Abstract
Lung cancer is one of the most commonly diagnosed malignancies and the leading cause of cancer-related mortality in Canada. The heterogeneity of nsclc and the importance of linking new targeted agents to the appropriate disease subtype require an individualized approach to treatment. In patients with EGFR (epidermal growth factor receptor gene) mutations, egfr tyrosine kinase inhibitors (tkis) provide a highly effective treatment option, with improved toxicity compared with standard chemotherapy. However, the identification of mutation-positive patients is limited by a lack of funding for testing. The length of time required to receive test results and insufficient tissue from biopsies are additional limitations. In Canada, the use of egfr-tkis varies based on differences in provincial funding for both testing and treatment. With improvements in testing and access to funding for treatment, targeted use of egfr-tkis may greatly improve outcomes in nsclc.
Keywords: nsclc, egfr, egfr-tkis, Canadian situation
1. BACKGROUND
Lung cancer is one of the most commonly diagnosed malignancies and the leading cause of cancer-related mortality in Canada 1. Non-small-cell lung cancer (nsclc) is the most common form of the disease, accounting for approximately 85% of lung cancers, and it includes a number of histologies—namely, adenocarcinoma, squamous cell carcinoma, large-cell anaplastic carcinoma, and adenosquamous carcinoma 2,3.
Traditionally, systemic chemotherapies have been used to treat nsclc, but improvements in outcomes have reached a plateau 4,5. Use of platinum-based doublet chemotherapy for advanced disease has improved median survival to 8–10 months from 4–5 months (if untreated), but the doublets are limited by significant toxicities. Recent advances in the understanding of cell signalling pathways have led to the development of targeted treatments, offering potential benefits in efficacy and safety. However, targeted agents are unlikely to offer advantages unless administered to patients with specific genetic subtypes, suggesting a need for adequate mutation testing 6.
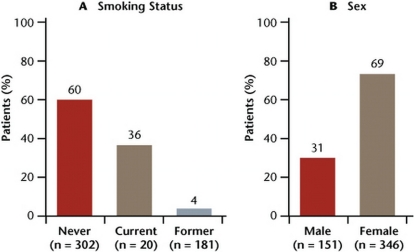
Examples of potentially targetable biomarkers associated with nsclc subtypes include mutations in the epidermal growth factor receptor (EGFR) and KRAS genes, and the fusion genes echinoderm microtubule-associated protein-like 4 and anaplastic lymphoma kinase (EML4-ALK) 7–9. Mutations in EGFR occur at exons 18–21, with approximately 90% occurring as short in-frame deletions in exon 19 or as point mutations in exon 21 10,11. These mutations are found in approximately 10% of patients from North America and in 33% of patients from East Asia, with most being found in female never-smokers with adenocarcinoma histology 11 (Figure 1). An ongoing study collecting data from a pan-Canadian EGFR mutation testing program found that 17.6% of samples (279 of 1588) were positive for exon 19 deletion and exon 21 L858R point mutation 12. In almost all cases, EGFR mutations are non-overlapping with other oncogenic mutations such as KRAS and ALK.
Figure 1.
Mutations in EGFR in adenocarcinoma patients by (A) smoking status and (B) sex. Adapted from D’Angelo et al., 2011 24.
Various clinical trials have demonstrated that egfr inhibitors are clinically efficacious in the management of solid tumour types, such as those of breast, colon, pancreas, head and neck, kidney, gastrointestinal stroma, and lung 13. The egfr tyrosine kinase inhibitors (egfr-tkis) impede phosphorylation of the intracellular tyrosine kinase component of egfr and thus block signal transduction pathways associated with the proliferation and survival of cancer cells 14. Erlotinib is a reversible egfr-tki that has been approved in the United States, Canada, and many other countries worldwide for use in nsclc after failure of chemotherapy, based on clinical trial results showing it to be safe and efficacious 15. Another reversible egfr-tki is gefitinib, which was recently granted marketing authorization by the European Medicines Agency and Canada for the first-line treatment of EGFR mutation–positive nsclc. Newer irreversible egfr-tkis in clinical development— such as afatinib (BIBW 2992), PF-00299804, and neratinib (HK-272)—may also prevent or delay the development of resistance by inhibiting the growth of tumours harboring a T790M mutation in exon 20 of the EGFR gene 16.
In unselected patients, response to monotherapy with egfr-tkis ranges from 4% to 27%; however, in subgroups of patients, such as never-smokers and people of Asian ethnicity, responses are achieved in approximately 40% 17–20. It is therefore important to test for biomarkers, such as the presence of EGFR mutations, that predict an optimal response to egfr-tkis. In trials that select patients based on the presence of activating EGFR mutations, responses to egfr-tkis occur in 30%–90% of patients 21.
The heterogeneity of the disease and the importance of linking new targeted agents to the appropriate disease subtype suggest the need for an individualized approach to the treatment of nsclc. Testing patients for biomarkers to identify the presence of disease-specific genes or gene profiles that control cancer growth can optimize the use of target-specific therapies such as egfr-tkis. The present paper sets out a Canadian perspective on the use of egfr-tkis in nsclc and addresses topics such as the need for EGFR mutation testing, the efficacy of egfr-tkis at various points in the treatment algorithm, and the use of egfr-tkis in Canada.
2. EGFR MUTATION TESTING
Given the heterogeneous nature of nsclc and the number of genetically distinct subtypes that exist, individualizing treatment is the next step in improving patient outcomes. If used in the appropriate patients, egfr-tkis can improve efficacy and reduce toxicity of treatment. Given the improved outcomes in patients with EGFR mutations, it is important to identify those patients up front and to treat them with egfr-tkis.
Certain tumour tissue characteristics—such as adenocarcinomas with non-mucinous bronchioloalveolar component, and papillary and micropapillary patterns—appear to occur more frequently with EGFR mutations 22,23. In addition, mutations occur more often in women and never-smokers 24 (Figure 1). Although phenotypic markers may aid in predicting the prevalence of EGFR mutation, using those markers to select patients for egfr-tkis would eliminate a number of patients who could benefit from such treatment. Currently, somatic mutations in the EGFR gene are the most robust biomarkers for egfr-targeted therapy selection 25. According to the 2011 provisional clinical opinion paper from the American Society of Clinical Oncology on EGFR testing in nsclc, all patients being considered for first-line treatment with an egfr-tki should be tested for EGFR mutations 26.
Although mutation testing is needed in Canada, a number of barriers exist, such as a lack of funding for testing, the length of time needed to obtain test results, and the inadequacy of biopsy tissue samples. Despite the approval of erlotinib and gefitinib in mutation-positive nsclc, funding for EGFR mutation testing is not readily available in Canada. Currently, only British Columbia and Alberta have access both to routine testing and to funding of gefitinib as initial treatment for advanced lung cancer patients with EGFR mutations 27. As a result, samples are typically sent to diagnostic laboratories, and it can take 3–4 weeks to receive results. Some patients may deteriorate while waiting, and others are too nervous to wait, which results in treatment with chemotherapy commencing before test results are received.
In an ongoing study using data from 5 regional diagnostic centres in a pan-Canadian mutation testing program, the median time for receipt of samples by test centres was 7 days and the time for centres to complete tests and report results was 11 days 12. That study suggests that EGFR mutation testing is feasible. However, if funding for on-site testing were to be made available to institutions, the length of time to obtain results might be reduced considerably. With the shift from conventional chemotherapy to an era of personalized medicine, there is also a need for increased involvement from pneumologists and thoracic surgeons in the testing process.
