Abstract
Differential regulation of gonadotropin hormone production in the pituitary is critical for fertility. Activin and progesterone signaling in gonadotrope cells is important for Fshb gene expression. Previously, we reported that synergy between activin and progestins required the binding of SMAD proteins and the progesterone receptor (PR) to the murine Fshb promoter. In this study, we demonstrate that the FOXL2 transcription factor is also necessary for the full synergistic response between activin and progestins. We show that this synergy occurs in a species-specific manner and that multiple elements in the Fshb promoter that bind forkhead box L2 (FOXL2), SMA/mothers against decapentaplegic homologs (SMAD), and PR are required. Furthermore, we demonstrate that FOXL2 can physically interact with PR and SMAD3. Thus, it is likely that protein-protein interactions among FOXL2, SMAD, and PR recruited to the Fshb promoter play a key role in facilitating Fshb transcription before the secondary FSH surge in rodents.
FSH and LH are essential for mammalian fertility because they are required for ovarian folliculogenesis, gametogenesis and steroidogenesis (1). Gonadotropins are heterodimeric glycoproteins composed of a common α-glycoprotein subunit and unique β-subunits that confer biological specificity. Transcription of the β-subunits is the rate-limiting step for production of the mature hormones (2, 3) and is differentially regulated during the estrous cycle. The LH surge is proceeded by an increase in Lhb and Fshb transcription in the afternoon of proestrus (4–6), while an independent secondary FSH surge occurs, after an increase in Fshb transcription during estrus, to promote follicular recruitment for the following cycle (3, 7, 8).
Multiple mechanisms have been proposed for the distinct regulation of Fshb vs. Lhb transcription (recently reviewed in Refs. 9 and 10). Pulsatile secretion of GnRH contributes to differential regulation of FSH and LH (2, 11, 12). In addition, an increase in activin levels during estrus, due to lower follistatin and/or inhibin, is thought to selectively favor Fshb synthesis (13, 14). Furthermore, steroid hormones such as progesterone have been shown to induce Fshb while inhibiting Lhb expression (15, 16).
Activin strongly induces Fshb expression in gonadotrope cells (17). Activin binding to the type II receptor leads to heterodimerization and phosphorylation of the type I receptor (18). Subsequently, the type I receptor phosphorylates SMA/mothers against decapentaplegic homologs (SMAD) 2 and SMAD3, which bind to SMAD4 and translocate into the nucleus to mediate transcription of specific target genes (18–20). The proximal murine Fshb promoter contains multiple activin responsive elements (reviewed in Refs. 21 and 22). The −267 SMAD-binding element (SBE) in the murine Fshb promoter is a consensus SBE comprised of a palindrome sequence GTCTAGAC (23–25). Two other elements at −153 and −120 are critical for activin induction in all species examined (26). The TALE homeodomain proteins, PBX1 and PREP1 bind the −120 site, which allows tethering of SMAD to the promoter (26, 27), whereas the forkhead box L2 (FOXL2) transcription factor was recently shown to bind the −153 element (28, 29). Indeed, Fshb mRNA levels are reduced in both FOXL2- and SMAD3-null mice, indicating these transcription factors are required for normal Fshb expression (30, 31).
Differential regulation of gonadotropin gene expression may be due to interactions among the hormone signaling pathways that regulate their synthesis. Cross talk between activin and progesterone may be important for the secondary FSH surge because FSH levels were reported to increase upon activin and progesterone cotreatment in primary rat pituitary cell cultures and a progesterone antagonist suppressed activin induction of Fshb gene expression in vivo in rats (32, 33). Another study revealed that progesterone stimulation of FSH secretion was prevented by follistatin treatment in rats (34). More recently, we showed synergistic induction of the Fshb promoter with activin and progestin cotreatment of murine primary pituitary cells as well as immortalized gonadotrope-derived LβT2 cells (35). We also demonstrated that SMAD can physically interact with progesterone receptor (PR) and that both transcription factors were required to bind to the Fshb promoter for the synergy (35).
In this study, we further investigated mechanisms of Fshb transcriptional regulation mediated by cross talk between activin and progestin signaling pathways using LβT2 cells. During the course of our investigations, we and others discovered that FOXL2 plays a role in activin induction of the Fshb promoter (28, 29). Therefore, we shifted our focus to understanding the potential role of FOXL2 in mediating activin and progestin synergy on the Fshb promoter. We show that the FOXL2 transcription factor is involved in activin and progestin synergy on the murine Fshb promoter and that the synergy occurs in a species-specific manner. We demonstrate that a −355 SMAD half-site and an adjacent −350 FOXL2 site are necessary for the full synergistic response along with a previously characterized −381 hormone response element (HRE) and −267 SBE. We also show that additional activin and progesterone-responsive elements are necessary for the maximal response. Furthermore, we demonstrate that FOXL2 physically interacts with PR, suggesting that interactions among FOXL2, SMAD, and PR may play a role in elevated Fshb synthesis before the secondary FSH surge in rodents.
Materials and Methods
Hormones
Promegestone (R5020) was purchased from NEN Life Science Products Life Sciences (Boston, MA), activin from Calbiochem (La Jolla, CA), and GnRH from Sigma-Aldrich (St. Louis, MO).
Plasmid constructs
The construction of the −1000 murine Fshb and −985 ovine FSHB luciferase (luc) reporter plasmids were described previously (16, 36). The −1028/+7 human FSHB-luc reporter plasmid was provided by Daniel Bernard, murine PRB by Dean Edwards, and murine FOXL2 and FOXL2 Δ133–375 by Louise Bilezikjian (37).
Mutagenesis
The QuikChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA) was used to generate deletions or mutations in plasmids containing the murine and human FSHB promoters. Insertion of the −381 HRE and/or the −267 SBE into the human FSHB promoter was performed with the following primers: 5′-TTTGTTTCTTCCTTCACAGTGTTCAATATGCTCTTGGAGCAATTT-3′ and 5′-AAAGATACAAAAGAAAAGTCTAGACTCTGGAGTCACAATTAATT-3′ (the mutated bases are indicated in bold). The sequences of the promoters were confirmed by dideoxyribonucleotide sequencing. The −381, −273, −230, −197, and −139 HRE mutations in the murine Fshb-luc reporter plasmid were described previously (16) as well as the −267, −153, −120, and 3xSBE mutations (35). The −355 SMAD, −350 FOXL2, −339 SF1, −355 SMAD/−350 FOXL2, −208 (5′ T), −153 (3′ T), and −106 mutations were also previously described (29, 38).
