Abstract
Loss of Pten in the KrasG12D;Amhr2-Cre mutant mice leads to the transformation of ovarian surface epithelial (OSE) cells and rapid development of low-grade, invasive serous adenocarcinomas. Tumors occur with 100% penetrance and express elevated levels of wild-type tumor repressor protein 53 (TRP53). To test the functions of TRP53 in the Pten;Kras (Trp53+) mice, we disrupted the Trp53 gene yielding Pten;Kras(Trp53−) mice. By comparing morphology and gene expression profiles in the Trp53+ and Trp53− OSE cells from these mice, we document that wild-type TRP53 acts as a major promoter of OSE cell survival and differentiation: cells lacking Trp53 are transformed yet are less adherent, migratory, and invasive and exhibit a gene expression profile more like normal OSE cells. These results provide a new paradigm: wild-type TRP53 does not preferentially induce apoptotic or senescent related genes in the Pten;Kras(Trp53+) cancer cells but rather increases genes regulating DNA repair, cell cycle progression, and proliferation and decreases putative tumor suppressor genes. However, if TRP53 activity is forced higher by exposure to nutlin-3a (a mouse double minute-2 antagonist), TRP53 suppresses DNA repair genes and induces the expression of genes that control cell cycle arrest and apoptosis. Thus, in the Pten;Kras(Trp53+) mutant mouse OSE cells and likely in human TP53+ low-grade ovarian cancer cells, wild-type TRP53 controls global molecular changes that are dependent on its activation status. These results suggest that activation of TP53 may provide a promising new therapy for managing low-grade ovarian cancer and other cancers in humans in which wild-type TP53 is expressed.
Ovarian cancer is a complex disease and remains the fifth most lethal cause of death by cancer in women. It is difficult to detect and hard to manage with current surgical and chemotherapeutic strategies (1, 2). Among all human ovarian cancers (excluding germ cell cancer), granulosa cell tumors account for approximately 10%, whereas epithelial ovarian carcinomas (EOCs) account for approximately 90% of known tumors. EOCs are subdivided into four major classes based on histological criteria as well as more recent molecular signatures (2). Approximately 70% of EOCs are serous adenocarcinomas followed by endometrioid, mucinous and clear cell cancers. EOC are also classified as low grade and high grade based on distinct pathogeneses, molecular phenotypes, and responses to conventional chemotherapy (1–5). One distinguishing and relevant feature of low-grade and high-grade ovarian cancer is the expression of the tumor repressor protein 53 (TP53; or p53): almost all high-grade serous adenocarcinomas (96%) have TP53 mutations, whereas low-grade tumors express elevated levels of wild-type TP53 (6–8). Normal ovarian surface epithelial cells or fallopian epithelial cells, the potential origins of low-grade serous ovarian carcinomas, do not normally express TP53 (9). DNA sequencing analysis has shown that most low-grade serous carcinomas of the ovary carry wild-type TP53 (10, 11). Based on immunohistochemistry analysis, low-grade ovarian serous carcinomas express wild-type TP53, although the expression is not as robust as mutated TP53 protein in high-grade serous ovarian carcinomas (7, 12). Thus, it will be interesting to determine the functional role of wild-type TP53 expression in low-grade ovarian carcinomas. Understanding the functional roles of mutant vs. wild-type TP53 is of critical importance for developing new and more effective therapies for each cancer subtype.
The task of understanding the functions of TP53 is challenging because the responses of cells to TP53 are complex, context specific, and dependent on its activation status (13–15). In addition, there is a complex interplay among phosphatase and tensin homolog deleted from chromosome 10, v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog, and TP53 in tumor development and progression (1, 2, 8). Many studies in other normal and cancer cell types as well as in other tissues during embryogenesis and development document that wild-type TP53 controls diverse cellular processes including cell cycle arrest, senescence, apoptosis, DNA repair, cell migration, autophagy, metabolism, differentiation, and reprogramming (7, 16–18). Because of its predominant role in preventing propagation of damaged DNA and in inducing cell death, TP53 has become known as the guardian of the genome (19). That mutations of TP53 are present in nearly 50% of all human cancers reinforces how critical the functions of this protein are to normal cell homeostasis. Due to this, restoration of TP53 is being investigated as a therapy for cancer treatment. Several recent animal studies document the effectiveness of restoring wild-type Trp53: it promotes regression of tumors containing wild-type Trp53 (20–22) and suppresses tumor growth in cells containing mutant Trp53 (23). Despite the presence of wild-type TP53 in low-grade serous ovarian cancer and the strikingly high prevalence of TP53 mutations in serous adenocarcinoma (24), little is yet known about the functional roles of wild-type or mutant TP53 in human ovarian cancer cells.
Mouse models provide efficient, reproducible systems in which to analyze the specific functions of oncogenes and tumor suppressors in vivo. Recently we generated a mouse model in which disruption of the Pten gene in the KrasG12D;Amhr2-Cre strain leads to the development of low-grade serous, papillary adenocarcinomas at an early age and with 100% penetrance (8, 25). We have shown further that the ovarian surface epithelial cells in these tumors express increased levels of wild-type TRP53 mRNA and protein (8) as is observed in human low-grade ovarian cancer (6, 7). The Pten;Kras; (Trp53+);Amhr2-Cre mutant mice therefore provide an ideal system in which to determine the functional roles of wild-type TRP53 in transformed serous adenocarcinoma cells that exhibit histological and functional similarities to human low-grade serous ovarian carcinomas. To accomplish this goal, we disrupted the Trp53 gene in the Ptenfl/fl;KrasG12D;Amhr2-cre mice. Because mutations in or loss of Trp53 are associated with high-grade ovarian cancer, we initially hypothesized that the loss of Trp53 in this context might lead to a more aggressive, metastatic tumor phenotype. However, we discovered quite the opposite. Growth of the Pten;Kras(Trp53−) ovarian surface epithelial (OSE) cell papillary-like structures was diminished dramatically and the tumors were less invasive in the ovarian stroma. By generating the Pten;Kras(Trp53−) mice, we have discovered further that wild-type TRP53 is critical for driving the Pten;Kras(Trp53+) mutant phenotype by regulating hundreds of genes identified by microarray. Furthermore, the results have revealed that the mutant Pten;Kras(Trp53+) OSE cell phenotype is dependent not only on levels of wild-typeTrp53 mRNA and protein but also on the degree to which TRP53 is activated by either endogenous factors or exogenous drugs.