Obtaining tissue samples sufficient for testing is essential for delivering appropriate treatment to patients. With educational initiatives providing a greater understanding of the need for upfront EGFR testing, pneumologists can play a key role in ordering tests and obtaining biopsies sufficient to fulfill testing needs. In addition, there is a need for re-testing at each new line of treatment, because new mutations that may require a specific individualized treatment approach may develop over time.
Gene sequencing by polymerase chain reaction (pcr) assay is the most widely used method for EGFR mutation testing. Unfortunately, the test involves multiple steps and typically requires several days to obtain results. In addition, 25% of the total dna must be mutant for detection; the sensitivity of the test may therefore be inadequate. False-negative results are therefore a concern with this method in the setting of nsclc 9,25.
In recent years, a number of new testing techniques that improve sensitivity and reduce testing time have been developed (Table i) 9. Single-stranded conformational polymorphism, denaturing high-performance liquid chromatography, and high-resolution melting analysis all rapidly screen for mutations in large numbers of samples with high sensitivity. However, those tests require direct sequencing to confirm the identity of the detected mutations. Other simple techniques are highly sensitive for the detection of specific EGFR mutations, such as the amplification refractory mutations system and the peptide nucleic acid–locked pcr clamping. Finally, the mutant-enriched pcr method selectively digests wild-type dna templates with restriction endonucleases to enrich mutant alleles.
TABLE I.
Techniques for detecting epidermal growth factor receptor mutations in lung cancer specimensa
| Technique | Sensitivity (% mutant dna) | Mutations identified | Comprehensive detection |
|---|---|---|---|
| Direct sequencing | 25 | Known and new | Yes |
| pcr-sscp | 10 | Known and new | Yes |
| TaqManbpcr | 10 | Known only | No |
| Loop-hybrid mobility shift assay | 7.5 | Known only | Yes |
| Cycleavecpcr | 5 | Known only | Yes |
| pcr-rflp and length analysis | 5 | Known only | Yes |
| maldi-tof ms-based genotyping | 5 | Known only | No |
| pna-lna pcr clamp | 1 | Known only | No |
| Scorpionsdarms | 1 | Known only | No |
| dhplc | 1 | Known and new | Yes |
| Single-molecule sequencing | 0.2 | Known and new | Yes |
| Mutant-enriched pcr | 0.2 | Known only | No |
| smap | 0.1 | Known only | No |
Adapted from Pao and Ladanyi, 2007 28.
Roche Molecular Systems, Pleasanton, CA, U.S.A.
Takara Bio, Otsu, Japan.
DxS Limited, Manchester, U.K.
pcr = polymerase chain reaction; sscp = single-strand conformation polymorphism; rflp = restriction fragment length polymorphism; maldi-tof ms = matrix-assisted laser desorption/ionization time-of-flight mass spectrometry; pna-lna = peptide nucleic acid– locked nucleic acid; arms = amplified refractory mutation system; dhplc = denaturing high-performance liquid chromatography; smap = smart amplification process.
3. EFFICACY OF EGFR-TKIS
3.1. First-Line Treatment
The addition of egfr-tkis to platinum-based chemotherapy has been examined in a number of trials (Table ii). Despite completion of a number of phase iii studies, no benefit was found with egfr-tkis compared with chemotherapy used alone. The lack of benefit shown in combination trials might be explained by the fact that targeted agents slow the proliferation of malignant cells that is needed for chemotherapy activity.
TABLE II.
Phase iii studies of epidermal growth factor receptor tyrosine kinase inhibitors as first-line treatment for advanced-stage non-small-cell lung cancera
| Reference (study name) | Treatment | Pts (n) | rr (%) | pfs (months) | Median os (months) |
|---|---|---|---|---|---|
| Combination | |||||
| Giaccone et al., 2004 30 (intact-1) | Gemcitabine/cisplatin/placebo | 363 | 47.2 | 6.0 | 10.9 |
| Gemcitabine/cisplatin/gefitinib (250 mg) | 363 | 51.2 | 5.8 | 9.9 | |
| Gemcitabine/cisplatin/gefitinib (500 mg) | 365 | 50.3 | 5.5 | 9.9 | |
| Herbst et al., 2004 31 (intact-2) | Paclitaxel/carboplatin/placebo | 345 | 28.7 | 5.0 | 9.9 |
| Paclitaxel/carboplatin/gefitinib (250 mg) | 345 | 30.4 | 5.3 | 9.8 | |
| Paclitaxel/carboplatin/gefitinib (500 mg) | 347 | 30.0 | 4.6 | 8.7 | |
| Herbst et al., 2005 32 (tribute) | Paclitaxel/carboplatin/placebo | 533 | 19.3 | 4.9 | 10.5 |
| Paclitaxel/carboplatin/erlotinib (150 mg) | 526 | 21.5 | 5.1 | 10.6 | |
| hr: 0.94; | hr: 0.99; | ||||
| 95% ci: nr | 95% ci: 0.86 to 1.16 | ||||
| Gatzemeier et al., 2007 33 (talent) | Gemcitabine/cisplatin/placebo | 586 | 29.9 | 24.6 weeks | 44.1 weeks |
| Gemcitabine/cisplatin/erlotinib (150 mg) | 586 | 31.5 | 23.7 weeks | 43.0 weeks | |
| hr: 0.98; | hr: 1.06; | ||||
| 95% ci: 0.86 to 1.11 | 95% ci: 0.90 to 1.23 | ||||
| Monotherapy | |||||
| Lee et al., 2009 34 (first-signal) | Gemcitabine/cisplatin | 150 | 46.3 | 6.1 | 23.3 |
| Gefitinib (250 mg) | 159 | 53.5 | 6.6b | 21.3 | |
| hr: 0.81; | hr: 1.0; | ||||
| 95% ci: 0.64 to 1.03 | 95% ci: 0.75 to 1.3 | ||||
| Mok et al., 2009 35 (ipass, all patients) | Paclitaxel/carboplatin | 608 | 32.0 | hr: 0.74; | hr: 0.91; |
| Gefitinib (250 mg) | 609 | 43.0b | 95% ci: 0.65 to 0.85 | 95% ci: 0.76 to 1.10 | |
| (ipass, egfr M+ patients) | Paclitaxel/carboplatin | 129 | 47.3 | hr: 0.48; | hr: 0.78; |
| Gefitinib (250 mg) | 132 | 71.2b | 95% ci: 0.36 to 0.64 | 95% ci: 0.50 to 1.20 | |
| Maemondo et al., 2010 36 (NEJS3, egfr M+) | Carboplatin/paclitaxel | 114 | 30.7 | 5.5 | 23.6 |
| Gefitinib | 114 | 73.7b | 10.4b | 30.5 | |
| hr: 0.3; | |||||
| 95% ci: 0.22 to 0.41 | |||||
| Mitsudomi et al., 2010 37 (wjtog 3405, egfr M+ patients) | Cisplatin/docetaxel | 89 | 32.2 | 6.3 | nr |
| Gefitinib (250 mg) | 88 | 62.1b | 9.2b | nr | |
| hr: 0.49; | |||||
| 95% ci: 0.34 to 0.71 | |||||
| Zhou et al., 2010 38 (optimal, egfr M+) | Gemcitabine/carbotaxel | 72 | 36 | 13.1 | nr |
| Erlotinib | 82 | 83b | 4.6b | nr | |
| hr: 0.16; | |||||
| 95% ci: 0.10 to 0.26 | |||||
| Rosell et al., 2011 39 (eurtac, egfr M+) | Platinum-based chemotherapy | 76 | 10.5 | 5.2 | 18.8 |
| Erlotinib | 77 | 54.5b | 9.4b | 22.9 | |
| hr: 0.42; | hr: 0.8; | ||||
| 95% ci: nr | 95% ci: nr | ||||
Adapted from Voon et al., 2010 29.
