Abstract
Context
The delineation of populations of cancer patients with complex symptoms can inform the planning and delivery of supportive care services.
Objectives
We explored the physical, psychosocial, and practical concerns experienced by patients attending an ambulatory oncology symptom control clinic.
Methods
Patients attending a Pain Clinic at a large tertiary cancer centre were invited to complete screening measures assessing distress, pain, fatigue, anxiety, depression, and practical and psychosocial problems. A matched sample of patients who did not attend the Pain Clinic were selected as a comparison group.
Results
Of all eligible Pain Clinic patients, 46 (77%) completed the measures; so did 46 comparison group patients. The percentages of patients reporting distress (78.3%), pain (93.5%), and fatigue (93.5%) were higher among Pain Clinic patients than among the comparison patients. A higher percentage of Pain Clinic patients also reported multiple, severe, concurrent symptoms: 87% scored 7 or higher in at least one of the pain, fatigue, or distress scales, and 30.4% of the patients scored 7 or higher on all three. The most common problem areas were feeling a burden to others, trouble talking with friends and family, spirituality, and sleep difficulties.
Conclusions
Higher levels of multiple, concurrent symptoms and psychosocial problems were found in Pain Clinic patients than in a group of patients who did not attend the Pain Clinic. Routine screening and triaging of cancer patients using a comprehensive and standardized panel of questions can facilitate symptom assessment and management, and can inform program planning.
Keywords: Screening for distress, pain, fatigue, common problems, oncology, neoplasms
1. INTRODUCTION
Distress in cancer patients has been defined as “an unpleasant emotional experience that interferes with [the] ability to cope with a diagnosis of cancer or its treatments” 1. Cross-sectional studies have documented that approximately 35%–45% of cancer patients in North America experience significant levels of distress 2,3, and in the advanced cancer population, the prevalence of distress may be as high as 60% 4,5.
Although initial conceptualizations of distress focused on anxiety and depression, recent models view distress more broadly as resulting from any, or a combination of, psychosocial, practical, and physical concerns 6. In advanced cancer populations, physical symptoms have been extensively examined 7–10, and research indicates that more severe symptoms are more distressing for patients 11. Most studies that include a broader construct of distress have focused to a greater extent on psychological and symptom distress in the advanced cancer population to the exclusion of practical and other psychosocial concerns. Some studies have been more inclusive in their definition of distress, using the Problem Checklist tool 1 in the assessment of lung cancer patients 12, bone marrow transplant patients 13, and heterogeneous cohorts of cancer patients 14,15; however, those studies did not specifically examine patients with advanced or progressive disease.
In the present study, we sought to prospectively examine distress from the broad perspective of psychosocial, practical, and physical concerns in a group of patients presenting to a Pain Clinic located at a tertiary cancer centre. This group was identified as potentially benefiting from a more thorough screening of their concerns beyond symptom screening, because they were identified as being likely to have higher and more complex symptoms than groups previously studied 3,16. A clearer delineation of the common problems in this group of patients would hold the potential to inform program planning within the cancer program.
2. METHODS
2.1. Participants
All participants were recruited as part of a larger randomized controlled trial examining distress and common problems experienced by cancer patients. The larger study was conducted over a 12-month period, and it screened 3113 patients at the Tom Baker Cancer Center in Calgary, Alberta, Canada. For the present study, baseline data were obtained from the subset of patients who were screened using the distress instrument on the day that they were waiting for appointments at the Pain Clinic. All reasons for nonparticipation in the study were recorded. Another 65 patients completed the distress instrument and were subsequently referred to and seen in the Pain Clinic on a different day; those patients are not included in the current study.
Additionally, a matched convenience sample of patients from the larger randomized controlled trial who had completed the same measures at a different clinic and time were selected as a comparison group. To obtain the matched comparison group, we first removed the 65 patients who had been initially screened then referred to the Pain Clinic from the 3113 patients in the randomized controlled trial sample. From among the remaining 3048 patients, we selected a subsample of 46 matched 1:1 to the Pain Clinic group for interval since diagnosis, age, sex, type of cancer, and treatments received.