Materials and Methods
Animal procedures
LSL-KrasG12D;Amhr2-Cre, Ptenfl/fl;Amhr2-Cre, and LSL-KrasG12D;Ptenfl/fl;Amhr2-Cre mice were derived and genotyped as previously described (25, 27). Trp53fl/fl mice (strain: FVB.129-Trp53tm1Brn) were obtained from the Mouse Models of Human Cancer Consortium, National Cancer Institute (Frederick, MD). Trp53fl/fl;LSL-KrasG12D;Ptenfl/fl;Amhr2-Cre mice are a mixture of strains derived by crossing the different genotypes. Animals were housed under a 16-h light, 8-h dark schedule in the Center for Comparative Medicine at Baylor College of Medicine and provided food and water ad libitum. Animals were treated in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, as approved by the Animal Care and Use Committee at the Baylor College of Medicine.
Histology and immunohistochemistry
Ovaries were collected and fixed in 4% paraformaldehyde, embedded in paraffin, and processed by routine procedures for immunohistochemistry (25) of Cytokeratin 8 (ab59400; Abcam, Cambridge, MA) and TRP53 (sc-6243; Santa Cruz Biotechnology, Inc., Santa Cruz, CA).
Cell lines and isolation and culture of primary OSE cells
The HOC-7 cell line was derived from the malignant ascite of a patient with stage III low-grade adenocarcinoma of the ovary (28, 29) and contains wild-type TP53 as determined by sequencing. OSE cells were isolated and cultured as previously described (8).
Migration and adhesion assays
Cell migration was assessed by plating 20,000 cells per chamber in 24-well BD Matrigel invasion chambers according to the manufacturer's instructions (BD Biosciences, Bedford, MA). Cell adhesion was analyzed by counting attached cells per field 1 h after the plating.
Inoculation of cells into syngenic mice
The 1 × 106 mutant OSE cells were injected sc into wild-type mice with the same genetic background along with Matrigel (BD Biosciences) according to the manufacturer's protocol. Tumors were harvested and fixed in 4% paraformaldehyde after 20 d.
Real-time RT-PCR for mRNA and mature microRNA (miRNA)
Total RNA was isolated using the RNeasy minikit (QIAGEN, Germantown, MD) and treated with deoxyribonuclease I (DNA free; Ambion, Inc. Austin, TX) according to the manufacturer's instructions. cDNA was synthesized with Moloney murine leukemia virus reverse transcriptase (Invitrogen, Grand Island, NY) primed with random hexamers. Real-time PCR was performed using the Light Cycler DNA master SYBR Green I kit (Roche Applied Sciences, Nutley, NJ). Primers (Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org) were used at a concentration of 0.25 μm and MgCl2 at 2.4 mm. Samples were denatured for 2 min at 95 C and then 35 cycles of 95 C for 15 sec, 60 C for 30 sec, and 72 C for 30 sec as previously described (25). Data were normalized to RPL19 using the comparative cycle threshold method. Data are presented as the mean ± sem of a representative of at least three experiments performed in triplicate.
Small RNA required for detecting and measuring mature miRNA were extracted using the mirVANA miRNA isolation kit (Ambion) according to the manufacturer's instructions. For quantification of mature miRNA, the TaqMan miRNA assay kit (Applied Biosystems, Foster City, CA) was used according to the manufacturer's instructions.
Microarray
Total RNA was prepared using the RNeasy mini kit (QIAGEN) from OSE cells isolated from wild-type control, Ptenfl/fl;KrasG12D;Amhr2-cre, and Ptenfl/fl;KrasG12D;Trp53fl/fl; Amhr2-cre mice at 3 months of age when tumor growth on the ovarian surface was highly evident. The quality of the RNA was verified by the MicroArray Core Facility at Baylor College of Medicine and hybridized to Mouse 420.3 Affymetrix Chips (Santa Clara, CA) using routine procedures.
Gene expression analysis
Fold changes between control and Ptenfl/fl;KrasG12D;Amhr2-cre mice and Ptenfl/fl;KrasG12D;Trp53fl/fl; Amhr2-cre mice were estimated, using the ratio of expression values. Microarray data were analyzed as previously reported using the Robust Multiarray Averaging function (30) [from the Affy package (version 1.5.8) through the BioConductor software (http://www.bioconductor.org/ (31)]. The hierarchical clustering is done through MeV (32, 33).
Results
TRP53 mediates the formation of elaborate papillary structures in low-grade serous adenocarcinoma tumors
Immunohistochemical staining for TRP53 shows that TRP53 is absent in the surface epithelial cells of normal ovaries (Fig. 1A). In contrast, TRP53 is highly induced in the OSE papillary structures of low-grade ovarian tumors from Pten;Kras (TRP53+) mice (Fig. 1A, middle column) as previously shown (8). Immunostaining forTRP53 is absent in the nonpapillary OSE cell layer in ovaries of the Pten;Kras (Trp53−) mice confirming the absence of Trp53 mRNA expression (Fig. 1A, last column). Histological sections from ovaries of control mice show that the ovarian surface epithelium is a single layer of mesoepithelial cells that stain for the epithelial-specific marker cytokeratin 8 (Fig. 1B). As previously shown, the OSE cell layer of ovaries from Pten fl/fl; KrasG12D;Amhr2-cre [Pten;Kras(Trp53+)] mice exhibit low-grade serous, papillary adenocarcinoma with elaborate papillary structures at 12 wk (8, 25) (Fig. 1, B and C, middle column). However, the OSE cells depleted of Trp53 from Pten fl/fl;KrasG12D; Trp53 fl/fl;Amhr2-cre [Pten;Kras(Trp53−)] mice exhibit a limited papillary morphology at a similar time point (Fig. 1, B and C, last column) providing evidence that wild-type TRP53 supports the formation of ovarian papillary structures in low-grade serous adenocarcinomas. The lack of papillary structures at later ages suggests Trp53 prevents progression of this phenotype (data not shown). The OSE cells were isolated and grown in culture to characterize the molecular and cellular effects of TRP53 (Fig. 1D). Similar to the transformed OSE cells from Pten;Kras(Trp53+) ovaries (8), OSE cells from Pten;Kras(Trp53−) ovaries have become an immortal cell line capable of continuous culture. The cells were plated in Matrigel to observe the morphological effects of a three-dimensional growth environment on the cells (Fig. 1E). Hematoxylin and eosin (H&E) staining of fixed colonies illustrates that wild-type OSE cells grew in tightly packed colonies. However, Trp53+ tumor OSE cells form structures similar to the papillary structures seen in vivo. The Trp53− OSE cells grew in solid colonies with both tightly packed and loosely packed cells, suggesting TRP53 is required for the formation of the papillary, tube-like structures in Matrigel.