Statistically significant.
Pts = patients; rr = response rate; pfs = progression-free survival; os = overall survival; intact = Iressa NSCLC Trial Assessing Combination Treatment; tribute = Tarceva Responses in Conjunction with Paclitaxel and Carboplatin; hr = hazard ratio; ci = confidence interval; talent = Tarceva Lung Cancer Investigation; ipass = Iressa Pan-Asia Study; egfr M+ = egfr mutation-positive; nr = not reported; wjtog = West Japan Thoracic Oncology Group; eurtac = European Randomised Trial of Tarceva vs. Chemotherapy.
Subsequently, a number of trials examined egfr-tki monotherapy in patients who were EGFR mutation–positive. In all studies pre-selecting patients with EGFR mutations, egfr-tkis achieved significantly improved response rates (rrs), ranging from 43%–83%, compared with those achieved by chemotherapy. Progression-free survival (pfs) outcomes were also improved with egfr-tkis, with durations ranging from 4.6 months to 10.4 months. However, thus far, no study has shown an overall survival (os) benefit with the use of egfr-tkis. Reasons for a lack of observed os benefit may include the high degree of crossover and the frequent use of subsequent therapies.
In Canada, gefitinib is the only egfr-tki currently approved by Health Canada and indicated for first-line treatment of nsclc 40. However, funding for up-front treatment with gefitinib is not available in all provinces across Canada, and this lack of funding is a major barrier to effective treatment. As described earlier, given the benefit in pfs and rrs shown with erlotinib, it is likely that other egfr-tkis will also prove reasonable options in EGFR mutation–positive patients.
A number of studies are examining the use of other egfr-tkis, such as PF-00299804 and afatinib as first-line treatment in nsclc. A phase ii study of first-line PF-00299804 in EGFR mutation–positive patients demonstrated a median pfs of 9.3 months [95% confidence interval (ci): 6.5 months to 11.2 months] 41. Afatinib has been examined in a phase ii study (LUX-Lung 2) as first- or second-line treatment in EGFR mutation–positive patients 42. After treatment with 40 mg or 50 mg of afatinib, the overall response rate was 67%, the disease control rate was 86%, the median pfs was 14 months, and the median os was 24 months. Comparable efficacy was observed in the first- and second-line settings. In addition, an ongoing phase iii study (LUX-Lung 6) is comparing first-line afatinib with cisplatin–gemcitabine in EGFR mutation–positive patients. Finally, a multicentre open-label phase iii study (LUX-Lung 3) is comparing afatinib with chemotherapy (cisplatin or pemetrexed) for the first-line treatment of EGFR mutation–positive nsclc patients. The accrual of LUX-Lung 3 has been completed, and results will be presented at the American Society of Clinical Oncology 2012 meeting.
Given the short survival times of patients with nsclc, 40%–60% of patients will receive only one line of treatment 43,44. It is therefore of key importance that the best treatment option be provided up front. In EGFR mutation–positive patients, it is clear that, compared with standard chemotherapy, egfr-tkis achieve superior treatment outcomes. Upfront mutation testing is therefore necessary to optimize outcomes by selecting the appropriate patients for egfr-tkis. If the cost of mutation testing and treatment were to be covered, many more patients would be tested, allowing for a more personalized approach to treatment.
3.2. Pretreated Patients
Data from phase iii studies in pretreated nsclc patients show rr ranges from 6.8% to 28.1%, and median pfs ranges from 2.0 months to 3.6 months (Table iii). Studies comparing egfr-tkis and chemotherapy have shown comparable pfs and os outcomes. In addition, non-inferiority was shown in interest (Iressa Non- Small-Cell Lung Cancer Trial Evaluating Response and Survival Against Taxotere), which demonstrated that os with gefitinib is non-inferior to that with docetaxel 45. Although no pfs or os advantage was shown for egfr-tkis in pretreated patients, the V-15- 32 and istana (Iressa as Second-Line Therapy in Advanced NSCLC–Asia) trials did show a significant improvement in rrs for gefitinib compared with those for docetaxel in Asiatic patients 46,47.
TABLE III.
Phase iii studies of epidermal growth factor receptor tyrosine kinase inhibitors in pretreated advanced-stage non-small-cell lung cancera
| Reference (study name) | Treatment | Pts (n) | rr (%) | pfs (months) | Median os (months) |
|---|---|---|---|---|---|
| Chemo-controlled | |||||
| Kim et al., 2008 45 (interest) | Docetaxel (75 mg/m2) | 733 | 7.6 | 2.2 | 7.6 |
| Gefitinib (250 mg daily) | 733 | 9.1 | 2.7 | 8.0 | |
| Non-inferiority design | hr: 1.04; | hr: 1.02; | |||
| 95% ci: 0.93 to 1.18 | 95% ci: 0.91 to 1.15 | ||||
| Maruyama et al., 2008 46 (V-15–32) | Docetaxel (60 mg/m2) | 244 | 12.8 | 2.0 | 14 |
| Gefitinib (250 mg daily) | 245 | 22.5b | 2.0 | 11.5 | |
| Non-inferiority design | hr: 0.79; | hr: 1.12; | |||
| 95% ci: 0.72 to 1.12 | 95% ci: 0.89 to 1.40 | ||||
| Lee et al., 2010 47 (istana) | Docetaxel (75 mg/m2) | 79 | 7.6 | hr: 0.73; | hr: 0.870; |
| Gefitinib (250 mg daily) | 82 | 28.1b | 95% ci: 0.53 to 0.99 | 95% ci: 0.613 to 1.236 | |
| Vamvakas et al., 2010 48 (NCT00440414) | Pemetrexed | 147 | 11.6 | 2.9 | 8.9 |
| Erlotinib (150 mg) | 150 | 6.8 | 3.6 | 7.7 | |
| Placebo-controlled | |||||
| Shepherd et al., 2005 18 (br.21) | Placebo | 243 | <1 | 1.8 | 4.7 |
| Erlotinib (150 mg daily) | 488 | 8.9b | 2.2b | 6.7b | |
| hr: 0.61; | hr: 0.7; | ||||
| 95% ci: 0.51 to 0.74 | 95% ci: 0.58 to 0.85 | ||||
| Thatcher et al., 2005 49 (isel) | Placebo | 563 | 1.3 | 2.6 | 5.1 |
| Gefitinib (250 mg daily) | 1129 | 8b | 3.0b | 5.6 | |
| hr: 0.82; | hr: 0.89; | ||||
| 95% ci: 0.73 to 0.92 | 95% ci: 0.77 to 1.02 | ||||
| Hirsh et al., 2011 50 (LUX-Lung 1) | Placebo | 195 | 0.5 | 1.1 | 11.7 |
| Afatinib (50 mg) | 390 | 7.0b | 3.3b | 11.0 | |
| hr: 0.38; | hr: 1.0 | ||||
| 95% ci: nr | |||||
Adapted from Voon et al., 2010 29.
