Neurons that produce GnRH serve as the final common pathway through which the brain regulates reproduction. Although GnRH neurons have spontaneous secretory activity, afferent input from kisspeptin (Kiss1) neurons is required to drive pulsatile GnRH secretion, initiate the onset of puberty, and regulate normal reproductive function (1–3). Kiss1 neurons in the arcuate nucleus (or infundibular nucleus in primates) coexpress at least two other neuropeptides, neurokinin B (NKB) and dynorphin A (4–6). However, the functional significance of these cotransmitters for reproduction remained a complete mystery until 2009, when it was discovered that patients bearing inactivating mutations in either the TAC3 or TACR3 gene (encoding NKB and its receptor, NK3R, respectively) fail to progress through puberty and exhibit profound hypogonadotropic hypogonadism (7–12). Indeed, this observation echoes the hallmarks seen in humans and mice with disabling mutations or deletions in KISS1R/Kiss1r. Paradoxically, earlier studies in Tacr3−/− mice, which were focused on the neurological functions of NK3R, mentioned anecdotally that mice bearing targeted deletions of the Tacr3 gene appeared to be fertile (13–15). At face value, this finding in mice seemed at odds with the phenotype of patients with TACR3 mutations. So, what's up with this apparent contradiction?
In the current issue of Endocrinology, Jasmine Yang and her colleagues (16) coax the puzzled parties toward reconciliation. In a compelling series of experiments, these authors demonstrate that notwithstanding their fertility, Tacr3-null mice exhibit a marked degree of reproductive impairment, (i.e. lower circulating levels of FSH, reduced testicular size, impaired ovulation, smaller uterine weight, and diminished fecundity compared with wild-type controls), reminiscent of the human phenotype. So the neuroendocrine community can breathe a collective sigh of relief; the TACR3/Tacr3 stories in humans and mice seem to be in broad agreement, despite a few awkward details. This is noteworthy, because having a mouse model that reasonably recapitulates the mutant human TACR3 phenotype offers the neuroendocrine community another tool to probe the mysteries of the Kiss1/GnRH pulse generator. But let's take a more careful look under the rug.
The TACR3 mutants and Tacr3−/− phenotypes are, in fact, quite different in several fundamental respects (e.g. puberty and fertility). How do we explain why some genetic lesions produce such different outcomes in mice and humans? First, different neuroendocrine mechanisms guide puberty and control reproduction in primates and rodents (17–19). Second, patterns of expression (and probably the functions) of TACR3/Tacr3 differ between humans and mice (20, 21). Third, the distribution of GnRH and Kiss1/NKB-expressing neurons are remarkably discordant between primates and rodents (22–24). Given these major anatomical differences, it should come as no surprise that genetic disruptions of an important G protein-coupled receptor should generate different phenotypes between mice and humans.
On the other hand, more comprehensive genetic studies in humans with more subtle reproductive disorders could blur the distinctions between human and mouse mutant TACR3/Tacr3 phenotypes even further. As we continue to learn more about the population of humans with mutations in TACR3, their deficits may not be quite as severe as initially described (7–12) (i.e. some patients seem either to spontaneously recover from their deficits or show recovery after sex steroid therapy). What might explain the different accounts of the TACR3 phenotype? Certainly, different mutations in the TACR3 gene can produce different degrees of G protein-coupled receptor impairment and thus different phenotypes. It could reflect the fact that the initial selection and description of the mutant TACR3 phenotype (collected from patients with severe idiopathic hypogonadotropic hypogonadism) represented one extreme of the bell-shaped curve of the phenotype of such individuals, skewed by the tyranny of small proband numbers, whose phenotype was the entrance criterion for the study. Perhaps, as we study more individuals with TACR3 mutations (or deletions), we'll discover that the reproductive lesion in humans is less dramatic (on a population basis) than originally thought, a possibility acknowledged by Yang and her colleagues (16).