Fig. 1.
Characterization of the OSE cell phenotypes of wild-type, Pten;Kras (Trp53+), and Pten;Kras (Trp53-) mice. TRP53 is not expressed in the OSE cell layer of ovaries collected from wild type or Pten;Kras (Trp53−) mice but is elevated in the OSE cells of papillary tumors present invading the ovaries of the Pten;Kras (Trp53+) mice. OSE cells from wild-type mice exhibit a mesothelial-like morphology, are cytokeratin-8 positive, and can proliferate in culture and in Matrigel but are not transformed. In contrast, Pten;Kras (Trp53+) OSE cells grow in elaborate papillary-like, cytokeratin-8-positive structures in vivo and when cultured in Matrigel. The Trp53 depleted OSE cells of the Pten;Kras (Trp53−) mice exhibit limited papillary-like structures in vivo and in Matrigel. A, Immunolabeling of TRP53 of sections from ovaries of WT and mutant mice. Areas of magnification (×40) are indicated by boxes. B, Immunolabeling of the epithelial specific marker cytokeratin-8 of sections from ovaries of wild-type and mutant mice. C, Higher magnification (×20) of the epithelial layer. D, Bright-field image of isolated OSE cells grown in cell culture dishes. E, H&E staining of sections from isolated OSE cells grown in Matrigel for 10 d.
TRP53 regulates genes in tumor OSE cells involved in ovarian cancer cell survival
To determine which genes are regulated by TRP53 in the tumor OSE cells, we performed gene array analyses on OSE cells isolated from wild-type, Pten;Kras(Trp53+), and Pten;Kras(Trp53-) mice. Compared with wild-type OSE cells, hundreds of genes were regulated in Pten;Kras(Trp53+) OSE cells (Fig. 2A). Consistent with the minimal morphological changes in OSE cells depleted of Trp53, the global gene expression pattern in the Pten;Kras(Trp53−) OSE cells is more similar to that of wild-type OSE cells than to the Trp53+ cell (Fig. 2, A and B). These comparisons show that many genes regulated by TRP53 in the mutant Pten/Kras(Trp53+) OSE cells promote DNA repair and stability (Birc5, Brca1, Rad51, Hells, Uhrf1), proliferation (Pcna), cell cycle progression (Ccnd1), and the mechanics of cell division (Table 1). Genes presumed to be stem cell markers (Ly6a, Cd24a) are markedly increased in the Trp53+ mutant OSE cells. Several genes (Cd200, Irx3, Egr1, Pdzd2, Igfpb2, Igfbp6 and Igfbp7, Capn2) that are regulated by wild-type TRP53 in the Pten/Kras OSE cells (Table 1) are also regulated in human low-grade ovarian cancer cells (Wong, K.-K., personal communication). The expression of several genes was confirmed by RT-PCR analysis of RNA from OSE cell lysates grown on a dish in regular culture conditions (Fig. 3A) and in cells grown in Matrigel (Fig. 3B). Similarly, the TRP53 targets mir-34a and mir-34c were up-regulated only in Trp53+ OSE cells (Fig. 3C). The tumor suppressor miRNA-31 was similarly expressed in Trp53+ and Trp53− OSE cells. Thus, rather than promoting cell cycle arrest or apoptosis, wild-type TRP53 in the Pten;Kras(Trp53+) OSE cells promotes the expression of genes involved in proliferation, cell survival, and cell migration and motility.
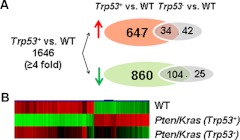
Fig. 2.
A, Venn analysis of microarray data comparing transcriptional profiles of Trp53+ and Trp53− OSE cells compared with wild-type OSE cells. Red arrow indicates the number of up-regulated genes and the green arrow indicates down-regulated genes. B, Heat map of representative microarray data from OSE cells isolated from ovaries of wild-type, Pten;kras;(Trp53+) and Pten;Kras (Trp53−) mice illustrates that TRP53 controls hundreds of genes and that the gene expression patterns of OSE cells from wild-type and Pten;Kras (Trp53−) ovaries are more similar. Red indicates up-regulation and green indicates down-regulation compared with wild type.
Table 1.