Cell culture and transient transfections
Cell culture and transient transfection experiments were performed using the LβT2 cell line (39, 40). The cells were maintained in 10-cm plates in DMEM from Mediatech Inc. (Herndon, VA) with 10% fetal bovine serum (Omega Scientific, Inc., Tarzana, CA) and penicillin/streptomycin antibiotics (Life Technologies, Inc./Invitrogen, Grand Island, NY) at 37 C and 5% CO2. Trypsin-EDTA (1×) (Sigma-Aldrich) was used for cell dissociation. Cells were split at 3 × 105 cells per well into 12-well plates and transfected 18 h later, using Fugene 6 reagent (Roche Molecular Biochemicals, Indianapolis, IN) following the manufacturer's instructions. For all experiments, the cells were transfected with 400 ng of the indicated reporter plasmid, 10 ng of mouse PRB expression vector, and 200 ng of a β-galactosidase reporter plasmid driven by the herpes virus thymidine kinase promoter to control for transfection efficiency. Cells were switched to serum-free DMEM containing 0.1% BSA, 5 mg/liter transferrin, and 50 mm sodium selenite 6 h after transfection. The cells were treated with various hormones after overnight starvation in serum-free media. Vehicle control for R5020 was 0.1% ethanol; for activin and GnRH, it was 0.1% BSA. The cells were treated with 10 ng/ml activin (unless stated otherwise), 10−9 m R5020 for 24 h or 10−8 m GnRH for 6 h.
Luciferase and β-galactosidase assays
After hormone treatment, cells were washed with 1× PBS and lysed with 0.1 m K-phosphate buffer (pH 7.8) containing 0.2% Triton X-100. Lysed cells were assayed for luciferase activity using a buffer containing 100 mm Tris-HCl (pH 7.8), 15 mm MgSO4, 10 mm ATP, and 65 μm luciferin. β-Galactosidase activity was assayed using the Tropix Galacto-light assay (Applied Biosystems, Foster City, CA), according to the manufacturer's protocol. Both assays were measured using a Veritas Microplate Luminometer (Promega, Madison, WI).
Glutathione S-transferase (GST) interaction assay
GST-FOXL2 was creating by ligating Flag-tagged murine FOXL2 (37) into the BamHI and EcoRI sites of the pGEX-5X-1 expression vector. GST-SMAD3 was provided by Rik Derynck. 35S-labeled proteins were produced using the TnT Coupled Reticulolysate System (Promega). Bacteria transformed with GST plasmids were grown to OD of 0.6 and induced with isopropyl β-D-1-thiogalactopyranoside overnight at 30 C (41). Bacterial pellets were sonicated in 0.1% Triton X-100, 5 mm EDTA in 1× PBS and centrifuged, and the supernatant was bound to glutathione Sepharose 4B resin (Amersham Pharmacia Biotech, Piscataway, NJ). Beads were washed 4× in PBS and in Hepes/NaCl/dithiothreitol (HND) buffer [10 mg/ml BSA, 20 mm HEPES (pH 7.8), 50 mm NaCl, 5 mm dithiothreitol, and 0.1% Nonidet P-40]. For the interaction assay, 40 μl of 35S-labeled in vitro transcribed-translated PR or 5 μl of green fluorescent protein (GFP) was added to the beads with 400 μl HND buffer. Beads were incubated overnight at 4 C and washed twice with HND buffer and twice with 0.1% Nonidet P-40 in PBS. Thirty microliters of 2× Laemmli load buffer were added, and the samples were boiled and electrophoresed on a 10% SDS-polyacrylamide gel. One tenth of the 35S-labeled in vitro transcribed-translated product was loaded onto the gel as input.
Statistical analysis
Transient transfection experiments were repeated independently at least three times. Data were normalized for transfection efficiency with luciferase activity relative to β-galactosidase. Data were also normalized to the pGL3 plasmid (to control for hormone effects on the vector DNA). In the figures, the error bars represent the sem. Data were analyzed by one-way ANOVA, followed by post hoc comparisons with the Tukey-Kramer honestly significant difference test or two-way ANOVA to determine synergy (as described in Ref. 42) using the statistical package JMP version 9.0 (SAS, Cary, NC). In all analyses, the result was considered significant if P ≤ 0.05.
Results
Low levels of exogenous PR are sufficient for progestin responsiveness and synergy between activin and progestins on the Fshb promoter
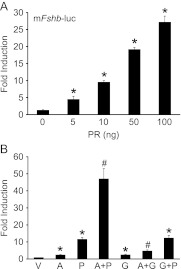
One concern from our previous studies was the high levels of exogenous PR transfected into LβT2 cells to measure progestin responsiveness (16, 35). In the current study, we performed a titration to determine the minimal effective level of PR for progestin induction of the murine Fshb promoter (Fig. 1A). Because 10 ng PR resulted in a robust induction, we used this amount of receptor for all of the subsequent experiments. Ten nanograms of PR resulted in an 11-fold response to R5020 (a synthetic progestin) on the Fshb promoter, whereas the synergistic response to activin and progestin was 47-fold (Fig. 1B).
Fig. 1.
In contrast to activin with progestins or activin with GnRH, R5020, and GnRH do not synergistically regulate Fshb gene expression. Panel A, The −1000 murine Fshb-luc reporter was transiently transfected into LβT2 cells along with increasing amounts of PR as indicated. After overnight starvation in serum-free media, cells were treated with vehicle or 10−9 m R5020. Panel B, The −1000 murine Fshb-luc reporter was transiently transfected into LβT2 cells along with 10 ng PR. After overnight starvation in serum-free media, cells were treated with vehicle (V), 10 ng/ml activin (A), 10−9 m R5020 (P), or 10 nm GnRH (G), individually or with the indicated combinations. The results represent the mean ± sem of at least three experiments. *, Significant difference from the vehicle-treated control using one-way ANOVA followed by Tukey's post hoc test; #, significant interaction as defined by two-way ANOVA (42).
GnRH and progestin signaling pathways do not synergize on the Fshb promoter
As shown previously (23, 35, 43), activin with progestins or activin with GnRH synergistically enhanced transcription of a Fshb-luc reporter (Fig. 1B). Because activin, GnRH, and progesterone are all present during the estrous cycle to regulate Fshb gene expression, we investigated whether GnRH and progestin signaling pathways interact to mediate Fshb gene expression. As shown in Fig. 1B, GnRH induced the Fshb promoter 2.6-fold and R5020 11-fold. However, GnRH and R5020 cotreatment up-regulated Fshb gene expression 12-fold, indicating that they do not interact to regulate the Fshb promoter.
Synergy between activin and progestins is not conserved on the ovine and human FSHB promoters
Activin has been shown to regulate ovine FSHB gene expression by SMAD and TGF-β-activated kinase-1-dependent signaling pathways (26, 44, 45). It is less clear whether progesterone modulates transcription of ovine FSHB in gonadotropes. FSHB mRNA levels decreased after progestin treatment in ovine primary pituitary cells (46). On the other hand, a reporter gene containing the proximal ovine FSHB promoter was induced by progesterone in ovine primary pituitary cells (47). Data regarding transcriptional regulation of the human FSHB gene by activin and progestins are limited. The human FSHB promoter has been reported to be relatively insensitive to activin treatment (48, 49). A polymorphism in the human FSHB promoter was mapped to a conserved region containing a putative HRE, suggesting that progestin regulation of FSHB synthesis may be conserved among mammals (50).
