Abstract
Ovarian cancer in women is a complex and deadly disease, where the molecular events that initiate and control tumor formation remain poorly defined. Therefore, mouse models provide one approach for determining the mechanisms by which specific oncogenic factors cause ovarian surface epithelial cell and granulosa cell transformation. This minireview summarizes the phenotypes of current mouse models that have been generated and some of the underlying mechanisms they have provided.
Ovarian cancer in women is the fifth most lethal cause of death (1). As indicated in the accompanying minireview, human ovarian cancer is a complex disease derived from three major histotypes (surface epithelia, sex-chord-stroma, and germ cells) and is associated with different, specific changes in molecular signatures and genomic stability (2). This disease remains hard to manage with current surgical and chemotherapeutic strategies, because it is difficult to detect, presents at a late stage, and often becomes drug resistant (3, 4). Approximately 3% of ovarian cancers, such as choriocarcinomas, are derived from germ cells, 7%, such as granulosa cell/Sertoli cell tumors, are from sex-chord-stromal cells, whereas about 90% are derived from either the ovarian surface epithelium (OSE) or fallopian tube epithelium (FTE), including serous, endometrioid, mucinous, and clear cell carcinomas (5, 6). Recent DNA sequencing of genomic DNA from human serous high-grade type II ovarian cancer specimens has documented unequivocally that almost all (96%) high-grade epithelial ovarian cancers (EOC) harbor mutations in, or loss of, the tumor repressor protein (TRP)53 (also known as p53) gene and exhibit genomic instability (7). Results from these same samples highlighted and confirmed that other specific oncogenic pathways are altered in the high-grade EOC. These include the retinoblastoma protein (RB) pathway (67%), the phosphatidylinositol 3 kinase (PI3K)/rat sarcoma viral oncogene pathways (45%), breast cancer 1 (23%) and breast cancer 2 (11%), and Notch signaling (22%) (7). DNA sequencing of low-grade EOC has not yet been done, but this subtype is characterized by elevated expression of wild-type TRP53 and mutations in Braf and Pten (8, 9). Although our understanding of what initiates ovarian epithelial cell transformation and drives ovarian cancer progression in each cancer subtype still remains limited, it is clear that multiple “hits” are required and that they appear to occur in a context-specific manner.
Genetically engineered mouse models provide efficient, reproducible systems, in which to analyze the specific functions and functional interactions of oncogenes and tumor suppressors during tumor development in vivo. Mouse models that have currently been generated verify that OSE cells can be transformed, that alterations in two or more oncogenes or tumor suppressors are essential for transformation to occur, and that levels of expression, activation, or mutation of Trp53 are involved (reviewed below). Although the number of mouse models of ovarian cancer remains rather limited, some major advances have been made in the last decade (Fig. 1). The laying hen is also a model of human ovarian cancer, because ovarian tumors with histological characteristics and stages similar to those in women occur spontaneously (Fig. 1) (10, 11). There are many excellent and detailed reviews on animal models of ovarian cancer (12–15). The goals of this minireview are to 1) highlight the major contributions that current mutant mouse models have provided, 2) discuss the limitations of the current models, and 3) outline what approaches might lead to future advances in this complex research field. We will focus primarily on EOC models but will also point out some key differences that exist in the response of granulosa cells to specific oncogenes as well as mutations that characterize granulosa cell tumors (GCT).
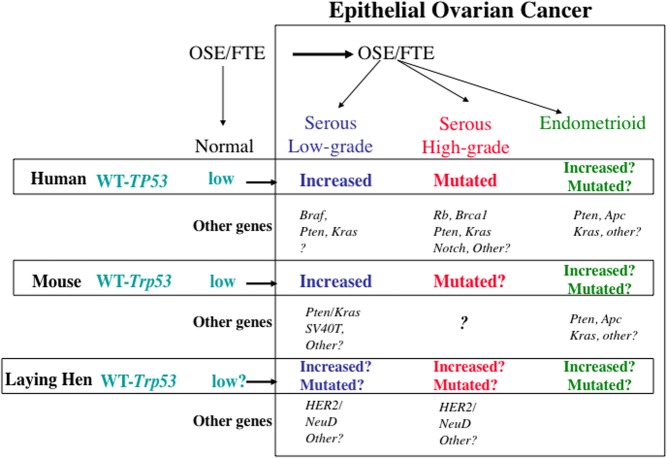
Fig. 1.
Schematic of genetic alterations in human ovarian cancers that have been studied in mouse and hen. See text for details. HER2, Human epidermal receptor 2/neutral tumor gene; WNT, Wingless integrated into the genome.
Epithelial Cell-Derived Ovarian Cancer
Mouse models
The first major leap forward in mouse models of ovarian cancer came from studies of Orsulic et al. (16), who systematically introduced one or more oncogenes into dispersed mouse ovarian cells that were either genotypically Trp53+/+ or Trp53−/−. This was accomplished by using mice expressing the avian retroviral receptor (TVA) selectively on the surface ovarian cells and exposing the cells to replication competent avian leukosis viral-derived vectors expressing c-Myc, Kras, or Ak mouse strain thymic lymphoma (Akt) or combinations thereof. The results of these studies documented that at least two factors were required to transform OSE cells and that this only occurred in Trp53 null cells and not in cells expressing wild-type Trp53. In addition, these studies documented that OSE cells and not other ovarian (granulosa cells or stromal) cells were susceptible to transformation by these factors. Additional studies have shown that Trp53−/−;c-myc;Akt or Trp53−/−;K-ras;Akt triple mutant cells are growth inhibited by rapamycin, whereas Trp53−/−;c-myc;Kras cells are resistant (17).
