Abstract
Arginine-phenylalanine-amide (RFamide)-related peptide 3 (RFRP-3, encoded by the Rfrp gene) is the mammalian ortholog of gonadotropin-inhibiting hormone and can inhibit GnRH neuronal activity and LH release. However, the development and regulation of the RFRP-3 system in both sexes is poorly understood. Using in situ hybridization, we examined changes in Rfrp-expressing neurons in mice of both sexes during development and under different adulthood hormonal milieus. We found no sex differences in Rfrp expression or cell number in adult mice. Interestingly, we identified two interspersed subpopulations of Rfrp cells (high Rfrp-expressing, HE; low Rfrp-expressing, LE), which have unique developmental and steroidal regulation characteristics. The number of LE cells robustly decreases during postnatal development, whereas HE cell number increases significantly before puberty. Using Bax knockout mice, we determined that the dramatic developmental decrease in LE Rfrp cells is not due primarily to BAX-dependent apoptosis. In adults, we found that estradiol and testosterone moderately repress Rfrp expression in both HE and LE cells, whereas the nonaromatizable androgen dihydrotestosterone has no effect. Using double-label in situ hybridization, we determined that approximately 25% of Rfrp neurons coexpress estrogen receptor-α in each sex, whereas Rfrp cells do not readily express androgen receptor in either sex, regardless of hormonal milieu. Lastly, when we looked at RFRP-3 receptors, we detected some coexpression of Gpr147 but no coexpression of Gpr74 in GnRH neurons of both intact and gonadectomized males and females. Thus, RFRP-3 may exert its effects on reproduction either directly, via Gpr147 in a subset of GnRH neurons, and/or indirectly, via upstream regulators of GnRH.
Members of the arginine-phenylalanine-amide (RFamide) peptide family have emerged as important regulators of reproductive function (1). RFamide-related peptide 3 (RFRP-3; the mammalian ortholog to avian gonadotropin-inhibiting hormone) has potent inhibitory actions on both GnRH neuronal activity and LH secretion in rodents (2–4). Encoded by the Rfrp gene, RFRP-3 is expressed in the dorsal-medial nucleus of the hypothalamus (DMN) (5–8). In rodents, RFRP-3-immunoreactivity (RFRP-3-ir) fibers project to several brain regions, including the paraventricular and arcuate nuclei, lateral hypothalamus, and the preoptic area, where some fibers appose GnRH neurons (6, 9–11). Matching the presence of some RFRP-3-ir fibers in non-GnRH areas, RFPR-3 has also been shown to modulate nociception, body temperature, and food intake (12–16), suggesting that RFRP-3 may have additional biological functions outside reproduction.
In rodents, Rfrp neurons in the DMN are born embryonically (17), and total Rfrp mRNA levels, quantified with quantitative PCR (qPCR), increase in juvenile rats before puberty and then subsequently decrease after puberty (18, 19). Similar data obtained by quantifying RFRP-3-ir in male mice showed a decrease in RFRP-3-ir cell number after sexual maturation (20, 21), but developmental changes in Rfrp mRNA were not measured in mice, and females were not studied. Moreover, to date, sex differences in developmental changes in Rfrp expression have not been directly assessed in any species.
Many neuropeptide systems are regulated by hormones, but contradictory outcomes currently exist regarding the roles of sex steroids in regulating Rfrp neurons. Estradiol (E2) treatment had no effect on total Rfrp mRNA levels in female rats (18) but decreased total Rfrp levels in female mice (22). Further clouding the issue, another study in female rats reported that E2 treatment increases total Rfrp mRNA levels (19). Likewise, the degree of coexpression of sex steroid receptors in RFRP-3 neurons is currently not well characterized. In female hamsters, approximately 40% of RFRP-3-ir neurons coexpress estrogen receptor α (ERα) (6), but in female mice, only 20% of Rfrp neurons coexpress ERα (22). At present, no studies have examined the effects of E2 on, or the degree of ERα coexpression in, RFRP-3 neurons of male rodents. Furthermore, the regulation of Rfrp specifically by androgen pathways has not yet been examined in either sex of any species, nor has the coexpression of androgen receptors (AR) in Rfrp neurons been quantified.
How RFRP-3 communicates with GnRH neurons is not clearly defined, and the expression of RFRP-3 receptors specifically in GnRH neurons has not been well characterized in mammals. RFRP-3 binds Gpr147 (also called Npffr1) with high affinity and Gpr74 (also called Npffr2) at lower affinity (5, 12, 23–27), leading to uncertainty regarding which receptor(s) RFRP-3 acts through to inhibit GnRH/LH secretion. In male Siberian hamsters, Gpr147-ir was recently detected in about 80% of GnRH neurons (28), similar to findings in birds (29). However, whether similar Gpr147 (or Gpr74) coexpression levels exist in other mammalian species of either sex remains undetermined.
This study addresses several important gaps of knowledge regarding RFRP-3 in rodents. Using mice, we determined 1) whether sex differences exist in adult Rfrp gene expression; 2) the developmental profile of Rfrp expression in postnatal/prepubertal mice; 3) whether the developmental pattern of RFRP-3 neurons differs between the sexes or is affected by BAX-mediated apoptosis; 4) whether sex steroids, including estrogens and androgens, can affect Rfrp neurons in both sexes; 5) whether any sex steroid effects are direct by assessing whether Rfrp neurons coexpress either ERα and/or AR in both sexes; and 6) whether GnRH neurons in male and females mice coexpress either of the two RFRP-3 receptors, Gpr147 and Gpr74. Lastly, we identified two subpopulations of Rfrp-expressing neurons interspersed within the DMN, and we evaluated several of the above issues for both subpopulations.
Materials and Methods
Animals
C57BL6/J mice were housed on a 12-h light, 12-h dark cycle (lights off at 1800 h) with food and water available ad libitum. The day of birth was designated as postnatal day (PND) 1. Weaned animals were grouped two to three per cage. All experiments were conducted in accordance with the National Institutes of Health Animal Care and Use Guidelines and with approval of the Animal Care and Use Committee of the University of California, San Diego. Experiment 3 used brain sections collected from adult Bax knockout (KO) mice from a previous study (30).
Gonadectomies, hormone treatments, and tissue collection
For some experiments, adult mice were anesthetized with isoflurane, bilaterally gonadectomized (GDX), and implanted sc with a SILASTIC (Dow Corning Corp., Midland, MI) capsule (internal diameter, 1.47 mm; external diameter, 1.96 mm) packed with E2 (4 mm, 1:4 with cholesterol), testosterone (T, 6 mm), or dihydrotestosterone (DHT, 8 mm), or received no implant. These implants have been shown previously to produce elevated physiological levels of hormone (87.9 ± 10.0 pg/ml for E2; 11.1 ± 0.8 ng/ml for T; 2.8 ± 0.7 ng/ml for DHT) (31, 32) and to significantly change gene expression of other neuropeptides in adult mice (30–33). One week after GDX, animals were anesthetized with isoflurane and rapidly decapitated. Brains were collected, frozen on dry ice, and stored at −80 C. Five coronal series of 20-μm brain sections were cut on a cryostat, thaw mounted onto Superfrost-plus slides, and stored at −80 C until in situ hybridization (ISH).
Single-label and double-label ISH
All ISH cRNA probes, except for Gnrh [described previously (34)], were cloned from adult mouse hypothalamic cDNA into pBluscript II SK(-) transcription plasmid (Stratagene, La Jolla, CA) between HindIII and KpnI restriction sites, and antisense probes transcribed with T7 polymerase. The Rfrp cRNA probe is complementary to bases 52–538 of the published mouse Rfrp mRNA sequence (GenBank accession no. NM_021892). The ERα cRNA and AR probes were designed against bases 1165–1982 and 2001–2312 of the murine ERα and AR sequences, respectively (GenBank accession nos. NM_007956 and NM_013476). The Gpr147 and Gpr74 cRNA probes bind bases 246–724 and 62–487 of the mouse Gpr147 and Gpr74 mRNA, respectively (GenBank accession nos. NM_001177511 and NM_133192). The Gpr54 probe was designed against bases 2276–2879 of the mouse sequence (NM_053244).