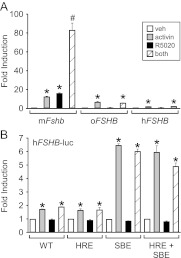
To determine whether the ovine and human promoters respond to progestins alone or synergistically with activin and progestins, −1000 murine, −985 ovine, or −1028/+7 human FSHB-luc reporter constructs were transiently transfected into LβT2 cells along with PR (Fig. 2A). In contrast to the murine promoter, which was induced 15-fold by progestin treatment and 82-fold by activin and progestins, the ovine and the human FSHB promoters did not exhibit significant progestin responsiveness alone or in the presence of 50 ng/ml activin.
Fig. 2.
Activin and progestin synergy is not conserved on the ovine and human FSHB promoters and addition of the −381 HRE and/or −267 SBE to the human FSHB promoter is not sufficient for synergy. A, The −1000 murine (m), −985 ovine (o), or −1028/+7 human (h) FSHB-luc reporter constructs were transiently transfected into LβT2 cells, as indicated. B, Wild-type (WT) −1028/+7 hFSHB-luc or mutants containing the −381 HRE, −267 SBE, or both were transiently transfected into LβT2 cells, as indicated. After overnight starvation in serum-free media, cells were treated for 24 h with vehicle (veh), 50 ng/ml activin, 10−9 m R5020, or both. The results represent the mean ± sem of at least three experiments. *, Significant difference from the vehicle-treated control using one-way ANOVA followed by Tukey's post hoc test; #, significant interaction as defined by two-way ANOVA.
Addition of the −381 HRE and/or the −267 SBE to the human FSHB promoter is not sufficient for activin and progestin synergy
The −267 SBE is present in rodent Fshb promoters but is not conserved in other mammalian species. Addition of the −267 SBE to the human FSHB promoter increased activin responsiveness (48). The −381 HRE is necessary for the synergistic induction of murine Fshb gene expression by activin and progestins, because mutating it reduced the synergy by 73% (35). We, therefore, investigated whether addition of the murine −381 HRE, −267 SBE, or both to the human FSHB promoter would be sufficient to observe synergy between activin and progestins. With the addition of the −267 SBE site, the human FSHB promoter responded to activin 3-fold more than the wild-type human promoter, as previously demonstrated (48). On the other hand, addition of the −381 HRE did not result in progestin responsiveness or synergy between activin and R5020, indicating that the −381 HRE is not sufficient (Fig. 2B). Furthermore, although addition of the −267 SBE to the human promoter resulted in substantial activin responsiveness, it was unable to facilitate activin and progestin synergy.
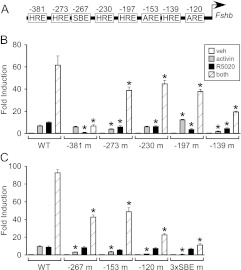
The −273, −230, −197, and −139 HRE are necessary for activin and progestin synergy
In addition to the −381 HRE, several other HREs on the murine Fshb promoter are necessary for the full progestin response (16) (Fig. 3A). Because the −381 HRE is not sufficient for the synergy between activin and progestins, mutations in these additional HRE sites were tested for responsiveness to activin, progestins, and cotreatment. The −273 and −139 HRE mutations resulted in a significant decrease in the activin response, perhaps due to proximity of these sites to the −267 and −153 activin responsive elements, respectively (Fig. 3B). The −197 HRE mutation increased the activin response by approximately 50%, probably due to enhancement of the adjacent −208 activin responsive element as an unintended consequence of the mutation. Indeed, an individual base-pair change at −193 instead of the double mutation at −196 and −193 did not exhibit an increased activin response (see Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). All HRE mutants showed a significant decrease in progestin responsiveness and synergistic induction by activin and R5020 cotreatment (Fig. 3B). The −381 HRE mutation resulted in a complete lack of responsiveness to R5020 alone or in combination with activin.
Fig. 3.
Multiple response elements are necessary for the full synergistic response between activin and progestins. A, Schematic of the mFshb promoter showing the position of previously identified HRE, SBE, and activin-responsive elements (ARE). B and C, The wild-type (WT) −1000 mFshb-luc reporter and mutations in specific response elements were transiently transfected into LβT2 cells, as indicated. After overnight starvation in serum-free media, cells were treated for 24 h with vehicle (veh), 10 ng/ml activin, 10−9 m R5020, or both. The results represent the mean ± sem of at least three experiments. *, Significant difference from the wild-type Fshb promoter using one-way ANOVA followed by Tukey's post hoc test.
Disruption of individual activin-responsive elements prevents cross talk between activin and progestins
The expression of a luciferase reporter containing three mutated activin-responsive elements in the murine −1000 Fshb proximal promoter (3xSBE) was previously studied for its responsiveness to activin and progestins (35). Here, we measured responsiveness to activin, R5020, and cotreatment of individual mutations in these elements. Mutations in the −267, −153, and −120 elements showed a significant decrease in activin responsiveness compared with the wild-type promoter (Fig. 3C). None of the mutated promoters showed any significant difference in their progestin response. The synergistic induction of the mutated promoters due to activin and R5020 cotreatment was significantly reduced (Fig. 3C). The 3xSBE mutation led to a complete lack of response to activin alone or cotreatment with activin and progestins, as previously reported (35).
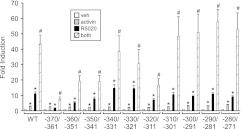
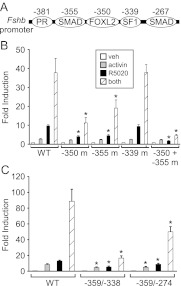
The region between the −381 HRE and the −267 SBE contains a FOXL2 site and SMAD half-site necessary for synergy between activin and progestins
Because it appeared, from our studies, that additional elements might be involved in the synergy between activin and progestins (Fig. 2) and that the −381 HRE, −273 HRE, and −267 SBE are important elements for this synergy (Fig. 3), we undertook a systematic analysis of the region between these elements. Internal deletions of 10 bp from −370 to −271 in the murine −1000 Fshb promoter were created because the helical periodicity of DNA is approximately 10 bp per turn, and the orientation of the promoter DNA would not be altered. The responsiveness of the 10-bp deletions to activin, progestin, or cotreatment was measured. The −370/−361 deletion overlapped the last 4 bp of the 3′ end of the −381 HRE and thus led to a significant reduction of R5020 responsiveness and synergy compared with the wild-type promoter (Fig. 4). Deletions at −360/−351, −350/−341, and −320/−311 also resulted in significant reductions in the induction of Fshb gene expression by R5020 and cotreatment (Fig. 4). The −320/−311 region was recently identified as playing a role in activin induction of the Fshb promoter (29), but it remains to be determined what transcription factors bind to this region.
Fig. 4.