Another major advance came from the studies of Flesken-Nikitin et al. (18), who ingeniously injected an adenoviral vector expressing cytomegalovirus (CMV)-Cre recombinase under the ovarian bursal sac of mice expressing conditional floxed-P alleles of Trp53, Rb, or both. Although only a few tumors were observed when either Trp53 or Rb was disrupted, depletion of both Trp53/Rb led to OSE tumor formation with a relatively long mean latency of 227 d (7.5 months) and with varied phenotypes (serous and poorly differentiated), suggestive that mutations or alterations in additional signaling pathways occur in the Trp53/Rb mutant cells. Although these studies provided a novel technical advance for delivering recombinase directly to the OSE cell layer, subsequent studies with other oncogenes have determined that the injection of the adenoviral vector under the bursa not only causes transformation of OSE cells, but it sometimes leaks and penetrates the ovary or alters oviductal or uterine tissues, perhaps due to the trajectory of the injection needle. More recently, these investigators have isolated OSE cells carrying conditional alleles for Trp3 or Rb from the ovaries of mutant mice and exposed the cells in culture to adenoviral CMV-Cre (19). Although this approach is useful for short-term mechanistic studies and documenting that the tyrosine kinase MET (MNNG HOS transforming gene; also known as hepatocyte growth factor receptor HGFR) may be a TRP53 target, it does not directly apply to cancer development in vivo.
In a third approach, transgenic mice expressing the simian virus 40T (SV40T) antigen driven by the Mullerian inhibitory substance type 2 receptor promoter (MISIIR) [also known as the anti-Mullerian hormone type 2 receptor (Amhr2)] were generated. The SV40T antigen was used because it is a known oncogene and has been shown to block the actions or activity of TRP53 and RB (20). In these mice, OSE tumors occurred with 50% penetrance. The OSE exhibited papillary-like structures, invaded the ovarian stroma, and metastasized to the peritoneal cavity. Thus, these tumors appear to be primarily like human low-grade, invasive EOC but also exhibit areas with poorly differentiated cells. Although Amhr2 is expressed in ovarian granulosa cells and uterine myometrial cells, tumors were not observed in granulosa/stromal cells, and only one mouse developed multiple leiomyomas. This model clearly established that mouse OSE cells express Amhr2 and are highly responsive to the oncogenic transformation potential of SV40T. These results also confirmed the results of Orsulic et al. (16), showing that granulosa cells are resistant to these same oncogenic factors. More recently, Quinn et al. (21) have used MOVCAR cell lines derived from ascites fluid of SV40T EOC-bearing mice to generate ectopic tumors in syngenic mice expressing low MISIIR-SV40T. To date, the number of studies using mouse models of ovarian cancer (with known oncogenic mutations) for analyzing the effects of the currently used chemotoxic anticancer drugs, cisplatin, and paclitaxel is limited (22). Therefore, these MOVCAR luciferase-expressing cells and mouse model will be important for testing therapeutic drugs that might be used to control and manage EOC (21).
Because the FTE is a potential source of ovarian cancer in women, targeting this tissue has relevance (3, 23). Miyoshi et al. (24) generated mice in which the mouse oviductal glycoprotein (OGP) promoter was used to drive expression of the SV40T. These mice spontaneously developed tumors of the reproductive tract (oviducts, uterus, and vagina). Strikingly, they observed that ovariectomy prevented tumor formation, whereas exogenous estradiol reversed this effect. Because the OGP promoter contains estradiol receptor response elements, the expression of SV40T appeared to be estrogen dependent and associated with the activation of the OGP promoter. Although this is a potentially useful model in which to target oviduct (FTE) cells, the type of tumor (serous, endometrioid, other) was not clearly defined and has not been analyzed further.
Hereditary germ-line mutations in or genetic/epigenetic inactivation of Brca1 and Brca2 occur in humans and appear to predispose women to ovarian cancer (as well as breast cancer), especially in combination with mutations of Trp53 (4). Therefore, several investigators have examined the effects of disrupting Brca1 or Brca2 alone or in combination with Trp53. When adenoviral Cre was injected under the ovarian bursa of mice expressing floxed-P alleles of Trp53, 100% of the mice developed tumors. Concomitant inactivation of Trp53 and Brca1, but not Trp53 and Rb, accelerated tumor growth. However, the tumors that developed were not EOC. Rather, they were leiomyosarcomas that likely were derived from ovarian bursal cells or other oviductal/uterine cell types (25). Quinn et al. (26) observed similar results for conditional disruption of Trp53 with adenoviral-Cre, although the incidence of tumor formation was less. Reasons for these differences in the effects of Trp53 depletion are not known. These results differ sharply from the OSE tumors generated by Flesken-Nikitin et al. (18) using a similar approach to disrupt the expression of Trp53. That Trp53 and Brca1 selectively target myometrial cells and not OSE cells in mice is supported by the evidence that leiomyosarcomas also develop when floxed alleles of the Trp53 and Brca1 genes were conditionally disrupted in vivo using the Amrh2-Cre mice (27). That these mouse models do not recapitulate the high incidence of ovarian cancer in women with Brca1 and Trp53 mutations highlights a clear difference in the susceptibility of mouse OSE (and FTE?) cells to transformation by these mutations and the need to determine what underlies this difference. The acceleration of tumor growth by disruption of Brca1, but not Rb, in the Trp53 mutant cells also differs from the mice expressing the SV40T antigen driven by the Amhr2 promoter, where presumably inactivation of both TRP53 and RB synergizes to promote tumor growth. One explanation for these differences is that the SV40T antigen effects transformation by mechanisms in addition to blocking TRP53 and RB activity. Thus, although highly useful, the SV40T antigen model also does not strictly mimic mutations of human ovarian cancers, and the mechanisms by which it is tumorigenic remain unclear.