Statistically significant.
Pts = patients; rr = response rate; pfs = progression-free survival; os = overall survival; interest = Iressa Non-Small-Cell Lung Cancer Trial Evaluating Response and Survival Against Taxotere; hr = hazard ratio; ci = confidence interval; istana = Iressa as Second Line Therapy in Advanced NSCLC–Asia; isel = Iressa Survival Evaluation in Lung Cancer; nr = not reported.
Placebo-controlled trials show significantly improved rr and pfs outcomes for egfr-tkis compared with those for chemotherapy (Table iii). In addition, the br.21 study showed an improved os for erlotinib compared with that for placebo (4.7 months vs. 6.7 months; p < 0.001) 18. Although other studies have not found an improved os in unselected patient populations, the isel (Iressa Survival Evaluation in Lung Cancer) study showed that subgroups of patients— such as never-smokers and people of Asian origin—experienced a significantly longer os. In addition, exploratory analyses showed an increased rr in patients with EGFR mutations compared with that in patients having wild-type disease (37.5% vs. 2.6%). There may therefore be an egfr-tki treatment advantage for pretreated patients who are EGFR mutation– positive. However, the br.21 study showed that the os advantage remained even in male current- or ex-smokers with squamous cell histology [6.1 months vs. 4.7 months; hazard ratio (hr): 0.66 (data on file)]. A benefit of egfr-tkis may therefore remain in second- and subsequent-line treatment for patients who typically do not have EGFR mutations.
A number of studies are examining the use of newer irreversible egfr-tkis, such as afatinib and PF- 00299804 in previously treated patients with nsclc. A phase iib/iii study (LUX-Lung 1) in patients failing chemotherapy and erlotinib or gefitinib found a tripling in median pfs to 3.3 months from 1.1 month (hr: 0.38; p < 0.0001) with the addition of afatinib (50 mg) to best supportive care 50 (Table iii). In addition, a subgroup analysis examined the benefit of afatinib in patients with the highest likelihood of EGFR mutations—that is, those with a complete or partial response on prior erlotinib or gefitinib, or with 48 weeks or more on treatment with erlotinib or gefitinib, or both 51. Those patients experienced an improved median pfs (4.4 months vs. 1.0 months; hr: 0.28; 95% ci: 0.21 to 0.36) and a trend toward an improved os (11.8 months vs. 11.2 months; hr: 0.9; 95% ci: 0.69 to 1.18). As discussed earlier, afatinib has also been examined in a phase ii study (LUX-Lung 2) as first- or second-line treatment in EGFR mutation–positive patients 42. In addition, a phase iii study (LUX-Lung 5) is examining afatinib in combination with paclitaxel after first-line treatment with afatinib in patients failing erlotinib or gefitinib.
Several clinical trials have also examined PF-00299804. An open-label phase ii study evaluated the safety and efficacy of PF-00299804 as first-line treatment in advanced nsclc. Of 29 evaluable patients, 1 experienced a complete response; 6, a partial response; and 16, stable disease at 6 weeks or more 41. Preliminary pfs rates at 3, 4, and 6 months were 90%, 79%, and 79% respectively. A number of ongoing studies are also examining the efficacy of PF-00299804. The phase iii br.26 study (NCT01000025) is comparing PF-00299804 with placebo in patients with stage iiib or iv nsclc that has not responded to standard therapy for advanced or metastatic cancer. In addition, the archer 1009 study (NCT01360554) is a phase iii study comparing PF-00299804 with erlotinib in patients with advanced nsclc who have been treated with at least one prior regimen. Several phase ii studies are also examining PF-00299804 as second-line therapy compared with erlotinib 52 and as subsequent treatment after chemotherapy and erlotinib 53.
4. THE CANADIAN EXPERIENCE
4.1. Mutation Testing
In Canada, mutation testing was supported by an AstraZeneca program until the middle of March 2011. Since then, the single greatest barrier to testing has been a lack of funding. Additional barriers include a lack of local pathology labs capable of performing the tests, insufficient tissue from biopsies, and long wait times. In some cases, local labs are available that can perform mutation testing, but in most cases, samples must be sent to a single centralized lab or to labs in other provinces. Advantages of a centralized lab are improved quality control and standardization of methods. However, by increasing the number of labs available locally, the sample transit time may be reduced, providing faster results.
In Atlantic Canada, no lab is currently able to test for EGFR or ALK mutations. In Halifax, about 20 selected nonsmoking patients were tested through the AstraZeneca program, of which 4 (20%) were EGFR mutation–positive. In the future, funding for ALK mutation testing in Atlantic Canada may become available through Pfizer. It is currently possible to send tissue samples to Montreal; however, if patients are mutation-positive for EGFR, they must pay for egfr-tkis themselves. In addition, KRAS mutation tests are sometimes performed for colon cancer, but need to be evaluated in lung cancer as well. In Halifax, advocacy discussions on mutation testing are ongoing, and the plan is to approach the provincial government for funding.
In Quebec, screening for EGFR mutations has declined since the termination of the AstraZeneca program. Until recently, no egfr-tkis were funded for first-line treatment of mutation-positive metastatic nsclc. With the recent approval of gefitinib by the provincial Ministry of Health, it is hoped that the number of patients tested for EGFR mutations will increase in Quebec. Despite recent funding restrictions, testing varies widely, depending on whether pathology labs are available locally. In centres with access to local pathology labs, 60%–70% of adenocarcinoma patients are tested for EGFR mutations and 30%–40% for ALK mutation, depending on tissue availability. Fewer than 20% of squamous patients undergo mutation testing. Where pathology labs are not available locally, mutation testing may occur in fewer than 10% of patients. On January 1, 2012, all patients newly diagnosed with non-squamous tumours at the McGill University Health Centre will undergo mutation testing when adequate biopsies are provided. In Montreal, only two labs perform EGFR mutation testing, and it takes about 3 weeks from the time a biopsy is taken to obtain the lab report.
As in other provinces, mutation testing in Ontario has declined since the termination of the AstraZeneca program. However, it is likely that testing will increase in the near future, because the Ministry of Health has recently agreed to fund up-front mutation testing. Currently, around 15% of patients are tested for EGFR mutations. Adenocarcinoma patients who are Asian, female, and nonsmokers are most likely to be tested. With funding now available for testing, screening of nsclc patients should increase, resulting in a greater percentage of patients being tested.
In Alberta, since the termination of the AstraZeneca program, testing has continued with provisional funding from Alberta Health Services. Approximately 50% of patients with nsclc (adenocarcinoma) are tested for EGFR mutations up front. It is still unclear whether one central lab in Calgary will continue testing for the Prairie provinces or whether a number of subsidiary labs will be available. The goal for turnaround of test results is 2 weeks from anywhere in the province from the time samples are sent. To increase efficiency further, the aim is for pathologists to order mutation tests at the time of initial review instead of waiting for medical oncologists to order tests.
In British Columbia, approximately 50% of patients with stage iv nsclc are tested for an EGFR mutation. Currently, a Vancouver lab performs mutation testing for the entire province. The goal for turnaround of test results is 2–3 weeks from the time samples are sent from anywhere in the province. Testing and tyrosine kinase therapy are both provided by the systemic drug program.