The 10-bp internal deletions between the −381 HRE and the −267 SBE reveal several regions involved in activin and progestin synergy on the murine Fshb promoter. The wild-type (WT) −1000 mFshb-luc reporter or mutants with the indicated 10-bp deletions were transiently transfected into LβT2 cells. After overnight starvation in serum-free media, cells were treated for 24 h with vehicle (veh), 10 ng/ml activin, 10−9 m R5020, or both. The results represent the mean ± sem of at least three experiments. *, Significant difference from the vehicle-treated control using one-way ANOVA followed by Tukey's post hoc test; #, significant interaction as defined by two-way ANOVA.
Closer examination of the 10-bp internal deletions from −360 to −341 found that a putative SMAD half-site at −355 bp and a newly characterized FOXL2 site at −350 bp described by Corpuz et al. (29) (Fig. 5A) had been deleted. The expression of specific mutations in each of the above-mentioned sites and a double mutant was measured to understand the effects of these sites on hormonal induction of the Fshb promoter. As expected from the deletion studies, mutation of a putative SF1 site did not significantly alter Fshb induction by activin, R5020, or cotreatment (Fig. 5B), indicating that this element does not play a significant role. The −355 SMAD half-site and the −350 FOXL2 site mutations did not significantly alter activin induction of the Fshb promoter. On the other hand, mutation of the −355 SMAD half-site reduced the progestin response by 53% and the synergistic induction by 49% of the wild-type promoter induction. Similarly, the −350 FOXL2 mutation reduced the progestin response by 58% and synergistic induction by 70% of the wild-type promoter. Interestingly, the SMAD half-site/FOXL2 double mutation decreased the progestin response and the synergy by approximately 80–90% of the wild-type promoter without significantly reducing activin induction.
Fig. 5.
The −355 SMAD half-site and −350 FOXL2 site on the murine Fshb promoter are necessary for the synergy between activin and progestins. A, Schematic illustrating the −381 PR binding site, −355 putative SMAD half-site, −350 FOXL2 site, and −339 putative SF1 site on the murine Fshb promoter. B, The wild-type (WT) −1000 mFshb-luc reporter or mutations in specific sites were transiently transfected into LβT2 cells, as indicated. C, The WT −1000 mFshb-luc reporter construct or deletions in the region between the −381 HRE and the −267 SBE were transiently transfected into LβT2 cells, as indicated. After overnight starvation in serum-free media, cells were treated for 24 h with vehicle (veh), 10 ng/ml activin, 10−9 m R5020, or both. The results represent the mean ± sem of at least three experiments. *, Significant difference from the wild-type Fshb promoter using one-way ANOVA followed by Tukey's post hoc test.
Because the −355 putative SMAD half-site and the −350 FOXL2 site are located between the −381 HRE and −267 SBE, we hypothesized that these sites may help to stabilize a ternary complex formed by PR, SMAD, and FOXL2. If this is the case, bringing the −381 HRE and −267 SBE closer together on the promoter may remove the necessity for the SMAD and FOXL2 sites. Deletions of 21 or 85 bp were created in the murine Fshb promoter from −359/−338 and −359/−274, respectively. Similarly to Fig. 5B, the −359/−338 deletion encompassing the SMAD half-site and FOXL2 site significantly reduced Fshb gene expression (Fig. 5C). Notably, because the −381 HRE and the −267 SBE were brought closer together with the 85-bp deletion, the progestin response and the synergy were partially rescued. For the 21- and 85-bp deletions, the progestin response was reduced by 66 and 30%, whereas the synergy was reduced by 81 and 44% of the wild-type promoter, respectively (Fig. 5C).
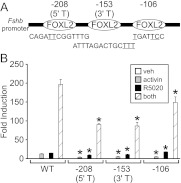
The −208, −153, and −106 FOXL2 sites are necessary for maximal synergistic response
In addition to the −350 FOXL2 site, several other FOXL2 sites have been recently characterized that play a role in activin induction of the murine Fshb promoter (28, 29, 51). We used specific mutations in the −208, −153, and −106 elements to determine whether they are also necessary for progestin responsiveness and the synergy between activin and progestins. In the −208 (5′ T) mutation, two thymines at −204 and −203 were changed to cytosines, whereas in the −153 (3′ T) mutation, three thymines at −142/−140 were altered to guanines (Fig. 6A) (29). The −208 (5′ T) mutation reduced the response to activin, progestins, and cotreatment by 69, 25, and 54% of the wild-type response, respectively, whereas the −153 (3′ T) mutation reduced the response to activin, progestins, and cotreatment by 61, 24, and 66% (Fig. 6B). The −106 mutation (38) reduced the response to activin and cotreatment by 60 and 25% of the wild-type response, whereas the response to progestins was increased by 23%.
Fig. 6.
The −208, −153, and −106 FOXL2 sites are necessary for the full synergistic response between activin and progestins on the murine Fshb promoter. A, Schematic of the FOXL2 sites on the mFshb promoter with the mutated bases underlined (29, 38). B, The −1000 mFshb-luc reporter or mutants were transiently transfected into LβT2 cells, as indicated. After overnight starvation in serum-free media, cells were treated for 24 h with vehicle (veh), 10 ng/ml activin, 10−9 m R5020, or both. The results represent the mean ± sem of at least three experiments. *, Significant difference from the wild-type Fshb promoter using one-way ANOVA followed by Tukey's post hoc test.
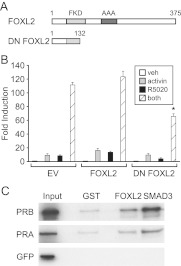
Overexpression of a dominant-negative (DN) FOXL2 reduces synergy between activin and progestins on the Fshb promoter
Because FOXL2 binding sites are necessary for activin and progestin synergy, we asked whether the FOXL2 transcription factor is required for the synergy to occur. Transient transfection of LβT2 cells with an expression plasmid containing murine FOXL2 resulted in only a slight increase in Fshb gene expression compared with the empty vector, likely due to the fact that the cells already contain high levels of FOXL2 protein. LβT2 cells were also transfected with an expression plasmid containing FOXL2 Δ133–375, which lacks part of the DNA-binding forkhead domain and the carboxyl-terminal alanine-rich region (Fig. 7A). Several mutations in patients with blepharophimosis-ptosis-epicanthus inversus syndrome produce truncated proteins lacking part of the forkhead-binding domain and/or a polyalanine tract (52, 53). Truncated FOXL2 proteins have been shown to exert DN effects on wild-type FOXL2 (54). Overexpression of the DN FOXL2 Δ133–375 resulted in 40% reduction in the synergy between activin and progestins (Fig. 7B).
Fig. 7.