Mutations or alterations (deletions, amplifications, copy number changes) in the phosphatase and tensin homolog (PTEN)/PI3K/AKT1/2 and the Kirsten rat sarcoma viral oncogene/v-RAF murine sarcoma viral oncogene (KRAS/BRAF) pathways also occur in EOC but with varying frequencies in different subtypes (3, 4, 7). Although activating mutations of Kras and Braf are more frequent in human low-grade serous and mucinous ovarian cancer subtypes, the loss of Pten or amplification of PI3K are more prevalent in the endometrioid ovarian cancer subtype. However, deregulation of Pten/PI3K and Kras pathways is observed in high-grade and low-grade serous adenocarcinomas (3, 7). Therefore, several investigators have used mouse models to analyze these pathways by determining the oncogenic effects of specific factors alone and in combination. Dinulescu et al. (28) were the first to report that adenoviral-Cre injections under the bursa of mice expressing conditional alleles of Pten and the oncogenic form of Kras (KrasG12D) led to the induction of endometrioid-like ovarian adenocarcinomas. However, the presence of some “cribriform”-like structures in these tumors indicates that papillary-like structures might also be a characteristic feature. Although the OSE cells are presumed to be the major target, other cell types might also be involved. Mutations in the Wnt/β-catenin (Ctnnb1) pathway also occur in endometrioid ovarian cancers. Wu et al. (29) documented that depletion of Apc and Pten in OSE cells via the adenoviral Cre approach, generated tumors that grew rapidly and were histologically similar to endometrioid-type ovarian adenocarcinomas. Mouse models that mimic mucinous ovarian cancer have not yet been generated.
More recently, Fan et al. (30) generated mice in which the Pten gene was disrupted and an oncogenic form of KrasG12D was expressed selectively in OSE cells using mice in which Cre recombinase was driven by the Amhr2 promoter. The resulting Ptenfl/fl;L-S-L-Kras G12D;Amhr2-Cre (Pten/Kras) mice developed low-grade, invasive serous adenocarcinomas with 100% penetrance at an early age (2 months). This Pten/Kras mutant mouse model has provided the first in vivo model clearly classified as similar to the low-grade serous human ovarian cancer subtype where levels of wild-type TRP53 are elevated and TRP53 target genes, such as Cdkn1a and micro-RNA 34a-c, are increased (31). Therefore, this mouse model provides an ideal system in which to understand the etiology of low-grade serous ovarian cancer and, as indicated in the accompanying review, to test various anticancer drugs. In this regard, we have shown that hyperactivation of wild-type TRP53 by the small molecule nutlin-3a causes the Pten/Kras mutant mouse cells and a TP53 positive human low-grade ovarian cancer cell line to undergo cell cycle arrest and apoptosis (32). This provides the first evidence that wild-type TRP53 promotes tumor growth at low levels of activity but causes cell cycle arrest at higher levels (32).
Quite surprisingly, the serous adenocarcinoma-like phenotype of the tumors in these Pten/Kras/Amhr2-Cre mice differs dramatically from the endometrioid phenotype observed when the same mutations were generated in OSE cells using intrabursal injections of the adenoviral CMV-Cre vector (28). Why would there be such differences? Perhaps the cell types responding to the adenoviral vector (OSE or bursal) or those expressing Amhr2 (OSE) are different. Or perhaps the cells were transformed at different developmental stages. The mice exposed to adenoviral Cre were primed with gonadotropic hormones leading to increased ovarian follicular production of estradiol. In contrast, the ovaries of the Pten/Kras/Amhr2-Cre mice are devoid of growing follicles and thus are essentially steroid hormone depleted (30, 33). Alternatively, the degree of recombination may differ between the exposure of cells to an adenoviral vector vs. exposure of cells to endogenous Cre.
The laying hen
The adult hen is also recognized as a relevant model for human ovarian cancer, because ovarian tumors arise spontaneously in approximately 40% of the hens around 4 yr of age (13). The ovarian tumors exhibit serous, endometrioid, mucinous, and clear cell histo-pathological features, express some genes present in human and mouse EOC, such as CA125 (Muc16), and about 48% harbor mutations in Trp53 and an increase in human epidermal growth factor receptor/neuronal tumor gene (10, 11, 31). Therefore, the hen provides another model in which to determine the progression of this disease and to test various anticancer drugs in vivo.
Future goals
But what is missing? What is needed? What future experiments will be required to help unravel the key molecular events that control ovarian cancer initiation and progression? First, because most ovarian cancers in women are high-grade serous adenocarcinomas, a model that faithfully recapitulates this ovarian subtype in vivo is clearly needed. Reasons why such a model has not yet been generated in mice are not entirely clear but may be due, in large part, to the fact that mutant forms of Trp53 have not yet been tested in any mouse model of ovarian cancer. It is becoming clear that cells with null Trp53 alleles may act differently than cells with mutant alleles, and different mutated forms of TRP53 may also exert different effects (34, 35). Furthermore, it may be necessary to express mutant Trp53 genes selectively in FTE. Differences in the susceptibility of mouse OSE cells to Brca1 and Trp53 mutations, as is indicated by the induction of leiomyomas or leiomyosarcomas but not EOC in current mouse models, may also contribute to the current lack of a mouse model of high-grade serous adenocarcinomas.
Genomic instability, gene amplifications, and changes in copy number alterations are prevalent in human high-grade EOC (7) but have not been detected or well characterized in the hen ovarian cancers or current mouse models of this disease. In general, murine epithelial cells appear to have greater genomic stability than human epithelial cells, perhaps because of greater telomere length (36). This may explain the differences in susceptibility of mouse and human OSE cells to disruptions of Brca1 and Trp53. Therefore, specific mutations need to be made to make mouse OSE cells genomically more unstable and thereby to recapitulate human ovarian cancer more closely. Of particular relevance in this regard might be the generation of mice in which telomerase RNA component (Terc) or Ataxia-telangiectasia mutated (Atm) are disrupted in OSE cells of mice that are also null for Trp53, Brca1, or other genes or are expressing a gain-of-function mutant of Trp53 (36, 37). Successful generation of a mouse model of high-grade ovarian cancer that harbors relevant oncogenic mutations, amplifications, or copy number alterations would push the field forward and pave the way for analyzing the early events in the development of this complex disease that cannot be studied in human samples derived from late-stage tumors. Furthermore, model(s) of high-grade ovarian cancer are highly relevant for testing the myriad of potential anticancer drugs that are available. In addition, mice that express cell-specific, inducible Cre are needed to permit more precise timing of expression of specific oncogenes relative to one another. Tumorigenesis is a multistep process, and therefore, the sequence in which different genes are mutated may dictate the final outcome (38, 39).