4.2. First-Line Treatment
In all provinces, EGFR mutation status (where available), performance status, and patient preference are considered in first-line treatment decisions. In Canada, gefitinib is the only egfr-tki currently approved by Health Canada for the first-line treatment of nsclc 40. Current data support the use of gefitinib up front, but if erlotinib were to become available, it would also be a reasonable option. Data from the eurtac (European Erlotinib Versus Chemotherapy) trial support the use of upfront erlotinib, but its use in the first-line setting awaits published data and Health Canada approval 39. In addition, before the use of irreversible egfr-tkis can be considered, further data from head-to-head studies are needed.
Currently, first-line treatment with egfr-tkis is not approved or funded provincially in Atlantic Canada by either special access programs or industry. However, for certain frail adenocarcinoma patients who are nonsmokers, it is possible to obtain first-line erlotinib by applying to Roche directly.
In Quebec, gefitinib was not funded until recently; therefore, only privately insured EGFR mutation– positive patients were able to receive egfr-tkis as first-line treatment. However, with the recent approval of gefitinib by the provincial Ministry of Health and Social Services, it is hoped that this treatment option will be available for more patients who are EGFR mutation–positive.
In Ontario, gefitinib was recently approved by the Ministry of Health for the first-line treatment of patients with EGFR mutation–positive nsclc. Currently, fewer than 5% of patients are prescribed gefitinib as first-line treatment. However, with the availability of testing and upfront gefitinib, it is expected that 10%–15% of patients with nsclc will be given gefitinib up front.
In Alberta, whenever possible, EGFR mutation status is determined in all adenocarcinoma patients. All patients who are EGFR mutation–positive are considered for treatment with gefitinib by a medical oncologist. For patients who are gefitinib-eligible, funding can be obtained through a bridging program until reimbursement has undergone final approval through Alberta Health and Wellness. Patients who are mutation-negative are typically given platinum doublet chemotherapy, when appropriate.
On June 1, 2011, the BC Cancer Agency approved first-line gefitinib for patients harbouring the EGFR mutation; those patients receive gefitinib per the BC Cancer Agency policy. A submission for erlotinib using similar patient selection is pending. For mutation-negative or unknown patients, platinum doublet or single-agent chemotherapy is given, depending on age and performance status.
4.3. Pretreated Patients
In Canada, erlotinib is currently approved for the second-line treatment of nsclc, regardless of mutational status, based on the significant os results shown in the br.21 study 54. Therefore, no mutation testing is needed for second-line treatment with egfr-tkis. Thus far, no other egfr-tkis have been approved, and other treatment options are being examined in ongoing clinical trials. In the future, it is hoped that the LUX-Lung trials will provide support for using afatinib as rescue treatment in patients who fail firstline gefitinib or erlotinib.
In Atlantic Canada, erlotinib is funded provincially for second- and third-line therapy. About 50% of patients are given erlotinib as second-line treatment. In patients failing second-line chemotherapy, about 20%–30% are given erlotinib.
In Quebec, patients can receive erlotinib or docetaxel as second- or third-line treatment. The decision to use erlotinib or docetaxel is based on patient eligibility, performance status, comorbidities, and toxicities after first-line therapy. Unfortunately, pemetrexed has been refused approval by the Ministry of Health and Social Services in Quebec. Thus, pemetrexed is available only under special circumstances, such as in patients who are ineligible for docetaxel. The lack of approval of pemetrexed in Quebec has resulted in frustration and discontent amongst oncologists, who are unable to use this option to treat their patients.
In Ontario, erlotinib is funded for second- and third-line treatment of nsclc. Patients failing firstline treatment are typically treated with pemetrexed, docetaxel, or erlotinib. About 20% of all patients who originally present with nsclc end up being treated with erlotinib. Patients typically prescribed erlotinib include those who refuse or are unable to tolerate chemotherapy, and those who are likely to benefit from erlotinib based on their clinical profile.
In Alberta, patients are not currently funded to receive maintenance therapy after completion of first-line therapy; however, they may receive erlotinib (non-selected), pemetrexed (adenocarcinomas only), or docetaxel (mainly squamous histology) as second-line treatment. Erlotinib is not funded for patients that have previously been treated with gefitinib in the first-line setting. After second-line therapy, patients are may be offered any of the first- or second-line treatment options that they have not already received.
In British Columbia, about 50% of patients with non-squamous histology are given erlotinib, and 50% are given pemetrexed. In patients with squamous histology, erlotinib or docetaxel are the treatment options. For patients with non-squamous histology, some patients prefer the oral administration of erlotinib; others prefer to receive pemetrexed chemotherapy every 3 weeks. After second-line therapy, patients are given any of the above treatment options that they have not already received.
5. OVERCOMING RESISTANCE TO EGFR-TKIs
As presented in the foregoing section, treatment with egfr-tkis demonstrates good rrs and pfss in EGFR mutation–positive patients with nsclc. However, acquired resistance to egfr-tkis usually occurs after a median of approximately 10 months from treatment initiation. To date, several mechanisms of acquired resistance have been discovered, such as secondary mutation of the EGFR gene, amplification of the MET gene, and overexpression of HGF16.
Studies using clinical specimens from patients with acquired resistance to egfr-tkis have found that about 50% have a secondary T790M mutation 16. This secondary mutation involves a threonine-to-methionine substitution in codon 790 (T790M) of the EGFR gene. In addition, MET gene amplification is present in approximately 20% of cases of acquired resistance and appears to occur independently of T790M mutations. MET causes phosphorylation of ErbB3, which in turn sustains activation of the phosphatidylinositol 3 kinase (pi3k)/akt signal downstream. Because of those phenomena, reversible egfr-tkis are unable to inhibit the proliferation signal because of the maintenance of phosphorylation of ErbB3 by MET, resulting in resistance. Finally, HGF has been shown to induce restoration of the pi3k/akt signalling pathway through phosphorylation of Met.
Given the development of resistance to first-generation egfr-tkis, a number of second-generation egfr-tkis are being developed. Unlike first-generation egfr-tkis, second-generation agents covalently and irreversibly bind a cysteine residue in EGFR to the amino acid position 797 16. That binding enables the inhibition of egfr kinase activity, even in the presence of an EGFR T790M mutation. Many of the irreversible inhibitors have demonstrated activity in preclinical studies against T790M mutations. The dual inhibitor HKI-272 [against egfr and the human epidermal growth factor receptor 2 (her2)], and PF-00299804 and afatinib (multi-inhibitors against egfr, her2, and her4) are agents currently undergoing clinical trials.
6. SAFETY OF EGFR-TKIs
Studies examining the addition of gefitinib or erlotinib to platinum chemotherapy have demonstrated that egfr-tkis do not increase the incidence or severity of the hematologic and pulmonary toxicities associated with the use of those chemotherapies 30–32. In addition, placebo-controlled trials showed that the overall incidence of adverse events, especially myelosuppression, alopecia, and fatigue are higher in patients given chemotherapy than in those given egfr-tkis 37,38,47.
Although targeted agents are generally less toxic than traditional antineoplastic agents, egfr-tkis are associated with a number of bothersome adverse effects that need to be managed in most patients. The two most common adverse events are rash and diarrhea. The incidence of rash varied from 37% to 78% in phase iii clinical trials and appears to be dose-dependent, with up to 25% of patients experiencing severe reactions (grade 3 or greater). In addition, the incidence of diarrhea varies from 27% to 87%, with up to 25% of patients experiencing severe reactions (grade 3 or greater, Table iv) 55. Despite the higher incidences of rash and diarrhea with egfr-tkis, these side effects can be effectively managed through early treatment, and they rarely result in treatment discontinuation.