Another possibility is that the Tacr3−/− mouse being studied retains some residual NK3R activity. If there were residual receptor expression and activity in the Tacr3 knockout, it might help to explain why the mice appear to be fertile and relatively normal (except under careful scrutiny). The original paper characterizing the mouse used by Yang et al. (16) presents some evidence, albeit inconclusive, that the Tacr3 gene has in fact been deleted (i.e. PCR analysis) (13). Two other Tacr3−/− models have also been described in the literature. The first was reported by Kung and collaborators (14), who noted that their Tacr3−/− mice were fertile. A second report by Nordquist and colleagues (15) makes no direct mention of fertility status. In these two reports, the absence of NK3R was tested by PCR (14) and Western blot and impaired responsiveness to senktide (an NKB agonist) of putative NKB targets in the ventral tegmental area of the brain (15). The conclusions of the Yang paper would be strengthened by establishing that the basic premise is true, i.e. the Tacr3−/− mice lack the Tacr3 transcript and NK3R protein and that senktide cannot induce GnRH/LH secretion in Tacr3−/− mice (6, 25–28).
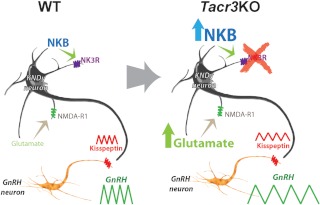
Putting aside the technical concerns about the completeness of the Tacr3 knockout, the fact remains that the Tacr3 knockout mice show normal pubertal maturation and become fertile as adults, reminiscent of some patients having similar genetic lesions (9). Does this mean that NKB-NK3R signaling isn't essential in normal physiology? Maybe only a trickle of GnRH is needed to support the hypothalamic-pituitary-gonadal axis, partly spontaneous and partly driven by constitutive Kiss1/Kiss1r signaling. It also seems plausible that NKB-NK3R signaling is part of a highly redundant control system bearing many fail-safes. Although redundancies make sense, they are difficult to test. It seems more plausible (and testable) that Tacr3/TACR3 lesions are detected by the developing brain and that the brain compensates for the loss by marshaling reinforcements, ramping up the expression of other factors that would allow this exquisitely tuned network to overcome this developmental adversity. How might the Kiss1/NKB/GnRH network ameliorate the loss of NKB/NK3R signaling? One possibility is that Kiss1 (and perhaps glutamate/glutamate receptor production by Kiss1 neurons) becomes up-regulated to compensate for the loss of stimulation from recurrent collaterals (5, 29), as suggested in the cartoon illustrated in Fig. 1.
Fig. 1.
Cartoon illustrating possible compensatory mechanisms that mitigate the sequelae of disruptions of TACR3/Tacr3 signaling in Kiss1 neurons (an NKB/NK3R autosynaptic, feed-forward loop). In this model, a congenital loss of Tacr3/NK3R results in an up-regulation of NKB production, which has no discernible effect because the receptor is lost. However, glutamate → N-methyl-D-aspartate R1 subunit (NMDA-R1), signaling is also up-regulated, which allows the ailing neuron to maintain its output of kisspeptin and sustain, at least partially, GnRH and gonadotropin secretion in amounts sufficient to support puberty and fertility. The Tacr3−/− mouse described by Yang and her colleagues (16) presents a unique opportunity to learn how the brain attempts to fix genetic accidents and could even point the way to unimagined solutions. WT, Wild type.
As with other benchmark studies, Yang et al. (16) is a launching pad. So, where to from here? First, it would be helpful to strengthen the foundation of the Tacr3 knockout itself, establishing beyond a reasonable doubt that the Tacr3 knockout is complete (i.e. neither mRNA nor protein can be found, as analyzed by quantitative RT-PCR, in situ hybridization, and immunocytochemistry, and there is no LH response to senktide). Second, if the Tacr3 knockout were indeed complete, it would be fascinating to learn whether (and by what mechanisms) other factors compensate for the loss of Tacr3. Third, because Tacr3 is expressed in Kiss1 neurons, the phenotype of global Tacr3/TACR3 knockout (or mutation) may be entirely explicable by the loss of NKB signaling in Kiss1 neurons. If so, knocking out Tacr3 specifically in Kiss1 neurons (by crossing Kiss1-Cre mice with a floxed Tacr3 allele) should recapitulate the phenotype of the global knockout. This is particularly important because Tacr3 (not to mention Tac2, encoding NKB in nonprimate species) is widely expressed in the brain (21, 26) and clearly has other functions beyond reproduction.
Acknowledgments
We are grateful for critical commentary provided by Don Clifton, Simina Popa, Paul Amieux, and Amy Oakley.
This work was supported in part by NIH/NICHD R01 HD049651 and the Marie Curie Outgoing International Fellowship within the 7th Framework Program of the European Union.
