Abstract
The Kiss1 gene, which encodes kisspeptin and is critical for reproduction, is sexually differentiated in the hypothalamic anteroventral periventricular (AVPV)/rostral periventricular (PeN) nuclei. Specifically, female rodents have higher AVPV/PeN Kiss1 expression than males, but how this Kiss1 sex difference is induced in early development is poorly understood. Here, we explored the contribution of epigenetic mechanisms to the establishment of the AVPV/PeN Kiss1 sex difference, focusing on histone deacetylation and DNA methylation. First, we utilized postnatal pharmacological blockade of histone deacetylation and analyzed Kiss1 expression in the AVPV/PeN. Postnatal disruption of histone deacetylase modestly increased AVPV Kiss1 cell number in both sexes but did not alter the Kiss1 sex difference. Next, we assessed whether the level of CpG methylation, which can influence transcription factor binding and gene expression, in the murine Kiss1 gene differs between males and females. We found significant sex differences in methylation at several CpG sites in the putative promoter and first intron of the Kiss1 gene in the AVPV/PeN, but not in the arcuate (which lacks adult Kiss1 sex differences), suggesting that differential methylation of the Kiss1 gene may influence sexually-dimorphic Kiss1 expression in the AVPV/PeN. Transgenic impairment of methyl CpG-binding protein-2 function did not eliminate the Kiss1 sex difference, indicating that other methylation factors are involved. Interestingly, CpG methylation in the AVPV/PeN was lower in males than females, suggesting that transcriptional repressors may contribute to the AVPV/PeN Kiss1 sex difference, a possibility supported by in silico identification of putative repressor binding sites near some of the sexually-dimorphic CpG.
The Kiss1 gene, and its neuropeptide product kisspeptin, is an important regulator of mammalian puberty and fertility. Kisspeptin directly stimulates neurons that secrete GnRH, thereby activating the reproductive axis (1–3). In rodents, Kiss1 is expressed in several discrete brain regions, including the medial amygdala (4) and two hypothalamic areas: the anatomical continuum comprising the anteroventral periventricular nucleus (AVPV) and neighboring rostral periventricular nucleus (PeN) and, more caudally, the arcuate nucleus (ARC) (5–7). In adult rodents, ARC Kiss1 neurons are inhibited by sex steroids and may mediate negative feedback effects of gonadal hormones on pulsatile GnRH secretion (1, 8, 9). In contrast, AVPV/PeN Kiss1 neurons are stimulated by sex steroids, primarily estradiol (E2), and may mediate E2's positive feedback induction of the preovulatory LH surge, a sexually-dimorphic event occurring only in females (1, 10, 11).
In rodents, the sex-specific ability of females, but not males, to display LH surges may reflect underlying sex differences in the AVPV/PeN Kiss1 population. In both rats and mice, the number of Kiss1-expressing neurons in the AVPV/PeN, as well as the amount of Kiss1 mRNA per cell in this region, is much greater in females than males (12–14). Similar female-biased sex differences have been reported for kisspeptin protein levels in the AVPV/PeN (15, 16) Thus, the ability to display E2-induced LH surges may depend, in part or completely, on the size of the AVPV/PeN kisspeptin population (17). Like many other sexually-dimorphic traits in the brain, the sex difference in AVPV/PeN Kiss1 neurons is permanently induced early in postnatal development by the actions of sex steroids, specifically by testosterone (T) signaling via estrogen receptors after its aromatization to E2 (12). In support of this, the Kiss1 sex difference can be permanently eliminated by either castrating newborn males (to remove circulating postnatal T) or treating newborn females with a single T or E2 injection (13, 15, 18).
Although sexual differentiation of the AVPV/PeN Kiss1 system is clearly governed by postnatal sex steroids, the mechanism by which this occurs is currently unknown. In mice, the overall size of the AVPV region is sexually differentiated due to Bax-dependent apoptosis (programmed cell death) (19). However, we showed that Bax-dependent apoptosis does not influence the sexual differentiation of the Kiss1 system (14), suggesting that other regulatory mechanisms underlie the development of higher Kiss1 expression in the AVPV/PeN of females than males. One possibility is that the Kiss1 sex difference is not induced solely by processes affecting the existence of Kiss1 cells (e.g. apoptosis, neurogenesis), but rather by mechanisms affecting the transcriptional activity of the Kiss1 gene (17). Epigenetic changes, such as histone modifications and DNA methylation, can be induced by sex steroids during development and have recently been implicated in the sexual differentiation of several brain and behavioral phenotypes (20–25). For example, inhibiting histone deacetylase (HDAC) during the early postnatal period in mice blocks the sexual differentiation of both the size of the bed nucleus of the stria terminalis (BNST) and also alters sexually-dimorphic adult olfactory behavior and vasopressin fiber projections (23, 24). Similar postnatal HDAC inhibition also alters sexual differentiation of male sexual behavior in rats (21). Other recent studies addressing the role of DNA methylation in brain sexual differentiation reported that the expression of DNA methyl transferase 3a in newborn rats is sexually differentiated in the amygdala (a sexually-dimorphic region) (26), and that sex differences in DNA methylation correlate with sexually differentiated expression of sex steroid receptor genes in the hypothalamus (20, 25). It is currently unknown whether epigenetic mechanisms, such as histone acetylation or DNA methylation, influence the AVPV/PeN Kiss1 sex difference.
Here, we investigated the contribution of several epigenetic mechanisms to the development of the sex difference in AVPV/PeN Kiss1expression. We focused on histone deacetylation, which serves to condense chromatin and decrease transcriptional activity, and DNA methylation, which typically blocks transcription factor binding and usually occurs at CpG dinucleotides within a gene. We used in vivo pharmacological blockade or transgenic impairment of these epigenetic processes and analyzed subsequent Kiss1 expression in the AVPV/PeN of adult mice to determine whether the Kiss1 sex difference was altered. We also combined in vivo manipulation with in vitro molecular analyses to assess whether the level of CpG methylation in the putative promoter, first intron, and CpG island of the murine Kiss1 gene differs in the AVPV/PeN between males and females, correlating with the known sex difference in AVPV/PeN Kiss1 expression.
Materials and Methods
Animals
Mice were housed at the University of California, San Diego on a 12-h light, 12-h dark cycle with food and water available ad libitum. Most experiments used C57Bl6 mice from our colony. Experiment 4 used adult B6.129S-Mecp2tm1Hzo/J and C57Bl6/J (control) mice purchased from Jackson Laboratories (Bar Harbor, ME). B6.129S-Mecp2tm1Hzo/J mice, originally created in the Zoghbi laboratory, are methyl CpG-binding protein-2 (Mecp2) mutant mice that display impaired Mecp-2 function and hyperacetylation of histone H3 (27). These mice display tremors in mid-to-late adulthood and were therefore studied at approximately 7 wk of age, before the tremor phenotype manifests. Experiments were conducted in accordance with the National Institutes of Health Animal Care and Use Guidelines and with approval of the local Animal Care and Use Committee of the University of California, San Diego.
Hormone treatments and tissue collection
Adult Kiss1 expression levels change with fluctuations in the sex steroid milieu: in the AVPV/PeN, sex steroids temporarily up-regulate Kiss1 gene expression (6, 13). To control for adulthood sex steroid levels, which may differ between individuals or sexes, E2 levels were equalized among groups using E2 implants. Adults of both sexes were anesthetized, gonadectomized (GDX), and given an sc E2-filled SILASTIC (Dow Corning Corp., Midland, MI) implant, constructed as previously described and shown to significantly regulate Kiss1 expression in the mouse brain (4, 6, 28). All mice were killed via rapid decapitation 1 wk after E2 implantation. At decapitation, brains were collected, frozen on dry ice, and stored at −80 C. Depending on the experiment, frozen brains were either micropunched for AVPV/PeN tissue collection and subsequent RNA/DNA analysis or cut on a cryostat into five series of 20-μm sections encompassing the entire AVPV/PeN region, thaw mounted onto Superfrost plus slides, and stored at −80 C until assaying by in situ hybridization (ISH).