2.2. Measures
2.2.1. Demographics and Cancer History
Relevant background characteristics and cancer history variables, including age, sex, marital status, education, ethnic or cultural background, income, source of income, type of cancer, and stage of the treatment process were collected by self-report. Type of cancer and time since diagnosis were obtained from electronic medical records.
2.2.2. The Distress Thermometer
The Distress Thermometer (dt) is a 0–10 visual analog scale vertically oriented in the form of a typical thermometer. A single cut-off score has not yet been defined for all clinical scenarios. A cut-off score of 4 or more has been shown to perform best in terms of sensitivity and specificity for labelling cancer patients with high psychological distress 13,14,17. When calculating concurrent multiple symptoms, a cut-off of 7 or more was used. Hoffman et al. 18 suggested that a score of between 4 and 6 on the dt could indicate possible distress, with a recommendation for referral, and that a score above 7 would indicate definite distress, requiring further assessment or intervention.
2.2.3. Pain
A numerical rating scale from 0–10 19,20 was used to quantify how much pain patients had been experiencing. A cut-off of 4 or more out of 10 has been recommended for use as a screening tool for pain within ambulatory cancer patients 21, and a cut-off of 7 or more was used to identify severe levels of pain.
2.2.4. Fatigue
Fatigue was evaluated on a 0–10 numerical rating scale (ft) similar to the dt; patients were asked to rate their average fatigue in the preceding week. Few studies have examined single-item screening tools for fatigue. One study reported a cut-off of 3 as optimal 22; another reported a cut-off of 5 21. The U.S. National Comprehensive Cancer Network recommends using a cut-off of 4 or more to identify cases of possible fatigue. A cut-off of 7 or more was used to identify severe levels of fatigue.
2.2.5. The Modified Problem Checklist
The Modified Problem Checklist 1 has been adapted to the Canadian setting from the original list published by the U.S. National Comprehensive Cancer Network. It includes 7 practical problems (accommodation, transportation, parking, drug coverage, work or school, income or finances, and groceries) and 13 psychosocial problems (burden to others, worry about family and friends, talking with family, talking with the medical team, treatment decisions, family conflict, changes in appearance; alcohol or drugs, smoking, coping, sexuality, spirituality, and sleep). Participants indicated the presence or absence of each problem in the week preceding completion of the measure.
2.2.6. The Psychological Screen for Cancer
Part C of the Psychological Screen for Cancer 23,24 consists of 10 items rated on a 5-point Likert scale (“not at all” to “very much so”) to measure anxiety and depression. Developed for screening in clinical practice and as a research tool, the Psychological Screen has been validated in two separate groups of cancer patients 23,24. A cut-off score of 11 or more indicates high anxiety and distress.
2.3. Procedure
All procedures were approved by the Conjoint Health Research Ethics Board of the University of Calgary, Faculty of Medicine/Tom Baker Cancer Centre. All patients attending the Pain Clinic for the first time were approached by a screening assistant in the waiting area. All patients in the comparison group were approached by a screening assistant in the waiting area of the outpatient clinics at the Tom Baker Cancer Center. All consenting participants completed the screening on a touch-screen computer.
2.4. Data Analysis
Independent t-tests and chi-square statistics were used to compare the groups on demographic and medical intervention variables and on the mean scores of the dt, pt, and ft. The chi-square statistic was used to analyze between-group differences for categorical outcomes—that is, the percentage of participants at risk for high distress, pain, and fatigue (using a cut-off score of 4 or more) and participants at risk for concurrent severe levels (using a cut-off of 7 or more). The chi-square statistic was used to analyze between-group differences for each individual problem and for two summed problem scores created from the Problem Checklist (practical problems and psychosocial problems). The data were analyzed using the Statistical Package for the Social Sciences, version 17 (SPSS, Chicago, IL, U.S.A.).