Single-label ISH was performed as previously described (30, 35–37). Briefly, slide-mounted DMN sections were fixed in 4% paraformaldehyde, pretreated with acetic anhydride, rinsed in 2 × SSC (sodium citrate, sodium chloride), delipidated in chloroform, dehydrated in ethanols, and air dried. Radiolabeled (33P) Rfrp antisense riboprobe (0.04 pmol/ml) was combined with tRNA, heat denatured, added to hybridization buffer, and applied to each slide (100 μl/slide). Slides were coverslipped and placed in a 55 C humidity chamber overnight. The slides were then washed in 4 × SSC and placed into RNase A treatment for 30 min at 37 C, then in RNase buffer without RNase at 37 C for 30 min. After washing in 2 × SSC at room temperature, slides were washed in 0.1 × SSC at 62 C for 1 h, dehydrated in ethanols, and air dried. Slides were then dipped in Kodak (Eastman Kodak, Rochester, NY) NTB emulsion, air dried, and stored at 4 C for 3–4 d (depending on the assay) before being developed and coverslipped. No staining was detected with sense probes.
For double-label ISH, slide-mounted brain sections were treated similarly to single-label ISH with the following modifications. Digoxigenin (DIG)-labeled antisense mouse Rfrp or Gnrh cRNA were synthesized with DIG labeling mix (Roche, Indianapolis, MN). Radiolabeled (33P) antisense ERα, AR, Gpr147, Gpr74, or Gpr54 (0.05 pmol/ml) and DIG-labeled Gnrh or Rfrp (1:500) riboprobes were combined with tRNA, heat denatured, and dissolved together in hybridization buffer. The probe mix was applied to slides (100 μl/slide), and slides were hybridized at 55 C overnight. After the 62 C washes on d 2, slides were incubated in 2 × SSC with 0.05% Triton X-100 containing 3% normal sheep serum for 1 h at 21 C. Slides were then incubated overnight at 21 C with anti-DIG antibody conjugated to alkaline phosphatase (Roche; diluted 1:500 in Buffer 1 containing 1% normal sheep serum and 0.3% Triton X-100). Slides were then washed with Buffer 1 and incubated with Vector Red alkaline phosphatase substrate (Vector Laboratories, Burlingame, CA) for 1 h at 21 C. The slides were then air dried, dipped in emulsion, stored at 4 C, and developed and coverslipped 9 d later.
Quantification and analysis of ISH data
ISH slides were analyzed with an automated image processing system (Dr. Don Clifton, University of Washington) by a person unaware of the treatment group of each slide. For single-label experiments, the software counted the number of silver grain clusters representing cells, as well as the number of silver grains over each cell (a semiquantitative index of mRNA content per cell) (30, 33, 39). Cells were considered Rfrp positive when the number of silver grains in a cluster exceeded that of background by 3-fold.
During our early examinations of single-label Rfrp staining, we noticed what appeared to be two obvious Rfrp cell populations interspersed within the DMN that dramatically differed in silver grain (i.e. mRNA) expression. Therefore, we divided the Rfrp cells into two categories based on mRNA expression levels. Rfrp cells were counted as high expressing (HE) cells if the grain cluster diameter at the longest axis was more than 20 μm whereas Rfrp cluster diameters less than 20 μm were counted as low expressing (LE) cells (silver grain number and silver grain cell area are highly dependent).
For double-label assays, DIG-containing cells (Rfrp or Gnrh cells) were identified under fluorescence microscopy and the grain-counting software was used to quantify silver grains (representing ERα, AR, Gpr147, or Gpr74 mRNA) overlying each cell. Signal-to-background ratios for individual cells were calculated, and a cell was considered double-labeled if its ratio was equal to or greater than 4. HE and LE Rfrp cell binning was not used in double-label quantification of Rfrp cells because the expression difference between them cannot be easily determined with only fluorescence.
Experimental design
Experiment 1: evaluation of possible sex differences in Rfrp gene expression in adult mice
Because the expression of several reproductive neuropeptide genes is sexually dimorphic (40), we tested whether Rfrp is expressed in a sex-specific manner in adult mice (8–10 wk). ISH of Rfrp mRNA in the DMN of adult gonadal-intact male and diestrous female mice was performed to determine whether overt sex differences exist in the number, expression, or distribution of Rfrp neurons (n = 4 animals per sex).
Experiment 2: assessment of the pattern of Rfrp mRNA expression during postnatal and prepubertal development in mice of both sexes
The developmental pattern of Rfrp expression in male and female mice during postnatal and peripubertal development is not well characterized. Here we determined if and when Rfrp expression changes during postnatal development in mice of both sexes at ages PND1, PND10, PND20, and adulthood (7–9 wk; females in diestrus). Brains from mice at these ages were assayed for Rfrp mRNA expression, including number of Rfrp-expressing cells and relative Rfrp mRNA per cell, using single-label ISH (n = 6–7 animals per group per sex).
Experiment 3: assessment of BAX-dependent apoptosis regulation of Rfrp neuron development
In experiment 2, we identified a significant decrease in total Rfrp cell number, primarily due to a robust decrease in LE cells, between birth and adulthood. We hypothesized that apoptosis (programmed cell death) may be responsible for this developmental decrease in Rfrp cells. To test this, brain sections from gonadectomized adult Bax KO males (which lack BAX-mediated apoptosis) were assayed and compared with wild types (WT) using single-label ISH for Rfrp expression (n = 7–8 animals per genotype).
Experiment 4: evaluation of the regulatory effects of E2 and androgens on Rfrp expression in adult gonadectomized males and females
The effects of E2 on Rfrp-expressing cells are unclear and not fully characterized. Moreover, the effects of E2 on Rfrp have not yet been examined in males, and the effects of nonaromatizable androgens (DHT) on Rfrp neurons have not been examined yet in any species. Therefore, we first tested whether Rfrp expression differs between GDX mice of each sex with and without E2 replacement. Adult male and female mice were GDX and implanted with steady-state E2 (or nothing). Brains were collected 1 wk later and assayed for Rfrp expression via ISH (n = 6 animals per group). Next, we tested whether Rfrp expression is regulated by androgens. Adult GDX males were given implants containing E2, T, or nonaromatizable DHT, or received no implant. Brains were collected 1 wk later and assayed for Rfrp expression using ISH (n = 6 animals per group).
Experiment 5: coexpression of sex steroid receptors in Rfrp neurons in males and females
If sex steroids act directly on Rfrp neurons, then sex steroid receptors should be coexpressed in Rfrp cells. We therefore tested whether Rfrp cells coexpress ERα or AR in males and females. Brains from both gonadally intact males and females (diestrous), as well as GDX+E2 males and females, were assayed for coexpression of ERα in Rfrp neurons using double-label ISH. In a separate experiment, alternate sections from the same gonadally intact and E2-treated groups were assayed for AR coexpression in Rfrp neurons by double-label ISH (n = 6–7 animals per group per assay).
Experiment 6: assessment of coexpression of RFRP-3 receptors, Gpr147 and Gpr74, in GnRH neurons
The inhibition of GnRH neurons by RFRP-3 is likely mediated through one of two receptors, Gpr147 or Gpr74. We therefore tested whether either receptor is coexpressed in GnRH neurons. Double-label ISH for Gpr147 or Gpr74 mRNA in GnRH neurons was performed on brain tissue from gonadally intact males and females (diestrous) (n = 5–7 animals per group). For comparison, we included a group of three diestrous female mice in which coexpression of Gpr54 (kisspeptin receptor) in GnRH neurons was assessed. To determine whether RFRP-3 receptor levels in GnRH neurons change depending on the sex steroid milieu, we also assessed coexpression of Gpr147 and Gpr74 in GnRH neurons of GDX males and females.
Statistical analysis
All data are expressed as the mean ± sem for each group. In all experiments, differences were analyzed by Student's t test or by two-way ANOVA, followed by post hoc comparisons for individual sex/age or sex/treatment groups via Fisher's (Protected) LSD, except for the difference between HE and LE cells, which was analyzed using the two-sample Kolmogorov-Smirnov nonparametric test. Statistical significance was P < 0.05. All analyses were performed in Statview 5.0.1 (SAS Institute, Cary, NC).