Selected Trp53-regulated genes in a gene array analyses of OSE cells
| Gene symbol | Pten/Kras (Trp53+)/WT | Pten/Kras (Trp53−)/WT |
|---|---|---|
| Cell cycle/apoptosis | ||
| Ccnd1 | 4.4 | 2.9 |
| Cdkn1a(p21)a | 1.9 | −2.1 |
| Cdkn2a(p16)a | −1.0 | 27.9 |
| Cdkn2b(p15) | −23.2 | 1.1 |
| Igfbp2 | −246.1 | −1.3 |
| Igfbp6b | −.3 | 1.3 |
| Igfbp7b | −11.8 | −1.6 |
| Mdm2a | 6.3 | 1.3 |
| Mki67(Ki67) | 4.5 | 1.7 |
| Pcnaa | 4.0 | 1.4 |
| Pdzd2b | −31.5 | −1.6 |
| Trp53b | 1.2 | −3.3 |
| DNA stability/repair | ||
| Birc5 | 10.7 | 3.1 |
| Brca1 | 10.1 | 2.7 |
| Hellsa | 13.5 | 2.5 |
| Hmgb2 | 6.6 | 2.2 |
| Mcm6 | 4.2 | 1.6 |
| Mcm7 | 4.6 | 1.2 |
| Mcm10 | 7.8 | 1.6 |
| Mdm2 | 6.3 | 1.3 |
| Rad51a | 13.4 | 2.6 |
| Metastasis/motility | ||
| Cadm4 | −9.0 | 1.3 |
| Capn2b | 1.3 | 1.2 |
| Cd200b | −22.1 | 2.3 |
| Cd44 | 2.6 | 2.2 |
| Perpa | 32.7 | 1.7 |
| Tubb3 | 26.2 | 1.3 |
| Stem cell-like | ||
| Cd24a | 35.8 | 1.5 |
| Ly6a | 56.8 | −1.0 |
| Thy1 | −15.4 | 1.9 |
| Transcription factors | ||
| Areg | 13.5 | −1.6 |
| Egr1b | −25.1 | −2.5 |
| Esr1 | −39.3 | −1.6 |
| Eya4b | 22.9 | −1.0 |
| Irx3b | −6.9 | 1.1 |
| Pgr | −4.6 | −1.9 |
| Tumor suppressors | ||
| Armcx1 | −3.1 | −1.2 |
| Armcx2 | −11.6 | −1.7 |
| Cd82 | −6.1 | 1.1 |
| Pdzd2 | −31.5 | −1.6 |
| Tshz2 | −23.1 | −1.2 |
| Uhrf1b | 11.8 | 2.7 |
WT, Wild type.
P53 target genes.
Genes expressed in human low-grade serous ovarian carcinomas.
Fig. 3.
RT-PCR analyses of RNA from wild-type, Pten;kras;(Trp53+), and Pten;Kras (Trp53−) mutant OSE cells. These results confirm the distinctly different expression patterns of genes involved in cell survival and cell migration. A, Expression of genes in RNA samples from purified OSE cells isolated from ovaries of wild-type, Pten;kras;(Trp53+), and Pten;Kras (Trp53−) mice grown in cell culture dishes in regular culture conditions. B, Expression profiles of genes in OSE cells grown in Matrigel. C, TRP53 target miRNA miR-34a, miR-34c, and the tumor suppressor miR-31 are regulated in Pten;Kras;(Trp53+)-mutant OSE cells as shown by RT-PCR.
TRP53 regulates OSE tumor cell adherence, migration, and invasion
The Trp53− mutant cells were grown in soft agar to determine whether the cells are transformed. The Trp53+ and Trp53− mutant cells formed a similar number of colonies, indicating the Trp53− cells are transformed (Fig. 4A). TRP53+ and TRP53− cells were also injected sc into syngeneic mice as another measure of transformation (Fig. 4B). As seen previously, the TRP53+ cells formed tumors (8) and invaded the dermal and epidermal layers of the skin after 20 d. Similarly, the TRP53− cells formed tumors, confirming these cells are transformed. However, the tumors derived from the Trp53− cells were smaller and did not invade the dermal layer, indicating TRP53 mediates OSE cell tumor invasion in the Pten;Kras(Trp53+) model (Fig. 4, B and C). To asses proliferation, OSE cells isolated from wild-type, Pten;Kras(Trp53+), and Pten;Kras(Trp53−) ovaries were grown in culture for 2 d. As previously described, the Trp53+ mutant cells had a significantly higher proliferation rate compared with wild-type cells (Fig. 5A). However, the proliferation rate in culture of cells lacking Trp53 was not altered, indicating that TRP53 is not obligatory in this context. To assess the impact of Trp53 on cell migration and adhesion, migration and adhesion assays were performed. Only a few wild-type cells had migratory activity, and no cells were attached to the cell culture dish 1 h after plating but were attached at 6 h (data not shown). In contrast, a large number of Trp53+ cells were migratory compared with wild-type cells (Fig. 5B). Cell migration was decreased in the absence of Trp53, suggesting that TRP53 controls OSE tumor cell migration. Similar to migration, Trp53− cells were less adherent compared with Trp53+ mutant cells (Fig. 5C). Thus, Trp53 appears to promote adhesion and migration of these transformed OSE cells in these contexts.
Fig. 4.
Pten;Kras;(Trp53+) and Pten;Kras;(Trp53−) mutant OSE cells are transformed, yet only Pten;Kras;(Trp53+) cells are locally invasive. A, Trp53+ and Trp53− OSE cells form similar numbers of colonies in soft agar. B, Pten;Kras;(Trp53+) and Pten;Kras;(Trp53−) mutant OSE cells form sc ectopic tumors, yet only the Trp53+ OSE cells invade the dermal and epidermal layers of the skin as shown by the damaged tissue and the presence of the cytokeratin-8 immunolabeled tumor cells in the dermal and epidermal layers of the skin. The black line demarcates the cytokeratin-8-positive tumors cells and cytokeratin-8-negative dermal and epidermal cells. C, Average volume of six ectopic tumors formed 20 d after sc injection of 1 × 106 Trp53+ or Trp53− OSE cells.
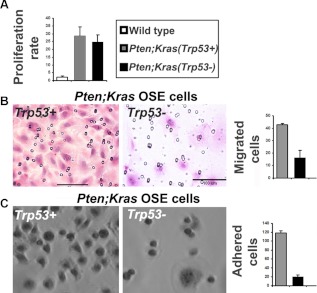
Fig. 5.
OSE-isolated cells from Pten;Kras(Trp53+) and Pten;Kras(Trp53−) mice are transformed and have similar proliferation rates in culture; however, the Trp53+ cells are more migratory and adherent compared with the Trp53− mutant OSE cells. A, Pten;Kras;(Trp53+) and Pten;Kras (Trp53−) OSE cells proliferate at a similar rate in cell culture. B, H&E staining of Trp53+ and Trp53− OSE cells on Matrigel invasion membrane membranes 24 h after plating. The numbers of cells per field were counted and plotted. C, Bright-field image of adhered cells in culture. Twenty thousand cells were plated in 24-well cell culture dishes and the number of cells adhered at 1 h were counted.
