Female mice, like premenopausal women, are generally protected from the insulin resistance and inflammation associated with obesity. With aging and menopause, levels of estrogens fall and women tend to gain body fat and become as susceptible as men to obesity-related metabolic diseases. In male mice, high-saturated-fat diets cause obesity, systemic insulin resistance, and adipose tissue inflammation, especially in gonadal fat depots where adipocyte death is associated with macrophage infiltration and activation. Female mice also gain body fat on high-fat diets, although they exhibit much lower levels of adipose inflammation and insulin resistance (1, 2). This sex difference is not likely entirely attributable to estrogens because high-fat feeding of ovariectomized female mice does not increase adipose death to levels seen in high-fat-fed males (1). Over and above female sex, a lower-body or gynoid fat distribution further protects women from metabolic disease (3). Thus, mechanisms linking the biology of specific fat depots to sex differences in metabolic risk are of great interest for translational research.
Lipocalin 2 (Lcn2), also known as 24p3 in mouse and neutrophil gelatinase-associated lipocalin (NGAL in humans), is a small secreted protein that binds a variety of hydrophobic ligands including retinoids, fatty acids, prostaglandins, various steroids, and bacterial siderophores. It is expressed in many tissues and has a number of roles, including apoptosis, cancer, inflammation, iron homeostasis, and innate immunity (4).
Lcn2 was fairly recently recognized as an adipokine that is secreted from adipose tissue of both mice and humans (5). Adipose Lcn2 expression is up-regulated in various models of mouse obesity and insulin resistance and in visceral compared with sc adipose tissue of obese humans. Addition of exogenous Lcn2 to 3T3-L1 adipocytes causes insulin resistance (6). Thus, several groups were motivated to investigate obesity-related phenotypes of Lcn2 knockout mice. Initial reports focused on Lcn2-null male mice and found discrepant results: either protecting from (7) or potentiating (5) high-fat-induced obesity and associated insulin resistance. However, a recent publication (8) that studied Lcn2−/− mice derived from the same strain of mice as Guo et al. (9) found no phenotype in female mice on chow or high-fat diets and only a limited phenotype in males on a high-fat diet.
In this issue of Endocrinology, Guo and colleagues (9) provide evidence for depot differences in Lcn2 expression in female mice and evidence for a potentially novel role of Lcn2 in modulating production and action of estrogens. They found that Lcn2 is expressed at much higher levels in inguinal sc than gonadal adipose tissue of females and is highly up-regulated in the inguinal depot of females compared with males. Inguinal Lcn2 protein increased further with aging and high-fat diet-induced obesity in wild-type females. Like the males they previously studied (5), female Lcn2−/− mice gained more body weight and fat pad weight on a high-fat diet. Knowing that Lcn2 expression is regulated by estrogens and conversely that Lcn2 can modulate estrogen receptor-α expression, the authors investigated these relationships in the context of aging and diet-induced obesity.
The data presented by Guo et al. (9) indicate that Lcn2 deficiency leads to decreases in serum estradiol. They also found lower estrogen receptor-α expression in gonadal adipocytes and stromal cells that contribute to the development of obesity and dysregulated lipid metabolism. Consistent with their hypothesis that Lcn2 regulates estrogens and their actions, the Lcn2-deficient female mice displayed lower expression of key transcription factors and genes involved in lipid metabolism (protein levels of peroxisome proliferator-activated receptor gamma, liver-x-receptor beta, and low density lipoprotein receptor protein levels, and the mRNA levels of Srebf1c, Scd1, Cd36, LPL, and Adipoq) in gonadal adipose tissue (inguinal was not studied). Consistent with this finding, 17β-estradiol increased LXRβ expression was significantly lower in bone marrow-derived macrophages from the knockouts. These data suggest that Lcn2 deficiency causes dysregulated systemic and adipose lipid metabolism through effects on production and action of estrogens.
The mechanisms through which Lcn2 deficiency decreases serum estradiol remain unclear. Lcn2 deficiency further decreased the age-related decline in the expression of aromatase, an enzyme that generates estradiol from testosterone, in adipose tissues but not ovary. The consequences of the lower aromatase expression in adipose tissues also remain an open question. Although the evidence accumulated so far is intriguing, potential depot differences in the conversion of testosterone to estrogens and tissue sex steroid concentrations and their relationship to potential alterations in lipid metabolism and inflammation were not studied. Given the known antiinflammatory effects of Lcn2 (10, 11), it would be especially interesting to know whether the high Lcn2 observed in inguinal adipose tissue normally protects this depot from inflammation.