3. RESULTS
Of 60 eligible Pain Clinic patients, 46 (76.7%) consented to the study. Of those not consenting, 1 was deemed too ill to participate, and 10 declined participation because of lack of interest (n = 3), unwillingness to consent to research (n = 3), feeling too ill (n = 1), or unspecified reasons (n = 3). Additionally, 3 others in the Pain Clinic were missed at the time of screening. Table i presents demographics for the 46 Pain Clinic patients and the matched comparison group. The groups showed no differences on any demographic or treatment variable.
TABLE I.
Demographics and interventions for participants screened and not screened in the Pain Clinic at baseline
| Variable |
Screened in Pain Clinic |
p Value | |||
|---|---|---|---|---|---|
|
Yes |
No |
||||
| (n) | (%) | (n) | (%) | ||
| Patients | 46 | 46 | |||
| Mean age (years) | 59.06±12.67 | 61.02±14.44 | 0.49 | ||
| Sex | |||||
| Men | 20 | 43.5 | 19 | 41.3 | 0.90 |
| Women | 26 | 56.5 | 27 | 58.7 | |
| Marital status | |||||
| Single | 16 | 34.8 | 17 | 37.0 | 0.71 |
| Married/committed relationship | 30 | 65.2 | 27 | 58.7 | |
| No response | 0 | 0 | 2 | 4.3 | |
| Education | |||||
| High school or less | 24 | 52.2 | 22 | 47.8 | 0.84 |
| More than high school | 22 | 47.8 | 24 | 52.2 | |
| Ethnicity | |||||
| Minority | 4 | 8.7 | 8 | 17.4 | 0.17 |
| Majority | 42 | 91.3 | 36 | 78.3 | |
| No response | 0 | 0 | 2 | 4.3 | |
| Family income | |||||
| <$50,000 | 16 | 34.8 | 20 | 43.5 | 0.34 |
| ≥$50,000 | 19 | 41.3 | 15 | 32.6 | |
| Prefer not to say | 11 | 23.9 | 11 | 23.9 | |
| Income source | |||||
| On support | 19 | 41.3 | 13 | 28.3 | 0.32 |
| Employment/retired | 23 | 50.0 | 25 | 54.3 | |
| Prefer not to say | 4 | 8.7 | 8 | 17.4 | |
| Diagnosis | |||||
| Breast | 8 | 17.4 | 9 | 19.6 | 0.90 |
| Gastrointestinal | 8 | 17.4 | 9 | 19.6 | 0.90 |
| Hematologic | 9 | 19.6 | 7 | 15.2 | 0.49 |
| Other | 21 | 45.6 | 21 | 45.6 | 0.99 |
| Treatment in the past montha | |||||
| Surgery | 2 | 4.3 | 7 | 15.2 | 0.08 |
| Chemotherapy | 11 | 23.9 | 6 | 13.0 | 0.18 |
| Radiation therapy | 7 | 15.2 | 5 | 10.9 | 0.54 |
| Hormone therapy | 2 | 4.3 | 0 | 0 | 0.15 |
| Other | 3 | 6.5 | 3 | 6.5 | 1.00 |
| None | 17 | 44.7 | 21 | 55.3 | 0.40 |
| Mean time since diagnosis (years) | 2.00±1.99 | 2.09±2.11 | 0.84 | ||
Percentages do not total to 100 because some patients (n = 4) received more than one treatment.
3.1. Distress, Pain, Fatigue, Anxiety, and Depression
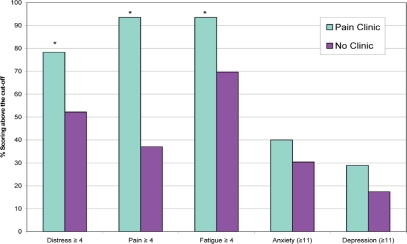
Figure 1 presents the percentage of people in each group who scored above the clinical cut-off for each outcome. A higher percentage of patients in the Pain Clinic group reported scores above the clinical cut-off for distress (χ2 =6.90, df =1, p < 0.01), pain (χ2 =32.39, df =1, p < 0.001), and fatigue (χ2 =8.73, df =1, p < 0.01). No between-group differences were found for anxiety and depression. Using the cut-off of 7 or more, a higher percentage of people in the Pain Clinic group reported severe distress (41.3% vs. 26.1%, p < 0.05), severe pain (73.9% vs. 23.9%, p < 0.001), and severe fatigue (60.9% vs. 34.8%, p < 0.01). There were no statistically significant between-group differences for severe distress (41.3% vs. 26.1%, p = 0.12).