DN FOXL2 inhibits activin and progestin synergy, and FOXL2 interacts directly with PR. A, Schematic showing the structure of FOXL2 and DN FOXL2 Δ133–375. B, The −1000 mFshb-luc reporter was transiently transfected into LβT2 cells along with empty vector (EV), wild-type or DN FOXL2, as indicated. After overnight starvation in serum-free media, cells were treated for 24 h with vehicle (veh), 10 ng/ml activin, 10−9 m R5020, or both. The results represent the mean ± sem of at least three experiments. *, Significant difference from the wild-type Fshb promoter using one-way ANOVA followed by Tukey's post hoc test. C, GST interaction assays were performed using bacterially expressed GST-fusion proteins (indicated above each lane) and 35S-labeled in vitro-transcribed and -translated PRB, PRA, and GFP (indicated on the left). GFP was used as a negative control. The GST-fusion proteins included GST alone, GST-FOXL2, and GST-SMAD3. One tenth of the protein used in the interaction assay was loaded in the lane marked input. The experiment was repeated several times with the same results, and a representative experiment is shown.
FOXL2 interacts with PR and SMAD3
Because FOXL2 is necessary for the maximal synergistic response between activin and progestins on the murine Fshb promoter, we investigated whether FOXL2 can physically interact with PR. FOXL2 has been reported to interact with SMAD3 (37), and we have shown that SMAD2, -3, and -4 can interact with PR (35). We tested whether FOXL2 interacts with PR by incubating a GST-FOXL2 fusion protein with in vitro-transcribed and -translated PR in pull-down experiments (Fig. 7C). FOXL2 bound to both the full-length PRB and PRA isoforms. As a positive control, we also showed that FOXL2 interacts with SMAD3, as previously reported (37). In contrast, there was minimal interaction between the GST-FOXL2 fusion protein and the negative control (GFP) or with GST alone incubated with PR (Fig. 7C).
Discussion
Interactions among multiple hormone signaling pathways are likely responsible for the differential regulation of Fshb synthesis before the secondary FSH surge. In investigating combinatorial interactions among activin, progestins, and GnRH, we first confirmed that there are synergistic interactions between activin and progestins or activin and GnRH on the murine Fshb promoter, as previously reported (23, 35, 43). In contrast, our studies demonstrate that there is no significant interaction between GnRH and progestins on the murine Fshb promoter in LβT2 cells. This is particularly interesting because cross talk with PR has been implicated as critical for GnRH self-priming in pituitary gonadotropes (55, 56), and progestins inhibit GnRH induction of Lhb gene expression (57), suggesting that interactions between GnRH and progesterone signaling pathways may be important for LH synthesis and secretion but not for Fshb transcriptional regulation.
Several of the HREs previously characterized in the murine Fshb promoter have a degree of conservation across multiple species, suggesting that steroid hormones such as progesterone may regulate other mammalian FSHB promoters (16). However, we demonstrated that neither the ovine nor human promoter exhibited a progestin response or synergistic induction of the promoter upon cotreatment with activin and progestins. This result suggests that progesterone does not directly regulate ovine or human FSHB transcription in gonadotrope cells due to the lack of species-specific regulatory elements present on the rodent promoter. Because our data differ from what was observed in ovine primary pituitary cells with an ovine FSHB-luc reporter (47), they suggest that the progesterone induction observed in ovine pituitary cells was due to paracrine influences from other cells in the primary cultures. On the other hand, our results agree with experiments in sheep and ovine pituitary cells showing that progestins do not up-regulate FSHB mRNA (46, 58).
To test whether elements in the murine promoter, not conserved in the ovine and human promoters, are sufficient to induce a progestin response and synergy with activin, we inserted the murine −381 HRE, −267 SBE, or both into the human FSHB promoter. Addition of the −267 SBE resulted in a substantial activin response, as previously described (48). In contrast, addition of the −381 HRE to the human FSHB promoter was not sufficient for a progestin response alone or in combination with activin, suggesting that additional regulatory elements are required.
Because the extent of steroid hormone response conveyed by a single HRE is often weak, multiple HRE are often found in the promoters of steroid-responsive genes (59). We confirmed that the −381 HRE is required for the progestin response and for synergistic induction of the promoter by activin and progestin cotreatment, in agreement with our previous studies (16, 35). We also showed that additional HRE previously characterized on the murine Fshb promoter at −273, −230, −197, and −139 as playing a role in the progestin response (16) are also necessary for maximal synergistic induction of the promoter by activin and progestins. It is noteworthy that mutation of the elements at −273, −197, and −139 also affected activin responsiveness, possibly due to the proximity or overlap of these sites with the −267 SBE, −208, and −153 FOXL2 sites, respectively. These results also indicate that there may be strong coregulation between elements that bind PR and FOXL2/SMAD.
Our previous studies showed that SMAD binding to the Fshb promoter was necessary for the synergistic interaction between activin and progestins (57). As previously reported, mutation of three activin-responsive elements (3xSBE) led to a lack of activin responsiveness and disappearance of the synergistic induction of the promoter. Individual disruption of the −267 SBE substantially reduced cross talk between activin and progestins and did not allow for maximal synergistic induction of the promoter. However, mutation of the −267 SBE did not completely abolish activin induction or synergy on the Fshb promoter, indicating that this hormonal regulation requires the binding of additional factors to multiple activin-responsive elements in the Fshb promoter. PBX1/PREP1 were reported to bind a CTGTCTATCCAA element encompassing a putative SMAD half-site (underlined) at −120 in the murine Fshb promoter and recruit SMAD3/4 to the promoter (26). Not surprisingly, mutation of the −120 element not only blocked activin induction, as previously described, but also greatly reduced the synergy between activin and progestins. These data confirm that activin signaling through SMAD proteins is critical for synergy between activin and progestins on the Fshb promoter.
In addition to transcription factors such as SMAD and PBX1/PREP1, recent studies have characterized the binding of the FOXL2 transcription factor at several elements in the murine Fshb promoter (28, 29). In the current experiments, mutation of the −350 FOXL2 site and a nearby −355 putative SMAD half-site had little effect on activin responsiveness, in contrast to our previous study, which showed a significant reduction with a mutation of the −350 FOXL2 site (29). It remains to be determined whether the −355 element is a bona fide SBE because SMAD4 did not supershift protein complexes bound to an oligo encompassing this site (29). On the other hand, deletion or disruption of these two sites greatly reduced the response to progestins, alone or in combination with activin (Figs. 4 and 5, B and C). Indeed, a double FOXL2/SMAD mutation almost completely abolished progestin responsiveness. These results indicate that the FOXL2 site and nearby SMAD half-site are necessary for progestin responsiveness and the synergy between activin and progestins on the murine Fshb promoter. They also suggest that these elements play a role in supporting progestin action at the nearby −381 HRE via protein-protein interactions among FOXL2, SMAD, and PR. The fact that the −359/−274 deletion (which moved the −273 HRE and −267 SBE much closer to the −381 HRE) partially restored progestin responsiveness and synergy between activin and progestins also suggests that multiple elements are necessary to stabilize interactions among SMAD, FOXL2, and PR on the Fshb promoter.
