Abstract
Secular trends toward a declining age at puberty onset with correlated changes in body weight have been reported in economically advanced countries. This has been attributed to excess calorie intake along with reduced physical activity in children. However, because the timing of puberty in humans is also influenced by other factors, such as genetic traits, living conditions, geographical location, and environmental chemicals, it is difficult to distinguish the effect of diet and body size from other factors in a human population. Here we report that feeding juvenile female rhesus monkeys born and raised at the Wisconsin National Primate Research Center with a high-calorie diet results in acceleration of body growth and precocious menarche. The monkeys fed a high-calorie diet also had an elevated body mass index. The most significant treatment effects on circulating hormones were increased leptin and IGF-I levels throughout the experiment. The findings of this study suggest the importance of close monitoring of juvenile feeding behaviors as an important intervention to reduce the prevalence of precocious development and metabolic diseases in adulthood.
The average age that girls reach first menstruation (menarche) has declined over the last 150 yr by 4 yr (1–3). Importantly, examination of this secular trend revealed that body weight at menarche was unchanged (4). This finding led to the critical body weight hypothesis stating that the transition to puberty is gated by a defined body weight. Several more recent studies (5–8) support secular trends toward a declining age at thelarche and/or menarche with correlated changes in body mass index (BMI). However, because the timing of puberty in humans is also influenced by other factors, such as genetic traits, living conditions, lifestyle, geographical location, and environmental chemicals (1, 3), distinguishing the effect of body size from these other factors is difficult in a human population.
We recently noticed that the age at menarche in our experimental rhesus monkeys had gradually declined over the past 30 yr, yet the body weight at menarche stayed the same. During this period, caloric intake in Wisconsin National Primate Research Center (WNPRC) colony animals increased, as a result of modifications made to enrich their environment. Consequently, WNPRC colony females were growing faster and also seemed to reach menarche at an earlier age. Given the similar secular trends between monkeys in the WNPRC colony and human populations, we decided to investigate whether nutrition can in fact directly influence the timing of puberty in female rhesus monkeys. With an increased prevalence of childhood obesity in the United States and economically advanced countries, it is imperative to learn whether this link exists between feeding behavior and the transition to puberty.
Accordingly, we conducted an experiment examining whether feeding female juvenile rhesus monkeys with a high-calorie diet causes an accelerated body weight increase and precocious menarche. Studying the effect of a nutritional influence on the timing of puberty in female rhesus monkeys born and raised in the WNPRC colony was an ideal situation because they are homogeneous compared with human populations and we could control social and living conditions, including photoperiod, and largely eliminate exposure to environmental chemicals. We found that intake of a high-calorie diet was related to accelerated body growth and precocious menarche. Analysis of circulating hormones further indicated that leptin and/or IGF-I appear to be peripheral signals to the brain that enable this early transition to puberty.
Materials and Methods
Historical data
Animals
Animals analyzed in this study were born and raised in the WNPRC. They were kept in rooms at 22 C with a lighting schedule of 12 h light (0600–1800 h) and 12 h dark (1800–0600 h). Water was available ad libitum. Housing conditions changed over the 30 yr: animals born in 1973–1975 were kept in a single cage (size 75 × 75 × 75 cm) and fed a standard Purina monkey chow (5038; Ralston Purina, St. Louis, MO) supplemented with fresh fruits/vegetables three times per week. Animals born in 1988–1991 were kept in pairs in a double-sized cage (172 × 86 × 86 cm) and fed Purina monkey chow 5038 once every morning, supplemented with fresh fruits/vegetables five times per week. Animals born in 2003–2005 were also kept in pairs in cages (172 × 86 × 86 cm). Purina chow was replaced by Harlan 20% protein diet (TD.2050; Harlan-Teklad, Madison, WI), which was fed twice a day (morning and afternoon) and supplemented with fresh fruits, vegetables, and/or sweet treats seven times per week.