FIGURE 1.
Percentage of patients experiencing symptoms and reporting scores above the clinical cut-off on the Distress Thermometer, the Pain Thermometer, the Fatigue Thermometer, and the Psychological Screen for Cancer anxiety and depression subscales. * p < 0.05.
3.2. Concurrent Symptoms
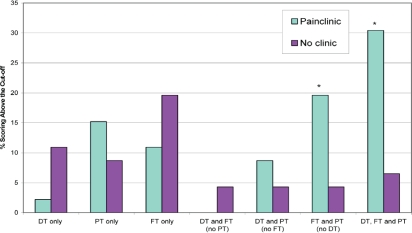
Figure 2 presents the percentage of patients in each group reporting multiple concurrent symptoms. Of the 46 Pain Clinic patients, 40 (87%) scored 7 or higher in at least one of the pain, fatigue, or distress scales; 14 (30.4%) scored 7 or higher in all three. For the comparison group, 27 (58.7%) scored 7 or higher in at least one of the pain, fatigue, or distress scales; only 3 patients (6.5%) scored 7 or higher in all three. When examining specific combinations of symptoms, a much higher percentage of patients in the Pain Clinic group reported experiencing fatigue and pain (χ2 =5.06, df =1, p < 0.05) and also distress, pain, and fatigue (χ2 =16.51, df =1, p < 0.001).
FIGURE 2.
Co-occurrence of symptoms (cut-off: ≥7): the overlap of severe distress [Distress Thermometer (dt)], pain [Pain Thermometer (pt)], and fatigue [Fatigue Thermometer (ft)]. * p < 0.05.
3.3. Common Problems
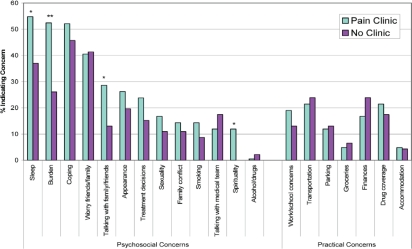
Figure 3 presents common problems endorsed in both groups. Compared with controls, patients in the Pain Clinic group showed a trend to endorse at least 1 psychosocial problem (90.5% vs. 76.1%, p = 0.07) and to endorse a greater overall number of psychosocial problems (t(86) = 1.88, p = 0.06). A higher percentage of patients in the Pain Clinic group endorsed feeling a burden to others and problems with spirituality (p < 0.05). Patients attending the Pain Clinic showed trends to endorse problems with talking with family and friends, and with sleep (p = 0.07). There were no differences between the groups in the practical problems endorsed.
FIGURE 3.
Concerns identified by patients: the percentage of patients endorsing each of the concerns in the Problem Checklist. * p < 0.07. ** p < 0.05.
4. DISCUSSION AND CONCLUSIONS
Our study describes the demographic and symptom profiles of two groups of cancer patients: those attending a Pain Clinic in a tertiary cancer centre, and those attending other clinics. Compared with a matched sample of patients who did not attend the Pain Clinic, the Pain Clinic attendees reported significantly higher levels of pain, distress, and fatigue. Compared with controls, patients in the Pain Clinic were also more likely to report multiple co-occurring severe symptoms, including pain, fatigue, and distress, and additional psychosocial problems. When approached about the results of this study, clinicians in the Pain Clinic indicated that patients commonly present in hopes of obtaining relief from one major symptom, and leave the Clinic with a comprehensive plan for addressing all major symptoms.