TABLE IV.
Incidence of rash and diarrhea with epidermal growth factor receptor (egfr) tyrosine kinase inhibitors (tkis) in non-small-cell lung cancer trialsa
| egfr-tki | Dosage | Description | Rash | Diarrhea | ||
|---|---|---|---|---|---|---|
| All grades (%) | Grade≥3 (%) | All grades (%) | Grade≥3 (%) | |||
| Erlotinib | 150 mg | All studies | 33–79 | 3–10 | 10–69 | 0–17 |
| Phase iii studies | 62–76 | 3–10 | 40–68 | 2–12 | ||
| Gefitinib | 250 mg and 500 mg | All studies | 34–75 | 0–13 | 27–75 | 0–25 |
| 250 mg | 34–66 | 0–4 | 27–58 | 0–10 | ||
| 500 mg | 57–75 | 4–13 | 51–75 | 5–25 | ||
| Phase iii studies | 37–67 | 2–13 | 27–69 | 3–25 | ||
| 250 mg | 37–66 | 2–4 | 27–58 | 3–10 | ||
| 500 mg | 57–67 | 12–13 | 51–69 | 12–25 | ||
| Afatinib (bibw 2992) | 20 mg, 40 mg, and 50 mg | All studies | 33–100 | 0–25 | 0–100 | 0–33 |
| 40 mg | 90–100 | 0–7 | 67–97 | 0–7 | ||
| 50 mg | 67–92 | 0–25 | 87–100 | 17–33 | ||
| Phase iii studies (50 mg) | 78 | 14 | 87 | 17 | ||
| PF-00299804b | 15 mg, 30 mg, and 45 mg | All studies (phase ii) | 68–100 | 0–15 | 77–97 | 0–15 |
| 30 mg | 69 | 0 | 77 | 0 | ||
| 45 mg | 68–85 | 15 | 81–97 | 13–15 | ||
Adapted from Hirsh and Cadranel, 2011 50.
To date, no phase iii study results for PF-00299804 are available.
7. BENEFITS OF EGFR-TKIs
As discussed earlier, egfr-tkis demonstrate a side effect profile superior to that seen with chemotherapy, despite the higher incidence of rash and diarrhea 37,38,47. In addition, evidence from clinical trials comparing chemotherapy with egfr-tkis shows improved quality of life (qol) with egfr-tkis. Results from the optimal trial demonstrated a clinically relevant improvement in scores on the Functional Assessment of Cancer Therapy–Lung, the Lung Cancer Symptom Scale, and the Trial Outcome Index with erlotinib (p < 0.0001) 38. Improved qol was also shown in the br.21 study, which showed reductions in dyspnea, pain, and cough, and improved physical functioning related to symptom improvement with erlotinib 18. In addition, results from the interest study showed that significantly more patients sustained a clinically relevant improvement in qol with gefitinib than with docetaxel, as assessed by the Functional Assessment of Cancer Therapy–Lung and Trial Outcome Index (p < 0.01) 45. Improved qol with gefitinib was also demonstrated in V-15-32 and ipass (Iressa Pan- Asian Study), which compared that agent with chemotherapy 35,46. Finally, results of the LUX-Lung 1 study showed significantly improved global qol, physical function, and fatigue with afatinib use. In addition, afatinib also improved lung cancer-related symptoms such as cough, dyspnea, and pain, and significantly delayed time to deterioration 50.
The oral administration of egfr-tkis confers other benefits in addition to improved qol. Because of the high demand for chemotherapy at oncology clinics, patients often wait for weeks to start intravenous treatment. Moreover, many patients are elderly and not able to come to the clinic by themselves to receive intravenous treatment. Other family members often act as caregivers and need to take time off work to transport patients for treatment. The oral administration of egfr-tkis is therefore far more convenient than intravenous chemotherapy. Finally, some patients, such as those with comorbidities or poor performance status, are often unable to tolerate chemotherapy. Less-aggressive treatments, such as the egfr-tkis, are therefore preferable for those patients.
8. CONCLUSIONS
In recent years, a plateau has been reached with the use of traditional chemotherapies for the treatment of nsclc. The development of agents that target key signalling pathways provides alternative treatment options that may improve the efficacy and reduce the toxicity associated with chemotherapy, thus improving qol. In patients with EGFR mutations, egfr-tkis provide a highly effective treatment option, with an improved toxicity profile compared with that for standard chemotherapy. However, identification of mutation-positive patients is limited by a lack of funding for testing in Canada.
In Canada, gefitinib is the only egfr-tki currently approved by Health Canada for first-line treatment of nsclc. Recent study results show that erlotinib may also prove to be a reasonable first-line option. In pretreated patients, erlotinib is currently approved for the second-line treatment of nsclc, regardless of mutational status. Results of ongoing studies will determine whether other egfr-tkis such as gefitinib, afatinib, or PF-00299804 are good or even better alternatives in pretreated patients. Despite the success of egfr-tkis, development of resistance typically occurs over time. To reduce the risk of resistance, a number of second-generation egfr-tkis such as afatinib, PF-00299804, and HKI-272 are under development. Lack of funding for both testing and treatment is a major barrier to the use of egfr-tkis in Canada.
Despite Health Canada approval for the use of gefitinib in mutation-positive nsclc, only patients in British Columbia and Alberta have access both to routine testing and to funding in that setting. Barriers to testing include the length of time required to receive test results and insufficient tissue from biopsies. Given the shift from conventional chemotherapy to individualized treatment, there is an increased need for education of respirologists and thoracic surgeons in the testing process to ensure that adequate tissue samples are obtained. Standardization and validation of testing methods used by local laboratories is also necessary to ensure the quality of test results.
The heterogeneity of nsclc and the importance of linking new targeted agents to the appropriate disease subtype require an individualized approach to the treatment of nsclc. With careful selection of patients, egfr-tkis may dramatically improve the efficacy and safety of nsclc treatment, bringing personalized medicine one step closer.
9 ACKNOWLEDGMENT
The authors acknowledge medical writing support from Anna Christofides msc rd of Sage Medica; this support was funded by Boehringer Ingelheim Canada.
Footnotes
10. CONFLICT OF INTEREST DISCLOSURES
VH has acted as an advisory board member for Hoffmann–La Roche, AstraZeneca, Amgen, Boehringer Ingelheim, Pfizer, Eli Lilly, Novartis, and Celgene. DM has acted as an advisory board member for AstraZeneca, Boehringer Ingelheim, and Pfizer, and has received grant funding from Pfizer. WM has received an educational grant and has acted as an advisory board member for AstraZeneca, Eli Lilly, Hoffmann–La Roche, Pfizer, and Boehringer Ingelheim. BM has received honoraria from Hoffmann–La Roche, Eli Lilly, and Boehringer Ingelheim. GG has acted as an advisory board member for AstraZeneca, Pfizer, and Boehringer Ingelheim.