Results
Experiment 1: Rfrp expression is not sexually dimorphic in adult mice
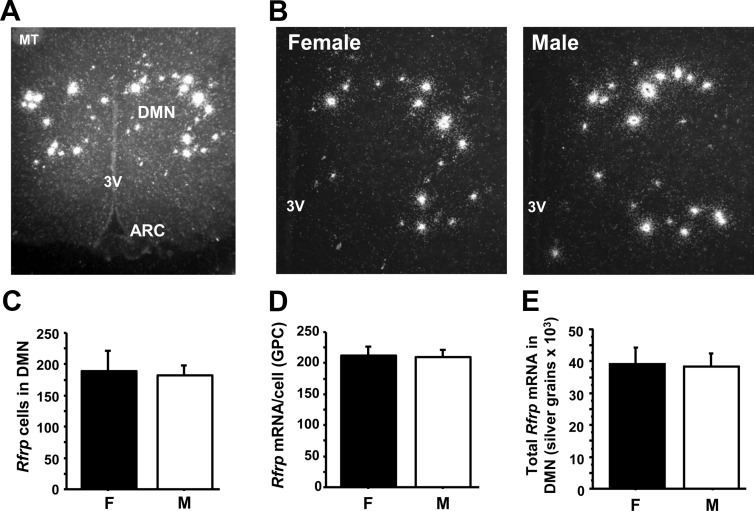
We determined whether Rfrp neuron number or expression in the DMN is sexually dimorphic in adult gonadally intact mice, as are some other reproductive neuropeptides. We found that neither the total number of Rfrp-expressing cells, nor the relative amount of Rfrp mRNA per cell, nor the total amount of Rfrp mRNA in the DMN is significantly different between adult males and females (Fig. 1).
Fig. 1.
Expression of Rfrp in adult male (M) and female (F) mice by ISH. A, Representative low-power photomicrograph of Rfrp expression in adult male mouse. MT, Mammilary tract; 3V, third ventricle; ARC, arcuate nucleus. B, Representative mid-power photomicrograph of Rfrp expression in an adult female (diestrus) and male. 3V, Third ventricle. C, Total number of Rfrp cells in the DMN is not significantly different between adult female and male mice. D, Mean Rfrp mRNA per cell [determined by silver grains per cell (GPC) and E, total Rfrp mRNA in DMN (total silver grains in the DMN) are not significantly different between adult females and males.
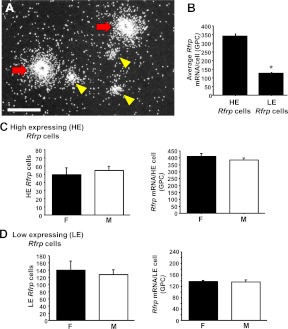
In analyzing Rfrp expression in this experiment, we identified two obvious subpopulations of Rfrp-expressing cells interspersed with each other in the DMN: one set of cells expressed extremely high levels of Rfrp mRNA, and another set expressed much lower Rfrp levels (∼3-fold lower; Fig 2, A and B). These two Rfrp subpopulations, termed high-expressing (HE) and low-expressing (LE) cells, were analyzed separately in adult mice to determine whether a sex difference exists in either cell type. In each sex, there were many more LE cells than HE cells. However, there were no sex differences in either the number of LE or HE Rfrp cells or in the relative mRNA content of either cell type (Fig. 2, C and D). We also examined the rostral-caudal distribution of LE and HE Rfrp neurons in the DMN and found no noticeable differences in the anatomical distribution of either Rfrp subpopulation: both HE and LE Rfrp cells are present throughout the DMN, with no preferential localization (data not shown).
Fig. 2.
A, Representative photomicrograph of HE (red arrows) and LE (yellow arrowheads) Rfrp cells in an adult male. Note that the central dark void observed in some HE Rfrp cells is actually an extremely dense clustering of silver grains that prevents transillumination under dark field, as determined under bright-field microscopy (data not shown). Scale bar, 50 μm. B, Average Rfrp mRNA per cell (determined by silver grains) between HE and LE population from a representative adult male. The two Rfrp subpopulations have significantly different (P < 0.05) amounts of Rfrp mRNA [silver grains per cell (GPC)]. C, Mean number of HE Rfrp cells in adult females (F) and males (M) and Rfrp mRNA per HE cell as determined by silver grains per HE cell. Neither measure was significantly different between sexes. D, Mean number of LE Rfrp cells in adult females and males and mRNA per cell as determined by grains per cell of LE cells. Neither measure was significantly different between sexes.
Experiment 2: Expression of Rfrp changes dramatically in both sexes during postnatal and prepubertal development
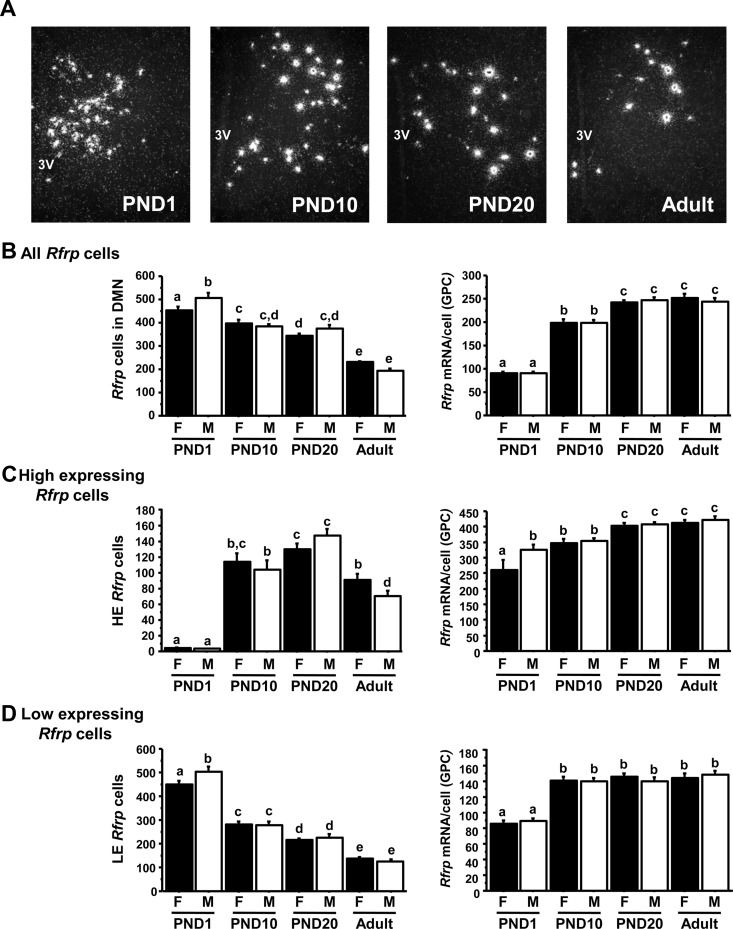
This experiment determined the developmental profile of Rfrp expression in the DMN of both sexes. Rfrp mRNA levels showed significant developmental changes between birth and adulthood in both males and females. In both sexes, total DMN Rfrp mRNA levels increased during early postnatal life and then decreased between PND20 and adulthood (Supplemental Fig. 1 published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). However, the total number of Rfrp cells significantly decreased between birth and adulthood, dropping nearly 2-fold in both sexes, whereas the overall relative amount of Rfrp mRNA per cell significantly increased between birth and adulthood, rising more than 3-fold (P < 0.05; Fig, 3, A and B). Specifically, the number of Rfrp cells was significantly lower on PND10 than PND1, did not differ between PND 10 and PND 20, and dropped significantly again between PND20 and adulthood (Fig. 3B). Conversely, the relative Rfrp mRNA per cell significantly increased at each subsequent age examined, except between PND 20 and adulthood (Fig. 3B). Overall, males and females showed the same general developmental pattern in total Rfrp cell number, relative Rfrp mRNA per cell, and total Rfrp mRNA.
Fig. 3.
Changes in Rfrp expression in male and female mice over postnatal development. A, Representative photomicrographs of Rfrp expression on PND1, PND10, PND20, and adult male mice. 3V, Third ventricle. B, Mean changes in the total number of Rfrp cells and Rfrp mRNA per cell [determined by silver grains per cell (GPC)] of all cells in males (M) and females (F) over development. C, Mean changes in the HE Rfrp cells and Rfrp mRNA per HE cell in M and F over development. D, Mean changes in the LE Rfrp cells and Rfrp mRNA per LE cell in M and F over development. Bars labeled with different letters differ significantly from each other (P < 0.05).
We next analyzed the developmental pattern of Rfrp expression in the HE and LE Rfrp subpopulations. At all ages, and in both sexes, there were more LE cells than HE cells. Interestingly, there were virtually no HE cells detected on PND1 in either sex. A dramatic increase in the number of HE cells occurred between PND1 and PND10 and PND20 (Fig. 3C, P < 0.05). The number of HE cells then decreased significantly between PND20 and adulthood. Unlike in Experiment 1, there was a very small sex difference in HE cell number in adult mice (P < 0.05). In both sexes, the Rfrp mRNA levels of HE cells increased over development, being significantly higher in PND20 and adult mice than PND1 mice (Fig. 3C, P < 0.05).