Distress in cancer patients is consistently reported as a highly prevalent and significant problem, with incidence rates estimated at 35%–55% 2,3,16. Similarly, pain and fatigue are frequently reported as the most common symptoms in the cancer population 3,25,26, and they have been endorsed as important components of distress 1. Research has focused primarily on identifying and managing these individual symptoms, but recently, there has been a push to move away from examining symptoms in isolation and toward exploring the relationships between multiple co-occurring symptoms 27. Experiencing multiple symptoms can have a negative impact on functional status, quality of life, and symptom distress of people with cancer 9. The presence of multiple symptoms can also increase the burden on the health system, because patients may access acute care services and require crisis intervention more frequently 28.
Ambulatory cancer patients attending a Pain Clinic were also experiencing significant levels of concurrent fatigue and distress and problems connected to feeling a burden, spirituality, sleep, and trouble talking with family and friends. Despite its name, the Pain Clinic was established to address all of the foregoing symptoms and problems within its scope of practice. It has been suggested that treating symptoms individually may not necessarily lead to improvements in patient outcomes, because other symptoms may be present and negatively affecting the patient at the same time 29. Focusing on a patient’s pain alone, without acknowledging the other concerns and the effects they may be having on pain levels, could hinder any potential benefit of a more focused approach to pain management. Thus, the role of comprehensive patient assessment is affirmed, mandating an interdisciplinary approach as the standard of care in a tertiary-level ambulatory Pain Clinic.
Although the present study provides insight into the pattern of symptoms and the common problems that may be experienced by cancer patients presenting with severe pain and other concomitant symptoms, further work using larger sample sizes, more robust study designs (cohort, case–control, or randomized controlled trials), and statistical analysis to better establish the nature of the relationship between distress, symptoms, and psychosocial problems is required. For example, what are the interdependencies between cancer pain and other concurrent symptoms? Is pain experienced by patients exacerbated by the additional symptoms and psychosocial problems? Or are the additional symptoms and psychological problems amplified by uncontrolled pain?
The small sample size did not allow for multivariate or symptom-cluster analyses because the study was intended only to explore the demographic and symptom profiles of patients attending the Pain Clinic. The comparison group was matched from a larger sample not specifically recruited for the purpose of comparison in this study. Even so, no significant differences were observed between the groups on key variables that may contribute to symptoms and psychosocial distress. Other studies on symptom clusters have examined a multitude of physical and psychological symptoms. The literature shows wide variation with respect to the symptoms that should be examined and the methodology that should be used to analyze the relationships 27,29.
The dt was not linked to the Problem Checklist as it was in the original National Comprehensive Cancer Network version 1, and the time period assessed by the Problem Checklist was altered. Hence, it is possible that the interpretations made by patients regarding the source and definition of distress varied considerably. Moreover, the authors acknowledge the need for further validation work on the modified Problem Checklist in a larger sample of patients than was included here. Similar validation work is required for the ft, which has not previously been used in this population.
Given the range of concerns patients were experiencing, the present study is congruent with the results of larger studies 3,16 recommending routine screening, assessment, and intervention for cancer patients to lower their distress and symptom burden. Routine screening for physical, psychosocial, and practical concerns may help to identify individuals who would most benefit from referrals to services to manage concerns 6. Rather than an approach to symptom management that targets each symptom individually, we recommend that co-occurring symptoms be identified so as to provide additional multidisciplinary resources to patients in a timely and efficient manner 29. Specifically, screening and triaging are needed to ensure that multiple concurrent symptoms in patients are managed comprehensively and in a coordinated manner by the appropriate services. To meet these complex patient needs, prospectively surveying patients about their distress can inform program planning and delivery.
5. ACKNOWLEDGMENTS
AW holds a Psychosocial Oncology Research Training Fellowship. LEC holds the Enbridge Research Chair in Psychosocial Oncology, co-funded by Enbridge Inc., the Alberta Cancer Foundation, and the Canadian Cancer Society–Alberta/NWT Division. She also holds an Alberta Heritage Foundation for Medical Research Health Scholar Award. The research reported here was funded in part by the Alberta Cancer Research Institute. The authors particularly thank screening assistants Paula Jones, Jassandre Adamyk, Sacha Bachor, Paula McQuaid, Andrea Williams, and Agnes Sroczynska.