Single-label ISH
Single-label ISH for Kiss1 was performed as previously described (7, 13, 28, 29), using a validated Kiss1 riboprobe (7). Briefly, slide-mounted AVPV/PeN sections were fixed in 4% paraformaldehyde, treated with acetic anhydride, rinsed in 2 × SSC (sodium citrate, sodium chloride), delipidated in chloroform, dehydrated in ethanols, and air dried. Radiolabeled (P33) antisense riboprobe (0.05 pmol/ml) was combined with 1/20 volume yeast tRNA, heat denatured, added to hybridization buffer, and applied to each slide (100 μl/slide). Slides were coverslipped and hybridized at 55 C for 17 h. The slides were then washed in 4 × SSC and placed into RNase A treatment for 30 min at 37 C, then in RNase-free RNase buffer at 37 C for 30 min. After washing in 2 × SSC at 22 C, slides were washed in 0.1 × SSC at 62 C, dehydrated in ethanols, and air dried. Slides were then dipped in Kodak NTB emulsion (Eastman Kodak, Rochester, NY), air dried, and stored at 4 C for 6–8 d (depending on the assay) before being developed and coverslipped. ISH slides were analyzed with an automated image-processing system and custom grain counting software (Dr. Don Clifton, University of Washington) by a person blind to the treatment group. The software counts the number of silver grain clusters representing Kiss1 cells, as well as the number of silver grains in each cell cluster (a semiquantitative index of mRNA content per cell) (30). Cells were considered Kiss1-positive when the number of silver grains in a cluster exceeded that of background by 3-fold.
RT-PCR and quantitative (real-time) PCR (qPCR)
For some experiments, total RNA was extracted from 400 μm-thick frozen micropunches (2 mm diameter) of the AVPV/PeN or lung or spleen tissue using the RNeasy Lipid Tissue Mini kit (QIAGEN, Chatsworth, CA). RNA (500 ng) was reverse transcribed using the Omniscript RT kit (QIAGEN) and cDNA stored at −20 C until use in either RT-PCR or qPCR. In experiment 4, RT-PCR was performed to assess the presence of Mecp2 mRNA in the AVPV/PeN of postnatal d 1 (PND1) males and females. Primers designed to amplify this mRNA predominantly yielded a 241-bp fragment corresponding to Mecp2: 5′-CAG CAG TGC CAG AAG CCT CG (forward) and 5′-GGG TCC AAG GAG GTG TCT CC (reverse), using a 62 C annealing temperature. In experiments 2 and 3, to detect Kiss1 in micropunch cDNA using qPCR, Kiss1-specific primers: 5′-CAA AAG TGA AGC CTG GAT CC (forward in exon 2) and 5′-GTT GTA GGT GGA CAG GTC C (reverse in exon 3) were used, as previously reported (4). qPCR was performed on each cDNA sample in duplicate using the Bio-Rad iCycler Detection System (Bio-Rad Laboratories, Inc., Hercules, CA) and Quantitect SYBR Green PCR kit. Standard curves were generated for each product using a dilution series of cloned cDNA for Kiss1 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (ranging from 10–108 copies) to quantify the abundance of cDNA in each sample. The qPCR cycling parameters were: one cycle of 95 C for 15 min, followed by 40 cycles of 94 C for 15 sec, 60 C for 30 sec, and 72 C for 30 sec. Data collection was taken at the 72 C extension phase of each cycle. To ensure the presence of a single product, a dissociation curve was performed after each run. Data were collected from threshold values using the automatic function of the Bio-Rad MyIQ software. Kiss1 values were normalized to GAPDH. A subset of cDNA samples used in qPCR was also used in RT-PCR using the same primers (both Kiss1 and GAPDH). Additionally, another Kiss1 primer set was also used in RT-PCR with the forward primer starting in the first exon of Kiss1: 5′-GAG TAA GCC CAG GAG CCA GTG and the same Kiss1 reverse primer as above, predictably yielding two variants of the Kiss1 gene due to alternative splicing of exon 2 (31).
Bisulfite treatments and pyrosequencing
Genomic DNA was isolated using the QIAGEN DNeasy Blood and Tissue Kit from 400-μm frozen micropunches of the AVPV/PeN or ARC. Bisulfite treatments were performed on 500 ng DNA using the EZ DNA Methylation Gold Kit (Zymo Research, Irvine, CA) and stored at −80 C until use. Bisulfite-treated DNA was then PCR amplified using primers designed by EpigenDX (Worcestor, MA) to isolate multiple short amplicons in three regions of the murine Kiss1 gene. The first region included the first CpG of intron 1, all of exon 1, and 938 bp upstream of transcription start site (the putative promoter) (assay numbers: ADS2132, ADS2133, ADS2134, ADS2135, ADS2136). The second region included the last 964 bp of intron 1, all of exon 2, and the first two CpG of intron 2 (assay nos.: ADS1802, ADS1813, ADS1814, ADS1815-1, ADS1815-2, ADS1816). Lastly, the third region encompassed the intron/exon junction of exon 3, including a large portion of the CpG island contained within exon 3 (414 bp) (assay numbers: ADS1817, ADS1706). PCR samples were analyzed with pyrosequencing by EpigenDX to determine methylation status of each CpG. All primer sequences are proprietary and owned by EpigenDX.
In silico analysis of putative transcription factor-binding sites
In silico analysis for identification of putative transcription factor-binding sites was conducted as previously described (32). Briefly, 20–30 bp encompassing the CpG site was entered as a search term in TFSearch v1.3 and verified with TESS. For the initial screen of transcription factors putatively binding to the input sequences, any factor for which the inputted sequence did not possess more than 60% homology to the predicted transcription factor-binding sequence was eliminated. Other parameters considered were transcription factor-binding sequences that matched a portion of the input sequence with high sequence similarity and in the correct succession of base pairs, as well as the likelihood of each particular transcription factor being expressed in mouse neurons.
Statistical analysis
All data are expressed as the mean ± sem for each group. Differences in group means were assessed via overall ANOVA or two-way ANOVA with post hoc analysis determined by Fisher's Least Significant Difference test. Statistical significance was set at P < 0.05. All analyses were performed with Statview 5.0.1 (SAS Institute, Cary, NC).
Experimental design
Experiment 1: effect of inhibition of postnatal histone deacetylase on sexually-dimorphic Kiss1 expression in the AVPV/PeN
This experiment examined whether early postnatal sex steroids sexually differentiate Kiss1 expression in the AVPV/PeN by influencing histone deacetylation; if so, then blocking postnatal histone deacetylation should alter Kiss1 sexual differentiation. Newborn C57BL6 mice of both sexes were treated on PND1 (day of birth) and PND2 with two sc injections per day (morning and evening) of valproic acid (50 mg/kg body weight; Sigma, St. Louis, MO), a histone deacetylase inhibitor (HDACi). The valproic acid dose was previously shown in mice to block the deacetylation of histone H3 in the postnatal brain and to prevent sexual differentiation of the BNST (23). Additional newborn control mice were injected sc with saline (vehicle) during the same times. At least three different litters were used for each group. The HDACi- and saline-injected pups (six to seven per group) were aged into adulthood (∼6 wk of age) and were then GDX and E2-treated for 1 wk before brain collection. Single label ISH was performed on brain slices to analyze the relative amount of Kiss1 expression per cell and the number of Kiss1 cells in both the AVPV and PeN regions in saline- or HDACi-treated males and females. To ensure that the HDACi treatment worked, an alternate set of tissue (from the same animals) encompassing the BNST was fixed in 4% paraformaldehyde and Nissl stained for 30 min using 0.5% cresyl violet acetate in 0.25% glacial acetic acid. Stained tissue was analyzed under bright-field microscopy, and BNST area was measured for each animal.
Experiment 2: CpG methylation of the Kiss1 gene in the AVPV/PeN of adult males and females
The extent of DNA methylation at CpG sites can influence transcription factor binding (activators or repressors). This experiment investigated whether differential levels of CpG methylation of the Kiss1 gene between males and females might contribute to sexually-dimorphic Kiss1 gene expression in the AVPV/PeN. Adult female and male C57BL6 mice were GDX and E2 treated for 1 wk, and their brains were collected (n = 7–11 per sex, depending on the gene region). DNA was isolated from micropunches of the AVPV/PeN and then bisulfite treated. PCR was performed on the bisulfite-treated DNA to isolate fragments covering the putative Kiss1 promoter, part of intron 1, and the intron/exon junction of exon 3, including most of the CpG island in exon 3. Pyrosequencing analysis determined the mean level of methylation at each CpG site within these three regions of the Kiss1 gene. The mean methylation status of each Kiss1 CpG site was then compared between males and females.