11. REFERENCES
- 1.Canadian Cancer Society’s Steering Committee . Canadian Cancer Statistics 2010. Toronto, ON: Canadian Cancer Society; 2010. [Google Scholar]
- 2.United States, National Institutes of Health, National Cancer Institute (nci) General Information About Non-Small Cell Lung Cancer [Web page] Bethesda, MD: nci; n.d. [Available online at: http://www.cancer.gov/cancertopics/pdq/treatment/non-small-cell-lung/healthprofessional; cited September 12, 2010]. [Google Scholar]
- 3.Pisters KM, Evans WK, Azzoli CG, et al. Cancer Care Ontario and American Society of Clinical Oncology adjuvant chemotherapy and adjuvant radiation therapy for stages i–iiia resectable non small-cell lung cancer guideline. J Clin Oncol. 2007;25:5506–18. doi: 10.1200/JCO.2007.14.1226. [DOI] [PubMed] [Google Scholar]
- 4.Cagle PT, Allen TC, Dacic S, et al. Revolution in lung cancer: new challenges for the surgical pathologist. Arch Pathol Lab Med. 2011;135:110–16. doi: 10.5858/2010-0567-RA.1. [DOI] [PubMed] [Google Scholar]
- 5.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–8. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 6.Cagle PT, Dacic S. Lung cancer and the future of pathology. Arch Pathol Lab Med. 2011;135:293–5. doi: 10.5858/2011-0037-ED.1. [DOI] [PubMed] [Google Scholar]
- 7.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 8.Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 9.Pao W, Miller V, Zakowski M, et al. egf receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A. 2004;101:13306–11. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li AR, Chitale D, Riely GJ, et al. EGFR mutations in lung adenocarcinomas: clinical testing experience and relationship to EGFR gene copy number and immunohistochemical expression. J Mol Diagn. 2008;10:242–8. doi: 10.2353/jmoldx.2008.070178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uramoto H, Mitsudomi T. Which biomarker predicts benefit from egfr-tki treatment for patients with lung cancer? Br J Cancer. 2007;96:857–63. doi: 10.1038/sj.bjc.6603665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsao MS, Ionescu G, Chong D, et al. Population-based pan-Canadian EGFR-mutation testing program [abstract e18017] J Clin Oncol. 2011;29 [Available online at: http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=102&abstractID=82932; cited February 8, 2012] [Google Scholar]
- 13.Harandi A, Zaidi AS, Stocker AM, Laber DA. Clinical efficacy and toxicity of anti-egfr therapy in common cancers. J Oncol. 2009;2009:567486. doi: 10.1155/2009/567486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukuoka M, Yano S, Giaccone G, et al. Multi-institutional randomized phase ii trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (the ideal 1 Trial) J Clin Oncol. 2003;21:2237–46. doi: 10.1200/JCO.2003.10.038. [Erratum in: J Clin Oncol 2004;22:4811] [DOI] [PubMed] [Google Scholar]
- 15.Hirsh V. Systemic therapies in metastatic non-small-cell lung cancer with emphasis on targeted therapies: the rational approach. Curr Oncol. 2010;17:13–23. doi: 10.3747/co.v17i2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kosaka T, Yamaki E, Mogi A, Kuwano H. Mechanisms of resistance to egfr tkis and development of a new generation of drugs in non-small-cell lung cancer. J Biomed Biotechnol. 2011;2011:165214. doi: 10.1155/2011/165214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jänne PA, Gurubhagavatula S, Yeap BY, et al. Outcomes of patients with advanced non-small cell lung cancer treated with gefitinib (ZD1839, “Iressa”) on an expanded access study. Lung Cancer. 2004;44:221–30. doi: 10.1016/j.lungcan.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 18.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–32. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 19.Mok TS, Wu YL, Yu CJ, et al. Randomized, placebo-controlled, phase ii study of sequential erlotinib and chemotherapy as firstline treatment for advanced non-small-cell lung cancer. J Clin Oncol. 2009;27:5080–7. doi: 10.1200/JCO.2008.21.5541. [DOI] [PubMed] [Google Scholar]
- 20.Mok T, Wu YL, Zhang L. A small step towards personalized medicine for non-small cell lung cancer. Discov Med. 2009;8:227–31. [PubMed] [Google Scholar]
- 21.Ellis PM, Morzycki W, Melosky B, et al. The role of the epidermal growth factor receptor tyrosine kinase inhibitors as therapy for advanced, metastatic, and recurrent non-small-cell lung cancer: a Canadian national consensus statement. Curr Oncol. 2009;16:27–48. doi: 10.3747/co.v16i1.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sartori G, Cavazza A, Sgambato A, et al. EGFR and K-ras mutations along the spectrum of pulmonary epithelial tumors of the lung and elaboration of a combined clinicopathologic and molecular scoring system to predict clinical responsiveness to egfr inhibitors. Am J Clin Pathol. 2009;131:478–89. doi: 10.1309/AJCPH0TRMPXVZW2F. [DOI] [PubMed] [Google Scholar]
- 23.Ninomiya H, Hiramatsu M, Inamura K, et al. Correlation between morphology and EGFR mutations in lung adenocarcinomas. Significance of the micropapillary pattern and the hobnail cell type. Lung Cancer. 2009;63:235–40. doi: 10.1016/j.lungcan.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 24.D’Angelo SP, Pietanza MC, Johnson ML, et al. Incidence of EGFR exon 19 deletions and L858R in tumor specimens from men and cigarette smokers with lung adenocarcinomas. J Clin Oncol. 2011;29:2066–70. doi: 10.1200/JCO.2010.32.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ontario, Ministry of Health and Long-Term Care, Medical Advisory Secretariat (mas) Epidermal Growth Factor Receptor (EGFR) Genetic Testing for Prediction of Response to EGFR-Targeted (TKI) Drugs in Patients with Advanced Non-Small-Cell Lung Cancer: An Evidence-Based Analysis. Toronto, ON: MAS; 2010. Ontario health technology assessment series. Vol. 10. Iss. 24. [Available online at: http://www.health.gov.on.ca/english/providers/program/mas/tech/reviews/pdf/EGFR_20101209.pdf; cited September 2011] [PMC free article] [PubMed] [Google Scholar]
- 26.Keedy VL, Temin S, Somerfield MR, et al. American Society of Clinical Oncology provisional clinical opinion: epidermal growth factor receptor (EGFR) mutation testing for patients with advanced non-small-cell lung cancer considering firstline egfr tyrosine kinase inhibitor therapy. J Clin Oncol. 2011;29:2121–7. doi: 10.1200/JCO.2010.31.8923. [DOI] [PubMed] [Google Scholar]
- 27.BC Cancer Agency (bcca) BCCA Protocol Summary for First-Line Treatment of Epidermal Growth Factor Receptor (EGFR) Mutation-Positive Advanced Non-Small Cell Lung Cancer (NSCLC) with Gefitinib. Vancouver, BC: BCCA; 2010. [Google Scholar]
- 28.Pao W, Ladanyi M. Epidermal growth factor receptor mutation testing in lung cancer: searching for the ideal method. Clin Cancer Res. 2007;13:4954–5. doi: 10.1158/1078-0432.CCR-07-1387. [DOI] [PubMed] [Google Scholar]