Disclosure Summary: The authors have nothing to disclose.
For article see page 1498
- Kiss1
- Kisspeptin
- NKB
- neurokinin B
- NK3R
- NKB receptor.
References
- 1. de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. 2003. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA 100:10972–10976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Funes S, Hedrick JA, Vassileva G, Markowitz L, Abbondanzo S, Golovko A, Yang S, Monsma FJ, Gustafson EL. 2003. The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem Biophys Res Commun 312:1357–1363 [DOI] [PubMed] [Google Scholar]
- 3. Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. 2003. The GPR54 gene as a regulator of puberty. N Engl J Med 349:1614–1627 [DOI] [PubMed] [Google Scholar]
- 4. Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CV, Jafarzadehshirazi MR, Pereira A, Iqbal J, Caraty A, Ciofi P, Clarke IJ. 2007. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology 148:5752–5760 [DOI] [PubMed] [Google Scholar]
- 5. Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. 2009. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci 29:11859–11866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ramaswamy S, Seminara SB, Ali B, Ciofi P, Amin NA, Plant TM. 2010. Neurokinin B stimulates GnRH release in the male monkey (Macaca mulatta) and is colocalized with kisspeptin in the arcuate nucleus. Endocrinology 151:4494–4503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Francou B, Bouligand J, Voican A, Amazit L, Trabado S, Fagart J, Meduri G, Brailly-Tabard S, Chanson P, Lecomte P, Guiochon-Mantel A, Young J. 2011. Normosmic congenital hypogonadotropic hypogonadism due to TAC3/TACR3 mutations: characterization of neuroendocrine phenotypes and novel mutations. PLoS One 6:e25614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fukami M, Maruyama T, Dateki S, Sato N, Yoshimura Y, Ogata T. 2010. Hypothalamic dysfunction in a female with isolated hypogonadotropic hypogonadism and compound heterozygous TACR3 mutations and clinical manifestation in her heterozygous mother. Horm Res Paediatr 73:477–481 [DOI] [PubMed] [Google Scholar]
- 9. Gianetti E, Tusset C, Noel SD, Au MG, Dwyer AA, Hughes VA, Abreu AP, Carroll J, Trarbach E, Silveira LF, Costa EM, de Mendonça BB, de Castro M, Lofrano A, Hall JE, Bolu E, Ozata M, Quinton R, Amory JK, Stewart SE, Arlt W, Cole TR, Crowley WF, Kaiser UB, Latronico AC, Seminara SB. 2010. TAC3/TACR3 mutations reveal preferential activation of gonadotropin-releasing hormone release by neurokinin B in neonatal life followed by reversal in adulthood. J Clin Endocrinol Metab 95:2857–2867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guran T, Tolhurst G, Bereket A, Rocha N, Porter K, Turan S, Gribble FM, Kotan LD, Akcay T, Atay Z, Canan H, Serin A, O'Rahilly S, Reimann F, Semple RK, Topaloglu AK. 2009. Hypogonadotropic hypogonadism due to a novel missense mutation in the first extracellular loop of the neurokinin B receptor. J Clin Endocrinol Metab 94:3633–3639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Ozbek MN, Imamoglu S, Akalin NS, Yuksel B, O'Rahilly S, Semple RK. 2009. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for neurokinin B in the central control of reproduction. Nat Genet 41:354–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Young J, Bouligand J, Francou B, Raffin-Sanson ML, Gaillez S, Jeanpierre M, Grynberg M, Kamenicky P, Chanson P, Brailly-Tabard S, Guiochon-Mantel A. 2010. TAC3 and TACR3 defects cause hypothalamic congenital hypogonadotropic hypogonadism in humans. J Clin Endocrinol Metab 95:2287–2295 [DOI] [PubMed] [Google Scholar]
- 13. Siuciak JA, McCarthy SA, Martin AN, Chapin DS, Stock J, Nadeau DM, Kantesaria S, Bryce-Pritt D, McLean S. 2007. Disruption of the neurokinin-3 receptor (NK3) in mice leads to cognitive deficits. Psychopharmacology (Berl) 194:185–195 [DOI] [PubMed] [Google Scholar]