LE Rfrp cells displayed a different developmental pattern than HE cells. The number of LE cells mirrored the total Rfrp cell population, decreasing steadily and significantly from PND1 thorough adulthood (Fig. 3D). In both sexes, there were significantly more LE cells on PND1 than other ages, with each older age group having fewer LE cells than previous ages (Fig. 3D, P < 0.05). Although there was a small sex difference in the number of LE cells on PND1 (P < 0.05), there were no sex differences at other ages. Similar to HE cells, the relative amount of Rfrp mRNA of LE cells increased during development in both males and females, being significantly lower on PND1 than older ages (Fig. 3D, P < 0.05).
Experiment 3: BAX-dependent apoptosis moderately affects Rfrp neurons development
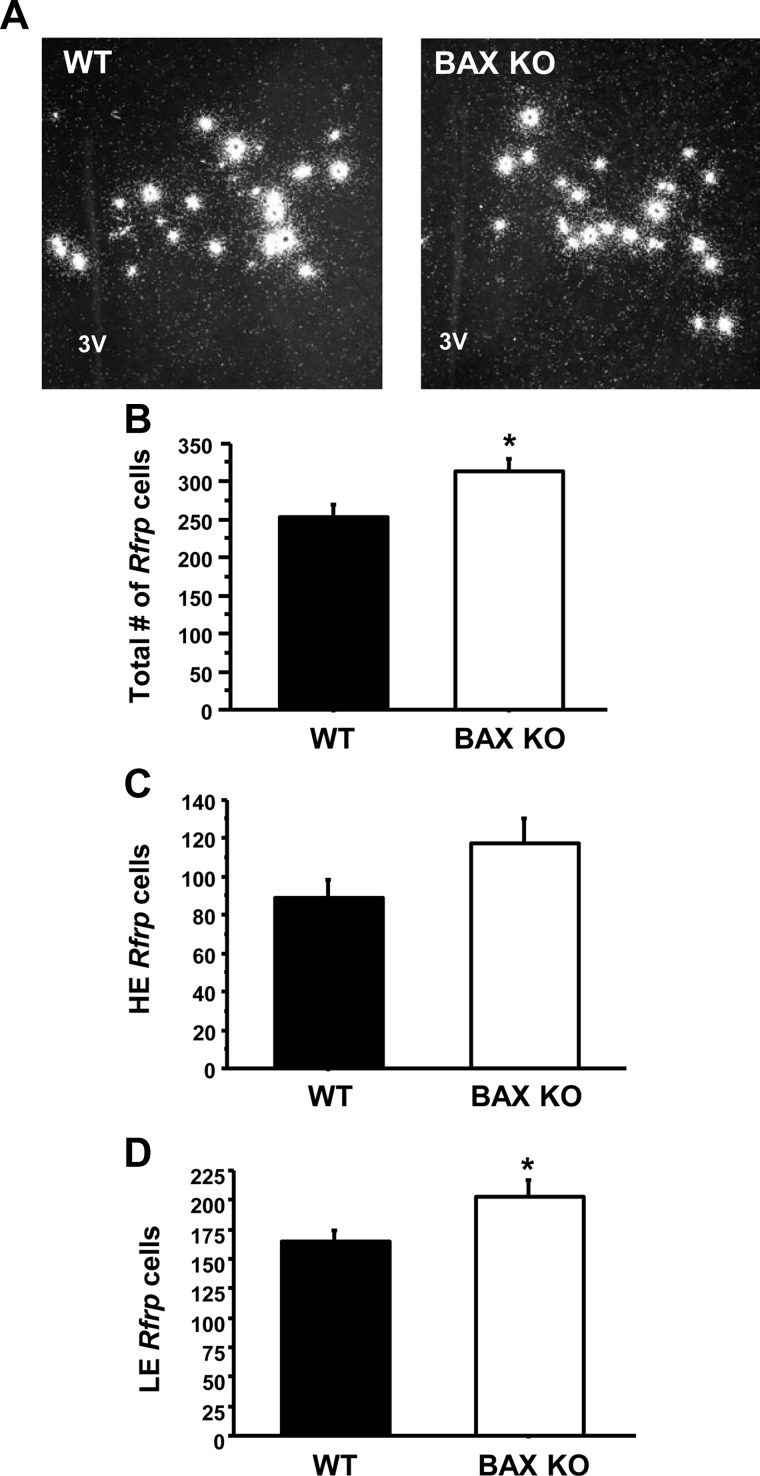
In Experiment 2, we observed a dramatic decrease in the total number of Rfrp cells, and LE cells, between birth and adulthood. Here we used Bax KO and WT mice to determine whether BAX-mediated apoptosis (cell death) underlies the robust developmental decrease in Rfrp cells. We found that Bax KO mice have a small but significant increase in the total number of Rfrp cells compared with WT mice (P < 0.05, Fig. 4B). The same general pattern was observed in both HE and LE Rfrp subpopulations, but only the number of LE cells was statistically different between Bax KO and WT (P < 0.05, Fig. 4D). There was no significant difference in Rfrp mRNA per cell between WT and KO mice in either subpopulation (data not shown). Although there are fewer Rfrp cells in WT than Bax KO animals, the observed difference in cell number (∼17% decrease) was not nearly as great as the difference seen between PND1 and adult C57BL6 mice (>55% decrease, Fig. 3), suggesting that BAX-mediated apoptosis is not fully responsible for the developmental decline in Rfrp cells.
Fig. 4.
Maturation of Rfrp neurons in Bax KO mice. A, Representative photomicrographs of Rfrp expression in adult-GDX WT and Bax KO male mice. 3V, Third ventricle. B, Mean number of total Rfrp cells between WT and KO animals. *, Significantly different (P < 0.05). C, Mean number of HE Rfrp cells between WT and KO animals. There was no significant difference between genotypes. D, Mean number of LE Rfrp cells between WT and KO animals. There were significantly more LE cells in Bax KO than WT animals (P < 0.05).
Experiment 4: Rfrp expression is moderately regulated by E2 in both sexes and unaffected by DHT
This experiment tested whether Rfrp expression or cell number is altered by E2 or androgens in adult mice of either sex. We found no significant difference in the total number of Rfrp neurons between GDX and GDX+E2 animals in either sex (Fig. 5B). However, E2 treatment significantly decreased the overall relative amount of Rfrp mRNA per cell, as well as the total amount of Rfrp mRNA in the DMN, in both males and females (P < 0.05, Fig. 5B and Supplemental Fig. 2A).
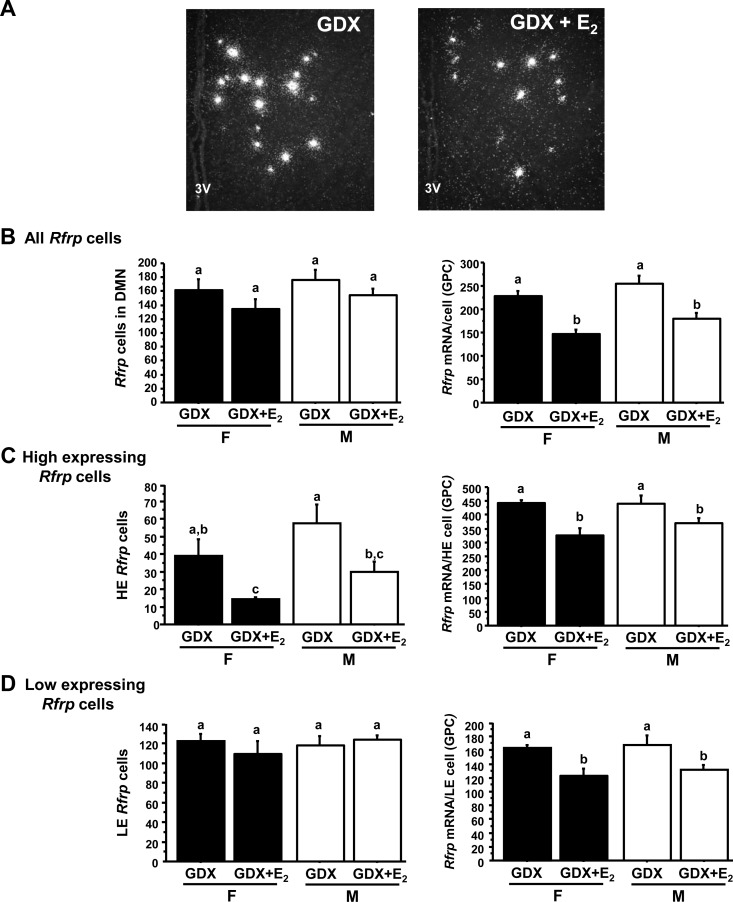
Fig. 5.
