Y54 (F54 is shown in blue) results in larger atomic fluctuations for loop L5,6 (green) and W79 (red), a key aromatic biotin contact. (Biotin is shown in orange.)