Abstract
Background
This phase ii clinical trial examined the activity of a metronomic dosing schedule of docetaxel and capecitabine chemotherapy in patients with advanced breast cancer. Patients also received daily oral celecoxib in an effort to improve outcome measures and to ameliorate some of the common side effects of chemotherapy.
Methods
Patients received docetaxel at a starting dose of 15 mg/m2 weekly, oral capecitabine 1250 mg/m2 once daily, and oral celecoxib 200 mg twice daily. The primary endpoint was clinical benefit: percentage of patients experiencing either an objective response or stable disease (sd) for more than 6 months. In the absence of significant neutropenia, the dose of docetaxel was escalated after 4 and 8 weeks of treatment. Therapy was given until disease progression or development of unacceptable toxicity. The level of thymidine phosphorylase expression in peripheral white blood cells of patients was measured before and during treatment to determine the effect on this capecitabine-activating enzyme.
Results
Of 47 patients enrolled, 38 (81%) completed treatment to a disease endpoint. No complete responses were achieved, but 13 of the 38 patients (34%) experienced a partial response, and another 3 patients (8%) experienced sd for more than 6 months. The clinical benefit rate was therefore 42% (95% confidence interval: 27% to 57%). The median time to disease progression for all evaluable patients was 3.6 months (range: 0.9–21.7 months). The most common nonhematologic toxicities were diarrhea, plantar– palmar erythrodysesthesia, fatigue, mucositis, and vomiting. Most patients (89%) received combination chemotherapy until disease progression.
Conclusions
The present study demonstrates that metronomic docetaxel–capecitabine chemotherapy with daily celecoxib can produce significant anticancer activity, with predictable toxicity. Efficacy fell short of expectations, with outcome measures being similar to those obtained when the study agents are given in conventional dosing. Furthermore, there is mounting evidence to indicate that a low dose of taxanes fails to induce thymidine phosphorylase expression, an effect believed to be important in achieving therapeutic synergism when taxanes are given concurrently with capecitabine.
Keywords: Metastatic breast cancer, metronomic chemotherapy, docetaxel, capecitabine
1. INTRODUCTION
Despite intensive research into novel therapies, metastatic breast cancer remains incurable, and the management, palliative. This dilemma persists despite the fact that breast cancer is sensitive (at least initially) to the effects of most classes of chemotherapeutic agents 1. The introduction of targeted agents into clinical practice has had only a modest impact on outcome measures.
New drug development is important, but patients might benefit by optimization of the use of existing agents. The availability of numerous antineoplastic drugs permits the design of studies using combinations of two or more existing agents in an effort to achieve therapeutic synergism. Many clinical trials have attempted to use the maximum tolerated doses of all the agents in the regimen. That approach has been of limited benefit in the palliative setting, because overlapping toxicities lead to diminished quality of life. Studies involving novel dosing schedules based on the concepts of either chronotherapy 2 or metronomic chemotherapy—antineoplastic agents given at low (non-toxic) doses in uninterrupted fashion 3—indicate that the therapeutic index of certain drugs can be improved.
In the present study, patients with metastatic breast cancer and prior anthracycline exposure were treated with a combination of docetaxel and capecitabine. This doublet was chosen because preclinical investigations indicate that taxanes and fluoropyrimidines have the potential for therapeutic synergy 4. Docetaxel induces expression of thymidine phosphorylase (tp, an enzyme essential for the metabolism of capecitabine to the active drug 5-fluorouracil) in tumour cells 5—a potential mechanism for synergism. Those two drugs were given to study patients in a metronomic schedule involving once-daily oral capecitabine and weekly infusions of docetaxel. Drug doses were chosen with the aim of permitting prolonged continuous administration while minimizing toxicity. Patients also received concurrent treatment with celecoxib (Celebrex: Pfizer Canada, Kirkland, QC) twice daily in an attempt to produce anticancer activity mediated through anti-angiogenic (and other) mechanisms 6.
2. METHODS
This phase ii study was conducted at the North-eastern Ontario Regional and Sunnybrook Odette cancer centres. Patients were eligible if they had histologic proof of breast cancer and had developed anthracycline-resistant or -refractory locally advanced or metastatic disease. The definition of anthracycline-resistant or -refractory was development of metastatic disease less than 2 years after anthracycline-based adjuvant chemotherapy or progression of locally advanced or metastatic disease after at least 2 cycles of anthracycline-based chemotherapy. Participants had to have an Eastern Cooperative Oncology Group performance status of 0–2, a life expectancy of at least 3 months, and measurable disease by the Response Evaluation Criteria in Solid Tumors (version 1.0). Tumours had to be negative for the human epidermal growth factor 2 (her2) by local testing.
Patients were ineligible if they had recently been exposed to a taxane (that is, as an adjuvant regimen within the preceding 6 months) or to any treatment with capecitabine of more than 1 week’s duration. They also must have discontinued any use of a Cox-2 inhibitor at least 1 week before enrolment. Eligible patients could not have received any form of chemotherapy within the preceding 4 weeks, and they could not have untreated central nervous system metastases or have received radiotherapy for central nervous system disease less than 1 month before participation.