We also determined whether additional FOXL2 binding elements that play a role in activin responsiveness on the murine Fshb promoter also play a role in progestin responsiveness alone or in combination with activin. Interestingly, these FOXL2 elements are in close proximity to other elements important for the synergy. Because the −208 FOXL2 element overlaps the −197 HRE by 2 bp, we mutated the 5′ T to try to separate the effect of FOXL2 vs. PR. The 5′ T mutation resulted in a decrease in progestin responsiveness and the synergy (Fig. 6B), in addition to the previously reported effect on activin induction (29). The −153 FOXL2 element (originally identified as a putative SMAD half-site at −149) (26) overlaps the −139 HRE by 2 bp. Mutation of residues in the SMAD half-site reduced activin response and, thus, the synergy (Fig. 3C), whereas the 3′ T mutation also decreased progestin induction of Fshb (Fig. 6B). Mutation of the −106 FOXL2 site increased progestin response, but the synergy was still reduced, presumably due to the role of the −106 element in activin induction (Fig. 6B). Similarly to the −350 FOXL2 site, these results suggest that the −208, −153, and −106 FOXL2 elements are necessary for a maximal progestin response and synergy between activin and progestins. This idea is supported by the fact that cotransfection with the DN FOXL2 Δ133–375 reduced the synergistic induction of Fshb gene expression by activin and progestins.
Because FOXL2 is necessary for the full synergistic response between activin and progestins, we tested whether there was a direct interaction between FOXL2 and PR. Using a GST interaction assay, we showed that FOXL2 can physically interact with PR as well as with SMAD3. Our data expand the set of proteins that can directly partner with FOXL2. Moreover, these results also suggest that FOXL2 may interact with PR directly and/or indirectly via SMAD3 and that a tripartite complex of FOXL2, SMAD, and PR may be important for the synergy between activin and progestins on the murine Fshb promoter.
In summary, our experimental results provide evidence that the FOXL2 transcription factor is involved in the cooperative interaction between activin and progesterone signaling pathways in pituitary gonadotrope cells. We demonstrate that the murine Fshb promoter contains a cluster of species-specific elements critical for activin and progestin synergy that may play a crucial role in the rapid elevation in Fshb mRNA before the secondary FSH surge in rodents. These elements are conserved on the rat promoter and likely function in a similar manner. In contrast, the ovine and human FSHB promoters are not induced by progestins alone or in combination with activin, suggesting that other mechanisms regulate FSHB gene expression in these species. Our data also indicate that the cooperation between activin and progesterone signaling pathways requires the binding of FOXL2, SMAD, and PR to their respective elements on the murine Fshb promoter and suggest that physical interactions among these transcription factors may be important for the synergy. Future experiments characterizing these interactions will shed light on how synergy between activin and progesterone signaling pathways regulates reproductive fitness. Cooperation among transcription factors such as FOXL2, SMAD, and PR may also prove to be important for the regulation of gene expression in other reproductive tissues such as the ovary (60).
Supplementary Material
Acknowledgments
We thank Djurdjica Coss, Kellie Breen, and Scott Kelley for their comments, suggestions, helpful discussions, and critical reading of the manuscript. We thank Susan Mayo for technical assistance. We are grateful to Malcolm Low, Dan Bernard, Dean Edwards, Louise Bilezikjian, Djurdjica Coss, Rik Derynck, and Douglass Forbes for providing reagents. We also acknowledge using the DNA Sequencing Shared Resource, UCSD Cancer Center, funded in part by NCI Cancer Center Support Grant P30 CA023100 for sequencing.
This work was supported by National Institutes of Health (NIH) Grant K01 DK080467 and R01 HD067448 (to V.G.T.) and Eunice Kennedy Shriver National Institute of Child Health and Human Development/NIH through a cooperative agreement (U54 HD012303) as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research and R01s HD020377 and DK044838 (to P.L.M.). P.L.M. was partially supported by P30 DK063491, P30 CA023100, and P42 ES010337. J.K.S. was supported by an Endocrine Society Student Summer Research Fellowship.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- DN
- Dominant-negative
- FOXL2
- forkhead box L2
- GFP
- green fluorescent protein
- GST
- glutathione S-transferase
- HND
- Hepes/NaCl/dithiothreitol
- HRE
- hormone response element
- PR
- progesterone receptor
- SBE
- SMAD-binding element
- SMAD
- SMA/mothers against decapentaplegic homologs.
References
- 1. Apter D. 1997. Development of the hypothalamic-pituitary-ovarian axis. Ann NY Acad Sci 816:9–21 [DOI] [PubMed] [Google Scholar]
- 2. Kaiser UB, Jakubowiak A, Steinberger A, Chin WW. 1997. Differential effects of gonadotropin-releasing hormone (GnRH) pulse frequency on gonadotropin subunit and GnRH receptor messenger ribonucleic acid levels in vitro. Endocrinology 138:1224–1231 [DOI] [PubMed] [Google Scholar]
- 3. Papavasiliou SS, Zmeili S, Khoury S, Landefeld TD, Chin WW, Marshall JC. 1986. Gonadotropin-releasing hormone differentially regulates expression of the genes for luteinizing hormone α and β subunits in male rats. Proc Natl Acad Sci USA 83:4026–4029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Butcher RL, Collins WE, Fugo NW. 1974. Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17β throughout the 4-day estrous cycle of the rat. Endocrinology 94:1704–1708 [DOI] [PubMed] [Google Scholar]
- 5. Zmeili SM, Papavasiliou SS, Thorner MO, Evans WS, Marshall JC, Landefeld TD. 1986. α and luteinizing hormone β subunit messenger ribonucleic acids during the rat estrous cycle. Endocrinology 119:1867–1869 [DOI] [PubMed] [Google Scholar]
- 6. Ortolano GA, Haisenleder DJ, Dalkin AC, Iliff-Sizemore SA, Landefeld TD, Maurer RA, Marshall JC. 1988. Follicle-stimulating hormone β subunit messenger ribonucleic acid concentrations during the rat estrous cycle. Endocrinology 123:2946–2948 [DOI] [PubMed] [Google Scholar]