Colony records analysis
To examine changes in the body weight growth curve over the past 30 yr, we used individual body weights from the WNPRC colony database. First, we averaged body weights at 3-month intervals after the date of birth. Second, three separate group averages were calculated from three successive years at 15-yr intervals: group 1: animals born January 1, 1973, through December 31, 1975 (n = 86 females, n = 83 males); group 2: animals born January 1, 1988, through December 31, 1990 (n = 109 females, n = 67 males); and group 3: animals born January 1, 2003, through December 31, 2005 (n = 77 females, n = 63 males). Menarcheal age was also obtained from the database of the WNPRC. The total number of monkeys used for 1973 through 1975, 1988 through 1990, and 2003 through 2005 were n = 23, n = 71, and n = 73, respectively.
High-calorie diet feeding study
Outline of experimental designs
Four pairs of female monkeys, matched by their birth date and body weight (high calorie group: 1.97 ± 0.08 kg; control group: 2.04 ± 0.02 kg) were randomly assigned to either the high-calorie diet group (TD.07802: 4.3 Calories/g, which consisted of 42.3% fat, 15.8% protein, and 41.9% carbohydrate; Harlan-Teklad) or control diet group (T.09796: 3.2 Calories/g, which consisted of 13.7% fat, 16.5% protein, and 69.8% carbohydrate; Harlan-Teklad). After close observation of physical growth and signs of puberty, we euthanized the pair when one of them exhibited menarche to obtain tissues, including the brain, for later analyses. Because it turned out that all high-calorie-diet females exhibited menarche before control diet monkeys (see Results), the control data for age and body weight at menarche were obtained from four experimental control females born in 2008 and 2009 (contemporary control group), assigned to another concurrent ongoing study (see Table 1). These animals were also kept in pairs in cages (172 × 86 × 86 cm) and fed a Harlan 20% protein diet (TD.2050) twice a day (morning and afternoon) supplemented with fresh fruits, vegetables, and/or sweet treats seven times per week.
Table 1.
Changes in the ages of menarche and first ovulation during the 30-yr course of our studies
| Experiments conducted (∼yr) | Number of animals | Menarche (months)a | BW (kg) | First ovulation (months)b | BW (kg) | Publications |
|---|---|---|---|---|---|---|
| 1978–1982c | 8 | 30.3 ± 3.1d | 3.1 ± 0.2 | 51.2 ± 3.3 | 4.6 ± 0.1 | Terasawa et al., 1984 (9) |
| 1993–1997c | 6 | 28.2 ± 2.3 | 3.1 ± 0.4 | 44.8 ± 1.8 | 4.3 ± 0.3 | Keen et al., 1999 (11) |
| 1999–2005c | 5 | 25.5 ± 0.9 | 3.2 ± 0.1 | 41.3 ± 1.3 | 5.2 ± 0.2 | Windsor-Engnell et al., 2007 (10) |
| 2009–2011c | 4 | 24.1 ± 1.6 | 3.3 ± 0.1 | NA | NA | Current (unpublished) |
NA, Not available; BW, body weight.
The age of menarche was obtained through daily inspection of vaginal bleeding.
The age of first ovulation was obtained by changes in circulating progesterone concentration.
The birth dates of animals were 12–16 months earlier, as these experiments were started at 12–16 months of age.
Mean ± sem.
Detailed descriptions
Female monkeys were weaned at 10 months of age and housed in pairs in double-sized cages (size: 172 × 86 × 86 cm). One month before commencing the differential feeding at 12 months of age, baseline food intake was assessed with a control diet (T.09796). The baseline intake levels were used to determine total grams of food available each day. To ensure accurate measurements of food intake for each animal, the pair was separated with a mesh sliding door in the middle of the cage whenever food was present (0700–1600 h). The mesh sliding door allowed visual and limited tactile contact between the pair mates. At 12 months of age, one of each pair was randomly assigned to be fed either the high calorie diet (TD. 07802) or control diet (T.09796) and the respective diet was started. Diets were given daily at 0700 h with an equal amount of supplemental fruits in the afternoon. Total intake was measured every day at the end of feeding time (1600 h) and excess food was removed from the cage and measured before repairing the animals. Animals were maintained on these diets throughout the entire study until the first animal of each pair exhibited first menstruation, at which point, both animals were euthanized to obtain necropsy data and later analysis of the brain. Other housing conditions including the lighting schedule were the same as described above with water available ad libitum. The protocol for this study was reviewed and approved by the Animal Care and Use Committee, University of Wisconsin, and all experiments were conducted under the guidelines established by the National Institutes of Health and the U.S. Department of Agriculture.