It is critical to distinguish the roles of mutant TRP53 vs. the null allele, because recent data indicate that wild-type TRP53 acts to promote cancer cell survival (32). Specifically, one distinguishing and relevant feature of type I and type II ovarian cancer is the expression of Trp53: almost all high-grade serous adenocarcinomas have Trp53 mutations, whereas low-grade tumors express elevated levels of wild-type Trp53 (7, 40). Therefore, understanding the mechanisms that control the expression and activation of wild-type TRP53 in low-grade ovarian cancer as well as that of mutant forms of TRP53 in high-grade ovarian cancer are essential for understanding the varied functions of this critical protein in ovarian cancer. Because Trp53 mutations are now questioned as diagnostic marker of high-grade ovarian cancer (41), we must identify what other mutations or alterations permit transformation and/or drive high-grade ovarian cancer vs. low-grade ovarian cancer. What other hits direct the more aggressive high-grade type II phenotype? Quite strikingly, wild-type TRP53 is elevated in human low-grade ovarian cancer (9) and in the low-grade Pten/Kras mutant mouse OSE cells (31). TRP53 is also elevated in the mouse ovarian tumors that express SV40T and exhibit a papillary-like morphology (20). Moreover, the SV40T “signature” defined by genes that are regulated by this oncogene in human breast, lung, and prostate cancer (42) indicate that the wild-type TRP53 may be active at a low level in the SV40T ovarian cancer cells (rather than being completely suppressed) and thereby may promote cancer cell survival in this context as in the Pten/Kras mutant OSE cells (32). This needs to be verified. It should also be noted that although 48% of ovarian tumors in the hen exhibit Trp53 mutations, most of the tumors appear to be low-grade serous papillary-like adenocarcinomas. This suggests that the Trp53 mutations in the hen may or may not recapitulate those in high-grade EOC in women or that high-grade EOC in women require other specific alterations that do not occur in the hen.
Because Notch signaling has recently been implicated in high-grade ovarian cancer (7), mouse models in which components of this pathway are disrupted or mutated need to be developed. Notch signaling in the hen ovarian cancers should also be analyzed.
Reproductive hormones (gonadotropins and steroids) also impact the incidence and progression of ovarian cancer (43). This may be associated with the frequency of ovulation, epithelial repair, and high levels of estradiol and inflammatory molecules associated with ovulation (13). Epidemiological and clinical evidence indicates that pregnant women and women on contraceptives for as few as 5 yr have a reduced incidence of ovarian cancer, whereas women on hormone replacement therapy (principally estradiol) exhibit an increased incidence of ovarian cancer (43–45). The few studies that have been done in mice indicate that estradiol (or ethinyl estradiol) enhances tumor growth (24, 46), whereas progesterone (or norethindrone) may be antagonistic (47, 48). Progesterone also reduces the incidence of ovarian cancer in the hen (10). However, the underlying mechanisms by which steroids exert these potent, long-term effects remain unclear. Therefore, more studies are required to determine the effects of contraceptives and steroid hormones on OSE and FTE cell functions and tumor growth.
As indicated in the accompanying review, animal models are critical for helping to identify new targets on the path to personalized therapy. This will require new mouse models that mimic high-grade ovarian cancer and that can be used to determine the effects of specific chemocytoxic drugs and the myriad of other anticancer drugs that are now in the pipeline. Because recurrence is so frequent in ovarian cancer patients, it is critical that we determine whether ovarian cancer stem cells or tumor initiating cells are present and whether their presence, number, or function changes as a consequence of cytotoxic or anticancer drug treatment.
Granulosa Cell Tumors
This review of ovarian cancer would not be complete without a brief mention of recent studies pertaining to GCT. In humans, these are subdivided by morphological and molecular characteristics as adult or juvenile (4). Although far less common than EOC in women, GCT occur frequently in primates and domestic animals (13). Moreover, as mentioned above, granulosa cells appear to be highly resistant to transformation by factors that are oncogenic in epithelial cells (16, 20, 30). What can we learn from this? First, recent studies have revealed that perhaps 100% of all human adult GCT harbor the same specific mutation in the gene encoding the transcription factor Foxl2 (49–51). Although this is a seminal observation and clearly points to a key role of this Foxl2 mutation in GCT, it does not by itself explain the etiology of GCT formation in the Foxl2 mutant cells. Therefore, it will be critical to generate mice expressing the specific mutant form of Foxl2.