Activation of TRP53 by nutlin-3a induces cell cycle arrest and apoptosis of Trp53+ OSE tumor cells
Similar to the tumor OSE cells in mice that develop low-grade serous adenocarcinoma, TP53 is elevated in tumor cells from human patients with low-grade serous adenocarcinoma (Fig. 6A). To determine whether TRP53 is fully activated in the mutant OSE cells, the cells were exposed to the TRP53 activator nutlin-3a (Fig. 6B). Nutlin-3a is a small molecule that disrupts the interactions of TRP53 with the E3 ubiquitin ligase mouse double minute-2 (MDM2) (34, 35) and thereby leads to TRP53 stabilization and increased activation. Indicative of elevated TRP53 activity, the expression of TRP53 target genes Mdm2 and those controlling the cell cycle arrest, p21 (Cdnk1a) and p16 (Cdnk2a), are elevated in nutlin-3a-treated cells. By contrast, the cell survival and DNA stability proteins Birc5 and Brca1, respectively, are decreased by nutlin-3a. Nutlin-3a also induced the expression of the apoptosis mediators Perp and Puma. We also treated the human low-grade serous adenocarcinoma cell line HOC-7 with nutlin-3a to confirm this effect in human ovarian cancer cells that contain wild-type TP53. As shown, nutlin-3a induced the expression of P21, P16, MDM2, PERP, and PUMA in the HOC-7 cells. Nultin-3a did not similarly regulate these genes or cause cell cycle arrest in mouse Pten;Kras(Trp53−) cells or human SKOV3 ovarian cancer cells that lack Tp53 (Fig. 6C). These results provide strong evidence that nutlin-3a may provide a novel therapy for low-grade serous ovarian carcinoma.
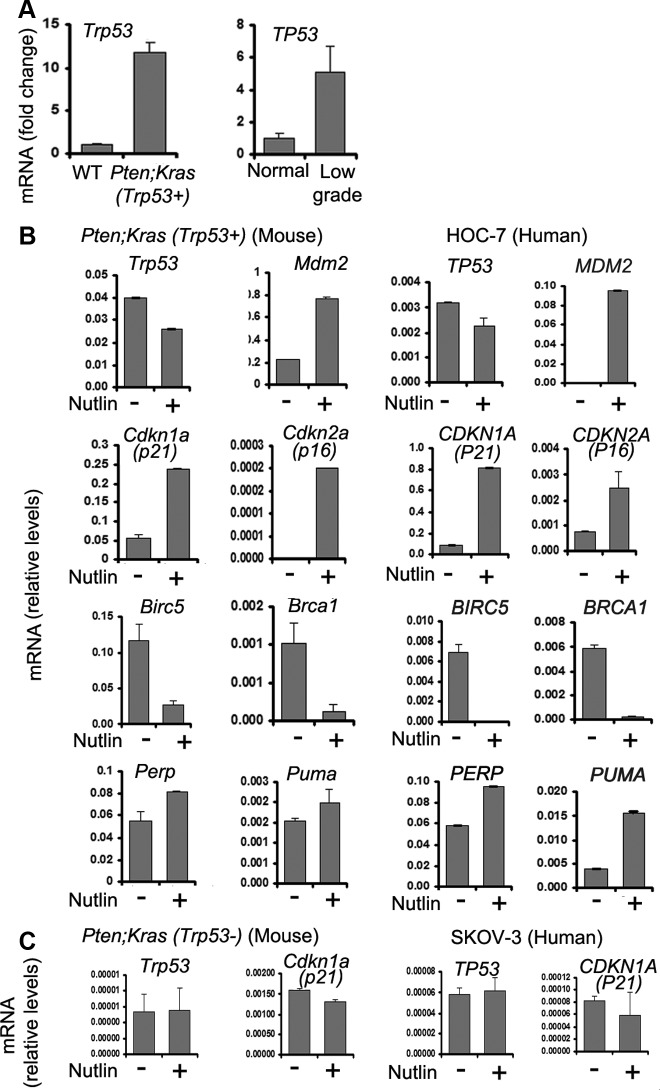
Fig. 6.
Nutlin-3a induces expression of cell cycle arrest genes and mediators of apoptosis in human and mouse cancer OSE cells that express wild-type TRP53 and Trp53, respectively. A, OSE cells from mouse and human serous adenocarcinoma express elevated levels of wild-type Trp53/TP53 as shown by RT-PCR. B, Nutlin-3a induces the expression of cell cycle control genes, DNA stability genes, and mediators of apoptosis in human and mouse serous adenocarcinoma OSE cells that express wild-type P53. C, Trp53/P53 is expressed at low levels and is not induced by nutin-3a in mouse Trp53− cells and human TP53− SKOV-3 cells.
Discussion
The results of these studies document that the expression of Trp53 in the Pten;Kras mutant OSE cells controls the morphology, functional activity (adhesion and migration), and gene expression profiles in the mutant OSE cells. By selectively disrupting Trp53 in the Pten;Kras OSE cells, we have unveiled a novel and powerful role for wild-type TRP53 as a major promoter of ovarian cancer cell survival and differentiation that, to our knowledge, has not been previously identified. The Pten;Kras mutant OSE cells lacking Trp53 are transformed yet are less adherent, migratory, and invasive in vivo and in vitro and exhibit a gene expression profile more like wild-type OSE cells (summarized in Fig 7). These results provide a new paradigm: wild-type TRP53 does not preferentially induce apoptotic or senescent related genes in the Pten;Kras(Trp53+) cells. Rather these cells express increased levels of genes regulating DNA repair, cell cycle progression, and proliferation and decreased levels of genes with putative tumor suppressor functions. In this regard, the role of wild-type TRP53 in the mutant Pten;Kras OSE cells appears to be similar to that in normal cells that are highly proliferative during embryogenesis and development (reviewed in Refs. 36 and 37). In this scenario, the levels of TRP53 and its activity, presumably low, are compatible with cell proliferation, survival, migration, and differentiation. Perhaps more intriguing and of relevance to various mouse cancer models, gene expression profiling in the Simian virus 40 (SV40)-T-transformed breast, lung, and prostate cells shows that expression of many DNA repair and cell survival genes, such as Rad51, Brca1, Birc5, and Hells to name a few, are elevated (38). These results combined with our observations suggest that although SV40T binds RB and TRP53 and has been presumed to inactivate them, there may be a sufficient low amount of active, wild-type TRP53 and/or RB to induce the expression of genes promoting proliferation and survival. Many of these genes have been shown to bind TP53 with high affinity compared with genes controlling apoptosis that bind TP53 with lower affinity (14, 15, 39, 40). Of further relevance, SV40T-transformed mouse OSE cells exhibit a phenotype similar to that of our Pten;Kras mutant mouse OSE cells (41). The SV40T-mutant OSE cells do exhibit some papillary structures and exhibit migratory activity. However, the gene expression profiles have not yet been published.