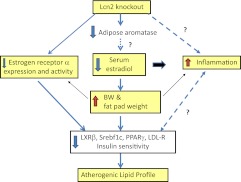
The importance of this work is that it reveals a potential new mechanistic link between a depot-specific alteration in adipose tissue biology and menopause-associated weight gain. However, several caveats should be considered in evaluating these new findings. As summarized in Fig. 1, there may be many intertwined connections between Lcn2 deficiency and obesity and inflammation and insulin resistance in female mice. Thus, instead of being a direct consequence of the Lcn2 deficiency, the lower estrogen status of the Lcn2−/− mice could result from their increased adiposity (12). Indeed, the authors of this manuscript have demonstrated that Lcn2 activates PPARγ and expression of adiponectin and that male Lcn2−/− mice exhibit increased inflammation (10). It will be important to determine whether systemic and adipose inflammation is also increased in female Lcn2 knockouts.
Fig. 1.
Potential relationships of Lcn2 deficiency, estrogen status, and metabolic phenotypes. Lcn2 deficiency leads to decreased serum estradiol levels and estrogen receptor-α activity. These changes in levels of and actions of estrogens may directly impact obesity and inflammation, leading to alterations in lipid metabolism. Although adipose tissue aromatase is decreased with Lcn2 deficiency, whether this change directly contributes to the low circulating estradiol levels and to inflammatory phenotypes remains an open question.
Another major question not addressed by the current report is whether the large increase in Lcn2 in the inguinal adipose tissue of female mice represents expression in mammary epithelium, adipocytes, preadipocytes, macrophages, or other stromal cells within this depot. Although the cellular source of this secreted protein may not matter from the physiological point of view, it will be important to assess the cellular origin to fully understand the mechanisms involved. It seems conceivable that the very high expression of Lcn2 seen was associated with the variations in expression in the mammary gland, which is known to increase Lcn2 expression during involution, which could conceivably result from reduced actions of estrogens. The consequences of the loss of this very high level of Lcn2 in inguinal sc adipose tissue on lipid metabolism, aromatase activity, estrogen action, and inflammation would be of great interest from the viewpoint of understanding the unique biology of this depot.
In a study of gene expression in male and female C56BL/6 mice on high-fat diets, it was also noted that Lcn2 gene expression was markedly up-regulated in male compared with female gonadal adipose tissue. The very high expression of the Lcn2 gene in gonadal (epididymal) fat of males occurred in association with adipocyte death and inflammation. Thus, high Lcn2 may be a salutary response to inflammation and in the pathophysiological setting might contribute to tissue remodeling as a result of stabilizing matrix metalloproteinase 9. In apparent contrast to the present results examining protein levels of Lcn2, we (1) detected no significant difference in Lcn2 gene expression in gonadal and inguinal sc adipose tissue of female mice of the same C57BL/6 background. This discrepancy could be due to posttranslational regulation as well as variable contamination of samples with mammary tissue, and this potentially important question for unraveling the role of Lcn2 in adipocyte biology also deserves further study.
Returning to the conundrum mentioned earlier, two groups studying the same Lcn2 knockout find different phenotypes. Jun et al. (8) found no evidence for obesity or insulin resistance in the same Lcn2 knockout females, whereas Guo et al. (9) now report a modest level of obesity they posit reflects low levels of estrogens and their actions. As discussed by Rosen's group, a likely contributor to discrepancies in the phenotypes includes the composition of the high-fat diets. Indeed, the fact that the diet used by Guo et al. (9) contained no dietary fiber deserves consideration as a factor that could affect the gut microbiome, which could instigate adipose inflammation and insulin resistance. The loss of intestinal Lcn2 antibacterial actions that deprive gut bacteria of iron and limit their growth may play a role here. Even differences in the fiber type present in chow diets and the levels of phytoestrogens known to variably contaminate these diets could affect the phenotype of the Lcn2-null animals observed in different labs. Alterations in iron status, established to be regulated by Lcn2, could also interact with diet or other environmental variables to affect the metabolic phenotypes observed in different labs, so studies that systematically alter variables may yield insights into the translational relevance of this mouse model.