Footnotes
6. CONFLICT OF INTEREST DISCLOSURES
There are no known financial conflicts of interest for the authors.
7. REFERENCES
- 1.National Comprehensive Cancer Network (NCCN) Distress Management. Fort Washington, PA: NCCN; 2002. Ver 1.2002. Practice Guidelines in Oncology. [Google Scholar]
- 2.Zabora J, BrintzenhofeSzoc K, Curbow B, Hooker C, Piantadosi S. The prevalence of psychological distress by cancer site. Psychooncology. 2001;10:19–28. doi: 10.1002/1099-1611(200101/02)10:1<19::AID-PON501>3.3.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 3.Carlson LE, Angen M, Cullum J, et al. High levels of untreated distress and fatigue in cancer patients. Br J Cancer. 2004;90:2297–304. doi: 10.1038/sj.bjc.6601887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Potash M, Breitbart W. Affective disorders in advanced cancer. Hematol Oncol Clin North Am. 2002;16:671–700. doi: 10.1016/S0889-8588(02)00013-8. [DOI] [PubMed] [Google Scholar]
- 5.Thekkumpurath P, Venkateswaran C, Kumar M, Bennett MI. Screening psychological distress in palliative care: a systematic review. J Pain Symptom Manage. 2008;36:520–8. doi: 10.1016/j.jpainsymman.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Bultz BD, Groff S, Fitch M, et al. on behalf of the Screening for Distress Toolkit Working Group Guide to Implementing Screening for Distress, the 6th Vital Sign. Part A: Background, Recommendations, and Implementation. Cancer Journey Action Group, Canadian Partnership Against Cancer; 2009 [Available online at: http://www.partnershipagainstcancer.ca/sites/default/files/Guide_CJAG.pdf; cited April 20, 2011]
- 7.Walsh D, Donnelly S, Rybicki L. The symptoms of advanced cancer: relationship to age, gender, and performance status in 1,000 patients. Support Care Cancer. 2000;8:175–9. doi: 10.1007/s005200050281. [DOI] [PubMed] [Google Scholar]
- 8.Kirkova J, Aktas A, Walsh D, Rybicki L, Davis MP. Consistency of symptom clusters in advanced cancer. Am J Hosp Palliat Care. 2010;27:342–6. doi: 10.1177/1049909110369869. [DOI] [PubMed] [Google Scholar]
- 9.Esther Kim JE, Dodd MJ, Aouizerat BE, Jahan T, Miaskowski C. A review of the prevalence and impact of multiple symptoms in oncology patients. J Pain Symptom Manage. 2009;37:715–36. doi: 10.1016/j.jpainsymman.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teunissen SC, Wesker W, Kruitwagen C, de Haes HC, Voest EE, de Graeff A. Symptom prevalence in patients with incurable cancer: a systematic review. J Pain Symptom Manage. 2007;34:94–104. doi: 10.1016/j.jpainsymman.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 11.Kirkova J, Walsh D, Rybicki L, et al. Symptom severity and distress in advanced cancer. Palliat Med. 2010;24:330–9. doi: 10.1177/0269216309356380. [DOI] [PubMed] [Google Scholar]
- 12.Graves KD, Arnold SM, Love CL, Kirsh KL, Moore PG, Passik SD. Distress screening in a multidisciplinary lung cancer clinic: prevalence and predictors of clinically significant distress. Lung Cancer. 2007;55:215–24. doi: 10.1016/j.lungcan.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ransom S, Jacobsen PB, Booth–Jones M. Validation of the Distress Thermometer with bone marrow transplant patients. Psychooncology. 2006;15:604–12. doi: 10.1002/pon.993. [DOI] [PubMed] [Google Scholar]
- 14.Jacobsen PB, Donovan KA, Trask PC, et al. Screening for psychologic distress in ambulatory cancer patients. Cancer. 2005;103:1494–502. doi: 10.1002/cncr.20940. [DOI] [PubMed] [Google Scholar]