Blood sampling
Weekly morning (0900 h) blood samples were taken from the femoral vein of nonfasted animals throughout the study. A single night (1900 h) blood sample was also taken after one of the monkeys in that pair reached first menstruation. Serum was separated by centrifugation and kept at −20 C until assayed for LH, FSH, estradiol, GH, leptin, IGF-I, insulin, and cortisol. Validation and quality controls for these hormone assays can be seen at http://www.primate.wisc.edu/assay.
Menstrual status
Occurrence of first menstruation, determined by trained observers, was documented at the first visualization of menstrual bleeding. Animals were visually inspected at least once every day between 0700 h and 1800 h in their home cage. When necessary, a vaginal swab was taken to confirm bleeding.
Somatometrics
Body dimensions (body weight, body lengths, circumferences of the body and limbs, skinfold thicknesses, and nipple volume) were measured 18 h after fasting every 4 wk with the following schedule: measurements were conducted after each dual-energy x-ray absorptiometry (DXA) scan (8 wk intervals; see below) and between DXA scanning body dimensions alone were measured under ketamine (10 mg, iv) sedation. Body lengths (crown-rump and crown-heel) were measured with the animal in the supine position on a calibrated rule with a fixed headrest. Circumferences of the left upper arm, left upper leg, chest, and abdomen were measured with a tape measure to the nearest 0.1 cm with the animal in lateral recumbancy. Skinfold thicknesses were measured with a Lange caliper at the left tricep, left pectoral, and two abdominal sites (2.5 cm above and below the umbilicus). Similarly, nipple volume (height and diameter) was measured with a caliper. BMI was calculated as body weight (kilograms)/crown heel length (meters)2.
Dual-energy x-ray absorptiometry
Total body DXA scanning was performed at 8-wk intervals starting at 12 months of age until one of the pair menstruated. Animals were sedated with ketamine (12 mg/kg, im) plus xylazine (1 mg/kg, im) and placed in a supine position with foam cushions for scanning (model DPX-L; GE/Lunar Corp., Madison, WI) in the small animal mode (version 1.0d) with 2.4- × 4.8-mm sample size and fine collimation. Scans were analyzed with DPX 4.0a pediatric software (GE/Lunar) to determine fat and lean mass percentages and distributions. Bone mineral content and bone mineral density were also measured.
Statistical analysis
In the middle of the study, we had to stop data collection from one of the four control animals in the high-calorie diet study because she was found to have a congenital heart problem, and this prevented us from conducting measurements under anesthesia. Consequently, all analyses were made with n = 4 for the high calorie diet group and n = 3 for the control diet group. Using GraphPad Prism 5 (La Jolla, CA), we performed two-way ANOVA with Bonferroni post hoc tests to determine statistical significance between the groups for both historical and high-calorie diet studies. The difference in menarche age was tested with a Student's t test. Significance was set at P < 0.05.
Results and Discussion
Historical data
The ages of menarche and first ovulation have gradually advanced among our experimental animals
Over the past 30 yr of research, we noticed that the age at menarche in our female rhesus monkey experimental controls had gradually declined, yet the body weight at menarche stayed the same (Table 1). Because all of our experimental animals were assigned from the WNPRC colony, we speculated that these trends in body weight and age at menarche might be a general phenomenon in the WNPRC colony.