In conclusion, the paper by Guo et al. (9) provides evidence linking Lcn2 to production of estrogens and action in sc adipose tissue, at least under the conditions that they studied the mice. It will be important to assess how Lcn2, levels of estrogens and their actions, inflammation, and obesity are interrelated. These findings suggest new avenues for research on the sex- and depot-related adipose tissue biology and once again caution us not to assume the findings in males will be applicable to females. Furthermore, we need to be cognizant of diet composition and housing conditions as contributors to obesity phenotypes.
Acknowledgments
A.S.G.'s work was supported by funding from the United States Department of Agriculture's Agricultural Research Service (58-1950-7-707), NIH DK082574, 1RC2ES01871, and R24DK0867669 (to A.S.G. and S.K.F.). S.K.F. is also supported by the National Institutes of Health (DK59823, P30 DK046299, DK080488), the Society for Women's Health Research, and the Evans Foundations for Interdisciplinary Research, Department of Medicine, Boston University School of Medicine.
Disclosure Summary: The authors have nothing to disclose.
For article see page 1183
- Lcn2
- Lipocalin 2.
References
- 1. Grove KL, Fried SK, Greenberg AS, Xiao XQ, Clegg DJ. 2010. A microarray analysis of sexual dimorphism of adipose tissues in high-fat-diet-induced obese mice. Int J Obes (Lond) 34:989–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Medrikova D, Jilkova ZM, Bardova K, Janovska P, Rossmeisl M, Kopecky J. 2011. Sex differences during the course of diet-induced obesity in mice: adipose tissue expandability and glycemic control. Int J Obes (Lond) 10.1038/ijo.2011.87 [DOI] [PubMed] [Google Scholar]
- 3. Snijder MB, Zimmet PZ, Visser M, Dekker JM, Seidell JC, Shaw JE. 2004. Independent and opposite associations of waist and hip circumferences with diabetes, hypertension and dyslipidemia: the AusDiab Study. Int J Obes Relat Metab Disord 28:402–409 [DOI] [PubMed] [Google Scholar]
- 4. Li C, Chan YR. 2011. Lipocalin 2 regulation and its complex role in inflammation and cancer. Cytokine 56:435–441 [DOI] [PubMed] [Google Scholar]
- 5. Guo H, Jin D, Zhang Y, Wright W, Bazuine M, Brockman DA, Bernlohr DA, Chen X. 2010. Lipocalin-2 deficiency impairs thermogenesis and potentiates diet-induced insulin resistance in mice. Diabetes 59:1376–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yan QW, Yang Q, Mody N, Graham TE, Hsu CH, Xu Z, Houstis NE, Kahn BB, Rosen ED. 2007. The adipokine lipocalin 2 is regulated by obesity and promotes insulin resistance. Diabetes 56:2533–2540 [DOI] [PubMed] [Google Scholar]
- 7. Law IK, Xu A, Lam KS, Berger T, Mak TW, Vanhoutte PM, Liu JT, Sweeney G, Zhou M, Yang B, Wang Y. 2010. Lipocalin-2 deficiency attenuates insulin resistance associated with aging and obesity. Diabetes 59:872–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jun LS, Siddall CP, Rosen ED. 2011. A minor role for lipocalin 2 in high-fat diet-induced glucose intolerance. Am J Physiol Endocrinol Metab 301:E825–E835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guo H, Zhang Y, Brockman D, Hahn WBD, Chen X. 2012. Lipocalin 2 deficiency alters estradiol production and estrogen receptor signaling in female mice. Endocrinology 153:1183–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jin D, Guo H, Bu SY, Zhang Y, Hannaford J, Mashek DG, Chen X. 2011. Lipocalin 2 is a selective modulator of peroxisome proliferator-activated receptor-γ activation and function in lipid homeostasis and energy expenditure. FASEB J 25:754–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang J, Wu Y, Zhang Y, Leroith D, Bernlohr DA, Chen X. 2008. The role of lipocalin 2 in the regulation of inflammation in adipocytes and macrophages. Mol Endocrinol 22:1416–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nilsson M, Dahlman I, Ryden M, Nordstrom EA, Gustafsson JA, Arner P, Dahlman-Wright K. 2007. Oestrogen receptor alpha gene expression levels are reduced in obese compared with normal weight females. Int J Obes (Lond) 31:900–907 [DOI] [PubMed] [Google Scholar]