- 7. DePaolo LV, Hirshfield AN, Anderson LD, Barraclough CA, Channing CP. 1979. Suppression of pituitary secretion of follicle-stimulating hormone by porcine follicular fluid during pro-oestrus and oestrus in the rat: effects on gonadotrophin and steroid secretion, follicular development and ovulation during the following cycle. J Endocrinol 83:355–368 [DOI] [PubMed] [Google Scholar]
- 8. Hoak DC, Schwartz NB. 1980. Blockade of recruitment of ovarian follicles by suppression of the secondary surge of follicle-stimulating hormone with porcine follicular field. Proc Natl Acad Sci USA 77:4953–4956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ciccone NA, Kaiser UB. 2009. The biology of gonadotroph regulation. Curr Opin Endocrinol Diabetes Obes 16:321–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Breen KM, Thackray VG, Coss D, Mellon PL. 2010. Runt-related transcription factors impair activin induction of the follicle-stimulating hormone β-subunit gene. Endocrinology 151:2669–2680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haisenleder DJ, Dalkin AC, Ortolano GA, Marshall JC, Shupnik MA. 1991. A pulsatile gonadotropin-releasing hormone stimulus is required to increase transcription of the gonadotropin subunit genes: evidence for differential regulation of transcription by pulse frequency in vivo. Endocrinology 128:509–517 [DOI] [PubMed] [Google Scholar]
- 12. Ferris HA, Shupnik MA. 2006. Mechanisms for pulsatile regulation of the gonadotropin subunit genes by GnRH1. Biol Reprod 74:993–998 [DOI] [PubMed] [Google Scholar]
- 13. Woodruff TK, Besecke LM, Groome N, Draper LB, Schwartz NB, Weiss J. 1996. Inhibin A and inhibin B are inversely correlated to follicle-stimulating hormone, yet are discordant during the follicular phase of the rat estrous cycle, and inhibin A is expressed in a sexually dimorphic manner. Endocrinology 137:5463–5467 [DOI] [PubMed] [Google Scholar]
- 14. Besecke LM, Guendner MJ, Sluss PA, Polak AG, Woodruff TK, Jameson JL, Bauer-Dantoin AC, Weiss J. 1997. Pituitary follistatin regulates activin-mediated production of follicle-stimulating hormone during the rat estrous cycle. Endocrinology 138:2841–2848 [DOI] [PubMed] [Google Scholar]
- 15. Burger LL, Haisenleder DJ, Dalkin AC, Marshall JC. 2004. Regulation of gonadotropin subunit gene transcription. J Mol Endocrinol 33:559–584 [DOI] [PubMed] [Google Scholar]
- 16. Thackray VG, McGillivray SM, Mellon PL. 2006. Androgens, progestins and glucocorticoids induce follicle-stimulating hormone β-subunit gene expression at the level of the gonadotrope. Mol Endocrinol 20:2062–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weiss J, Guendner MJ, Halvorson LM, Jameson JL. 1995. Transcriptional activation of the follicle-stimulating hormone β-subunit gene by activin. Endocrinology 136:1885–1891 [DOI] [PubMed] [Google Scholar]
- 18. Attisano L, Wrana JL. 2002. Signal transduction by the TGF-β superfamily. Science 296:1646–1647 [DOI] [PubMed] [Google Scholar]
- 19. Massagué J. 1998. TGF-β signal transduction. Annu Rev Biochem 67:753–791 [DOI] [PubMed] [Google Scholar]
- 20. Shi Y, Wang YF, Jayaraman L, Yang H, Massagué J, Pavletich NP. 1998. Crystal structure of a Smad MH1 domain bound to DNA: insights on DNA binding in TGF-β signaling. Cell 94:585–594 [DOI] [PubMed] [Google Scholar]
- 21. Coss D, Mellon PL, Thackray VG. 2010. A FoxL in the Smad house: activin regulation of FSH. Trends Endocrinol Metab 21:562–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ho CC, Bernard DJ. 2010. Bone morphogenetic protein 2 acts via inhibitor of DNA binding proteins to synergistically regulate follicle-stimulating hormone β transcription with activin A. Endocrinology 151:3445–3453 [DOI] [PubMed] [Google Scholar]
- 23. Gregory SJ, Lacza CT, Detz AA, Xu S, Petrillo LA, Kaiser UB. 2005. Synergy between activin A and gonadotropin-releasing hormone in transcriptional activation of the rat follicle-stimulating hormone-β gene. Mol Endocrinol 19:237–254 [DOI] [PubMed] [Google Scholar]
- 24. Suszko MI, Balkin DM, Chen Y, Woodruff TK. 2005. Smad3 mediates activin-induced transcription of follicle-stimulating hormone β-subunit gene. Mol Endocrinol 19:1849–1858 [DOI] [PubMed] [Google Scholar]
- 25. Bernard DJ. 2004. Both SMAD2 and SMAD3 mediate activin-stimulated expression of the follicle-stimulating hormone β subunit in mouse gonadotrope cells. Mol Endocrinol 18:606–623 [DOI] [PubMed] [Google Scholar]
- 26. Bailey JS, Rave-Harel N, McGillivray SM, Coss D, Mellon PL. 2004. Activin regulation of the follicle-stimulating hormone β-subunit gene involves Smads and the TALE homeodomain proteins Pbx1 and Prep1. Mol Endocrinol 18:1158–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Suszko MI, Lo DJ, Suh H, Camper SA, Woodruff TK. 2003. Regulation of the rat follicle-stimulating hormone β-subunit promoter by activin. Mol Endocrinol 17:318–332 [DOI] [PubMed] [Google Scholar]
- 28. Lamba P, Fortin J, Tran S, Wang Y, Bernard DJ. 2009. A novel role for the forkhead transcription factor FOXL2 in activin A-regulated follicle-stimulating hormone β subunit transcription. Mol Endocrinol 23:1001–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Corpuz PS, Lindaman LL, Mellon PL, Coss D. 2010. FoxL2 is required for activin induction of the mouse and human follicle-stimulating hormone β-subunit genes. Mol Endocrinol 24:1037–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Coss D, Thackray VG, Deng CX, Mellon PL. 2005. Activin regulates luteinizing hormone β-subunit gene expression through Smad-binding and homeobox elements. Mol Endocrinol 19:2610–2623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Justice NJ, Blount AL, Pelosi E, Schlessinger D, Vale W, Bilezikjian LM. 2011. Impaired FSHβ expression in the pituitaries of Foxl2 mutant animals. Mol Endocrinol 25:1404–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Miyake T, Irahara M, Shitukawa K, Yasui T, Aono T. 1993. Interaction of activin A and gonadal steroids on FSH secretion from primary cultured rat anterior pituitary cells. Biochem Biophys Res Commun 194:413–419 [DOI] [PubMed] [Google Scholar]
- 33. Szabo M, Kilen SM, Saberi S, Ringstrom SJ, Schwartz NB. 1998. Antiprogestins suppress basal and activin-stimulated follicle-stimulating hormone secretion in an estrogen-dependent manner. Endocrinology 139:2223–2228 [DOI] [PubMed] [Google Scholar]
- 34. Bohnsack BL, Szabo M, Kilen SM, Tam DH, Schwartz NB. 2000. Follistatin suppresses steroid-enhanced follicle-stimulating hormone release in vitro in rats. Biol Reprod 62:636–641 [DOI] [PubMed] [Google Scholar]