Changes in Rfrp expression with E2 treatment. A, Representative photomicrographs of Rfrp expression in GDX and GDX with E2-replaced (GDX+E2) adult females. 3V, Third ventricle. B, Mean changes in the total number of Rfrp cells and Rfrp mRNA per cell of all cells in GDX and GDX+E2 males (M) and females (F). C, Mean changes in the HE Rfrp cells and Rfrp mRNA per HE cell in GDX and GDX+E2 M and F. D, Mean changes in the LE Rfrp cells and Rfrp mRNA per LE cell in GDX and GDX+E2 M and F. Bars labeled with different letters designate significantly different groups (P < 0.05). GPC, Grains per cell.
When Rfrp cells were subdivided into HE and LE cells, both the number of HE cells and the amount of Rfrp mRNA per HE cell were significantly reduced by E2 treatment in both sexes (Fig. 5C, P < 0.05). E2 also significantly reduced the relative amount of Rfrp mRNA per cell of LE cells in both sexes (Fig. 5D, P < 0.05), but had no effect on LE cell number. No sex differences in Rfrp expression were detected in GDX or GDX+E2 animals for any measures.
Like E2, androgens can influence gene expression, either by acting through AR or acting via ER after aromatization to E2. We therefore tested whether either T or DHT could, like E2, regulate Rfrp expression in adult males. The inhibitory effect of E2 on Rfrp levels observed above was reproduced in this assay, with E2 having no effect on total cell number but significantly reducing the relative amount of Rfrp mRNA per cell (P < 0.05, Fig. 6B). Likewise, T treatment did not change Rfrp cell number but induced a small, but significant, reduction in the relative amount of Rfrp mRNA per cell and total Rfrp mRNA levels (P < 0.05 relative to GDX controls, Fig. 6B and Supplemental Fig. 2B). In contrast, DHT treatment had no effect on either the total number of Rfrp cells, the relative amount of Rfrp mRNA per cell, or total Rfrp levels (Fig. 6B and Supplemental Fig. 2B).
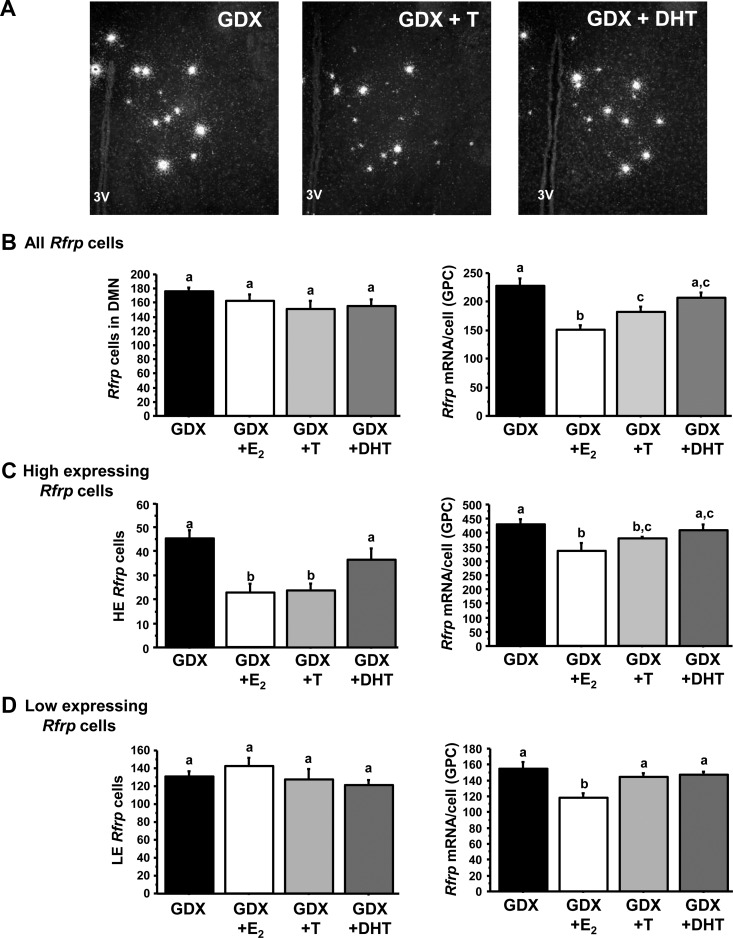
Fig. 6.
Changes in Rfrp expression with androgen treatment. A, Representative photomicrographs of Rfrp expression between GDX and GDX with T replaced (GDX + T) and GDX with DHT (GDX + DHT) replaced adult males. 3V, Third ventricle. B, Mean changes in the total number of Rfrp cells and Rfrp mRNA per cell of all cells. C, Mean changes in the HE Rfrp cells and Rfrp mRNA per HE cell. D, Mean changes in the LE Rfrp cells and Rfrp mRNA per LE cell. Bars labeled with different letters designate significantly different groups (P < 0.05). GPC, Grains per cell.
When examining only HE cells, both E2 and T significantly reduced the number of HE cells and relative amount of Rfrp mRNA/HE cell (Fig. 6C, P < 0.05 compared with GDX controls), whereas DHT had no effect on either measure. LE cell numbers were not changed by any hormone treatment, although E2 (but not T or DHT) significantly reduced relative Rfrp mRNA per cell of LE cells (Fig. 6D, P < 0.05). Thus, unlike E2, DHT had no effect on any Rfrp measure.
Experiment 5: a small proportion of Rfrp neurons coexpress ERα in both sexes but virtually no Rfrp cells coexpress AR
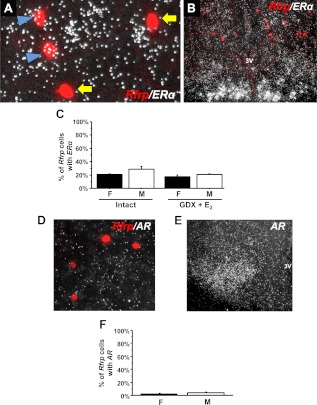
Only two studies have examined ERα coexpression in Rfrp cells of female rodents, but the results were not similar, leaving the issue unresolved. Additionally, ERα/Rfrp coexpression in males has not been determined, and coexpression levels of AR in Rfrp cells have not been reported in either sex. Here we determined that ERα mRNA is expressed in approximately 25% of Rfrp neurons, and the degree of coexpression does not significantly differ between sexes or hormonal treatments (Fig. 7C). The number of ERα silver grains in Rfrp cells was low, with only about nine silver grains per cell, suggesting that ERα is only weakly expressed in these cells. In contrast, we observed robust ERα expression in the arcuate nucleus of the same animals (Fig. 7B). Unlike ERα, AR mRNA was virtually undetectable in Rfrp cells, with less than 3% of Rfrp neurons coexpressing AR in both sexes (Fig. 7, D–F). High AR expression was readily detected in the same animals elsewhere in the brain, such as the ventromedial nucleus (Fig. 7E).
Fig. 7.
Expression of sex steroid receptors in Rfrp neurons by double-label ISH. A, Representative photomicrographs of double-label ISH of Rfrp (red fluorescence) and ERα (silver grains) in a diestrous female. Rfrp neurons coexpressing ERα (blue arrowhead) and Rfrp neurons with no coexpression of ERα (yellow arrows). 3V, Third ventricle. B, Low-power magnification photomicrograph of extensive ERα expression (silver grains) in the arcuate nucleus, with red Rfrp neurons labeled in the DMN (same animal as panel A). C, Quantification of the percent coexpression of ERα in Rfrp neurons between gonadally intact females (F) and males (M), as well as in GDX+E2 F and M. There were no significant differences in coexpression between any of the groups. D, Representative photomicrographs of the lack of AR expression in Rfrp neurons. E, Significant AR expression in the hypothalamic ventromedial nucleus of a male mouse from the same assay. F, Quantification of the percent coexpression of AR in Rfrp neurons between F and M.
Experiment 6: Gpr147 is weakly expressed in a subset of GnRH neurons, whereas Gpr74 is not readily coexpressed in GnRH neurons
Gpr147 and/or Gpr74 likely mediate the inhibitory actions of RFRP-3 on GnRH neurons, but the expression of either of these receptors in GnRH neurons of mice of either sex has not been demonstrated. Using double-label ISH, we determined that Gpr147 mRNA is present at low levels in approximately 15% of GnRH neurons (Fig. 8, A and F). In the same assay, we observed strong Gpr147 expression in the dorsal septal nuclei (Fig. 8B), a known region of Gpr147 expression in rodents (23). There were no significant differences in Gpr147/GnRH coexpression between males and females or between hormonal conditions (Fig. 8F).