As in other cells, it is likely that more than one hit (i.e. the Foxl2 mutation) is required. But what are these other alterations/mutations? Based on mouse models of GCT that have been published, other factors could involve the levels and activation of specific mothers against decapentaplegic homolog (Drosophila) SMA protein (C. elegens) family of transcription factors (52, 53), the activin/bone morphogenic protein receptors (54), inhibin α (55), or nuclear receptors (56, 57). Furthermore, granulosa cells appear to be highly susceptible to changes in the expression of the wingless integrated into the genome pathway transcription factor, Ctnnb1. Specifically, when mice express of a stable mutant form of Ctnnb1 that lacks exon 3 (Ctnnb1exon3fl/fl) in granulosa cells (driven by Amhr2- or Cyp19a1-Cre), GCT occur but infrequently (58). Likewise, the disruption of Pten alone in granulosa cells rarely leads to GCT (59, 60). However, when Pten is disrupted in the Ctnnb1 mutant strain, GCT appear in the ovaries at an early age and with 100% penetrance (60). The tumors grow aggressively and rapidly lead to lethality. GCT also occur in the testis of the Ctnnb1/Pten mutant mice (61). When KrasG12D is expressed in granulosa cells (driven by Amhr2 or Cyp19a1), it derails follicular development (33). The KrasG12D-expressing granulosa cells cease dividing, fail to undergo apoptosis, and do not differentiate. As a consequence, abnormal follicle-like structures devoid of oocytes accumulate in the ovaries of the mutant mice. The potent antiproliferative effects of KrasG12D in granulosa cells are completely overridden by mutant Ctnnb1. Granulosa cells expressing mutant Ctnnb1 and KrasG12D form GCT that phenotypically are identical to tumors in the Ctnnb1/Pten mice (62). More dramatically, the gene expression profiles of the Ctnnb1/Pten and Ctnnb1/KrasG12D mutant granulosa cells are remarkably similar. These results help explain why neither the loss of Pten nor expression of KrasG12D causes granulosa cell transformation in other studies. In granulosa cells, mutant Ctnnb1 is the driver and either loss of Pten or gain of mutant Kras enhances the genetic program set in motion by Ctnnb1 (62). These results indicate that alterations in Ctnnb1, Pten, or Kras or other pathways may also impact the transformation in granulosa cells expressing mutant Foxl2.
To resolve what controls adult GCT, additional studies are required to determine what pathways are altered and interact with mutant Forkhead box L2 transcription factor. Loss of Foxl2 could alter the balance of estrogen receptor (ESR) 2 and ESR1, leading to reduced levels of ESR2 and increased ESR1 that are associated with to GCT formation (56). The molecular basis for juvenile GCT formation remains to be determined but could be related to altered expression and activation of mothers against decapentaplegic homolog (Drosophila) SMA protein (C. elegens) (52).
Conclusions
Collectively, the current mouse models provide exciting evidence that OSE cells can be transformed by various oncogenic factors and exhibit histological appearances similar to various ovarian cancer subtypes in women (Fig. 1). These are important milestones and clearly validate the use of mouse models for determining the oncogenic potential and interactions of oncogenic factors in OSE cells in vivo. Moreover, the mice provide a more uniform genetic background (than that in humans) in which to dissect the key molecular events that are driving oncogenic transformation in each context. Although a mouse model that closely mimics low-grade serous adenocarcinomas is now available (31), it is also clear that mouse models recapitulating events associated with high-grade ovarian cancer have not yet been generated. Therefore, this void needs to be filled before our understanding of the etiology of the most prevalent form of human ovarian cancer can be pushed forward. Defining the roles of wild-type and mutated forms of Trp53 will provide major advances toward managing ovarian cancer and designing new therapeutic strategies. The laying hen also provides the important evidence that Trp53 is a central player in ovarian and oviductal tumors in this species. Lastly, because contraceptives have such a profound effect on reducing the incidence of ovarian cancer, the impact of steroid hormones on OSE (and FTE) cells during tumorigenesis needs to be analyzed rigorously and systematically in all ovarian cancer subtypes.
Acknowledgments
This work was supported by Grant NIH-HD-16229 (National Institutes of Child Health and Human Development), U54-NIH-HD-07495 (National Institutes of Child Health and Human Development), the Eunice Kennedy Shriver National Institutes of Child Health and Human Development through a cooperative agreement (U54-7495), as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research.
Disclosure Summary: The authors have nothing to disclose.
Note Added in Proof
Since the submission of this minireview, a paper has been published describing the generation of mice in which Pten and Dicer were conditionally depleted using the Amhr2-Cre mouse strain. These mice develop tumors that appear to arise from the fallopian tube and subsequently spread to the ovary and ultimately after 8–9 months metastasize to the peritoneal cavity. Hence, these mice provide one model for human high-grade ovarian cancer (63).
Footnotes
- AKT
- Ak mouse strain thymic lymphoma
- Amhr2
- anti-Mullerian hormone type 2 receptor
- CMV
- cytomegalovirus
- Ctnnb1
- β-catenin
- EOC
- epithelial ovarian cancer
- ESR
- estrogen receptor
- FTE
- fallopian tube epithelium
- GCT
- granulosa cell tumor
- KRAS
- Kirsten rat sarcoma viral oncogene
- OGP
- oviductal glycoprotein
- OSE
- ovarian surface epithelium
- PI3K
- phosphatidylinositol 3 kinase
- PTEN
- phosphatase and tensin homolog
- RB
- retinoblastoma protein
- SV40T
- simian virus 40T
- TRP
- tumor repressor protein.
References
- 1.American Cancer Society. What are the key statistics about ovarian cancer? 2009. Available at http://www.cancer.org/Cancer/OvarianCancer/DetailedGuide/ovarian-cancer-key-statistics.