Participants required adequate organ function characterized as follows: bone marrow (hemoglobin > 80 g/L, absolute neutrophil count > 1.5×109/L, platelet count > 100×109/L), heart (absence of unstable angina, congestive heart failure, and life-threatening dysrhythmia), lungs (oxygen dependency < 2 L/min), gastrointestinal system (absence of chronic diarrhea or gastrointestinal hemorrhage within the preceding 12 months, liver transaminases < 3 times the upper limit of normal, bilirubin < 1.5 times the upper limit of normal), and kidneys (creatinine < 150 μmol/L and not on dialysis). Patients could not have a previous diagnosis of coronary artery or cerebrovascular disease, but were permitted to be on low-dose cardioprotective oral aspirin (<325 mg daily). Local research ethics board approval for the study was obtained.
All patients were initially treated with once-weekly intravenous docetaxel 15 mg/m2 in combination with once-daily oral capecitabine 1250 mg/m2 starting on day 1. Treatment was administered for 4 weeks before consideration of docetaxel dose escalation to 20 mg/m2, which was permitted if no grade 3 or 4 neutropenia developed. All patients received twice-daily oral celecoxib 200 mg starting on day 1, to be taken continuously throughout the duration of therapy. A second escalation of the docetaxel to 25 mg/m2 (maximum) was permitted at 8 weeks. All patients received oral dexamethasone 4 mg every 12 hours for 6 doses starting the day before each docetaxel infusion. Patients were allowed to receive all forms of supportive therapy according to the attending oncologist’s recommendations. Palliative radiation could be administered to sites that would not subsequently be used to determine response to systemic therapy.
At enrolment, baseline complete blood count, electrolytes, creatinine, and liver function were determined. The complete blood count was repeated each week before the docetaxel administration for the first 2 months, and then every 2–4 weeks if counts were stable. Every 8 weeks, patients were monitored for compliance to treatment and for response of their measurable disease by the Response Evaluation Criteria in Solid Tumors.
The primary endpoint for the study was clinical benefit: percentage of patients experiencing either an objective response or disease stabilization for more than 6 months. Secondary endpoints included time to disease progression, overall survival, and toxicities according to the U.S. National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 3.0.
2.1. Sample Size and Statistical Considerations
The study was designed to determine a point estimate for the clinical benefit rate in the study population receiving this novel therapy. The sample size in this prospective cohort study was 38.
According to published tables 7, the clinical benefit rate was measured with a precision that extends to ±15 percentage points with a 95% probability, assuming a benefit rate of 50%. All outcomes are presented as descriptive statistics (means, medians, or proportions). Median time to progression and overall survival from the first cycle of chemotherapy was determined using the Kaplan–Meier method, with the date of re-imaging (or death) as the censoring date. An interim analysis of the primary endpoint was performed after enrolment of 14 patients to ensure that at least 1 response was achieved before proceeding further with the study.
2.2. Determination of TP Levels in Circulating White Blood Cells
Venous blood samples were collected from the participants in PAXgene Blood RNA tubes (Qiagen, Mississauga, ON) at baseline and after 4 and 8 weeks of therapy. Total rna was isolated from the blood sample using the PAXgene Blood RNA kit (Qiagen), and the amount of rna was measured using spectroscopy. Integrity of rna was determined using RNA 6000 Nano RNAchips in a 2100 Bioanalyzer (Agilent Technologies Canada, Mississauga, ON). Samples with rna concentrations of more than 0.05 μg/mL and a rna integrity number of at least 5 were used for analysis.
The rna (1 μg) was reverse-transcribed by application of the MMLV-RT enzyme (SuperScript II) purchased from InVitrogen (Burlington, ON) using Oligo(dT)18 Primer. Quantitative polymerase chain reaction was carried out on the resulting complementary dna (cdna) using specific TaqMan Gene Expression assays on an AB17700 Real-Time PCR System (Applied Biosystems: Life Technologies, Carlsbad, CA, U.S.A.). Expression of tp and of beta-actin (the calibrator) was determined using specific primers and 6-carboxy-fluorescein (fam)–labelled probes in the TaqMan methodology, with comparison to a standard curve. The polymerase chain reaction was carried out using 40 cycles of 95°C for 15 seconds and of 60°C for 60 seconds. The fam-dependent fluorescence was determined, and the amounts of tp and beta-actin cdna were calculated using the ABI Prism 7700 Sequence Detector system software (Life Technologies). The relative level of tp expression was normalized by comparison with the levels of the beta-actin calibrator in each sample.
3. RESULTS
3.1. Patient Characteristics
Table i summarizes the characteristics of the study population. All participants were female, and the median age of the cohort was 54.6 years (range: 32–76 years). Most of these women (87%) were postmenopausal and had a good performance status (range: 0–1). More than 50% had at least 3 sites of metastatic involvement. All but 1 patient had prior anthracycline exposure, either in the adjuvant setting or as first-line treatment of their metastatic disease, and 11% had prior exposure to a taxane in the adjuvant setting. The median number of prior lines of chemotherapy was 2 (range: 1–4). Of the 47 patients enrolled, 38 (81%) completed treatment to a disease-response endpoint (that is, week 8). The reasons for early discontinuation of therapy in 9 patients who were not evaluable for response were excess toxicity (n = 5), rapid clinical deterioration (n = 2), and death (n = 2).
TABLE I.