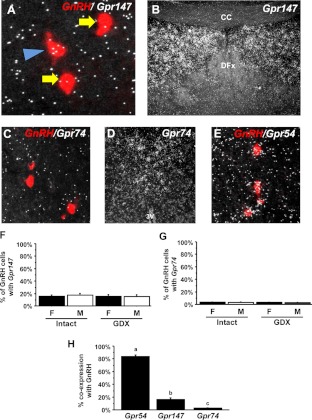
Fig. 8.
Low expression of RFamide receptors in GnRH neurons of mice, determined by double-label ISH. A, Representative photomicrographs of Gpr147 mRNA (silver grains) in a GnRH neuron (red fluorescence; indicated by blue arrowhead) from a male mouse. GnRH neurons not coexpressing Gpr147 are indicated with yellow arrows. B, Substantial Gpr147 expression in the dorsal septum in the same assay. CC, Corpus callosum; DFx, dorsal fornix. C, Representative photomicrograph of GnRH neurons (red fluorescence) and Gpr74 mRNA (silver grains) in a female mouse. D, Significant Gpr74 expression detectable in the thalamic nucleus of reunions in the same assay. 3V, Third ventricle. E, Representative photomicrograph of GnRH neurons (red fluorescence) and Gpr54 mRNA (silver grains) in a female mouse. All GnRH neurons pictured have significant coexpression of Gpr54 mRNA. F, Quantification of the percent coexpression of Gpr147 in GnRH neurons in intact and GDX females (F) and males (M). There were no significant differences between any groups. G, Quantification of the percent coexpression of Gpr74 in GnRH neurons in intact and GDX females and males. There were no significant differences between any groups. H, Comparison of the percent coexpression of Gpr54, Gpr147, and Gpr74 in GnRH neurons of intact (diestrous) females.
Gpr74 was weakly expressed in only about 3% of GnRH neurons (Fig. 8C). There were no significant differences in Gpr74/GnRH coexpression between the sexes or between hormone treatments (Fig. 8G). Despite virtually no coexpression in GnRH neurons, strong Gpr74 expression was observed in the nucleus reunions (Fig. 8D) and nucleus accumbens, consistent with previous reports in mice (23, 41, 42).
In stark contrast to Gpr147 and Gpr74, Gpr54 (kisspeptin receptor) was highly coexpressed in the majority of GnRH neurons of adult female mice, with greater than or equal to 85% of GnRH neurons expressing Gpr54 (Fig. 8, E and H), consistent with previous reports (43, 44).
Discussion
RFRP-3 has emerged as a potent regulator of GnRH and gonadotropin release, but the regulation of Rfrp neurons in mice has not been thoroughly examined. Here, we describe for the first time two subpopulations of Rfrp-producing neurons in the DMN with significant differences in their Rfrp mRNA levels. These two Rfrp subpopulations, as well as the total Rfrp population, are not sexually dimorphic, but the two subpopulations display unique developmental profiles and steroidal regulation. Additionally, we determined that in both sexes, E2 has a moderate inhibitory effect on Rfrp expression whereas nonaromatizable androgens have no effect on Rfrp expression, supported by a relative lack of AR in Rfrp cells. Some of the inhibitory effects of E2 may be achieved by direct action in Rfrp cells because a small proportion of Rfrp neurons coexpress ERα in both sexes. Lastly, we determined that Gpr147 is expressed in small subset of GnRH neurons in mice of both sexes, whereas Gpr74 is not readily coexpressed in GnRH cells, suggesting that any direct effects of RFRP-3 on GnRH neurons likely occur via Gpr174 (or another yet-to-be-identified receptor).
Previous RFRP-3 studies examined either only one sex or males and females separately (18–21). This is the first study to directly assess possible sex differences in the mammalian RFRP-3 system. We found no major differences in Rfrp cell number or mRNA levels between adult males and females. This is in sharp contrast to some other RFamide systems, such as kisspeptin, which are robustly sexually dimorphic (36). Although a previous study in hamsters proposed that RFRP-3 neurons are involved in the sexually dimorphic LH surge in female rodents (11), the absence of Rfrp sex differences in our present study suggests that RFRP-3 may not be a key component of the sexually dimorphic aspect of the positive feedback mechanism.
This is the first study to identify subsets of Rfrp cells in the DMN, with the finding of high Rfrp-expressing and low Rfrp-expressing cells. The presence of these Rfrp subpopulations, and their differential development and hormonal regulation, was likely missed in previous studies that used either qPCR analysis of homogenized brain tissue or fluorescent immunohistochemistry, which may not be sensitive enough to easily distinguish differences in staining levels between individual RFRP-3 cells. Our data demonstrate obvious differences in the number, development, and hormonal regulation of HE and LE cells, and in some cases, these subpopulation differences are obscured when examining the entire Rfrp population as a whole. Importantly, similar HE and LE cells are apparent in published Rfrp staining in sheep (although this was not studied) (45), as well as some other neuropeptides in the DMN, such as TRH (46). Whether the HE and LE Rfrp cells have different or overlapping functions remains to be determined.
We found that total Rfrp mRNA levels rise similarly in both sexes during postnatal/prepubertal development and then decline again between PND20 and adulthood. This pattern is consistent with qPCR findings in developing rats (18, 19) and may possibly reflect a developmental mechanism that restrains activation of the reproductive axis before puberty and then reduces inhibition of GnRH neurons during the pubertal transition. However, when we subsequently analyzed Rfrp cell number and relative Rfrp expression per cell, in both the total Rfrp population and the HE and LE subpopulations, we uncovered divergent and unique developmental patterns not previously detected using only qPCR. Intriguingly, the HE and LE cells showed dramatically different developmental patterns in cell number, although both subpopulations increased their cellular Rfrp levels throughout with age. The significance of these specific developmental changes is currently unknown. Although there are dramatic developmental decreases in the total number of Rfrp cells (specifically, LE cells) in both sexes, this change is likely not primarily due to BAX-dependent apoptosis, the main mechanism of neuronal cell death (47). Although we found that BAX-mediated apoptosis regulates Rfrp cell development to a minor degree (∼17% change in cell number), this small change cannot fully explain the dramatic (>55%) developmental decrease in Rfrp cells. Whether transcriptional and/or epigenetic changes underlie the observed decrease in Rfrp cells is currently unknown.
Previous reports examined E2 regulation of Rfrp only in females, with inconsistent results (19, 22). We found that E2 moderately represses Rfrp expression in mice of both sexes, including reducing expression levels in both LE and HE cells, as well as cell number. A similar decrease in LE cell number may have been masked by some original HE cells reducing their Rfrp expression levels to become LE cells. The purpose of the E2-mediated repression of Rfrp is unclear but does not support a role for RFRP-3 in E2-mediated negative feedback, because such a model would predict that E2 up-regulates Rfrp to increase inhibition of GnRH. Although E2-induced repression of Rfrp could possibly relate to reducing RFRP-3-mediated inhibition of GnRH during the LH surge, it is unclear why E2 would act similarly in males (who lack positive feedback). Alternatively, E2 may regulate the involvement of RFRP-3 in other nonreproductive functions, such as temperature or energy homeostasis (14–16) or cognitive behaviors (48).
We found that only about 25% of Rfrp neurons coexpress ERα mRNA in both males and females, regardless of the sex steroid milieu, consistent with about 20% coexpression detected previously in female mice by immunohistochemistry (22). The inhibitory effects of E2 on Rfrp expression may therefore be mediated directly in only a small subset of Rfrp neurons, or conversely, E2 may regulate other upstream circuits that signal to Rfrp neurons. Whether or not ERα coexpression is restricted to only HE or LE cells is unknown, although both subpopulations were affected by E2 treatment.
The role of androgens in regulating Rfrp in mice has not been previously assessed. In contrast to E2 and T, DHT had no discernable effect on Rfrp cell number or mRNA levels, suggesting that androgen-signaling pathways are not important regulators of RFRP-3 neurons. This conclusion is supported by our finding of virtually no coexpression of AR in Rfrp cells of either sex.