- 2. Romero IN, Bast RC., Jr 2012. Human ovarian cancer: biology, current management and paths to personalizing therapy. Endocrinology 153:1593–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bast RC, Hennessy B, Mills GB. 2009. The biology of ovarian cancer: new opportunities for translation. Nature Rev Cancer 9:415–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cho KR, Shih I-M. 2009. Ovarian cancer. Ann Rev Path 4:287–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Auersperg N. 2011. The origin of ovarian carcinomas: a unifying hypothesis. In J Gynecol Pathol 30:12–21 [DOI] [PubMed] [Google Scholar]
- 6. Auersperg N, Wong AS, Choi KC, Kang SK, Leung PC. 2001. Ovarian surface epithelium: biology, endocrinology and pathology. Endocr Rev 22:255–288 [DOI] [PubMed] [Google Scholar]
- 7. Cancer Genome Atlas Research Network 2011. Integrated genomic analyses of ovarian carcinoma. Nature 474:609–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wong KK, Tsang YT, Deavers MT, Mok SC, Zu Z, Sun C, Malpica A, Wolf JK, Lu KH, Gershenson DM. 2010. BRAF mutation is rare in advanced stage low-grade ovarian serous carcinomas. Am J Pathol 177:1611–1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bonome T, Lee JY, Park DC, Radonovich M, Pise-Masison C, Brady J, Gardner GJ, Hao K, Wong WH, Barrett JC, Lu KH, Sood AK, Gershenson DM, Mok SC, Birrer MJ. 2005. Expression profiling of serous low grade malignant potential, low-grade, and high grade tumors of the ovary. Cancer Res 65:10602–10612 [DOI] [PubMed] [Google Scholar]
- 10. Barua A, Bitterman P, Abramowicz JS, Dirks AL, Bahr JM, Hales DB, Bradaric MJ, Edassery SL, Rotmensch J, Luborsky JL. 2009. Histopathology of ovarian tumors in laying hens, a preclinical model of human ovarian cancer. Int J Gynecol Cancer 19:531–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Treviño LS, Giles JR, Wang W, Urick ME, Johnson PA. 2010. Gene expression profiling reveals differentially expressed genes in ovarian cancer in the hen: support for oviductal origin? Horm Cancer 1:177–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garson K, Shaw TJ, Clark KV, Yao DS, Vanderhyden BC. 2005. Models of ovarian cancer—are we there yet? Mol Cell Endocrinol 239:15–26 [DOI] [PubMed] [Google Scholar]
- 13. King SM, Burdette JE. 2011. Evaluating the progenitor cells of ovarian cancer: analysis of current mouse models. BMB Rep 44:435–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cheon D-J, Orsulic S. 2011. Mouse models of cancer. Ann Rev Pathol Mech Dis 6:95–119 [DOI] [PubMed] [Google Scholar]
- 15. Vanderhyden BC, Shaw TJ, Ethier JF. 2003. Animal models of ovarian cancer. Reprod Biol Endocrinol 1:67–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Orsulic S, Li Y, Soslow RA, Vitale-Cross LA, Gutkind JS, Varmus HE. 2002. Induction of ovarian cancer by defined multiple genetic changes in a mouse model system. Cancer Cell 1:53–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xing D, Orsulic S. 2005. A genetically defined mouse ovarian carcinoma model for molecular characterization of pathway-targeted therapy and tumor resistance. Proc Natl Acad Sci USA 102:6936–6941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Flesken-Nikitin A, Choi KC, Eng JP, Shmidt EN, Nikitin AY. 2003. Induction of carcinogenesis by concurrent inactivation of p53 and Rb1 in the mouse ovarian surface epithelium. Cancer Res 63:3459–3463 [PubMed] [Google Scholar]
- 19. Hwang CI, Matoso A, Corney DC, Flesken-Nikitin A, Körner S, Wang W, Boccaccio C, Thorgeirsson SS, Comoglio PM, Hermeking H, Nikitin AY. 2011. Wild-type p53 controls cell motility and invasion by dual regulation of MET expression. Proc Natl Acad Sci USA 108:14240–14245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Connolly DC, Bao R, Nikitin AY, Stephens KC, Poole TW, Hua X, Harris SS, Vanderhyden BC, Hamilton TC. 2003. Female mice chimeric for expression of the Simian virus 40T Ag under the control of the MISRIIR promoter develop epithelial ovarian cancer. Cancer Res 63:1389–1397 [PubMed] [Google Scholar]
- 21. Quinn BA, Xiao F, Bickel L, Martin L, Hua X, Klein-Szanto A, Connolly DC. 2010. Development of a syngenic mouse model of epithelial ovarian cancer. J Ovarian Res 3:24–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Paraskar AS, Soni S, Chin KT, Chaudhuri P, Muto KW, Berkowitz J, Handlogten MW, Alves NJ, Bilgicer B, Dinulescu DM, Mashelkar RA, Sengupta S. 2010. Harnessing structure-activity relationship to engineer a cisplatin nanoparticle for enhanced antitumor efficacy. Proc Natl Acad Sci USA 107:12435–12440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee Y, Miron A, Drapkin R, Nucci MR, Medeiros F, Saleemuddin A, Garber J, Birch C, Mou H, Gordon RW, Cramer DW, McKeon FD, Crum CP. 2007. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J Pathol 211:26–35 [DOI] [PubMed] [Google Scholar]
- 24. Miyoshi I, Takahashi K, Kon Y, Okamura T, Mototani Y, Araki Y, Kasai N. 2002. Mouse transgenic for murine oviduct-specific glycoprotein promoter-driven simian virus 40 large T antigen: tumor formation and its hormonal regulation. Mol Reprod Devel 63:168–176 [DOI] [PubMed] [Google Scholar]