Characteristics of the study patients
| Characteristic | Value |
|---|---|
| Patients (n) | 47 |
| Age (years) | |
| Median | 54.6 |
| Range | 32–76 |
| Ethnicity [n (%)] | |
| Caucasian | 41 (87.2) |
| Asian | 6 (12.8) |
| Menopausal status [n (%)] | |
| Pre | 6 (12.8) |
| Post | 41 (87.2) |
| ecog performance status [n (%)] | |
| 0–1 | 40 (85.1) |
| 2 | 6 (12.8) |
| 3 | 1 (2.1) |
| Metastases sites [n (%)] | |
| Location | |
| Liver | 30 (63.8) |
| Lung | 29 (61.7) |
| Bone | 22 (46.8) |
| Lymph nodes | 21 (44.7) |
| Ascites/pleural effusion | 10 (21.3) |
| Brain | 1 (2.1) |
| Number | |
| 1 | 6 (12.8) |
| 2 | 14 (29.8) |
| ≥3 | 27 (57.4) |
| Receptor status [n (%)] | |
| Positive | 26 (55.3) |
| Negative | 19 (40.4) |
| Unknown | 2 (4.3) |
| Prior anthracycline exposure [n (%)] | |
| Adjuvant only | 23 (48.9) |
| Adjuvant taxane | 5 (10.6) |
| Metastatic only | 16 (34.0) |
| Both adjuvant and metastatic | 7 (14.9) |
ecog = Eastern Cooperative Oncology Group.
3.2. Efficacy
Among the 38 patients evaluable for response, 76% received dose escalation of docetaxel to 20 mg/m2, and 32% went on to the second dose escalation to 25 mg/m2. No patient achieved a complete response; however, of the 38 evaluable patients, 13 (34%) had a partial response (9 confirmed, 4 unconfirmed), and another 3 (8%) experienced disease stabilization for at least 6 months. Hence, the primary endpoint of clinical benefit was 42% (95% confidence interval: 27% to 57%). The median time on treatment for these evaluable patients was 94 days. Of the 38 evaluable patients, 34 (89%) remained on both docetaxel and capecitabine for the duration of therapy (at the end of study, 3 patients were on docetaxel alone, and 1 patient was on capecitabine alone). The median time to disease progression for all evaluable patients was 3.6 months (range: 0.9–21.7 months); for those experiencing clinical benefit, it was 8.4 months (range: 3.7–21.7 months). The median overall survival for all study patients was 9.8 months (range: 0.5–57.7 months).
3.3. Toxicity
Table ii summarizes the observed hematologic toxicities for all enrolled patients. Fewer than 10% of patients experienced grade 3 anemia, leucopoenia, or thrombocytopenia (no grade 4 events occurred). Only 1 episode of febrile neutropenia was reported (none of the participants received support with granulocyte colony-stimulating factor).
TABLE II.
Hematologic adverse events
| Event |
Gradea[n (%) patients] |
||
|---|---|---|---|
| 1 | 2 | 3 | |
| Anemia | 16 (34) | 12 (26) | 3 (6) |
| Febrile neutropenia | 0 (—) | 0 (—) | 1 (2) |
| Leukopenia | 5 (11) | 5 (11) | 4 (9) |
| Neutropenia | 9 (19) | 3 (6) | 2 (4) |
| Thrombocytopenia | 13 (28) | 1 (2) | 1 (2) |
Evaluated according to the Common Terminology Criteria for Adverse Events, version 3.0.
The most common serious nonhematologic toxicity was diarrhea (grade 3), which was observed in 15% of patients (no grade 4 events were reported, Table iii). Other serious toxicities occurring in at least 5% of patients were plantar–palmar erythrodysesthesia (13%), fatigue (13%), mucositis (9%), nail changes (6%), thrombosis (6%), and vomiting (6%). During the 777 patient–weeks of treatment, 3 thromboembolic events occurred (1 at grade 3, 2 at grade 4) without any events of acute myocardial infarction or cerebrovascular accident. The study treatment was discontinued because of toxicities in 12 of the 47 patients (25%): in 4 because of composite toxicity; in 6 because of grade 3 vomiting or diarrhea (3 each); and in 2 because of progressive fatigue. One death was attributable to treatment: it involved a fatal gastrointestinal hemorrhage in a patient receiving therapeutic anticoagulation with warfarin for deep-vein thrombosis. Capecitabine is known to enhance the anticoagulant effect of warfarin, but the affected patient had a normal international normalized ratio 2 days before death.
TABLE III.