RFRP-3 has agonist activity for both Gpr74 and Gpr147 (12, 23–26), and the antagonist RF-9, which stimulates LH secretion, can antagonize either receptor (38, 49). We found that only Gpr147 is significantly coexpressed in GnRH neurons, whereas Gpr74 is virtually nonexistent in GnRH cells. However, the degree of Gpr147/GnRH coexpression was low, being detectable in only small subsets of GnRH neurons in either sex, regardless of the hormonal milieu. This contrasts with the kisspeptin receptor, which is coexpressed in the vast majority of GnRH cells. This is the first report of possible coexpression of Gpr147 or Gpr74 in GnRH neurons of mice of either sex. Our Gpr147 data disagree with another report in male Siberian hamsters, in which Gpr147-ir was observed in 80% of GnRH neurons (28). However, only about 25% of GnRH neurons in mice are inhibited by RFRP-3 (3), matching our present coexpression data. Whether the discrepancy in coexpression between our mouse data and the hamster data reflects species or technical differences (in situ hybridization vs. immunohistochemistry) is unknown. If Gpr147 is indeed only lowly expressed in mouse GnRH cells, some of RFRP-3 inhibitory actions may be achieved indirectly via other GnRH-regulating pathways.
In summary, we determined that Rfrp expression in mice is not sexually dimorphic, but there are two subpopulations of Rfrp-expressing neurons, present in both sexes, that display unique developmental and steroidal regulation. The function(s) of these subpopulations remains to be determined. Additionally, we determined that E2 has a moderate inhibitory effect on Rfrp cells in both sexes, potentially achieved by direct ERα activity in a subset of Rfrp cells. Conversely, AR pathways do not influence Rfrp expression. Lastly, we determined that many GnRH cells lack RFRP-3 receptors, suggesting that RFRP-3 directly regulates only a subset of GnRH cells (via Gpr147) and/or that some of the actions of RFRP-3 on GnRH neurons are achieved indirectly.
Supplementary Material
Acknowledgments
This research was supported by National Science Foundation Grant IOS-1025893, a Diabetes and Endocrinology Research Center (DERC) Pilot and Feasibility grant (P30 DK063491), and the Eunice Kennedy Shriver National Institutes of Child Health and Human Development Grant U54-HD012303.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AR
- Androgen receptor
- DHT
- dihydrotestosterone
- DIG
- digoxigenin
- DMN
- dorsal-medial nucleus of the hypothalamus
- E2
- estradiol
- ERα
- estrogen receptor α
- GDX
- gonadectomized
- HE
- high expressing
- ISH
- in situ hybridization
- KO
- knockout
- LE
- low expressing
- PND
- postnatal day
- qPCR
- quantitative PCR
- RFamide
- arginine-phenylalanine-amide
- RFRP
- RFamide-related peptide
- RFRP-3-ir
- RFRP-3 immunoreactivity
- SSC
- sodium citrate, sodium chloride
- T
- testosterone
- WT
- wild type.
References
- 1. Tsutsui K. 2009. A new key neurohormone controlling reproduction, gonadotropin-inhibitory hormone (GnIH): Biosynthesis, mode of action and functional significance. Prog Neurobiol 88:76–88 [DOI] [PubMed] [Google Scholar]
- 2. Anderson GM, Relf HL, Rizwan MZ, Evans JJ. 2009. Central and peripheral effects of RFamide-related peptide-3 on luteinizing hormone and prolactin secretion in rats. Endocrinology 150:1834–1840 [DOI] [PubMed] [Google Scholar]
- 3. Ducret E, Anderson GM, Herbison AE. 2009. RFamide-related peptide-3, a mammalian gonadotropin-inhibitory hormone ortholog, regulates gonadotropin-releasing hormone neuron firing in the mouse. Endocrinology 150:2799–2804 [DOI] [PubMed] [Google Scholar]
- 4. Wu M, Dumalska I, Morozova E, van den Pol AN, Alreja M. 2009. Gonadotropin inhibitory hormone inhibits basal forebrain vGluT2-gonadotropin-releasing hormone neurons via a direct postsynaptic mechanism. J Physiol 587:1401–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hinuma S, Shintani Y, Fukusumi S, Iijima N, Matsumoto Y, Hosoya M, Fujii R, Watanabe T, Kikuchi K, Terao Y, Yano T, Yamamoto T, Kawamata Y, Habata Y, Asada M, Kitada C, Kurokawa T, Onda H, Nishimura O, Tanaka M, Ibata Y, Fujino M. 2000. New neuropeptides containing carboxy-terminal RFamide and their receptor in mammals. Nat Cell Biol 2:703–708 [DOI] [PubMed] [Google Scholar]
- 6. Kriegsfeld LJ, Mei DF, Bentley GE, Ubuka T, Mason AO, Inoue K, Ukena K, Tsutsui K, Silver R. 2006. Identification and characterization of a gonadotropin-inhibitory system in the brains of mammals. Proc Natl Acad Sci USA 103:2410–2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ubuka T, Morgan K, Pawson AJ, Osugi T, Chowdhury VS, Minakata H, Tsutsui K, Millar RP, Bentley GE. 2009. Identification of human GnIH homologs, RFRP-1 and RFRP-3, and the cognate receptor, GPR147 in the human hypothalamic pituitary axis. PLoS One 4:e8400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Smith JT, Shahab M, Pereira A, Pau KY, Clarke IJ. 2010. Hypothalamic expression of KISS1 and gonadotropin inhibitory hormone genes during the menstrual cycle of a non-human primate. Biol Reprod 83:568–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ukena K, Tsutsui K. 2001. Distribution of novel RFamide-related peptide-like immunoreactivity in the mouse central nervous system. Neurosci Lett 300:153–156 [DOI] [PubMed] [Google Scholar]
- 10. Yano T, Iijima N, Kakihara K, Hinuma S, Tanaka M, Ibata Y. 2003. Localization and neuronal response of RFamide related peptides in the rat central nervous system. Brain Res 982:156–167 [DOI] [PubMed] [Google Scholar]
- 11. Gibson EM, Humber SA, Jain S, Williams WP, III, Zhao S, Bentley GE, Tsutsui K, Kriegsfeld LJ. 2008. Alterations in RFamide-related peptide expression are coordinated with the preovulatory luteinizing hormone surge. Endocrinology 149:4958–4969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Quelven I, Roussin A, Zajac JM. 2005. Comparison of pharmacological activities of neuropeptide FF1 and neuropeptide FF2 receptor agonists. Eur J Pharmacol 508:107–114 [DOI] [PubMed] [Google Scholar]
- 13. Moulédous L, Mollereau C, Zajac JM. 2010. Opioid-modulating properties of the neuropeptide FF system. Biofactors 36:423–429 [DOI] [PubMed] [Google Scholar]
- 14. Moulédous L, Barthas F, Zajac JM. 2010. Opposite control of body temperature by NPFF1 and NPFF2 receptors in mice. Neuropeptides 44:453–456 [DOI] [PubMed] [Google Scholar]
- 15. Johnson MA, Tsutsui K, Fraley GS. 2007. Rat RFamide-related peptide-3 stimulates GH secretion, inhibits LH secretion, and has variable effects on sex behavior in the adult male rat. Horm Behav 51:171–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Murakami M, Matsuzaki T, Iwasa T, Yasui T, Irahara M, Osugi T, Tsutsui K. 2008. Hypophysiotropic role of RFamide-related peptide-3 in the inhibition of LH secretion in female rats. J Endocrinol 199:105–112 [DOI] [PubMed] [Google Scholar]
- 17. Legagneux K, Bernard-Franchi G, Poncet F, La Roche A, Colard C, Fellmann D, Pralong F, Risold PY. 2009. Distribution and genesis of the RFRP-producing neurons in the rat brain: comparison with melanin-concentrating hormone- and hypocretin-containing neurons. Neuropeptides 43:13–19 [DOI] [PubMed] [Google Scholar]
- 18. Quennell JH, Rizwan MZ, Relf HL, Anderson GM. 2010. Developmental and steroidogenic effects on the gene expression of RFamide related peptides and their receptor in the rat brain and pituitary gland. J Neuroendocrinol 22:309–316 [DOI] [PubMed] [Google Scholar]
- 19. Iwasa T, Matsuzaki T, Murakami M, Kinouchi R, Osugi T, Gereltsetseg G, Yoshida S, Irahara M, Tsutsui K. 2011. Developmental changes in the mammalian gonadotropin-inhibitory hormone (GnIH) ortholog RFamide-related peptide (RFRP) and its cognate receptor GPR147 in the rat hypothalamus. Int J Dev Neurosci 30:31–37 [DOI] [PubMed] [Google Scholar]