- 15.Shim EJ, Shin YW, Jeon HJ, Hahm BJ. Distress and its correlates in Korean cancer patients: pilot use of the Distress Thermometer and the Problem List. Psychooncology. 2008;17:548–55. doi: 10.1002/pon.1275. [DOI] [PubMed] [Google Scholar]
- 16.Carlson LE, Groff SL, Maciejewski O, Bultz BD. Screening for distress in lung and breast cancer outpatients: a randomized controlled trial. J Clin Oncol. 2010;28:4884–91. doi: 10.1200/JCO.2009.27.3698. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell AJ. Pooled results from 38 analyses of the accuracy of Distress Thermometer and other ultra-short methods of detecting cancer-related mood disorders. J Clin Oncol. 2007;25:4670–81. doi: 10.1200/JCO.2006.10.0438. [DOI] [PubMed] [Google Scholar]
- 18.Hoffman BM, Zevon MA, D’Arrigo MC, Cecchini TB. Screening for distress in cancer patients: the nccn rapid-screening measure. Psychooncology. 2004;13:792–9. doi: 10.1002/pon.796. [DOI] [PubMed] [Google Scholar]
- 19.Dworkin RH, Turk DC, Farrar JT, et al. Core outcome measures for chronic pain clinical trials: immpact recommendations. Pain. 2005;113:9–19. doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 20.Cleeland CS, Ryan KM. Pain assessment: global use of the brief pain inventory. Ann Acad Med Singapore. 1994;23:129–38. [PubMed] [Google Scholar]
- 21.Butt Z, Wagner LI, Beaumont JL, et al. Use of a single-item screening tool to detect clinically significant fatigue, pain, distress, and anorexia in ambulatory cancer practice. J Pain Symptom Manage. 2008;35:20–30. doi: 10.1016/j.jpainsymman.2007.02.040. [DOI] [PubMed] [Google Scholar]
- 22.Kirsh KL, Passik S, Holtsclaw E, Donaghy K, Theobald D. I get tired for no reason: a single item screening for cancer-related fatigue. J Pain Symptom Manage. 2001;22:931–7. doi: 10.1016/S0885-3924(01)00350-5. [DOI] [PubMed] [Google Scholar]
- 23.Linden W, Yi D, Barroetavena MC, MacKenzie R, Doll R. Development and validation of a psychosocial screening instrument for cancer. Health Qual Life Outcomes. 2005;3:54. doi: 10.1186/1477-7525-3-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linden W, Andrea Vodermaier A, McKenzie R, Barroetavena MC, Yi D, Doll R. The psychosocial screen for cancer (psscan): further validation and normative data. Health Qual Life Outcomes. 2009;7:16. doi: 10.1186/1477-7525-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morrow GR, Shelke AR, Roscoe JA, Hickok JT, Mustian K. Management of cancer-related fatigue. Cancer Invest. 2005;23:229–39. doi: 10.1081/CNV-200055960. [DOI] [PubMed] [Google Scholar]
- 26.Holland JC, Bultz BD, on behalf of the National Comprehensive Cancer Network (nccn) The nccn guideline for distress management: a case for making distress the sixth vital sign. J Natl Compr Canc Netw. 2007;5:3–7. [PubMed] [Google Scholar]
- 27.Miaskowski C, Aouizerat BE, Dodd M, Cooper B. Conceptual issues in symptom clusters research and their implications for quality-of-life assessment in patients with cancer. J Natl Cancer Inst Monogr. 2007:39–46. doi: 10.1093/jncimonographs/lgm003. [DOI] [PubMed] [Google Scholar]
- 28.Gilbertson–White S, Aouizerat BE, Jahan T, Miaskowski C. A review of the literature on multiple symptoms, their predictors, and associated outcomes in patients with advanced cancer. Palliat Support Care. 2011;9:81–102. doi: 10.1017/S147895151000057X. [DOI] [PubMed] [Google Scholar]
- 29.Fan G, Filipczak L, Chow E. Symptom clusters in cancer patients: a review of the literature. Curr Oncol. 2007;14:173–9. doi: 10.3747/co.2007.145. [DOI] [PMC free article] [PubMed] [Google Scholar]