Treatment-related nonhematologic adverse events reported by 5% or more patients
| Event |
Gradea[n (%) patients] |
|||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Alopecia | 12 (26) | 1 (2) | — | — |
| Anorexia | 6 (13) | 2 (4) | 2 (4) | 0 (—) |
| Blurred vision | 2 (4) | 1 (2) | 1 (2) | 0 (—) |
| Diarrhea | 11 (23) | 4 (9) | 7 (15) | 0 (—) |
| Dizziness | 4 (9) | 0 (—) | 0 (—) | 0 (—) |
| Dry mouth | 5 (11) | 0 (—) | 0 (—) | — |
| Dry skin | 3 (6) | 0 (—) | 0 (—) | — |
| Dyspepsia | 1 (2) | 2 (4) | 1 (2) | — |
| Dyspnea | 2 (4) | 3 (6) | 1 (2) | 0(—) |
| Edema | 19 (40) | 4 (9) | 0 (—) | 0 (—) |
| Epistaxis | 19 (40) | 0 (—) | 0 (—) | 0 (—) |
| Fatigue | 6 (13) | 16 (34) | 5 (11) | 1 (2) |
| Hand–foot | 5 (11) | 9 (19) | 6 (13) | — |
| Infectiona | — | 9 (19) | 1 (2) | 0 (—) |
| Mucositis | 12 (26) | 4 (9) | 4 (9) | 0 (—) |
| Muscle weakness | 4 (9) | 1 (2) | 1 (2) | 0 (—) |
| Nail changes | 3 (6) | 6 (13) | 3 (6) | — |
| Nausea | 10 (21) | 2 (4) | 2 (4) | 0 (—) |
| Other neurologicb | 3 (6) | 0 (—) | 0 (—) | 0 (—) |
| Pain | 14 (30) | 5 (11) | 0 (—) | 0 (—) |
| Pigment change | 4 (9) | 0 (—) | — | — |
| Rash | 3 (6) | 0 (—) | 0 (—) | 0 (—) |
| Sensory neuropathy | 13 (28) | 2 (4) | 1 (2) | 0 (—) |
| Taste alteration | 12 (26) | 1 (2) | — | — |
| Tearing | 11 (23) | 2 (4) | 0 (—) | — |
| Thrombosis | — | 0 (—) | 1 (2) | 2 (4) |
| Vomiting | 10 (21) | 2 (4) | 3 (6) | 0 (—) |
Normal or unknown absolute neutrophil count.
Memory impairment, trouble focusing, restlessness.
3.4. Effects of Treatment on White Blood Cell TP Expression
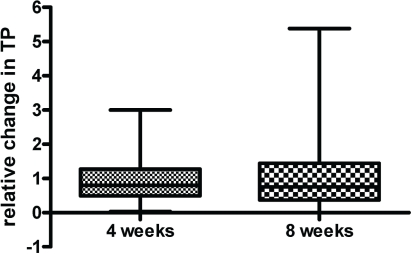
The analyzed rna preparations (n = 87) showed that the isolated rna was usually not significantly degraded: mean rna integrity numbers were 6.9 ± 1.4 (standard deviation), and the selected acceptable samples had rna integrity numbers greater than 5. In 4 preparations at baseline, 15 at 4 weeks of treatment, and 17 at 8 weeks of treatment, rna was unacceptable or unavailable (rna preparations in 6 participants were unacceptable at both 4 and 8 weeks of treatment). Compared with baseline levels, the normalized tp/beta-actin ratio after 4 weeks of treatment was 1.1 (95% confidence interval: 0.71–1.4; n = 29), and after 8 weeks, the relative change was 1.3 (95% confidence interval: 0.68–2.0, n = 24), which indicated no treatment-dependent changes in tp expression (Figure 1).
FIGURE 1.
Thymidine phosphorylase (tp) expression levels measured during the early phase of chemotherapy. Levels of tp expression in whole blood samples taken from subjects at baseline, at 4 weeks of treatment, and at 8 weeks of treatment were determined using quantitative polymerase chain reaction. Levels were normalized for beta-actin expression.
4. DISCUSSION
This study examined the clinical benefit and associated toxicities of docetaxel–capecitabine chemotherapy when given using a metronomic schedule and in combination with celecoxib. It attempted to combine therapeutic synergy with anti-angiogenic therapy in an effort to achieve anticancer activity with minimal toxicity. Indeed, compared with three-weekly dosing, weekly administration of paclitaxel is increasingly becoming a global standard of care in both adjuvant and metastatic breast cancer because of enhanced efficacy and reduced toxicity. Studies using experimental tumour models 4,8 have empirically shown that docetaxel–fluoropyrimidine combinations exhibit synergistic antineoplastic activity. The rationale for combining taxanes with capecitabine has been reviewed by Maher and Villalona–Calero 9. Docetaxel and capecitabine have significant activity against breast cancer when given as single agents; however, the enhanced activity with combination therapy appears to be mediated by upregulation of tp in neoplastic cells by taxanes 5,9.
Studies examining human breast cancer specimens have shown that tp is frequently expressed in these tumours 10–12, and levels are higher in malignant tumours than in benign lesions 11. Thymidine phosphorylase is believed to be involved in tumour angiogenesis, as its expression correlates with tumour vessel density 10–12. Studies using murine tumour models have shown that levels of tp expression are influenced by a number of agents, including cytokines 13, chemotherapeutic drugs (including taxanes 5,14), and radiation 15. Two studies involving neoadjuvant therapy 16,17 showed that treatment with docetaxel (60–75 mg/m2 every 21 days) was associated with increased expression of tp in serial biopsies from patients with locally advanced breast cancer.