- 20. Sethi S, Tsutsui K, Chaturvedi CM. 2010. Temporal phase relation of circadian neural oscillations alters RFamide-related peptide-3 and testicular function in the mouse. Neuroendocrinology 91:189–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sethi S, Tsutsui K, Chaturvedi CM. 2010. Age-dependent variation in the RFRP-3 neurons is inversely correlated with gonadal activity of mice. Gen Comp Endocrinol 168:326–332 [DOI] [PubMed] [Google Scholar]
- 22. Molnar CS, Kalló I, Liposits Z, Hrabovszky E. 2011. Estradiol down-regulates RF-amide-related peptide (RFRP) expression in the mouse hypothalamus. Endocrinology 152:1684–1690 [DOI] [PubMed] [Google Scholar]
- 23. Liu Q, Guan XM, Martin WJ, McDonald TP, Clements MK, Jiang Q, Zeng Z, Jacobson M, Williams DL, Jr, Yu H, Bomford D, Figueroa D, Mallee J, Wang R, Evans J, Gould R, Austin CP. 2001. Identification and characterization of novel mammalian neuropeptide FF-like peptides that attenuate morphine-induced antinociception. J Biol Chem 276:36961–36969 [DOI] [PubMed] [Google Scholar]
- 24. Mollereau C, Mazarguil H, Marcus D, Quelven I, Kotani M, Lannoy V, Dumont Y, Quirion R, Detheux M, Parmentier M, Zajac JM. 2002. Pharmacological characterization of human NPFF(1) and NPFF(2) receptors expressed in CHO cells by using NPY Y(1) receptor antagonists. Eur J Pharmacol 451:245–256 [DOI] [PubMed] [Google Scholar]
- 25. Yoshida H, Habata Y, Hosoya M, Kawamata Y, Kitada C, Hinuma S. 2003. Molecular properties of endogenous RFamide-related peptide-3 and its interaction with receptors. Biochim Biophys Acta 1593:151–157 [DOI] [PubMed] [Google Scholar]
- 26. Gouardères C, Mazarguil H, Mollereau C, Chartrel N, Leprince J, Vaudry H, Zajac JM. 2007. Functional differences between NPFF1 and NPFF2 receptor coupling: high intrinsic activities of RFamide-related peptides on stimulation of [35S]GTPγS binding. Neuropharmacology 52:376–386 [DOI] [PubMed] [Google Scholar]
- 27. Talmont F, Moulédous L, Piedra-Garcia L, Schmitt M, Bihel F, Bourguignon JJ, Zajac JM, Mollereau C. 2010. Pharmacological characterization of the mouse NPFF2 receptor. Peptides 31:215–220 [DOI] [PubMed] [Google Scholar]
- 28. Ubuka T, Inoue K, Fukuda Y, Mizuno T, Kazyoshi U, Kriegsfeld LJ, Tsutsui K. 2012. Identification, expression, and physiological functions of siberian hamster gonadotropin-inhibitory hormone. Endocrinology 153:373–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ubuka T, Kim S, Huang YC, Reid J, Jiang J, Osugi T, Chowdhury VS, Tsutsui K, Bentley GE. 2008. Gonadotropin-inhibitory hormone neurons interact directly with gonadotropin-releasing hormone-I and -II neurons in European starling brain. Endocrinology 149:268–278 [DOI] [PubMed] [Google Scholar]
- 30. Semaan SJ, Murray EK, Poling MC, Dhamija S, Forger NG, Kauffman AS. 2010. BAX-dependent and BAX-independent regulation of Kiss1 neuron development in mice. Endocrinology 151:5807–5817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. 2005. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 146:3686–3692 [DOI] [PubMed] [Google Scholar]
- 32. Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA. 2005. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology 146:2976–2984 [DOI] [PubMed] [Google Scholar]
- 33. Kim J, Semaan SJ, Clifton DK, Steiner RA, Dhamija S, Kauffman AS. 2011. Regulation of Kiss1 expression by sex steroids in the amygdala of the rat and mouse. Endocrinology 152:2020–2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Finn PD, Steiner RA, Clifton DK. 1998. Temporal patterns of gonadotropin-releasing hormone (GnRH), c-fos, and galanin gene expression in GnRH neurons relative to the luteinizing hormone surge in the rat. J Neurosci 18:713–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. 2004. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology 145:4073–4077 [DOI] [PubMed] [Google Scholar]
- 36. Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown A, Clifton DK, Hoffman GE, Steiner RA, Tena-Sempere M. 2007. Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology 148:1774–1783 [DOI] [PubMed] [Google Scholar]
- 37. Robertson JL, Clifton DK, de la Iglesia HO, Steiner RA, Kauffman AS. 2009. Circadian regulation of Kiss1 neurons: implications for timing the preovulatory gonadotropin-releasing hormone/luteinizing hormone surge. Endocrinology 150:3664–3671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pineda R, Garcia-Galiano D, Sanchez-Garrido MA, Romero M, Ruiz-Pino F, Aguilar E, Dijcks FA, Blomenröhr M, Pinilla L, van Noort PI, Tena-Sempere M. 2010. Characterization of the potent gonadotropin-releasing activity of RF9, a selective antagonist of RF-amide-related peptides and neuropeptide FF receptors: physiological and pharmacological implications. Endocrinology 151:1902–1913 [DOI] [PubMed] [Google Scholar]
- 39. Kauffman AS, Navarro VM, Kim J, Clifton D, Steiner RA. 2009. Sex differences in the regulation of Kiss1/NKB neurons in juvenile mice: implications for the timing of puberty. Am J Physiol Endocrinol Metab 297:E1212–E1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Semaan SJ, Kauffman AS. 2010. Sexual differentiation and development of forebrain reproductive circuits. Curr Opin Neurobiol 20:424–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gouardères C, Kieffer BL, Zajac JM. 2004. Opposite alterations of NPFF1 and NPFF2 neuropeptide FF receptor density in the triple MOR/DOR/KOR-opioid receptor knockout mouse brains. J Chem Neuroanat 27:119–128 [DOI] [PubMed] [Google Scholar]
- 42. Gouardères C, Puget A, Zajac JM. 2004. Detailed distribution of neuropeptide FF receptors (NPFF1 and NPFF2) in the rat, mouse, octodon, rabbit, guinea pig, and marmoset monkey brains: a comparative autoradiographic study. Synapse 51:249–269 [DOI] [PubMed] [Google Scholar]
- 43. Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. 2004. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology 80:264–272 [DOI] [PubMed] [Google Scholar]
- 44. Kauffman AS, Park JH, McPhie-Lalmansingh AA, Gottsch ML, Bodo C, Hohmann JG, Pavlova MN, Rohde AD, Clifton DK, Steiner RA, Rissman EF. 2007. The kisspeptin receptor GPR54 is required for sexual differentiation of the brain and behavior. J Neurosci 27:8826–8835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Smith JT, Coolen LM, Kriegsfeld LJ, Sari IP, Jaafarzadehshirazi MR, Maltby M, Bateman K, Goodman RL, Tilbrook AJ, Ubuka T, Bentley GE, Clarke IJ, Lehman MN. 2008. Variation in kisspeptin and RFamide-related peptide (RFRP) expression and terminal connections to gonadotropin-releasing hormone neurons in the brain: a novel medium for seasonal breeding in the sheep. Endocrinology 149:5770–5782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sugrue ML, Vella KR, Morales C, Lopez ME, Hollenberg AN. 2010. The thyrotropin-releasing hormone gene is regulated by thyroid hormone at the level of transcription in vivo. Endocrinology 151:793–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. White FA, Keller-Peck CR, Knudson CM, Korsmeyer SJ, Snider WD. 1998. Widespread elimination of naturally occurring neuronal death in Bax-deficient mice. J Neurosci 18:1428–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kaewwongse M, Takayanagi Y, Onaka T. 2011. Effects of RFamide-related peptide (RFRP)-1 and RFRP-3 on oxytocin release and anxiety-related behaviour in rats. J Neuroendocrinol 23:20–27 [DOI] [PubMed] [Google Scholar]
- 49. Simonin F, Schmitt M, Laulin JP, Laboureyras E, Jhamandas JH, MacTavish D, Matifas A, Mollereau C, Laurent P, Parmentier M, Kieffer BL, Bourguignon JJ, Simonnet G. 2006. RF9, a potent and selective neuropeptide FF receptor antagonist, prevents opioid-induced tolerance associated with hyperalgesia. Proc Natl Acad Sci USA 103:466–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.