Experiment 3: CpG methylation of the Kiss1 gene in the ARC of adult males and females
Because experiment 2 found significant sex differences in several CpG in the AVPV/PeN Kiss1 gene, we determined whether these particular CpG were also sexually-dimorphic in the ARC Kiss1 gene, which unlike the AVPV/PeN, is not highly sexually-dimorphic in adults. DNA from micropunches of the ARC of E2-treated males and females was analyzed for methylation status of the specific sexually-dimorphic CpG from experiment 2 (3 CpG from promoter and 1 from intron 1) using the same bisulfate treatment and pyrosequencing procedure in experiment 2 (n = 7–8 per sex).
Experiment 4: involvement of Mecp2 in the sexual differentiation of AVPV/PeN Kiss1 expression
Experiment 2 suggested that factors affecting DNA methylation might influence the Kiss1 sex difference. One such factor, MeCP2, is neurally expressed, can influence gene transcription by linking DNA methylation to chromatin structure (33), and has recently been linked to sexual differentiation of the brain and behavior in rodents (34, 35). This experiment investigated whether MeCP2 activity contributes to the AVPV/PeN Kiss1 sex difference. First, we performed RT-PCR on cDNA isolated from micropunches of the AVPV/PeN of PND1 female and male C57BL6 mice (n = 2–3 per sex) to assess the presence of Mecp2 mRNA during the postnatal period (when sexual differentiation of Kiss1 occurs). Next, brains from adult Mecp2 mutant mice and age-matched C57BL6/J mice of both sexes were examined (n = 6–7 per group) for Kiss1 expression in the AVPV and PeN via ISH. As in experiment 1, all adult mice were GDX and E2 treated for 1 wk before brain collection.
Results
Experiment 1: blocking histone deacetylation during the critical period does not alter Kiss1 sexual differentiation
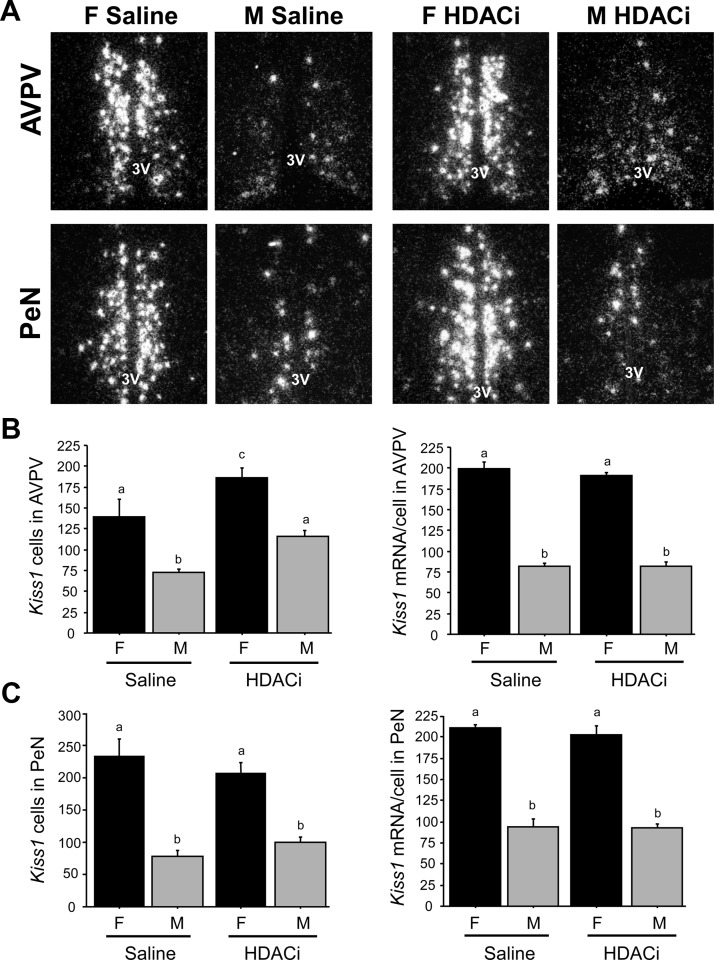
This experiment investigated whether blocking histone deacetylation with an HDACi during the first two postnatal days reduces or eliminates the AVPV/PeN Kiss1 sex difference. Adult male and female control mice that were postnatally treated with saline exhibited the predicted AVPV and PeN sex differences in Kiss1 expression, with females having more Kiss1 cells and higher Kiss1 mRNA per cell than males in both regions (P < 0.01; Fig. 1). Postnatal HDACi treatment significantly increased the number of detectable Kiss1 cells in the adult AVPV in each sex (P < 0.05 relative to saline-treated controls). Despite this increase in Kiss1 cell number, the sex difference in Kiss1 was not eliminated: HDACi-treated females still had significantly more Kiss1 neurons and higher Kiss1 mRNA per cell in the AVPV than HDACi-treated males (P < 0.01; Fig. 1). Likewise, the sex differences in Kiss1 cell number and Kiss1 mRNA/cell were still present in the PeN of HDACi-treated mice, being significantly higher in females (P < 0.001; Fig. 1). Neither the number of Kiss1 cells nor the relative amount of Kiss1 mRNA/cell in the PeN was different between saline- and HDACi-treated mice (Fig. 1).
Fig. 1.
A, Representative photomicrographs of Kiss1 ISH labeling in the AVPV and PeN of adult female (F) and male (M) mice that had been treated postnatally with either saline or an HDACi. All animals were GDX approximately 6 wk of age and treated with E2 for 1 wk before brain collection. 3V, Third ventricle. Mean number of Kiss1 cells in the AVPV (B) and PeN (C) of adult mice that were treated postnatally with saline or HDACi. Kiss1 gene expression in both the AVPV and PeN was significantly higher in females than males in both saline-treated and HDACi-treated groups. In the AVPV, there were significantly more Kiss1 cells in HDACi-treated animals compared with saline-treated animals. Different letters signify significantly different groups (P < 0.05).
To assess efficacy of the postnatal HDACi treatment, the size of the BSNT, a sexually-dimorphic area (M>F), was also examined. Vehicle-treated males had larger BNST than vehicle-treated females (0.366 ± 0.009 vs. 0.267 ± 0.003 mm2; P < 0.001), whereas this sex difference was abolished in HDACi-treated mice (0.255 ± 0.009 vs. 0.241 ± 0.021 mm2, for HDACi-treated male and females, respectively; P = 0.49).
Experiment 2: methylation status of the Kiss1 gene in the AVPV/PeN differs between males and females
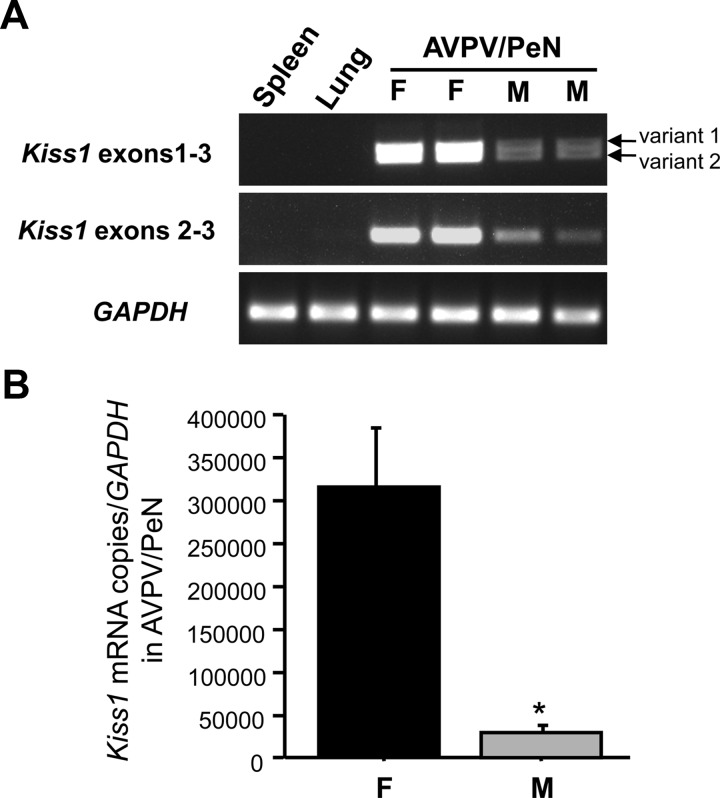
CpG methylation levels of some sexually-dimorphic genes in the brain, such as estrogen receptor α, are different between males and females (20, 25). This experiment determined whether CpG methylation of different regions of the murine Kiss1 gene in the AVPV/PeN differs between adult males and females, correlating with higher AVPV/PeN Kiss1 expression in females. First, we performed qPCR on cDNA obtained from AVPV/PeN micropunches of adult mice to confirm sex differences in Kiss1 mRNA expression in these micropunches. As expected, adult females had significantly higher Kiss1 levels in AVPV/PeN micropunches than adult males (P < 0.05; Fig. 2).