- 35. Thackray VG, Mellon PL. 2008. Synergistic induction of follicle-stimulating hormone β-subunit gene expression by gonadal steroid hormone receptors and Smad proteins. Endocrinology 149:1091–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Strahl BD, Huang HJ, Pedersen NR, Wu JC, Ghosh BR, Miller WL. 1997. Two proximal activating protein-1-binding sites are sufficient to stimulate transcription of the ovine follicle-stimulating hormone-β gene. Endocrinology 138:2621–2631 [DOI] [PubMed] [Google Scholar]
- 37. Blount AL, Schmidt K, Justice NJ, Vale WW, Fischer WH, Bilezikjian LM. 2009. FoxL2 and Smad3 coordinately regulate follistatin gene transcription. J Biol Chem 284:7631–7645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McGillivray SM, Thackray VG, Coss D, Mellon PL. 2007. Activin and glucocorticoids synergistically activate follicle-stimulating hormone β-subunit gene expression in the immortalized LβT2 gonadotrope cell line. Endocrinology 148:762–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Graham KE, Nusser KD, Low MJ. 1999. LβT2 gonadotroph cells secrete follicle stimulating hormone (FSH) in response to activin A. J Endocrinol 162:R1–R5 [DOI] [PubMed] [Google Scholar]
- 40. Pernasetti F, Vasilyev VV, Rosenberg SB, Bailey JS, Huang HJ, Miller WL, Mellon PL. 2001. Cell-specific transcriptional regulation of FSHβ by activin and GnRH in the LβT2 pituitary gonadotrope cell model. Endocrinology 142:2284–2295 [DOI] [PubMed] [Google Scholar]
- 41. Zappavigna V, Sartori D, Mavilio F. 1994. Specificity of HOX protein function depends on DNA-protein and protein-protein interactions, both mediated by the homeo domain. Genes Dev 8:732–744 [DOI] [PubMed] [Google Scholar]
- 42. Slinker BK. 1998. The statistics of synergism. J Mol Cell Cardiol 30:723–731 [DOI] [PubMed] [Google Scholar]
- 43. Coss D, Hand CM, Yaphockun KK, Ely HA, Mellon PL. 2007. p38 mitogen-activated kinase is critical for synergistic induction of the FSHβ gene by gonadotropin-releasing hormone and activin through augmentation of c-Fos induction and Smad phosphorylation. Mol Endocrinol 21:3071–3086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Safwat N, Ninomiya-Tsuji J, Gore AJ, Miller WL. 2005. Transforming growth factor β activated kinase1 (TAK1) is a key mediator of ovine follicle stimulating hormone β subunit expression. Endocrinology 146:4814–4824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dupont J, McNeilly J, Vaiman A, Canepa S, Combarnous Y, Taragnat C. 2003. Activin signaling pathways in ovine pituitary and LβT2 gonadotrope cells. Biol Reprod 68:1877–1887 [DOI] [PubMed] [Google Scholar]
- 46. Phillips CL, Lin LW, Wu JC, Guzman K, Milsted A, Miller WL. 1988. 17 β-estradiol and progesterone inhibit transcription of the genes encoding the subunits of ovine follicle-stimulating hormone. Mol Endocrinol 2:641–649 [DOI] [PubMed] [Google Scholar]
- 47. Webster JC, Pedersen NR, Edwards DP, Beck CA, Miller WL. 1995. The 5′-flanking region of the ovine follicle-stimulating hormone-β gene contains six progesterone response elements: three proximal elements are sufficient to increase transcription in the presence of progesterone. Endocrinology 136:1049–1058 [DOI] [PubMed] [Google Scholar]
- 48. Lamba P, Santos MM, Philips DP, Bernard DJ. 2006. Acute regulation of murine follicle-stimulating hormone β subunit transcription by activin A. J Mol Endocrinol 36:201–220 [DOI] [PubMed] [Google Scholar]
- 49. Wang Y, Fortin J, Lamba P, Bonomi M, Persani L, Roberson MS, Bernard DJ. 2008. Activator protein-1 and smad proteins synergistically regulate human follicle-stimulating hormone β-promoter activity. Endocrinology 149:5577–5591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Grigorova M, Punab M, Ausmees K, Laan M. 2008. FSHB promoter polymorphism within evolutionary conserved element is associated with serum FSH level in men. Hum Reprod 23:2160–2166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tran S, Lamba P, Wang Y, Bernard DJ. 2011. SMADs and FOXL2 synergistically regulate murine FSHβ transcription via a conserved proximal promoter element. Mol Endocrinol 25:1170–1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Crisponi L, Deiana M, Loi A, Chiappe F, Uda M, Amati P, Bisceglia L, Zelante L, Nagaraja R, Porcu S, Ristaldi MS, Marzella R, Rocchi M, Nicolino M, Lienhardt-Roussie A, Nivelon A, Verloes A, Schlessinger D, Gasparini P, Bonneau D, Cao A, Pilia G. 2001. The putative forkhead transcription factor FOXL2 is mutated in blepharophimosis/ptosis/epicanthus inversus syndrome. Nat Genet 27:159–166 [DOI] [PubMed] [Google Scholar]
- 53. De Baere E, Dixon MJ, Small KW, Jabs EW, Leroy BP, Devriendt K, Gillerot Y, Mortier G, Meire F, Van Maldergem L, Courtens W, Hjalgrim H, Huang S, Liebaers I, Van Regemorter N, Touraine P, Praphanphoj V, Verloes A, Udar N, Yellore V, Chalukya M, Yelchits S, De Paepe A, Kuttenn F, Fellous M, Veitia R, Messiaen L. 2001. Spectrum of FOXL2 gene mutations in blepharophimosis-ptosis-epicanthus inversus (BPES) families demonstrates a genotype-phenotype correlation. Hum Mol Genet 10:1591–1600 [DOI] [PubMed] [Google Scholar]
- 54. Pisarska MD, Bae J, Klein C, Hsueh AJ. 2004. Forkhead l2 is expressed in the ovary and represses the promoter activity of the steroidogenic acute regulatory gene. Endocrinology 145:3424–3433 [DOI] [PubMed] [Google Scholar]
- 55. Turgeon JL, Waring DW. 1994. Activation of the progesterone receptor by the gonadotropin-releasing hormone self-priming signaling pathway. Mol Endocrinol 8:860–869 [DOI] [PubMed] [Google Scholar]
- 56. Waring DW, Turgeon JL. 1992. A pathway for luteinizing hormone releasing-hormone self-potentiation: cross-talk with the progesterone receptor. Endocrinology 130:3275–3282 [DOI] [PubMed] [Google Scholar]
- 57. Thackray VG, Hunnicutt JL, Memon AK, Ghochani Y, Mellon PL. 2009. Progesterone inhibits basal and GnRH induction of luteinizing hormone β-subunit gene expression. Endocrinology 150:2395–2403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Di Gregorio GB, Nett TM. 1995. Estradiol and progesterone influence the synthesis of gonadotropins in the absence of gonadotropin-releasing hormone in the ewe. Biol Reprod 53:166–172 [DOI] [PubMed] [Google Scholar]
- 59. Geserick C, Meyer HA, Haendler B. 2005. The role of DNA response elements as allosteric modulators of steroid receptor function. Mol Cell Endocrinol 236:1–7 [DOI] [PubMed] [Google Scholar]
- 60. Richards JS, Pangas SA. 2010. The ovary: basic biology and clinical implications. J Clin Invest 120:963–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.