- 29.Voon PJ, Chul Cho B, Yeo WL, Soo RA. The role of epidermal growth factor receptor tyrosine kinase inhibitors in the treatment of advanced stage non-small cell lung cancer. J Thorac Dis. 2010;2:144–53. doi: 10.3978/j.issn.2072-1439.2010.02.03.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giaccone G, Herbst RS, Manegold C, et al. Gefitinib in combination with gemcitabine and cisplatin in advanced nonsmall- cell lung cancer: a phase iii trial—intact 1. J Clin Oncol. 2004;22:777–84. doi: 10.1200/JCO.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Herbst RS, Giaccone G, Schiller JH, et al. Gefitinib in combination with paclitaxel and carboplatin in advanced nonsmall- cell lung cancer: a phase iii trial—intact 2. J Clin Oncol. 2004;22:785–94. doi: 10.1200/JCO.2004.07.215. [DOI] [PubMed] [Google Scholar]
- 32.Herbst RS, Prager D, Hermann R, et al. tribute: a phase iii trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2005;23:5892–9. doi: 10.1200/JCO.2005.02.840. [DOI] [PubMed] [Google Scholar]
- 33.Gatzemeier U, Pluzanska A, Szczesna A, et al. Phase iii study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: the Tarceva Lung Cancer Investigation Trial. J Clin Oncol. 2007;25:1545–52. doi: 10.1200/JCO.2005.05.1474. [DOI] [PubMed] [Google Scholar]
- 34.Lee JS, Park K, SW K, et al. A randomized phase iii study of gefitinib (Iressa) versus standard chemotherapy (gemcitabine plus cisplatin) as a first-line treatment for never-smokers with advanced or metastatic adenocarcinoma of the lung [abstract PRS.4] J Thorac Oncol. 2009;4(suppl 1):S283. [Google Scholar]
- 35.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–57. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 36.Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–8. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 37.Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–8. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 38.Zhou C, Wu YL, Chen G, et al. Efficacy results from the randomized phase iii optimal (ctonG 0802) study comparing first-line erlotinib versus carboplatin (cbdca) plus gemcitabine (gem), in Chinese advanced non-small-cell lung cancer (nsclc) patients (pts) with EGFR activating mutations [abstract LBA13] Ann Oncol. 2010;21(suppl 8):viii6. doi: 10.1093/annonc/mdp507. [DOI] [Google Scholar]
- 39.Rosell R, Gervais R, Vergnenegre A, et al. Erlotinib versus chemotherapy (ct) in advanced non-small cell lung cancer (nsclc) patients (p) with epidermal growth factor receptor (EGFR) mutations: Interim results of the European Erlotinib Versus Chemotherapy (eurtac) phase iii randomized trial [abstract 7503] J Clin Oncol. 2011;29 [Available online at: http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=102&abstractID=78285; cited February 8, 2012] [Google Scholar]
- 40.AstraZeneca Canada . IRESSA (Gefitinib Tablets) Mississauga, ON: AstraZeneca Canada; 2009. [product monograph]. [Google Scholar]
- 41.Mok TSK, Spigel DR, Park K, et al. Efficacy and safety of PF299804 as first-line treatment (tx) of patients (pts) with advanced (adv) nsclc selected for activating mutation (mu) of epidermal growth factor receptor (egfr) Ann Oncol. 2010;21(suppl 8):viii1–12. [Google Scholar]
- 42.Yang C, Shih J, Su W, et al. A phase ii study of BIBW 2992 in patients with adenocarcinoma of the lung and activating egfr/ her1 mutations (LUX-Lung 2) [abstract 7521] J Clin Oncol. 2010;28 [Available online at: http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=74&abstractID=50532; cited February 8, 2012] [Google Scholar]
- 43.Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18:2095–103. doi: 10.1200/JCO.2000.18.10.2095. [DOI] [PubMed] [Google Scholar]
- 44.Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase iii trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–97. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 45.Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (interest): a randomised phase iii trial. Lancet. 2008;372:1809–18. doi: 10.1016/S0140-6736(08)61758-4. [DOI] [PubMed] [Google Scholar]
- 46.Maruyama R, Nishiwaki Y, Tamura T, et al. Phase iii study, V-15-32, of gefitinib versus docetaxel in previously treated Japanese patients with non-small-cell lung cancer. J Clin Oncol. 2008;26:4244–52. doi: 10.1200/JCO.2007.15.0185. [DOI] [PubMed] [Google Scholar]
- 47.Lee DH, Park K, Kim JH, et al. Randomized phase iii trial of gefitinib versus docetaxel in non-small cell lung cancer patients who have previously received platinum-based chemotherapy. Clin Cancer Res. 2010;16:1307–14. doi: 10.1158/1078-0432.CCR-09-1903. [DOI] [PubMed] [Google Scholar]
- 48.Vamvakas L, Agelaki S, Kentepozidis NK, et al. Pemetrexed (mta) compared with erlotinib (erl) in pretreated patients with advanced non-small cell lung cancer (nsclc): results of a randomized phase iii Hellenic Oncology Research Group trial [abstract 7519] J Clin Oncol. 2010;28 [Available online at: http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=74&abstractID=43881; cited February 8, 2012] [Google Scholar]
- 49.Thatcher N, Chang A, Parikh P, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer) Lancet. 2005;366:1527–37. doi: 10.1016/S0140-6736(05)67625-8. [DOI] [PubMed] [Google Scholar]
- 50.Hirsh V, Cadranel J, Cong J, et al. Symptom and health-related quality of life benefit of afatinib (BIBW 2992) in advanced nsclc patients previously treated with erlotinib or gefitinib: results of a randomized phase iii trial (LUX-Lung 1) [abstract 011.07] J Thorac Oncol. 2011;6(suppl 2):S324. doi: 10.1097/JTO.0b013e3182773fce. [DOI] [PubMed] [Google Scholar]
- 51.Miller VA, Hirsh V, Cadranal J, et al. Subgroup analysis of LUX-Lung 1: a randomized phase iii trial of afatinib (BIBW 2992) + best supportive care (bsc) versus placebo + bsc in patients failing 1–2 lines of chemotherapy and erlotinib or gefitinib [abstract LBPL3] J Thorac Oncol. 2010;5(suppl 7):S557. doi: 10.1097/JTO.0b013e3182016287. [DOI] [Google Scholar]
- 52.Ramalingam SS, Boyer MJ, Park K, et al. Randomized phase 2 study of PF299804, an irreversible human epidermal growth factor receptor (egfr) inhibitor, versus (v) erlotinib (e) in patients (pts) with advanced non-small cell lung cancer (nsclc) after chemotherapy (ct) failure: quantitative and qualitative benefits [abstract 365PD] Ann Oncol. 2010;21(suppl 8):viii122. doi: 10.1093/annonc/mdq518. [DOI] [Google Scholar]
- 53.Janne PA, Reckamp K, Koczywas M, et al. Efficacy and safety of PF-00299804 (PF299) in patients (pt) with advanced nsclc after failure of at least one prior chemotherapy regimen and prior treatment with erlotinib (e): a two-arm, phase ii trial [abstract 8063] J Clin Oncol. 2009;27 [Available online at: http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=65&abstractID=32872; cited February 8, 2012] [Google Scholar]
- 54.Hoffmann–La Roche . Product Monograph: Tarceva (Erlotinib Hydrochloride Tablets) Mississauga, ON: Hoffmann–La Roche; 2005. [Google Scholar]
- 55.Hirsh V. Managing treatment-related adverse events associated with egfr tyrosine kinase inhibitors in advanced non-small-cell lung cancer. Curr Oncol. 2011;18:126–38. doi: 10.3747/co.v18i3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]