Fig. 2.
Confirmation of sexually differentiated Kiss1 mRNA expression from AVPV/PeN micropunches in adult male (M) and female (F) mice. Animals were GDX and E2 treated for 1 wk in adulthood. A, Representative ethidium bromide-stained gel showing AVPV/PeN Kiss1 expression is higher in females than males. Gapdh expression is shown as a loading control. B, Mean Kiss1 expression normalized to Gapdh in micropunches taken from the AVPV/PeN of males and females, as determined by qPCR. Females express significantly more Kiss1 mRNA from AVPV/PeN micropunches than males. *, Significantly different from females (P < 0.05).
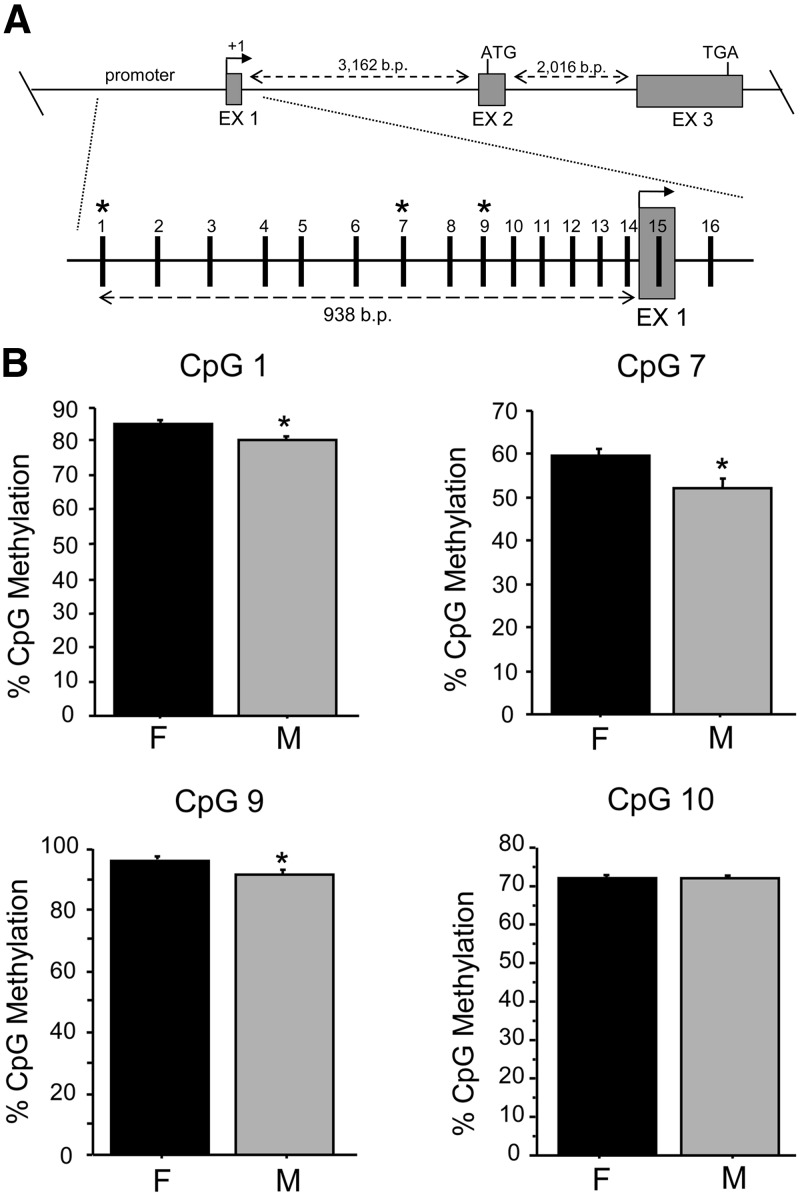
Next, we used pyrosequencing of bisulfite-treated DNA from adult male and female AVPV/PeN micropunches to quantitatively measure the percent methylation of CpG sites in several regions of the Kiss1 gene. We first measured methylation levels of 16 CpG sites in the putative Kiss1 promoter region, including those CpG sites contained in the first exon (Fig. 3). In comparing overall methylation of all 16 promoter CpGs, we detected a significant difference in percent methylation between males and females, with the female Kiss1 promoter in the AVPV/PeN being significantly more methylated than that of males (P < 0.05). Post hoc analysis of the percent methylation of specific CpG sites within the putative Kiss1 promoter identified three sexually differentiated CpGs (CpG 1, 7, and 9), with all three sites being significantly more methylated in females than males (P < 0.05; Fig. 3).
Fig. 3.
CpG methylation analysis of the putative murine Kiss1 promoter region in the AVPV/PeN. A, Map of pyrosequenced CpG sites in the putative Kiss1 promoter region using bisulfite-treated DNA derived from AVPV/PeN micropunches of adult males (M) and females (F) that were E2 treated for 1 wk before tissue collection. B, Mean percentage of methylation of sexually-dimorphic CpG (CpG 1, 7, 9) in the Kiss1 promoter, as well as mean methylation levels in a representative nonsexually-dimorphic CpG (CpG 10). *, Significantly different than females (P < 0.05). EX, Exon.
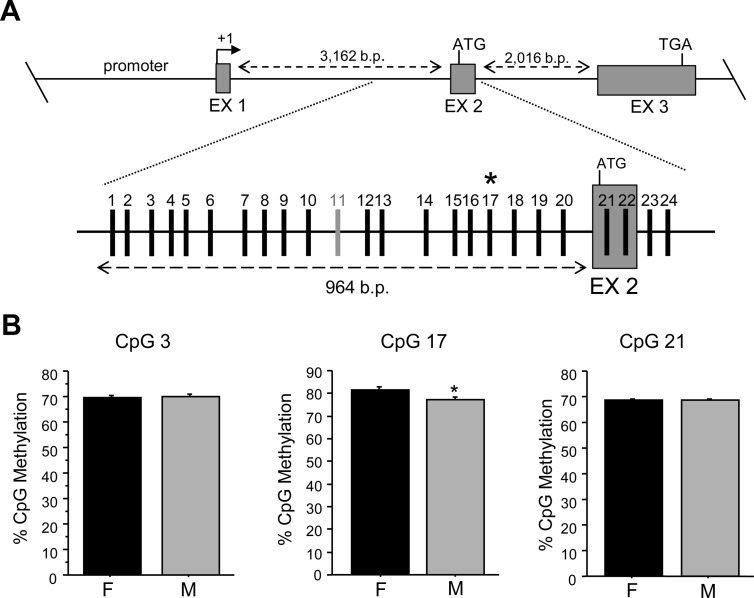
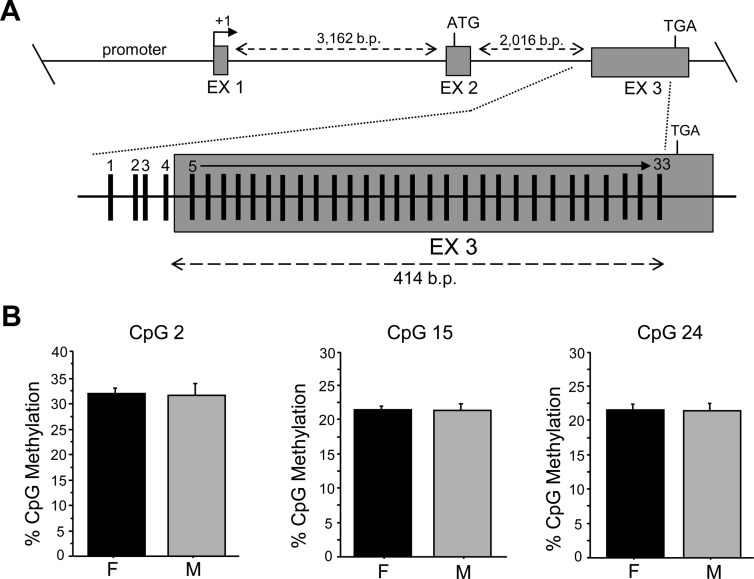
Because the first intron of a gene also commonly contains important regulatory elements (36, 37), we next analyzed the methylation status of 24 CpGs in the first intron of the Kiss1 gene, directly upstream of and including the second exon (Fig. 4). In this region of the Kiss1 gene, we found one sexually differentiated site, CpG 17, which, similar to the sexually-dimorphic CpG in the promoter region, was significantly more methylated in females; P < 0.05) (Fig. 4). We also assessed methylation levels in the intron/exon junction of the third exon of Kiss1, which contains a large CpG island. Interestingly, of the 33 CpG sites analyzed in this region, no significant sex differences were observed in percent methylation (Fig. 5).