Phase i clinical trials using combined docetaxel–capecitabine chemotherapy have demonstrated significant anticancer activity without unexpected toxicity 18. A large phase iii clinical trial involving patients with anthracycline-resistant metastatic breast cancer demonstrated a significant overall survival advantage (3 months) for patients who received combined docetaxel–capecitabine chemotherapy compared with those who received single-agent docetaxel 19. This survival advantage was achieved at the cost of substantially increased toxicity, which necessitated frequent dose reductions or omissions. Importantly, 31% of patients randomized to the combined therapy arm discontinued one of the agents before disease progression (18% discontinued docetaxel and 13% discontinued capecitabine 20). The impact of the required dose reductions and drug discontinuations on outcome measures is unknown. Statistically significant improvements in response rates, time to tumour progression, and overall survival were also demonstrated in a subsequent randomized study 21 involving patients treated either with docetaxel– capecitabine or with single-agent docetaxel as firstline treatment for metastatic breast cancer.
In the current study, we attempted to minimize the toxicity of docetaxel–capecitabine chemotherapy by using lower doses of the drugs and giving them more frequently and without interruption. The potential value of metronomic chemotherapy in the treatment of breast cancer has been reviewed 22. A predefined dose escalation schedule for docetaxel was used in our study in an effort to avoid excess initial toxicity. The relative dose intensities of docetaxel and capecitabine during the initial 4 weeks of therapy (docetaxel 15 mg/m2 weekly, and oral capecitabine 1250 mg/m2 daily) compared with those in the phase iii study reported by O’Shaughnessy et al. 19 were 0.6 and 0.75 respectively. Weekly docetaxel is an accepted form of palliative chemotherapy in the treatment of metastatic carcinomas and, compared with administration every 3 weeks, is associated with lower incidences of myelosuppression, fatigue, nausea and vomiting, mucositis, diarrhea, neurotoxicity, and fluid retention 23. The commonly encountered toxicities tend to develop gradually, typically are not life-threatening, and generally resolve after docetaxel is withheld. The duration of treatment with capecitabine is generally limited by acute toxicity or disease progression. To promote compliance and permit continuous administration in the current study, capecitabine was given once daily (as opposed to the twice-daily standard dosing). The pharmacokinetics of orally administered capecitabine is complex, given this agent’s dependence on absorption and its need for several enzymatic steps in the production of metabolites with varying half-lives (one reaction occurring preferentially in the tumour cell population) 24. It appears unlikely that the once-daily administration of capecitabine used in the present study resulted in “underdosing,” given that a significant proportion of the patients experienced the toxicities (mucositis, palmar–plantar erythrodysesthesia) that are characteristic for this agent and that were found to resolve after capecitabine was withheld.
The objective response rate observed in the assessable patients (34%) was consistent with the literature for single-agent docetaxel 1 or weekly docetaxel given with intermittent capecitabine 25,26. In the study by O’Shaughnessy et al. 19, the objective response rate seen in patients randomized to the docetaxel– capecitabine arm was 42% (5% complete response rate), and the time to disease progression 6.1 months (compared with 3.6 months in our patients). Caution must be used when comparing the efficacy of these two regimens, because the outcome measures are influenced by patient populations and trial methodologies. In retrospect, the decision to use docetaxel dose escalation may have been a weakness of our trial design. An important principle of metronomic chemotherapy is to administer drugs at doses that are minimally toxic. Escalation of the docetaxel dose in our study was an attempt to ensure that early disease progression did not occur as a consequence of underdosing. However, that design ultimately led to increased toxicity, with 25% of patients discontinuing therapy because of side effects (Table iv). An alternative approach would have been to use docetaxel at 15 mg/m2 in combination with capecitabine and, at the time of disease progression, to consider “salvage” therapy with a taxane given in conventional fashion.
TABLE IV.
Comparison of treatment-related grades 3 and 4 adverse events occurring in 5% or more patients
| Event |
Patients (%) in |
|
|---|---|---|
| Present studya(n=47) | O’Shauhnessy et al., 200219,b(n=251) | |
| Alopecia | —c | 6 |
| Diarrhea | 15 | 14 |
| Fatigue/asthenia | 13 | 8 |
| Febrile neutropenia | 2 | 16 |
| Hand–foot syndrome | 13 | 24 |
| Mucositis/stomatitis | 9 | 17 |
| Nausea | 4 | 6 |
| Neutropenia | 4 | 16 |
Evaluated according to the Common Terminology Criteria for Adverse Events, version 3.0.
Evaluated according to the Common Toxicity Criteria (probably the 1991 version).
No grade 3 or 4 defined.
The data in Figure 1 suggest that metronomic docetaxel–capecitabine does not induce expression of tp in the circulating white blood cells of patients. Levels of tp activity in circulating white blood cells have previously been studied in cancer patients receiving interferon therapy 27, in which a marked and prolonged induction of tp activity was observed after a single dose of that cytokine. The apparent lack of induction of tp expression in white blood cells in our study has several potential explanations. The most likely is that the doses of docetaxel administered in the first 2 months (15 mg/m2 and 20 mg/m2) were insufficient to induce tp expression. Bartsch et al. 28 obtained similar results in patients with metastatic breast cancer treated with weekly paclitaxel at 80 mg/m2. They used a colorimetric assay to measure tp protein levels in peripheral mononuclear cells as a function of time on treatment and observed no evidence for induction. This explanation is further supported by data reported by Layman et al. 29, who measured tp levels in tumour specimens taken from breast cancer patients receiving low-dose (weekly) docetaxel neoadjuvant chemotherapy. They found no increased expression of tp (as assayed by quantitative immunofluorescence analysis) in 24 patients who underwent a repeat biopsy 5 days after a single dose of docetaxel (36 mg/m2). That finding contrasts with the results of the two previously mentioned studies 16,17 that used a similar methodology, but higher doses of docetaxel (60–75 mg/m2), and recorded enhanced tp expression in most cases. The data from the study by Layman et al. 29 and our own results in the present work (Figure 1) suggest that lower doses of docetaxel may not achieve the therapeutic synergism desired with co-administration of capecitabine.