Changes in body weight and menarcheal age in WNPRC colony animals over 30 yr
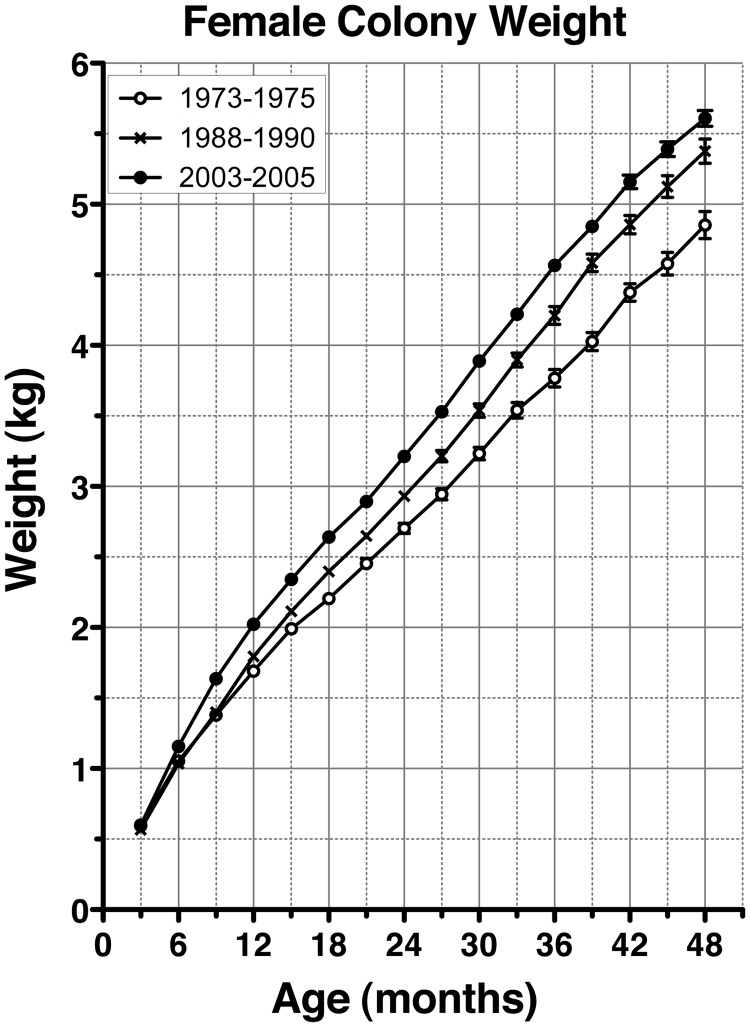
Accordingly, we analyzed body weights of rhesus monkeys born between 1973 and 2005 at the WNPRC (3 yr periods at 15 yr intervals, i.e. 1973 through 1975, 1988 through 1990, and 2003 through 2005). As seen in Fig. 1, the rate of the body weight growth of females gradually accelerated over 30 yr. That is, despite the fact that there was no difference in the body weights at 3 months of age, the body weight growth curves in females had a gradual upward shift. Two-way ANOVA indicated the differences in growth curves for females born between 1973–1975 and 1988–1990 and between 1988–1990 and 2003–2005 were both highly significant (P < 0.0001). Interestingly, the average weight at 24 months of age in females born in 1973–1975 was already achieved by 18 months of age in animals born in 2003–2005. Similar secular trends of accelerated body weight growth curves were also seen in males (see Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org).
Fig. 1.
Accelerated growth among WNPRC colony females over the past 30 yr. Body weight growth curves of female rhesus monkeys born between 1973 and 2005 at the WNPRC are shown. Each line represents the average (±sem) growth curve of females during 3-year periods at 15-yr intervals, i.e. 1973 to1975 (n = 86), 1988 to1990 (n = 109), and 2003 to 2005 (n = 77). Although body weights at 3 months of age were not different, the rate of body weight growth of females gradually accelerated over 30 yr. Two-way ANOVA indicated that differences in growth curves in females born between 1973 and 1975 and 1988 and 1990 and between 1988 and 1990 and 2003 and 2005 were both highly significant (P < 0.0001).