Fig. 4.
CpG methylation analysis of the first Kiss1 intron in the AVPV/PeN of mice that were GDX and E2 treated in adulthood before brain collection. A, Map of pyrosequenced CpG sites in intron 1 using bisulfite-treated DNA derived from AVPV/PeN micropunches. CpG 11 (light gray dash) was not included in analysis due to assay limitations. B, Of the CpG in the intron 1 region that were analyzed, only CpG 17 was found to be sexually dimorphic in the level of methylation, with males (M) being less methylated than females (F). For comparison, several other nonsexually-dimorphic CpG from flanking areas are shown. *, Significantly different from female (P < 0.05). EX, Exon.
Fig. 5.
CpG methylation analysis of exon 3 CpG island in the AVPV/PeN of mice that were GDX and E2 treated in adulthood before brain collection. A, Map of pyrosequenced CpG sites in exon 3 using bisulfite-treated DNA derived from AVPV/PeN micropunches. B, Mean CpG methylation percentage of representative CpG across the CpG island in exon 3. No significant sex differences in CpG methylation were detected in this region of the Kiss1 gene. EX, Exon; F, female; M, male.
Because there were several Kiss1 CpG sites that were sexually dimorphic in methylation status, in silico analysis was used to identify candidate transcription factors that might act at these sexually-dimorphic CpGs. Putative binding sites for several regulatory factors, both activators and repressors, were identified for all four sexually-dimorphic CpG sites (Table 1).
Table 1.
In silico analysis of putative transcription factor sites at sexually differentiated CpGs in the Kiss1 gene.
| Kiss1 CpG site | Putative factor | Known function(s) | References |
|---|---|---|---|
| Promoter | |||
| CpG 1 | Gfi-1 | Repressor | 42–44 |
| Cdx-1 | Repressor/activator | ||
| CpG 7 | ERE | Mainly activator | 45–47 |
| SRY | Activator/repressor | ||
| CREB/CRE-BP | Activators | ||
| CpG 9 | MZF-1 | Repressor | 48, 49 |
| Intron 1 | |||
| CpG 17 | Nkx-2 | Involved in neural development | 49, 50 |
| CdxA | Activator | ||
| MZF1 | Repressor |
Experiment 3: methylation status of Kiss1 CpG sites in the ARC is not sexually dimorphic
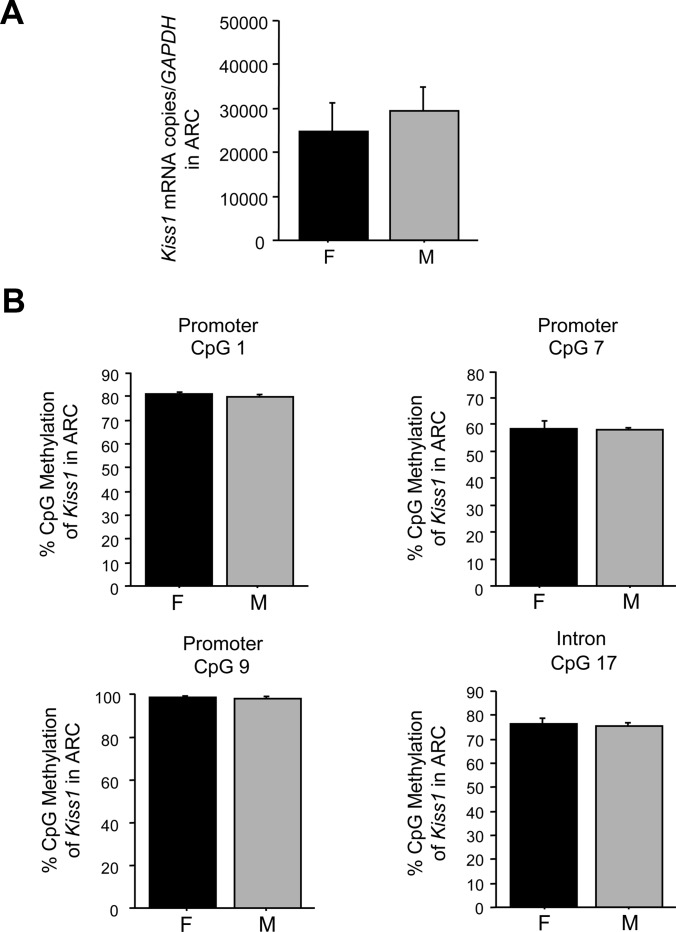
Because we found significant sex differences in several CpGs in the AVPV/PeN Kiss1 gene, we determined whether these particular Kiss1 CpGs are also sexually dimorphic in the ARC, which unlike the AVPV/PeN, is not sexually dimorphic in Kiss1 expression in adults. Of the four sexually-dimorphic CpGs identified in the AVPV/PeN Kiss1 gene (CpG 1, 7, 9 in the promoter; CpG 17 in intron 1), none were sexually dimorphic in the ARC (Fig. 6). Similarly, there were no methylation sex differences in several other nearby CpG in the ARC Kiss1 gene that were included in our analysis (data not shown). Thus, the sex difference in methylation status of the Kiss1 gene appears specific to the AVPV/PeN.
Fig. 6.
CpG methylation analysis of Kiss1 CpG in the ARC of mice that were GDX and E2 treated in adulthood before brain collection. A, Mean Kiss1 expression normalized to Gapdh as determined by qPCR of micropunches taken from the ARC of males (M) and females (F). No sex difference in Kiss1 gene expression was detected in the ARC micropunches. B, Mean CpG methylation levels in the ARC Kiss1 gene of four CpG that were found to be sexually dimorphic in the AVPV/PeN. Unlike in the AVPV/PeN, no significant sex differences were detected in the Kiss1 methylation levels of any of the four CpG in the ARC region.