The pivotal role that Cox-2 plays in carcinogenesis and tumour progression has been reviewed 6,30. This enzyme is frequently expressed in both invasive and in situ breast cancers 31,32. Inhibitors of Cox-2 may interfere with tumour growth by a number of mechanisms, including inhibition of angiogenesis or lymphangiogenesis 33. Extensive preclinical data indicate that Cox-2 inhibition can enhance the cytotoxic effects of chemotherapeutic agents 34. Studies using cultured monocytes and macrophages indicate that exposure to taxanes may actually stimulate expression of Cox-2 35. That observation is supported by data from a study involving lung cancer patients treated with preoperative taxane-based chemotherapy 36. It should also be noted that the anti-inflammatory properties of celecoxib may reduce the severity of some of the common toxicities of docetaxel and capecitabine, namely arthralgias and hand–foot syndrome.
Studies examining the potential anticancer activity of celecoxib (either with or without standard systemic therapy) in the treatment of breast cancer 37,38 have used a higher dose of this agent (400 mg orally twice daily) than the dose used for our patients. That higher dose may provide increased anticancer activity, given that it has been shown to be more effective at preventing colonic polyp formation than either 100 mg or 200 mg twice daily 39. Sauter et al. 40 reported that treatment of healthy women with oral celecoxib 400 mg (but not 200 mg) twice daily resulted in lower levels of prostaglandin E2 (a product of Cox-2 activity) in breast nipple aspirates. The optimal therapeutic dose of celecoxib in cancer patients has not yet been determined because the dose required for inhibition of carcinogenesis may be different from that needed for treatment of established tumours.
5. CONCLUSIONS
To summarize, weekly docetaxel and daily oral capecitabine can be safely administered to patients with metastatic breast cancer. The regimen is associated with significant anticancer activity when given in metronomic fashion. No attempt was made in the present study to evaluate the effects of therapy on tumour angiogenesis; however, for most of our patients, the anticancer activity achieved was not as dramatic as that observed in tumour-bearing mice treated with metronomic chemotherapy. Mounting evidence suggests that, in human cancers, lower doses of docetaxel may not be sufficient to induce tp expression, an effect thought to be important for therapeutic synergism when docetaxel is being given concurrently with capecitabine. It should be noted that, in the present study, tp expression was examined in the white blood cells of patients as a surrogate of what might be occurring in the target population (endothelial cells involved in tumour angiogenesis or actual tumour cells). The contribution of celecoxib to outcome measures is not assessable from the study data, given that the study was uncontrolled. However, no unexpected serious side effects attributable to this Cox-2 inhibitor occurred. The most important finding of the study is that this metronomic schedule for docetaxel and oral capecitabine did not appear to increase the therapeutic index of those agents over that achieved with standard dosing.
6. ACKNOWLEDGMENTS
This research was conducted with grants from Roche Canada and The Northern Cancer Research Foundation. The authors extend their gratitude to the patients and their families; to Suzanne Cecchetto and Anne Jack (clinical trials nurses); to Jennifer Dumont (clinical research associate); and to Dr. Kathleen Pritchard for their commitment to this trial. We are grateful to Dr. J. Noble for his insightful comments on the manuscript. We also thank Heather Panas (administrative secretary) for her invaluable efforts in the preparation of the manuscript.
Footnotes
7. CONFLICT OF INTEREST DISCLOSURES
SDY has received research funding (Roche), an honorarium (Roche), and travel grants (Roche, Aventis). MJC has received research funding (Roche) and honoraria (Roche, Aventis). RML has no financial conflicts of interest to disclose.