Colony records for menarche data further indicated that the average (±sem) age of menarche in females significantly (P = 0.002) decreased from 31.2 ± 1.1 months of age in 1973–1975 (n = 23) to 29.0 ± 0.5 and 27.3 ± 0.6 months of age in 1988–1990 (n = 71) and 2003–2005 (n = 73), respectively. The older menarche age in colony females compared with experimental control females of comparable time periods shown in Table 1 is due to the difference in observers. Although the data of experimental control females were obtained by well-trained scientists in our laboratory, the data of colony animals were obtained by animal caretakers, who could easily miss a small amount of blood. Therefore, menarche could easily be missed, and subsequent menses (heavier bleeding) may have been recorded as menarche.
Effects of a high-calorie diet on the body weight growth curve and the age of menarche (High calorie diet feeding study)
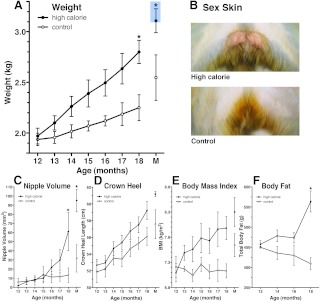
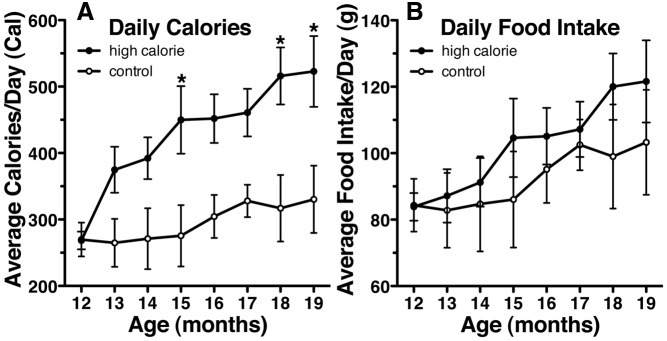
Feeding prepubertal female monkeys with the high-calorie diet induced a striking upward shift of the body weight growth curve (Fig. 2A, P < 0.0001). The high-calorie diet also increased the height growth curve (Fig. 2D, P < 0.001). Clearly, this is due to a higher calorie intake by the high-calorie-diet monkeys (Fig. 3A, P < 0.0001); the mass of food intake did not differ between the groups (Fig. 3B). The high-calorie diet also induced a precocious increase in nipple volume (Fig. 2C, P < 0.01), sex-skin swelling (Fig. 2B), and precocious menarche (age at menarche: 19.8 ± 0.9 months of age, body weight: 3.1 ± 0.1 kg, n = 4). None of the control diet monkeys exhibited menarche or perineal swelling (Fig. 2B) during the entire course of the study. The age of menarche in the high-calorie diet group was significantly younger (P < 0.05) than in a group of our most recent (2008–2011) controls from another study (menarche age: 25.5 ± 0.9 months of age, body weight: 3.3 ± 0.1 kg, n = 4, Table 1) and the control animals of our experiments conducted between 1999 and 2005 (menarche age: 24.1 ± 1.6 months of age, body weight: 3.3 ± 0.1 kg, n = 5, Table 1).
Fig. 2.
Somatometric differences between high-calorie- and control-fed monkeys during prepubertal development. Feeding prepubertal female monkeys with the high-calorie diet induced a striking upward shift of the body weight growth curve (A, P < 0.0001) and precocious sex-skin swelling (B). Precocious increases in nipple volume (C, P < 0.01) were also evident in monkeys fed a high-calorie diet. The high-calorie diet also increased body (crown heel) length (D, P < 0.001), BMI (E, P < 0.01), and total body fat (F, P < 0.001) as assessed by DXA scanning. Data shown are mean ± sem with high calorie (n = 4) and control (n = 3). P values above represent group differences across the entire study (two way ANOVA). *, On graphs represent differences (P < 0.05) at individual points based on Bonferroni post hoc analyses. M, Average day of menarche (19.8 ± 1.1 months of age) of the four high-fat-diet animals. Measurements were taken from both animals of each pair on the day of first menstruation, although only high-fat-diet animals reached menarche. The blue box represents the mean body weights of experimental control animals at menarche shown in Table 1.
Fig. 3.