Fig. 7.
In the absence of Trp53, Pten;Kras OSE cells are transformed yet do not differentiate into extensive papillary structures. Pten;Kras OSE tumor cells that express low levels of active TRP53 are similarly transformed yet form extensive papillary structures in addition to having increased migration and invasion properties in vitro and in vivo. Additional modifications are required for secondary tumors at distant sites. Nutlin-3a activation of TRP53 in transformed OSE cells triggers cell cycle arrest and apoptosis. Thus, activation of TP53 may provide an innovative therapeutic approach for low-grade serous adenocarcinomas and other cancers that express wild-type TP53.
Our studies document further that in the Pten;Kras(Trp53+)-mutant mouse OSE cells and likely in human low-grade ovarian cancer cells, wild-type TRP53 controls global, molecular changes that are dependent not only on the level of wild-type TRP53 expression but also on its activation status. The levels of TRP53 that are present in the Pten;Kras(Trp53+) cells in vivo promote tumor cell survival and growth, migration, and adhesion. Clearly in this context, wild-type TRP53 is not mediating apoptosis or cell cycle arrest, two outcomes of TRP53 activation when cells are stressed by cytotoxic, DNA damaging agents and TRP53 is highly activated (19). Accordingly, when TRP53/TP53 activity was increased dramatically in the mouse and human mutant OSE cells in culture by exposure to nutlin-3a, TRP53/TP53 induced the expression of genes controlling cell cycle arrest and apoptosis and suppressed genes controlling DNA repair and survival. This major switch in gene expression by elevated TRP53 activity indicates clearly that the effects of endogenous wild-type TPR53 are dependent not only on the level of its expression but also acutely on its activation status. Therefore, activation of TP53 in vivo with nutlin-3a may provide a promising new therapy for managing low-grade serous ovarian cancer and other cancers in which wild-type TP53 is expressed by switching growth-promoting to growth-inhibitory pathways (6, 7, 42). Because we have shown that the loss of Pten increases Trp53 (Ref. 8) and herein) and because others have shown that overexpression and activation of TRP53 can suppress phosphatidylinositol 3-kinase activity (43), this feedback loop may be critical for increasing or suppressing tumor growth.
The results of these studies shed new light and thus may also help explain why the focus of TP53 as a tumor suppressor has overshadowed the results of studies showing that TP53 also promotes cell survival and proliferation. For example, the expression of some oncogenes in some tissues may cause DNA damage that not only leads to increased expression of Tp53 but may also lead to changes in cell function that promote a high level of TP53 activation. In this context, TP53 would induce cell cycle arrest and apoptosis. Furthermore, when cells are exposed to UV radiation and chemotoxic agents, TP53 expression and activation are increased to cause cell cycle arrest. However, when the activation status of TP53 declines, it can again promote cell survival. This may be one mechanism by which cancer stem cells lead to the recurrence of ovarian cancer.
TRP53 also appears to regulate genes involved in adhesion and migration. The movement of cells in developing tissues and during tumor cell metastasis are likely similar multifactorial events that require tight control of many processes including cell proliferation, migration, changes in cell shape, and adhesion. Tumors in Trp53-depleted OSE cells do not metastasize, suggesting other signaling pathways are required (44). However, although the data are limited, there is some evidence that TP53 regulates cell motility (26, 45, 46). Our data suggest that TRP53 plays a role in cell adhesion and migration, yet other modifying events are required for growth of tumors at secondary sites.
In summary, the results of these studies document that TRP53 can promote ovarian cancer cell survival and the differentiation of OSE cell papillary structures. This, in addition to the evidence that TP53 is induced by oncogenes in many cancers and the emerging role for TP53 regulation of cell differentiation and self-renewal, emphasizes the relevance of the impact of timing of expression and degree of activity on TP53 action.
Supplementary Material
Acknowledgments
We acknowledge the assistance of the Integrated Microscopy Core, which is supported by the Specialized Cooperative Centers Program in Reproduction Grants U54 HD-007495 (to B. W. O'Malley), P30 DK-56338 (to M. K. Estes), and P30 CA-125123 (to C. K. Osborne), and the Dan L. Duncan Cancer Center of Baylor College of Medicine. We also acknowledge the Baylor College of Medicine Diabetes and Endocrinology Research Center and the Microarray Core Facility, The University of Texas MD Anderson Cancer Center Specialized Program of Research Excellence in Ovarian Cancer (P50 CA08369) and advice from Dr. Robert Bast.
This work was supported by Grant NIH-HD-16229 and the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health through Cooperative Agreement U54-NIH-HD-07495 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- EOC
- Epithelial ovarian carcinoma
- H&E
- hematoxylin and eosin
- MDM2
- mouse double minute-2
- miRNA
- microRNA
- OSE
- ovarian surface epithelial
- SV40
- Simian virus 40
- TP53
- tumor repressor protein 53.