- 25. Clark-Knowles KV, Senterman MK, Collins O, Vanderhyden BC. 2009. Conditional inactivation of Brca1, p53 and Rb in mouse ovaries results in the development of leiomyosarcomas. PLoS One 4:e8534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Quinn BA, Brake T, Hua X, Baxter-Jones K, Litwin S, Ellenson LH, Connolly DC. 2009. Induction of ovarian leiomyosarcomas in mice by conditional inactivation of Brca1 and p53. PLoS One 4:e8404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xing D, Scangas G, Nitta M, He L, Xu X, Ioffe YJ, Aspuria PJ, Hedvat CY, Anderson ML, Oliva E, Karlan BY, Mohapatra G, Orsulic S. 2009. A role for BRCA1 in uterine leiomyosarcoma. Cancer Res 69:8231–8235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dinulescu DM, Ince TA, Quade BJ, Shafer SA, Crowley D, Jacks T. 2005. Role of K-ras and Pten in the development of mouse models of endometriosis and endometrioid ovarian cancer. Nat Med 11:63–70 [DOI] [PubMed] [Google Scholar]
- 29. Wu R, Hendrix-Lucas N, Kuick R, Zhai Y, Schwartz DR, Akyol A, Hanash S, Misek DE, Katabuchi H, Williams BO, Fearon ER, Cho KR. 2007. Mouse model of human ovarian endometrioid adenocarcinoma based on somatic defects in the Wnt/b-catenin and PI3K/Ptten pathways. Cancer Cell 11:321–333 [DOI] [PubMed] [Google Scholar]
- 30. Fan HY, Liu Z, Paquet M, Wang J, Lydon JP, DeMayo FJ, Richards JS. 2009. Cell type specific targeted mutation of Kras and Pten document proliferation arrest in granulosa cells versus oncogenic insult in ovarian surface epithelial cells. Cancer Res 69:6463–6472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mullany LK, Fan HY, Liu Z, White LD, Marshall A, Gunaratne P, Anderson ML, Creighton CJ, Xin L, Deavers M, Wong KK, Richards JS. 2011. Molecular characterization of ovarian surface epithelial cells transformed by oncogenes. Oncogene 30:3522–3536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mullany LK, Liu Z, King E, Wong KK, Richards JS. 2012. Wild type tumor repressor protein 53 (TRP53) promotes ovarian cancer survival. Endocrinology 153:1638–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fan HY, Shimada M, Liu Z, Cahill N, Noma N, Wu Y, Gossen J, Richards JS. 2008. Selective expression of KrasG12D in granulosa cells of the mouse ovary causes defects in follicular development and ovulation. Development 135:2127–2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang Y, Suh YA, Fuller MY, Jackson JG, Xiong S, Terzian T, Quintás-Cardama A, Bankson JA, El-Naggar AK, Lozano G. 2011. Restoring expression of wild-type p53 suppresses tumor growth but does not cause tumor regression in mice with a p53 missense mutation. J Clin Invest 121:893–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jackson JG, Post SM, Lozano G. 2011. Regulation of tissue- and stimulus- specific cell fate decisions by p53 in vivo. J Pathol 223:127–136 [DOI] [PubMed] [Google Scholar]
- 36. Maser RS, Choudhury B, Campbell PJ, Feng B, Wong KK, Protopopov A, O'Neil J, Gutierrez A, Ivanova E, Perna I, Lin E, Mani V, Jiang S, McNamara K, Zaghlul S, Edkins S, Stevens C, Brennan C, Martin ES, Wiedemeyer R, Kabbarah O, Nogueira C, Histen G, Aster J, Mansour M, Duke V, Foroni L, Fielding AK, Goldstone AH, Rowe JM, Wang YA, Look AT, Stratton MR, Chin L, Futreal PA, DePinho RA. 2007. Chromosomally unstable mouse tumours have genomic alterations similar to diverse human cancers. Nature 447:966–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wong KK, Maser RS, Bachoo RM, Menon J, Carrasco DR, Gu Y, Alt FW, DePinho RA. 2003. Telomere dysfunction and Atm deficiency compromises organ homeostasis and accelerates ageing. Nature 421:643–648 [DOI] [PubMed] [Google Scholar]
- 38. Feldser DM, Kostova KK, Winslow MM, Taylor SE, Cashman C, Whittaker CA, Sanchez-Rivera FJ, Resnick R, Bronson R, Hemann MT, Jacks T. 2010. Stage-specific sensitivity to p53 restoration during lung cance progression. Nature 468:572–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Young NP, Crowley D, Jacks T. 2011. Uncoupling cancer mutations reveals critical timing of p53 loss in sarcomagenesis. Cancer Res 71:4040–4047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gershenson DM, Deavers M, Diaz S, Tortolero-Luna G, Miller BE, Bast RC, Jr, Mills GB, Silva EG. 1999. Prognostic significance of p53 expression in advanced-stage ovarian serous borderline tumors. Clin Cancer Res 5:4053–4058 [PubMed] [Google Scholar]
- 41. Ahmed AA, Etemadmoghadam E, Temple J, Lynch AG, Riad M, Sharma R, Stewart C, Fereday S, Caldas C, Defazio A, Bowtell D, Brenton JD. 2010. Driver mutations in TP53 are ubiquitous in high grade serous adenocarcinomas of the ovary. J Pathol 22:49–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Deeb KK, Michalowska AM, Yoon CY, Krummey SM, Hoenerhoff MJ, Kavanaugh C, Li MC, Demayo FJ, Linnoila I, Deng CX, Lee EY, Medina D, Shih JH, Green JE. 2007. Identification of an integrated SV40T/t-antigen cancer signature in aggressive human breast, prostate and lung carcinomas with poor prognosis. Cancer Res 67:8065–8080 [DOI] [PubMed] [Google Scholar]
- 43. Salehi F, Dunfield L, Philips KP, Krowski D, Vanderhyden BC. 2008. Risk factors for ovarian cancer: an overview with emphasis on hormonal factors. J Toxicol Environ Health 11:301–321 [DOI] [PubMed] [Google Scholar]
- 44. Schildkraut JM, Calingaert B, Marchbanks PA, Moorman PG, Rodriguez GC. 2002. Impact of progestin and estrogen potency in oral contraceptives on ovarian cancer risk. J Natl Cancer I 94:32–38 [DOI] [PubMed] [Google Scholar]