8. REFERENCES
- 1.Vogel CL, Nabholtz JM. Monotherapy of metastatic breast cancer: a review of newer agents. Oncologist. 1999;4:17–33. [PubMed] [Google Scholar]
- 2.Levi F. Chronotherapeutics: the relevance of timing in cancer therapy. Cancer Causes Control. 2006;17:611–21. doi: 10.1007/s10552-005-9004-7. [DOI] [PubMed] [Google Scholar]
- 3.Pasquier E, Kavallaris M, André N. Metronomic chemotherapy: new rationale for new directions. Nat Rev Clin Oncol. 2010;7:455–65. doi: 10.1038/nrclinonc.2010.82. [DOI] [PubMed] [Google Scholar]
- 4.Smorenburg CH, Sparreboom A, Bontenbal M, Verweij J. Combination chemotherapy of the taxanes and antimetabolites: its use and limitations. Eur J Cancer. 2001;37:2310–23. doi: 10.1016/S0959-8049(01)00309-4. [DOI] [PubMed] [Google Scholar]
- 5.Sawada N, Ishikawa T, Fukase Y, Nishida M, Yoshikubo T, Ishitsuka H. Induction of thymidine phosphorylase activity and enhancement of capecitabine efficacy by Taxol/Taxotere in human cancer xenografts. Clin Cancer Res. 1998;4:1013–19. [PubMed] [Google Scholar]
- 6.Méric JB, Rottey S, Olaussen K, et al. Cyclooxygenase-2 as a target for anticancer drug development. Crit Rev Oncol Hematol. 2006;59:51–64. doi: 10.1016/j.critrevonc.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Lwanga SK, Lemeshow S. Sample Size Determination in Health Studies. A Practical Manual. Geneva, Switzerland: World Health Organization; 1991. [Google Scholar]
- 8.Takeda Y, Yoshizaki I, Nonaka Y, Yanagie H, Matsuzawa A, Eriguchi M. Docetaxel alone or orally combined with 5-fluorouracil and its derivatives: effects on mouse mammary tumor cell line MM2, in vitro and in vivo. Anticancer Drugs. 2001;12:691–8. doi: 10.1097/00001813-200109000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Maher JF, Villalona–Calero MA. Taxanes and capecitabine in combination: rationale and clinical results. Clin Breast Cancer. 2002;2:287–93. doi: 10.3816/CBC.2002.n.004. [DOI] [PubMed] [Google Scholar]
- 10.Toi M, Inada K, Hoshina S, Suzuki H, Kondo S, Tominaga T. Vascular endothelial growth factor and platelet-derived endothelial cell growth factor are frequently coexpressed in highly vascularized human breast cancer. Clin Cancer Res. 1995;1:961–4. [PubMed] [Google Scholar]
- 11.Moghaddam A, Zhang HT, Fan TP, et al. Thymidine phosphorylase is angiogenic and promotes tumor growth. Proc Natl Acad Sci U S A. 1995;92:998–1002. doi: 10.1073/pnas.92.4.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fox SB, Westwood M, Moghaddam A, et al. The angiogenic factor platelet-derived endothelial cell growth factor/thymidine phosphorylase is up-regulated in breast cancer epithelium and endothelium. Br J Cancer. 1996;73:275–80. doi: 10.1038/bjc.1996.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eda H, Fujimoto K, Watanabe S, et al. Cytokines induce thymidine phosphorylase expression in tumor cells and make them more susceptible to 5′-deoxy-5-fluorouridine. Cancer Chemother Pharmacol. 1993;32:333–8. doi: 10.1007/BF00735915. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto S, Kurebayashi J, Kurosumi M, et al. Combined effects of docetaxel and fluoropyrimidines on tumor growth and expression of interleukin-6 and thymidine phosphorylase in breast cancer xenografts. Cancer Chemother Pharmacol. 2001;48:283–8. doi: 10.1007/s002800100325. [DOI] [PubMed] [Google Scholar]
- 15.Sawada N, Ishikawa T, Sekiguchi F, Tanaka Y, Ishitsuka H. X-Ray irradiation induces thymidine phosphorylase and enhances the efficacy of capecitabine (Xeloda) in human cancer xenografts. Clin Cancer Res. 1999;5:2948–53. [PubMed] [Google Scholar]
- 16.Kurosumi M, Tabei T, Suemasu K, et al. Enhancement of immunohistochemical reactivity for thymidine phosphorylase in breast carcinoma cells after administration of docetaxel as a neoadjuvant chemotherapy in advanced breast cancer patients. Oncol Rep. 2000;7:945–8. doi: 10.3892/or.7.5.945. [DOI] [PubMed] [Google Scholar]
- 17.Toi M, Bando H, Horiguchi S, et al. Modulation of thymidine phosphorylase by neoadjuvant chemotherapy in primary breast cancer. Br J Cancer. 2004;90:2338–43. doi: 10.1038/sj.bjc.6601845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nadella P, Shapiro C, Otterson GA, et al. Pharmacobiologically based scheduling of capecitabine and docetaxel results in antitumor activity in resistant human malignancies. J Clin Oncol. 2002;20:2616–23. doi: 10.1200/JCO.2002.22.030. [DOI] [PubMed] [Google Scholar]
- 19.O’Shaughnessy J, Miles D, Vukelja S, et al. Superior survival with capecitabine plus docetaxel combination therapy in anthracycline-pretreated patients with advanced breast cancer: phase iii trial results. J Clin Oncol. 2002;20:2812–23. doi: 10.1200/JCO.2002.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Miles D, Vukelja S, Moiseyenko V, et al. Survival benefit with capecitabine/docetaxel versus docetaxel alone: analysis of therapy in a randomized phase iii trial. Clin Breast Cancer. 2004;5:273–8. doi: 10.3816/CBC.2004.n.030. [DOI] [PubMed] [Google Scholar]