Experiment 4: MeCP2 is not required for the sexual differentiation of AVPV/PeN Kiss1 gene expression
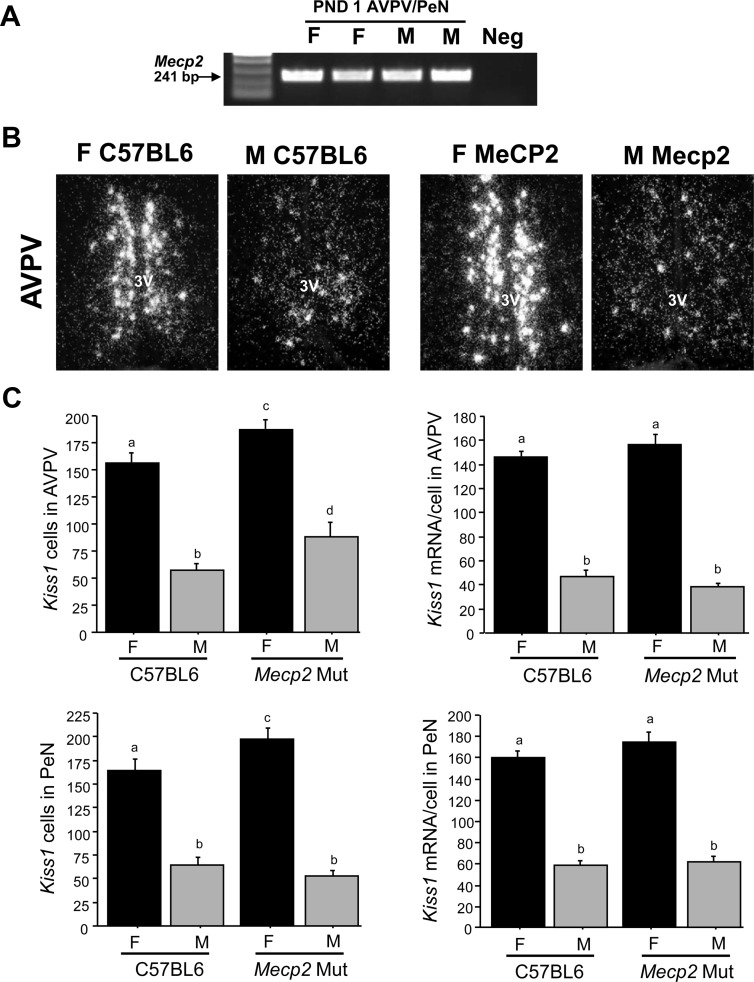
This experiment investigated the possible involvement of MeCP2, which can bind to methylated DNA and repress gene expression, in the sexual differentiation of the AVPV/PeN Kiss1 system. First, we used RT-PCR to confirm that Mecp2 mRNA is expressed in the AVPV/PeN of newborn males and females, when sex steroids sexually differentiate Kiss1 (Fig. 7A). We next assessed Kiss1 levels in the AVPV/PeN of adult transgenic mice harboring a mutated Mecp2 gene. As expected, control C57Bl6/J females had significantly more Kiss1 neurons and greater Kiss1 mRNA per cell in both the AVPV and PeN than control C57Bl6/J males (P < 0.001; Fig. 7, B and C). Interestingly, the Kiss1 sex difference was maintained in the absence of functional MeCP2: Mecp2 mutant female mice had significantly greater AVPV and PeN Kiss1 cell numbers, along with greater Kiss1 mRNA/cell, than Mecp2 mutant males (P < 0.001; Fig. 7C). Similar to HDACi treatment in experiment 1, there were significantly more Kiss1 cells in the AVPV between Mecp2 mutant mice and C57Bl6/J controls (P < 0.05; Fig. 7C), as well as in the PeN (P < 0.05; Fig. 7C). Levels of Kiss1 mRNA per cell in the AVPV and PeN were not different between genotypes (Fig. 7).
Fig. 7.
A, Image of ethidium bromide-stained gel of RT-PCR showing Mecp2 expression in the AVPV/PeN of PND1 male and female mice. B, Representative photomicrographs of Kiss1 ISH labeling in the AVPV and PeN of adult female (F) and male (M) C57BL6 or Mecp2 mutant (Mut) mice. All animals were GDX at approximately 6 wk of age and treated with E2 for 1 wk before brain collection. 3V, Third ventricle. C, Mean number of Kiss1 cells in the AVPV and PeN of adult C57BL6 controls or Mecp2 mutant (Mut) mice. Kiss1 gene expression in both regions was significantly higher in females than males in both genotypes. There were significantly more AVPV Kiss1cells in mutant mice than wild-type mice. Different letters signify significantly different groups (P < 0.05). neg, Negative control.
Discussion
The sexual differentiation of Kiss1 neurons in the AVPV/PeN of rodents may underlie several aspects of sexually-dimorphic neuroendocrine physiology, including the preovulatory LH surge (positive feedback) and, perhaps, puberty onset. However, despite evidence that the early postnatal sex steroid milieu permanently establishes the sex difference in AVPV/PeN Kiss1 expression, the specific mechanisms underlying these effects of postnatal sex steroids are unknown. Here, we provide new evidence about the contributions of histone deacetylation and DNA methylation to the AVPV/PeN Kiss1 sex difference. Although blocking histone deacetylation during the early postnatal period caused a significant increase in detectable AVPV Kiss1 cells in adult mice of both sexes, this impairment of postnatal histone deacetylation ultimately did not affect the Kiss1 sex difference. On the other hand, DNA methylation levels in the putative Kiss1 promoter region and first intron were found to be sexually dimorphic in the AVPV/PeN of adult mice, with several CpG sites being more highly methylated in females than males. Importantly, these AVPV/PeN sex differences in Kiss1 methylation were not observed in the ARC, which is not sexually dimorphic for Kiss1 cell number in adulthood. Thus, DNA methylation processes may contribute, fully or in part, to sexually-dimorphic Kiss1 expression in the AVPV/PeN, whereas histone deacetylation of histone H3 does not. Finally, because transgenic impairment of MeCP2 action did not alter the Kiss1 sex difference, other non-MeCP2-dependent processes involved in DNA methylation likely influence Kiss1 sex differences.
We recently reported that Bax-dependent apoptosis, the main mechanism for programmed neuronal death, does not influence the sexual differentiation of AVPV/PeN Kiss1 neurons (14). Thus, the Kiss1 sex difference may not reflect differences in absolute number of Kiss1 cells, but rather, differences in Kiss1 gene transcription between males and females, with higher transcription in female Kiss1 neurons. If so, epigenetic processes that affect gene transcription might contribute, fully or in part, to the AVPV/PeN Kiss1 sex difference, as is hypothesized for several other sexually-dimorphic brain phenotypes (22). Because histone H3 deacetylation reduces gene expression, we first hypothesized that the Kiss1 chromatin complex in males experiences higher histone deacetylation, resulting in decreased Kiss1 expression. If so, inhibiting histone deacetylase in males might increase Kiss1 transcriptional activation, resulting in elevated, female-like Kiss1 levels. However, we found that blocking histone deacetylase just after birth, when sexual differentiation of Kiss1 normally occurs, does not eliminate the Kiss1 sex difference. Thus, the AVPV/PeN Kiss1 sex difference, unlike the sex difference in BNST size and cell number (Ref. 23, and present results), is not influenced by histone acetylation/deactylation. We did find, however, that postnatal HDACi treatment moderately increased the number of detectable AVPV Kiss1 neurons in both sexes, indicating our HDACi treatment has some effect on the Kiss1 system. This increase in AVPV Kiss1 cell number in HDACi-treated mice may reflect alterations in apoptosis pathways because this pattern mimics the previously observed increase in AVPV Kiss1 cells in Bax KO mice (14). Conversely, it is possible that HDACi treatment activated gene expression in a general manner in AVPV/PeN cells that, under normal conditions, would not significantly express Kiss1.
One way in which DNA methylation can regulate gene expression is by inhibiting the ability of transcription factors to bind and either activate or repress gene transcription. We found significant sex differences in the CpG methylation of the AVPV/PeN Kiss1 gene, predominantly in the putative promoter region. In all cases, these sexually-dimorphic CpG sites were more methylated in females than males. Although the magnitude of these methylation differences were small to moderate, previous studies have also reported methylation differences of small magnitude, which have been proposed to relate to known sex differences in neural gene expression (20, 25). Moreover, because our DNA samples were obtained from AVPV/PeN micropunches, some other nonKiss1 cells in this region, in addition to Kiss1 neurons, were also likely included, thereby diluting the Kiss1 neurons in the samples. Based on this, it is likely that the sex differences in Kiss1 methylation levels are even more robust and of greater magnitude than what we actually detected. Importantly, the four Kiss1 CpGs that were sexually dimorphic in the AVPV/PeN were not sexually dimorphic in the ARC, matching the lack of Kiss1 sex differences in the latter nucleus and suggesting regional specificity in the methylation regulation of Kiss1.
Generally, higher DNA methylation levels are associated with suppressed gene expression due, in part, to DNA methylation reducing the binding of transcriptional activators. It was therefore initially surprising that sexually-dimorphic CpG sites of the Kiss1 gene were more methylated in females than in males, despite higher Kiss1 expression in females. However, although DNA methylation often reduces activator binding, DNA methylation can also serve to block binding of transcriptional repressors (38–40). Thus, we speculate that higher Kiss1 methylation levels in females may contribute to decreased repressor binding and activity, ultimately leading to higher Kiss1 expression. Conversely, lower Kiss1 methylation levels in males may allow for higher binding of transcriptional repressors, thereby decreasing Kiss1 expression. This possibility is supported by recent evidence showing that the human Kiss1 gene is regulated by several transcriptional repressors (41). To begin to assess this possibility in mice, we performed in silico analysis to identify candidate transcription factors, including repressors, that might act at or near the four sexually-dimorphic CpGs in the Kiss1 gene (Table 1). For the three sexually-dimorphic CpG in the Kiss1 promoter (CpG 1, 7, 9), we found binding sites for several possible regulatory factors, including several repressors: near CpG 1 are putative transcription factor-binding sites for the repressor Gfi-1 (42) and Cdx1, which can act as a repressor for some genes (43, 44). Between CpG 6 and 7, there are putative estrogen response elements as well as an SRY-binding site, and CpG 7 sits directly in putative cAMP response element binding protein- or cAMP-response element-binding protein-binding sites (usually associated with activation) (45–47). CpG 9 is located within a binding site for MZF1, which also acts as a repressor (48, 49). Finally, near CpG 17 in intron 1, which was also more methylated in females, are binding sites for Nkx-2, which is involved in neural development and morphogenesis (50), as well as MZF1, which, as stated above, is a transcriptional repressor, especially in introns (49). The actual involvement of any of these particular transcription factors in the development and sexual differentiation of the Kiss1 gene will be the focus of future investigations.