- 14. Kung TT, Crawley Y, Jones H, Luo B, Gilchrest H, Greenfeder S, Anthes JC, Lira S, Wiekowski M, Cook DN, Hey JA, Egan RW, Chapman RW. 2004. Tachykinin NK3-receptor deficiency does not inhibit pulmonary eosinophilia in allergic mice. Pharmacol Res 50:611–615 [DOI] [PubMed] [Google Scholar]
- 15. Nordquist RE, Durkin S, Jacquet A, Spooren W. 2008. The tachykinin NK3 receptor agonist senktide induces locomotor activity in male Mongolian gerbils. Eur J Pharmacol 600:87–92 [DOI] [PubMed] [Google Scholar]
- 16. Yang J, Caligioni CS, Chan YM, Seminara SB. 2012. Uncovering novel reproductive defects in neurokinin B receptor null mice: closing the gap between mice and men. Endocrinology 153:1498–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Plant TM, Barker-Gibb ML. 2004. Neurobiological mechanisms of puberty in higher primates. Hum Reprod Update 10:67–77 [DOI] [PubMed] [Google Scholar]
- 18. Terasawa E, Fernandez DL. 2001. Neurobiological mechanisms of the onset of puberty in primates. Endocr Rev 22:111–151 [DOI] [PubMed] [Google Scholar]
- 19. Ojeda SR, Advis JP, Andrews WW. 1980. Neuroendocrine control of the onset of puberty in the rat. Fed Proc 39:2365–2371 [PubMed] [Google Scholar]
- 20. Rance NE. 2009. Menopause and the human hypothalamus: evidence for the role of kisspeptin/neurokinin B neurons in the regulation of estrogen negative feedback. Peptides 30:111–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Duarte CR, Schütz B, Zimmer A. 2006. Incongruent pattern of neurokinin B expression in rat and mouse brains. Cell Tissue Res 323:43–51 [DOI] [PubMed] [Google Scholar]
- 22. Chawla MK, Gutierrez GM, Young WS, 3rd, McMullen NT, Rance NE. 1997. Localization of neurons expressing substance P and neurokinin B gene transcripts in the human hypothalamus and basal forebrain. J Comp Neurol 384:429–442 [DOI] [PubMed] [Google Scholar]
- 23. Herbison A. 2006. Physiology of the GnRH neuronal network. In: Neill J, Knobil E, eds. Physiology of reproduction. San Diego, CA: Academic Press; 1415–1482 [Google Scholar]
- 24. Oakley AE, Clifton DK, Steiner RA. 2009. Kisspeptin signaling in the brain. Endocr Rev 30:713–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Billings HJ, Connors JM, Altman SN, Hileman SM, Holaskova I, Lehman MN, McManus CJ, Nestor CC, Jacobs BH, Goodman RL. 2010. Neurokinin B acts via the neurokinin-3 receptor in the retrochiasmatic area to stimulate luteinizing hormone secretion in sheep. Endocrinology 151:3836–3846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Navarro VM, Castellano JM, McConkey SM, Pineda R, Ruiz-Pino F, Pinilla L, Clifton DK, Tena-Sempere M, Steiner RA. 2011. Interactions between kisspeptin and neurokinin B in the control of GnRH secretion in the female rat. Am J Physiol Endocrinol Metab 300:E202–E210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Navarro VM, Gottsch ML, Wu M, García-Galiano D, Hobbs SJ, Bosch MA, Pinilla L, Clifton DK, Dearth A, Ronnekleiv OK, Braun RE, Palmiter RD, Tena-Sempere M, Alreja M, Steiner RA. 2011. Regulation of NKB pathways and their roles in the control of Kiss1 neurons in the arcuate nucleus of the male mouse. Endocrinology 152:4265–4275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ramaswamy S, Seminara SB, Plant TM. 2011. Evidence from the agonadal juvenile male rhesus monkey (Macaca mulatta) for the view that the action of neurokinin B to trigger gonadotropin-releasing hormone release is upstream from the kisspeptin receptor. Neuroendocrinology 94:237–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gottsch ML, Popa SM, Lawhorn JK, Qiu J, Tonsfeldt KJ, Bosch MA, Kelly MJ, Rønnekleiv OK, Sanz E, McKnight GS, Clifton DK, Palmiter RD, Steiner RA. 2011. Molecular properties of kiss1 neurons in the arcuate nucleus of the mouse. Endocrinology 152:4298–4309 [DOI] [PMC free article] [PubMed] [Google Scholar]