- 21.Beslija S, Obralic H, Basic A, et al. Randomized trial of sequence vs. combination of capecitabine (x) and docetaxel (t): xt vs. t followed by x after progression as first-line therapy for patients (pts) with metastatic breast cancer (mbc) [abstract 571] J Clin Oncol. 2006;24 [Available online at: http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=40&abstractID=30696; cited February 4, 2012] [Google Scholar]
- 22.Tonini G, Schiavon G, Silletta M, Vincenzi B, Santini D. Antiangiogenic properties of metronomic chemotherapy in breast cancer. Future Oncol. 2007;3:183–90. doi: 10.2217/14796694.3.2.183. [DOI] [PubMed] [Google Scholar]
- 23.Hainsworth JD. Practical aspects of weekly docetaxel administration schedules. Oncologist. 2004;9:538–45. doi: 10.1634/theoncologist.9-5-538. [DOI] [PubMed] [Google Scholar]
- 24.Blesch KS, Gieschke R, Tsukamoto Y, Reigner BG, Burger HU, Steimer JL. Clinical pharmacokinetic/pharmacodynamic and physiologically based pharmacokinetic modeling in new drug development: the capecitabine experience. Invest New Drugs. 2003;21:195–223. doi: 10.1023/A:1023525513696. [DOI] [PubMed] [Google Scholar]
- 25.Mackey JR, Tonkin KS, Koski SL, et al. Final results of a phase ii clinical trial of weekly docetaxel in combination with capecitabine in anthracycline-pretreated metastatic breast cancer. Clin Breast Cancer. 2004;5:287–92. doi: 10.3816/CBC.2004.n.032. [DOI] [PubMed] [Google Scholar]
- 26.Puglisi F, Cardellino GG, Crivellari D, et al. Thymidine phosphorylase expression is associated with time to progression in patients receiving low-dose, docetaxel-modulated capecitabine for metastatic breast cancer. Ann Oncol. 2008;19:1541–6. doi: 10.1093/annonc/mdn165. [DOI] [PubMed] [Google Scholar]
- 27.Makower D, Wadler S, Haynes H, Schwartz EL. Interferon induces thymidine phosphorylase/platelet-derived endothelial cell growth factor expression in vivo. Clin Cancer Res. 1997;3:923–9. [PubMed] [Google Scholar]
- 28.Bartsch R, Steger GG, Forstner B, et al. Expression of thymidine phosphorylase in peripheral blood cells of breast cancer patients is not increased by paclitaxel. BMC Clin Pharmacol. 2007;7:7. doi: 10.1186/1472-6904-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Layman RM, Thomas DG, Griffith KA, et al. Neoadjuvant docetaxel and capecitabine and the use of thymidine phosphorylase as a predictive biomarker in breast cancer. Clin Cancer Res. 2007;13:4092–7. doi: 10.1158/1078-0432.CCR-07-0288. [DOI] [PubMed] [Google Scholar]
- 30.Mazhar D, Ang R, Waxman J. Cox inhibitors and breast cancer. Br J Cancer. 2006;94:346–50. doi: 10.1038/sj.bjc.6602942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Half E, Tang XM, Gwyn K, Sahin A, Wathen K, Sinicrope FA. Cyclooxygenase-2 expression in human breast cancers and adjacent ductal carcinoma in situ. Cancer Res. 2002;62:1676–81. [PubMed] [Google Scholar]
- 32.Watanabe O, Shimizu T, Imamura H, et al. Expression of cyclooxygenase-2 in malignant and benign breast tumors. Anticancer Res. 2003;23:3215–21. [PubMed] [Google Scholar]
- 33.Barnes NL, Warnberg F, Farnie G, et al. Cyclooxygenase-2 inhibition: effects on tumour growth, cell cycling and lymphangiogenesis in a xenograft model of breast cancer. Br J Cancer. 2007;96:575–82. doi: 10.1038/sj.bjc.6603593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liao Z, Mason KA, Milas L. Cyclo-oxygenase-2 and its inhibition in cancer: is there a role? Drugs. 2007;67:821–45. doi: 10.2165/00003495-200767060-00001. [DOI] [PubMed] [Google Scholar]
- 35.Cassidy PB, Moos PJ, Kelly RC, Fitzpatrick FA. Cyclooxygenase-2 induction by paclitaxel, docetaxel and taxane analogues in human monocytes and murine macrophages: structure– activity relationships and their implications. Clin Cancer Res. 2002;8:846–55. [PubMed] [Google Scholar]
- 36.Altorki NK, Port JL, Zhang F, et al. Chemotherapy induces the expression of cyclooxygenase-2 in non-small cell lung cancer. Clin Cancer Res. 2005;11:4191–7. doi: 10.1158/1078-0432.CCR-05-0108. [DOI] [PubMed] [Google Scholar]
- 37.Dirix LY, Ignacio J, Nag S, et al. Treatment of advanced hormone-sensitive breast cancer in postmenopausal women with exemestane alone or in combination with celecoxib. J Clin Oncol. 2008;26:1253–9. doi: 10.1200/JCO.2007.13.3744. [DOI] [PubMed] [Google Scholar]
- 38.Ueno T, Chow LW, Toi M. Increases in circulating vegf levels during Cox-2 inhibitor treatment in breast cancer patients. Biomed Pharmacother. 2006;60:277–9. doi: 10.1016/j.biopha.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 39.Steinbach G, Lynch PM, Phillips RK, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342:1946–52. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 40.Sauter ER, Qin W, Hewett JE, et al. Celecoxib concentration predicts decrease in prostaglandin E2 concentrations in nipple aspirate fluid from high risk women. BMC Cancer. 2008;8:49. doi: 10.1186/1471-2407-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]