The Kiss1 gene also contains an intragenic CpG island spanning a large portion of the third exon. Of the 33 CpG sites examined within this CpG island, there were no significant methylation sex differences. Although recent work has indicated that intragenic CpG islands contain features of promoters (51), methylation of the Kiss1 CpG island does not appear to relate to sexually differentiated Kiss1 expression. Although we examined methylation within the first approximately 1 kb of the putative Kiss1 promoter, primarily because this is where most transcriptional regulation would likely occur (52), we cannot rule out that additional CpG further upstream, either in the promoter or as-yet-unidentified enhancer regions (53), may also contribute to sexually-dimorphic Kiss1 gene expression, along with one or more of the sexually-dimorphic methylated CpG we identified in the present study.
Similar to our HDACi findings and previous Bax KO results (14), we found that sexual differentiation of AVPV/PeN Kiss1 expression was unaffected by impairments in MeCP2 function. MeCP2 can bind to methylated DNA and decrease gene transcription by either condensing the chromatin, interacting with other proteins to form inhibitory complexes, or directly blocking transcription factors (27, 33, 54). Impairments in MeCP2 function could therefore lead to increased gene expression via multiple mechanisms. Despite this, Mecp2 mutant mice still exhibited normal female-biased sex differences in AVPV/PeN Kiss1 expression. However, MeCP2 is only one of many factors that can participate in DNA methylation processes, and a lack of MeCP2 effect does not contradict or negate our findings of differential Kiss1 CpG methylation. Future experiments will be aimed at studying the potential involvement of other methylation-related processes and factors.
In summary, we provide evidence indicating that although histone acetylation contributes to the overall development of AVPV Kiss1 neuron number in both sexes, this epigenetic process likely does not regulate the sexual differentiation of the Kiss1 neurons. However, DNA methylation in the putative Kiss1 promoter region (and first intron) correlates with the sex difference in Kiss1 expression exclusively in the AVPV/PeN, and not the ARC, and may be linked to regulating sexually-dimorphic Kiss1 expression. The consistent presence of higher Kiss1 methylation levels in males than females suggests that differential binding of transcriptional repressors may contribute to the sex difference in Kiss1 expression, a possibility supported by repressor-binding sites at sexually-dimorphic CpG. This contribution of DNA methylation to sexually-dimorphic Kiss1 gene expression may be just one aspect of a complicated system involving multiple types of epigenetic interactions that ultimately determine Kiss1levels. Collectively, these findings provide new insight into possible contributions of epigenetic mechanisms to the AVPV/PeN Kiss1 sex difference and perhaps other sexually differentiated populations.
Acknowledgments
This research was supported by National Institutes of Health Grants R01 HD065856 and F32 HD066849. Additional support was provided by the National Institute of Child Health and Human Development through cooperative agreement U54 HD012303, as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AVPV
- Anteroventral periventricular nucleus
- ARC
- arcuate nucleus
- BNST
- bed nucleus of the stria terminalis
- E2
- estradiol
- GAPDH
- glyceraldehyde 3-phosphate dehydrogenase
- GDX
- gonadectomized
- HDAC
- histone deacetylase
- HDACi
- histone deacetylase inhibitor
- ISH
- in situ hybridization
- MeCP2
- methyl CpG-binding protein-2
- PeN
- periventricular nuclei
- PND
- postnatal day
- qPCR
- quantitative (real-time) PCR
- SSC
- sodium citrate, sodium chloride
- T
- testosterone.
References
- 1. Kauffman AS. 2010. Coming of age in the kisspeptin era: sex differences, development, and puberty. Mol Cell Endocrinol 324:51–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. 2003. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA 100:10972–10976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. 2003. The GPR54 gene as a regulator of puberty. N Engl J Med 349:1614–1627 [DOI] [PubMed] [Google Scholar]
- 4. Kim J, Semaan SJ, Clifton DK, Steiner RA, Dhamija S, Kauffman AS. 2011. Regulation of Kiss1 expression by sex steroids in the amygdala of the rat and mouse. Endocrinology 152:2020–2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kauffman AS, Park JH, McPhie-Lalmansingh AA, Gottsch ML, Bodo C, Hohmann JG, Pavlova MN, Rohde AD, Clifton DK, Steiner RA, Rissman EF. 2007. The kisspeptin receptor GPR54 is required for sexual differentiation of the brain and behavior. J Neurosci 27:8826–8835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. 2005. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 146:3686–3692 [DOI] [PubMed] [Google Scholar]
- 7. Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. 2004. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology 145:4073–4077 [DOI] [PubMed] [Google Scholar]
- 8. Oakley AE, Clifton DK, Steiner RA. 2009. Kisspeptin signaling in the brain. Endocr Rev 30:713–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Smith JT. 2009. Sex steroid control of hypothalamic Kiss1 expression in sheep and rodents: comparative aspects. Peptides 30:94–102 [DOI] [PubMed] [Google Scholar]
- 10. Khan AR, Kauffman AS. 2012. The role of kisspeptin and RFRP-3 neurons in the circadian-timed preovulatory luteinizing hormone surge. J Neuroendocrinol 24:131–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Herbison AE. 2008. Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: the case for the rostral periventricular area of the third ventricle (RP3V). Brain Res Rev 57:277–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kauffman AS, Navarro VM, Kim J, Clifton DK, Steiner RA. 2009. Sex differences in the regulation of Kiss1/NKB neurons in juvenile mice: implications for the timing of puberty. Am J Physiol Endocrinol Metab 297:E1212–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown A, Clifton DK, Hoffman GE, Steiner RA, Tena-Sempere M. 2007. Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology 148:1774–1783 [DOI] [PubMed] [Google Scholar]
- 14. Semaan SJ, Murray EK, Poling MC, Dhamija S, Forger NG, Kauffman AS. 2010. BAX-dependent and BAX-independent regulation of Kiss1 neuron development in mice. Endocrinology 151:5807–5817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Homma T, Sakakibara M, Yamada S, Kinoshita M, Iwata K, Tomikawa J, Kanazawa T, Matsui H, Takatsu Y, Ohtaki T, Matsumoto H, Uenoyama Y, Maeda K, Tsukamura H. 2009. Significance of neonatal testicular sex steroids to defeminize anteroventral periventricular kisspeptin neurons and the GnRH/LH surge system in male rats. Biol Reprod 81:1216–1225 [DOI] [PubMed] [Google Scholar]