References
- 1. Bast RC, Jr, Hennessy B, Mills GB. 2009. The biology of ovarian cancer: new opportunities for translation. Nat Rev Cancer 9:415–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cho KR, Shih IeM. 2009. Ovarian cancer. Annu Rev Pathol 4:287–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jackson JG, Post SM, Lozano G. 2011. Regulation of tissue- and stimulus-specific cell fate decisions by p53 in vivo. J Pathol 223:127–136 [DOI] [PubMed] [Google Scholar]
- 4. Karst AM, Drapkin R. 2010. Ovarian cancer pathogenesis: a model in evolution. J Oncol 2010:932371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Köbel M, Kalloger SE, Boyd N, McKinney S, Mehl E, Palmer C, Leung S, Bowen NJ, Ionescu DN, Rajput A, Prentice LM, Miller D, Santos J, Swenerton K, Gilks CB, Huntsman D. 2008. Ovarian carcinoma subtypes are different diseases: implications for biomarker studies. PLoS Med 5:e232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mishra SK, Crasta JA. 2010. An immunohistochemical comparison of P53 and Bcl-2 as apoptotic and MIB1 as proliferative markers in low-grade and high-grade ovarian serous carcinomas. Int J Gynecol Cancer 20:537–541 [DOI] [PubMed] [Google Scholar]
- 7. O'Neill CJ, Deavers MT, Malpica A, Foster H, McCluggage WG. 2005. An immunohistochemical comparison between low-grade and high-grade ovarian serous carcinomas: significantly higher expression of p53, MIB1, BCL2, HER-2/neu, and C-KIT in high-grade neoplasms. Am J Surg Pathol 29:1034–1041 [PubMed] [Google Scholar]
- 8. Mullany LK, Fan HY, Liu Z, White LD, Marshall A, Gunaratne P, Anderson ML, Creighton CJ, Xin L, Deavers M, Wong KK, Richards JS. 2011. Molecular and functional characteristics of ovarian surface epithelial cells transformed by KrasG12D and loss of Pten in a mouse model in vivo. Oncogene 30:3522–3536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Folkins AK, Jarboe EA, Saleemuddin A, Lee Y, Callahan MJ, Drapkin R, Garber JE, Muto MG, Tworoger S, Crum CP. 2008. A candidate precursor to pelvic serous cancer (p53 signature) and its prevalence in ovaries and fallopian tubes from women with BRCA mutations. Gynecol Oncol 109:168–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Salani R, Kurman RJ, Giuntoli R, 2nd, Gardner G, Bristow R, Wang TL, Shih IM. 2008. Assessment of TP53 mutation using purified tissue samples of ovarian serous carcinomas reveals a higher mutation rate than previously reported and does not correlate with drug resistance. Int J Gynecol Cancer 18:487–491 [DOI] [PubMed] [Google Scholar]
- 11. Wong KK, Tsang YT, Deavers MT, Mok SC, Zu Z, Sun C, Malpica A, Wolf JK, Lu KH, Gershenson DM. 2010. BRAF mutation is rare in advanced-stage low-grade ovarian serous carcinomas. Am J Pathol 177:1611–1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wong KK, Lu KH, Malpica A, Bodurka DC, Shvartsman HS, Schmandt RE, Thornton AD, Deavers MT, Silva EG, Gershenson DM. 2007. Significantly greater expression of ER, PR, and ECAD in advanced-stage low-grade ovarian serous carcinoma as revealed by immunohistochemical analysis. Int J Gynecol Pathol 26:404–409 [DOI] [PubMed] [Google Scholar]
- 13. Liebermann DA, Hoffman B, Vesely D. 2007. p53 induced growth arrest versus apoptosis and its modulation by survival cytokines. Cell Cycle 6:166–170 [DOI] [PubMed] [Google Scholar]
- 14. Ronen D, Schwartz D, Teitz Y, Goldfinger N, Rotter V. 1996. Induction of HL-60 cells to undergo apoptosis is determined by high levels of wild-type p53 protein whereas differentiation of the cells is mediated by lower p53 levels. Cell Growth Differ 7:21–30 [PubMed] [Google Scholar]
- 15. Weinberg RL, Veprintsev DB, Bycroft M, Fersht AR. 2005. Comparative binding of p53 to its promoter and DNA recognition elements. J Mol Biol 348:589–596 [DOI] [PubMed] [Google Scholar]
- 16. Maddocks OD, Vousden KH. 2011. Metabolic regulation by p53. J Mol Med (Berl) 89:237–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Muller PA, Vousden KH, Norman JC. 2011. p53 and its mutants in tumor cell migration and invasion. J Cell Biol 192:209–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vousden KH, Lane DP. 2007. p53 in health and disease. Nat Rev Mol Cell Biol 8:275–283 [DOI] [PubMed] [Google Scholar]
- 19. Lane DP. 1992. Cancer. p53, guardian of the genome. Nature 358:15–16 [DOI] [PubMed] [Google Scholar]
- 20. Martins CP, Brown-Swigart L, Evan GI. 2006. Modeling the therapeutic efficacy of p53 restoration in tumors. Cell 127:1323–1334 [DOI] [PubMed] [Google Scholar]
- 21. Ventura A, Kirsch DG, McLaughlin ME, Tuveson DA, Grimm J, Lintault L, Newman J, Reczek EE, Weissleder R, Jacks T. 2007. Restoration of p53 function leads to tumour regression in vivo. Nature 445:661–665 [DOI] [PubMed] [Google Scholar]
- 22. Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, Cordon-Cardo C, Lowe SW. 2007. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 445:656–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang Y, Suh YA, Fuller MY, Jackson JG, Xiong S, Terzian T, Quintás-Cardama A, Bankson JA, El-Naggar AK, Lozano G. 2011. Restoring expression of wild-type p53 suppresses tumor growth but does not cause tumor regression in mice with a p53 missense mutation. J Clin Invest 121:893–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cancer Genome Atlas Research Network 2011. Integrated genomic analyses of ovarian carcinoma. Nature 474:609–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fan HY, Liu Z, Paquet M, Wang J, Lydon JP, DeMayo FJ, Richards JS. 2009. Cell type-specific targeted mutations of Kras and Pten document proliferation arrest in granulosa cells versus oncogenic insult to ovarian surface epithelial cells. Cancer Res 69:6463–6472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sablina AA, Chumakov PM, Kopnin BP. 2003. Tumor suppressor p53 and its homologue p73α affect cell migration. J Biol Chem 278:27362–27371 [DOI] [PubMed] [Google Scholar]