- 45. Spillman MA, Manning NG, Dye WW, Sartorius CA, Post MD, Harrell JC, Jacobsen BM, Horwitz KB. 2010. Tissue-specific pathways for estrogen regulation of ovarian cancer growth and metastasis. Cancer Res 70:8927–8936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Laviolette LA, Garson K, Macdonald EA, Senterman MK, Courville K, Crane CA, Vanderhyden BC. 2010. 17b-Estradiol accelerates tumor onset and decreases survival in a transgenic mouse model of ovarian cancer. Endocrinology 151:929–938 [DOI] [PubMed] [Google Scholar]
- 47. Murdoch WJ, Van Kirk EA, Isaak DD, Shen Y. 2008. Progesterone facilitates cisplatin toxicity in epithelial ovarian cance cells and xenografts. Gynecol Oncol 110:251–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Romero IL, Gordon IO, Jagadeeswaran S, Mui KL, Lee WS, Dinulescu DM, Krausz TN, Kim HH, Gilliam ML, Lengyel E. 2009. Effects of Oral Contraceptives or a Gonadotropin-Releasing Hormone Agonist on Ovarian Carcinogenesis in Genetically Engineered Mice. Cancer Prev Res 2:792–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shah SP, Köbel M, Senz J, Morin RD, Clarke BA, Wiegand KC, Leung G, Zayed A, Mehl E, Kalloger SE, Sun M, Giuliany R, Yorida E, Jones S, Varhol R, Swenerton KD, Miller D, Clement PB, Crane C, Madore J, Provencher D, Leung P, DeFazio A, Khattra J, Turashvili G, Zhao Y, Zeng T, Glover JN, Vanderhyden B, Zhao C, Parkinson CA, Jimenez-Linan M, Bowtell DD, Mes-Masson AM, Brenton JD, Aparicio SA, Boyd N, Hirst M, Gilks CB, Marra M, Huntsman DG. 2009. Mutation of FOXL2 in granulosa cell tumors for the ovary. N Engl J Med 360:2719–2729 [DOI] [PubMed] [Google Scholar]
- 50. Jamieson S, Butzow R, andersson N, alexiadis M, Unkila-Kallio L, Heikinheimo M, Fuller PJ, Anttonen M. 2010. The FOXL2 C134W mutation is characteristic of adult granulosa cell tumors of the ovary. Modern Pathol 23:1477–1485 [DOI] [PubMed] [Google Scholar]
- 51. Fleming NI, Knower KC, Lazarus KA, Fuller PJ, Simpson ER, Clyne CD. 2010. Aromatase is a direct target of FOXL2:C134W in granulosa cell tumors via a single highly conserved binding site in the ovarian specific promoter. PLoS One 5:e14389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Middlebrook BS, Eldin K, Li X, Shivasankaran S, Pangas SA. 2009. Smad1-Smad5 ovarian conditional knockout mice develop a disease profile similar to the juvenile form of human granulosa cells tumors. Endocrinology 150:5208–5217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pangas SA. 7 July 2011. Bone morphogenetic protein signaling transcription factor (SMAD) function in granulosa cells. Mol Cell Endocrinol 10.1016/j.mce.2011.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Edson MA, Nalam RL, Clementi C, Franco HL, Demayo FJ, Lyons KM, Pangas SA, Matzuk MM. 2010. Granulosa cell-expresed BMPR1A and BMPR1B have unique functions in regulating fertility but act redundantly to suppress ovarian tumor development. Mol Endocrinol 24:1251–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Matzuk MM, Finegold MJ, Su JG, Hsueh AJ, Bradley A. 1992. a-Inhibin is a tumour-suppressor gene with gonadal specificity in mice. Nature 360:313–319 [DOI] [PubMed] [Google Scholar]
- 56. Fan X, Gabbi C, Kim HJ, Cheng G, Andersson LC, Warner M, Gustafsson JA. 2010. Gonadotropin-positive pituitary tumors accompanied by ovarian tumors in aging female ERb−/− mice. Proc Natl Acad Sci USA 107:6453–6458 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Alexiadis M, Eriksson N, Jamieson S, Davis M, Drummond AE, Chu S, Clyne CD, Muscat GE, Fuller PJ. 2011. Nuclear receptor profiling of ovarian granulosa cell tumors. Horm Cancer 2:157–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Boerboom D, Paquet M, Hsieh M, Liu J, Jamin SP, Behringer RR, Sirois J, Taketo MM, Richards JS. 2005. Misregulated Wnt/b-catenin signaling leads to ovarian granulosa cell tumor development. Cancer Res 65:9206–9215 [DOI] [PubMed] [Google Scholar]
- 59. Fan HY, Liu Z, Cahill N, Richards JS. 2008. Targeted disruption of Pten in ovarian granulosa cells enhances ovulation and extends the life span of luteal cells. Mol Endocrinol 22:2128–2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Laguë MN, Paquet M, Fan HY, Kaartinen MJ, Chu S, Jamin SP, Behringer RR, Fuller PJ, Mitchell A, Doré M, Huneault LM, Richards JS, Boerboom D. 2008. Synergistic effects of Pten loss and WNT/CTNNB1 signaling pathway activation in granulosa cell tumor development and progression. Carcinogenesis 29:2062–2072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Boyer A, Paquet M, Laguë MN, Hermo L, Boerboom D. 2009. Dysregulation of WNT/CTNNB1 and PI3K/AKT signaling in testicular stromal cells causes granulosa cell tumors of the testis. Carcinogenesis 30:869–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Richards JS, Fan HY, Liu Z, Tsoi M, Lague MN, Boyer A, Boerboom D. 22 August 2011. Either Kras activation or Pten loss similarly enhances the dominant-stable CTNNB1-induced genetic program to promote granulosa cell tumor development in the ovary and testis. Oncogene 10.1038/onc.2011.1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kim J, Coffey DM, Creighton CJ, Yu Z, Hawkins SM, Matzuk M.M. 2012. High-grade serous ovarian cancer arises from fallopian tube in a mouse model. Proc Natl Acad Sci 10.1073/pnas.1117135109 [DOI] [PMC free article] [PubMed] [Google Scholar]