- 16. Clarkson J, Herbison AE. 2006. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology 147:5817–5825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Semaan SJ, Kauffman AS. 2010. Sexual differentiation and development of forebrain reproductive circuits. Curr Opin Neurobiol 20:424–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bateman HL, Patisaul HB. 2008. Disrupted female reproductive physiology following neonatal exposure to phytoestrogens or estrogen specific ligands is associated with decreased GnRH activation and kisspeptin fiber density in the hypothalamus. Neurotoxicology 29:988–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Forger NG, Rosen GJ, Waters EM, Jacob D, Simerly RB, de Vries GJ. 2004. Deletion of Bax eliminates sex differences in the mouse forebrain. Proc Natl Acad Sci USA 101:13666–13671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kurian JR, Olesen KM, Auger AP. 2010. Sex differences in epigenetic regulation of the estrogen receptor-α promoter within the developing preoptic area. Endocrinology 151:2297–2305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Matsuda KI, Mori H, Nugent BM, Pfaff DW, McCarthy MM, Kawata M. 2011. Histone deacetylation during brain development is essential for permanent masculinization of sexual behavior. Endocrinology 152:2760–2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McCarthy MM, Auger AP, Bale TL, De Vries GJ, Dunn GA, Forger NG, Murray EK, Nugent BM, Schwarz JM, Wilson ME. 2009. The epigenetics of sex differences in the brain. J Neurosci 29:12815–12823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Murray EK, Hien A, de Vries GJ, Forger NG. 2009. Epigenetic control of sexual differentiation of the bed nucleus of the stria terminalis. Endocrinology 150:4241–4247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Murray EK, Varnum MM, Fernandez JL, de Vries GJ, Forger NG. 2011. Effects of neonatal treatment with valproic acid on vasopressin immunoreactivity and olfactory behaviour in mice. J Neuroendocrinol 23:906–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schwarz JM, Nugent BM, McCarthy MM. 2010. Developmental and hormone-induced epigenetic changes to estrogen and progesterone receptor genes in brain are dynamic across the life span. Endocrinology 151:4871–4881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kolodkin MH, Auger AP. 2011. Sex difference in the expression of DNA methyltransferase 3a in the rat amygdala during development. J Neuroendocrinol 23:577–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shahbazian M, Young J, Yuva-Paylor L, Spencer C, Antalffy B, Noebels J, Armstrong D, Paylor R, Zoghbi H. 2002. Mice with truncated MeCP2 recapitulate many Rett syndrome features and display hyperacetylation of histone H3. Neuron 35:243–254 [DOI] [PubMed] [Google Scholar]
- 28. Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA. 2005. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology 146:2976–2984 [DOI] [PubMed] [Google Scholar]
- 29. Robertson JL, Clifton DK, de la Iglesia HO, Steiner RA, Kauffman AS. 2009. Circadian regulation of Kiss1 neurons: implications for timing the preovulatory gonadotropin-releasing hormone/luteinizing hormone surge. Endocrinology 150:3664–3671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chowen JA, Argente J, Vician L, Clifton DK, Steiner RA. 1990. Pro-opiomelanocortin messenger RNA in hypothalamic neurons is increased by testosterone through aromatization to estradiol. Neuroendocrinology 52:581–588 [DOI] [PubMed] [Google Scholar]
- 31. Lapatto R, Pallais JC, Zhang D, Chan YM, Mahan A, Cerrato F, Le WW, Hoffman GE, Seminara SB. 2007. Kiss1−/− mice exhibit more variable hypogonadism than Gpr54−/− mice. Endocrinology 148:4927–4936 [DOI] [PubMed] [Google Scholar]
- 32. Semaan SJ, Li Y, Nickells RW. 2010. A single nucleotide polymorphism in the Bax gene promoter affects transcription and influences retinal ganglion cell death. ASN Neuro 2:e00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Guy J, Cheval H, Selfridge J, Bird A. 2011. The role of MeCP2 in the brain. Annu Rev Cell Dev Biol 27:631–652 [DOI] [PubMed] [Google Scholar]
- 34. Kurian JR, Forbes-Lorman RM, Auger AP. 2007. Sex difference in mecp2 expression during a critical period of rat brain development. Epigenetics 2:173–178 [DOI] [PubMed] [Google Scholar]
- 35. Kurian JR, Bychowski ME, Forbes-Lorman RM, Auger CJ, Auger AP. 2008. Mecp2 organizes juvenile social behavior in a sex-specific manner. J Neurosci 28:7137–7142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Melkonyan H, Hofmann HA, Nacken W, Sorg C, Klempt M. 1998. The gene encoding the myeloid-related protein 14 (MRP14), a calcium-binding protein expressed in granulocytes and monocytes, contains a potent enhancer element in the first intron. J Biol Chem 273:27026–27032 [DOI] [PubMed] [Google Scholar]
- 37. Charron M, Chern JY, Wright WW. 2007. The Cathepsin L first intron stimulates gene expression in rat Sertoli cells. Biol Reprod 76:813–824 [DOI] [PubMed] [Google Scholar]
- 38. Eden S, Constancia M, Hashimshony T, Dean W, Goldstein B, Johnson AC, Keshet I, Reik W, Cedar H. 2001. An upstream repressor element plays a role in Igf2 imprinting. EMBO J 20:3518–3525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jones PA, Takai D. 2001. The role of DNA methylation in mammalian epigenetics. Science 293:1068–1070 [DOI] [PubMed] [Google Scholar]
- 40. Nugent BM, McCarthy MM. 2011. Epigenetic underpinnings of developmental sex differences in the brain. Neuroendocrinology 93:150–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mueller JK, Dietzel A, Lomniczi A, Loche A, Tefs K, Kiess W, Danne T, Ojeda SR, Heger S. 2011. Transcriptional regulation of the human KiSS1 gene. Mol Cell Endocrinol 342:8–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wallis D, Hamblen M, Zhou Y, Venken KJ, Schumacher A, Grimes HL, Zoghbi HY, Orkin SH, Bellen HJ. 2003. The zinc finger transcription factor Gfi1, implicated in lymphomagenesis, is required for inner ear hair cell differentiation and survival. Development 130:221–232 [DOI] [PubMed] [Google Scholar]
- 43. Rath B, Pandey RS, Debata PR, Maruyama N, Supakar PC. 2008. Molecular characterization of senescence marker protein-30 gene promoter: identification of repressor elements and functional nuclear factor binding sites. BMC Mol Biol 9:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Guo RJ, Huang E, Ezaki T, Patel N, Sinclair K, Wu J, Klein P, Suh ER, Lynch JP. 2004. Cdx1 inhibits human colon cancer cell proliferation by reducing Î2-Catenin/T-cell factor transcriptional activity. J Biol Chem 279:36865–36875 [DOI] [PubMed] [Google Scholar]
- 45. Montminy MR, Sevarino KA, Wagner JA, Mandel G, Goodman RH. 1986. Identification of a cyclic-AMP-responsive element within the rat somatostatin gene. Proc Natl Acad Sci USA 83:6682–6686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yamamoto KK, Gonzalez GA, Biggs III WH, Montminy MR. 1988. Phosphorylation-induced binding and transcriptional efficacy of nuclear factor CREB. Nature 334:494–498 [DOI] [PubMed] [Google Scholar]
- 47. Shaywitz AJ, Greenberg ME. 1999. CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem 68:821–861 [DOI] [PubMed] [Google Scholar]
- 48. Morris JF, Rauscher III FJ, Davis B, Klemsz M, Xu D, Tenen D, Hromas R. 1995. The myeloid zinc finger gene, MZF-1, regulates the CD34 promoter in vitro. Blood 86:3640–3647 [PubMed] [Google Scholar]
- 49. Takahashi K, Nishiyama C, Hasegawa M, Akizawa Y, Ra C. 2003. Regulation of the human high affinity IgE receptor Î2-chain gene expression via an intronic element. J Immunol 171:2478–2484 [DOI] [PubMed] [Google Scholar]
- 50. Price M, Lazzaro D, Pohl T, Mattei MG, Rüther U, Olivo JC, Duboule D, Di Lauro R. 1992. Regional expression of the homeobox gene Nkx-2.2 in the developing mammalian forebrain. Neuron 8:241–255 [DOI] [PubMed] [Google Scholar]
- 51. Maunakea AK, Nagarajan RP, Bilenky M, Ballinger TJ, D'Souza C, Fouse SD, Johnson BE, Hong C, Nielsen C, Zhao Y, Turecki G, Delaney A, Varhol R, Thiessen N, Shchors K, Heine VM, Rowitch DH, Xing X, Fiore C, Schillebeeckx M, Jones SJ, Haussler D, Marra MA, Hirst M, Wang T, Costello JF. 2010. Conserved role of intragenic DNA methylation in regulating alternative promoters. Nature 466:253–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Maston GA, Evans SK, Green MR. 2006. Transcriptional regulatory elements in the human genome. Annu Rev Genomics Hum Genet 7:29–59 [DOI] [PubMed] [Google Scholar]
- 53. Ong CT, Corces VG. 2011. Enhancer function: new insights into the regulation of tissue-specific gene expression. Nat Rev Genet 12:283–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Skene PJ, Illingworth RS, Webb S, Kerr AR, James KD, Turner DJ, Andrews R, Bird AP. 2010. Neuronal MeCP2 is expressed at near histone-octamer levels and globally alters the chromatin state. Mol Cell 37:457–468 [DOI] [PMC free article] [PubMed] [Google Scholar]