- 27. Fan HY, Shimada M, Liu Z, Cahill N, Noma N, Wu Y, Gossen J, Richards JS. 2008. Selective expression of KrasG12D in granulosa cells of the mouse ovary causes defects in follicular development and ovulation. Development 135:2127–2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Buick RN, Pullano R, Trent JM. 1985. Comparative properties of five human ovarian adenocarcinoma cell lines. Cancer Res 45:3668–3676 [PubMed] [Google Scholar]
- 29. King ER, Zu Z, Tsang YT, Deavers MT, Malpica A, Mok SC, Gershenson DM, Wong KK. 2011. The insulin-like growth factor 1 pathway is a potential therapeutic target for low-grade serous ovarian carcinoma. Gynecol Oncol 123:13–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP. 2003. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4:249–264 [DOI] [PubMed] [Google Scholar]
- 31. Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, Hornik K, Hothorn T, Huber W, Iacus S, Irizarry R, Leisch F, Li C, Maechler M, Rossini AJ, Sawitzki G, Smith C, Smyth G, Tierney L, Yang JY, Zhang J. 2004. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 5:R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Eisen MB, Spellman PT, Brown PO, Botstein D. 1998. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA 95:14863–14868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Saeed AI, Sharov V, White J, Li J, Liang W, Bhagabati N, Braisted J, Klapa M, Currier T, Thiagarajan M, Sturn A, Snuffin M, Rezantsev A, Popov D, Ryltsov A, Kostukovich E, Borisovsky I, Liu Z, Vinsavich A, Trush V, Quackenbush J. 2003. TM4: a free, open-source system for microarray data management and analysis. Biotechniques 34:374–378 [DOI] [PubMed] [Google Scholar]
- 34. Xia M, Knezevic D, Vassilev LT. 2011. p21 does not protect cancer cells from apoptosis induced by nongenotoxic p53 activation. Oncogene 30:346–355 [DOI] [PubMed] [Google Scholar]
- 35. Tovar C, Rosinski J, filipovic Z, Higgins B, Kolinsky K, Hilton H, Zhao X, Vu BT, Qing W, Packman K, Myklebost O, Heimbrook DC, Vassilev LT. 2006. Small-molecule MDM2 antagonists reveal aberrant p53 signaling in cancer: implications for therapy. Proc Natl Acad Sci USA 103:1888–1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Molchadsky A, Rivlin N, Brosh R, Rotter V, Sarig R. 2010. p53 is balancing development, differentiation and de-differentiation to assure cancer prevention. Carcinogenesis 31:1501–1508 [DOI] [PubMed] [Google Scholar]
- 37. Spike BT, Wahl GM. 2011. p53, stem cells, and reprogramming: tumor suppression beyond guarding the genome. Genes Cancer 2:404–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Deeb KK, Michalowska AM, Yoon CY, Krummey SM, Hoenerhoff MJ, Kavanaugh C, Li MC, Demayo FJ, Linnoila I, Deng CX, Lee EY, Medina D, Shih JH, Green JE. 2007. Identification of an integrated SV40 T/t-antigen cancer signature in aggressive human breast, prostate, and lung carcinomas with poor prognosis. Cancer Res 67:8065–8080 [DOI] [PubMed] [Google Scholar]
- 39. Chen X, Ko LJ, Jayaraman L, Prives C. 1996. p53 levels, functional domains, and DNA damage determine the extent of the apoptotic response of tumor cells. Genes Dev 10:2438–2451 [DOI] [PubMed] [Google Scholar]
- 40. Qian H, Wang T, Naumovski L, Lopez CD, Brachmann RK. 2002. Groups of p53 target genes involved in specific p53 downstream effects cluster into different classes of DNA binding sites. Oncogene 21:7901–7911 [DOI] [PubMed] [Google Scholar]
- 41. Connolly DC, Bao R, Nikitin AY, Stephens KC, Poole TW, Hua X, Harris SS, Vanderhyden BC, Hamilton TC. 2003. Female mice chimeric for expression of the simian virus 40 TAg under control of the MISIIR promoter develop epithelial ovarian cancer. Cancer Res 63:1389–1397 [PubMed] [Google Scholar]
- 42. Oshima K, Naoi Y, Kishi K, Nakamura Y, Iwamoto T, Shimazu K, Nakayama T, Kim SJ, Baba Y, Tamaki Y, Noguchi S. 2011. Gene expression signature of TP53 but not its mutation status predicts response to sequential paclitaxel and 5-FU/epirubicin/cyclophosphamide in human breast cancer. Cancer Lett 307:149–157 [DOI] [PubMed] [Google Scholar]
- 43. Astanehe A, Arenillas D, Wasserman WW, Leung PC, Dunn SE, Davies BR, Mills GB, Auersperg N. 2008. Mechanisms underlying p53 regulation of PIK3CA transcription in ovarian surface epithelium and in ovarian cancer. J Cell Sci 121:664–674 [DOI] [PubMed] [Google Scholar]
- 44. Attardi LD, Jacks T. 1999. The role of p53 in tumour suppression: lessons from mouse models. Cell Mol Life Sci 55:48–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lefort K, Mandinova A, Ostano P, Kolev V, Calpini V, Kolfschoten I, Devgan V, Lieb J, Raffoul W, Hohl D, Neel V, Garlick J, Chiorino G, Dotto GP. 2007. Notch1 is a p53 target gene involved in human keratinocyte tumor suppression through negative regulation of ROCK1/2 and MRCKα kinases. Genes Dev 21:562–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mukhopadhyay UK, Eves R, Jia L, Mooney P, Mak AS. 2009. p53 suppresses Src-induced podosome and rosette formation and cellular invasiveness through the upregulation of caldesmon. Mol Cell Biol 29:3088–3098 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.