Food intake among high-calorie- and control-diet-fed animals. Monkeys fed a high-calorie diet eat more calories (A, P < 0.0001) but a similar mass of food (B) each day. Data shown are mean ± sem with high calorie (n = 4) and control (n = 3). P values above represent group differences across the entire study (two way ANOVA). *, On graphs represent differences (P < 0.05) at the individual points based on Bonferroni post hoc analyses.
The body weight increase at 18 months of age and older in the high-calorie group was related to elevated total fat deposition assessed by DXA scanning (Fig. 2F, P < 0.001). Similarly, both the abdominal skinfold and abdominal fat assessed by DXA scanning at 18 months of age and older were also significantly larger in the high-calorie group compared with the control group (P < 0.001), whereas the skinfold and fat deposition assessed by DXA scanning in other parts of the body, such as the chest, arm, and leg and percentage fat were not significantly different throughout the experiment (Supplemental Fig. 2). Finally, BMI in high-calorie-diet monkeys was significantly (P < 0.01) higher than in controls (Fig. 2E), and the high-calorie diet increased bone mineral content (P < 0.02) but not bone mineral density (Supplemental Fig. 3).
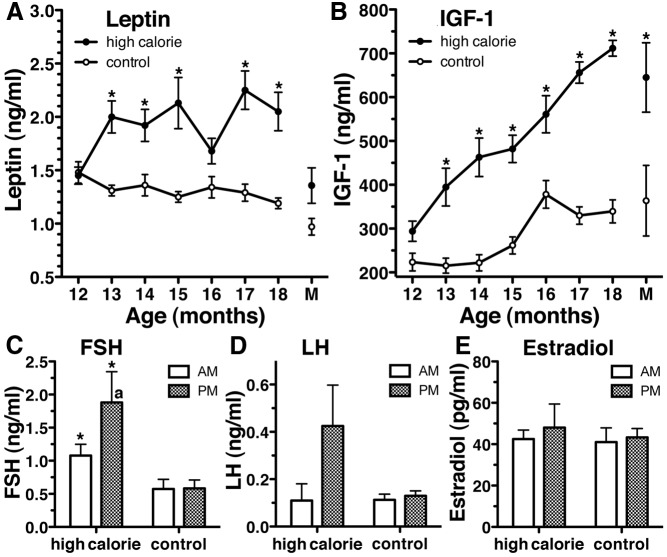
The most significant treatment effects observed among circulating hormones analyzed were elevated leptin and IGF-I levels throughout the experiment. In fact, at most points after 12 months of age (the age at initiation of the feeding program), leptin and IGF-I levels in the high-calorie group were higher than those in the control group (Fig. 4, A and B). In contrast, insulin and cortisol levels were not different (Supplemental Fig. 4). At 18 months of age and older, morning FSH levels in the high calorie group were significantly higher than those in the control group at the corresponding time (Fig. 4C, P < 0.01). Importantly, in the high-calorie group but not the control group, evening FSH levels became significantly higher than morning FSH levels (Fig. 4C, P < 0.02). Although a similar trend toward a nocturnal increase in bioactive LH levels was observed in the high calorie diet group (P = 0.14, Fig. 4D), a statistical difference was not attained. Also, despite precocious increases in the nipple volume and perineal swelling, estradiol levels were not different (Fig. 4E). Although there was a clear developmental difference in GH levels, no treatment effects were observed (Supplemental Fig. 4).
Fig. 4.
Circulating hormone levels in high-calorie- and control-diet-fed animals. The most significant differences between high-calorie- and control-diet-fed animals were circulating levels of leptin (A, P < 0.01) and IGF-I (B, P < 0.001). At most points after initiation of the feeding program, leptin and IGF-I levels in the high-calorie group were higher than those in the control group. At 18 months of age and older, morning FSH levels (C) in the high-calorie group were significantly higher than those in the control group (*, P < 0.01) at the corresponding time. Importantly, in the high-calorie group, but not the control group, evening FSH levels (C) became significantly higher than morning FSH levels (a, P < 0.02). Although a similar trend toward a nocturnal increase in bioactive LH levels was also observed in the high-calorie group (D), a statistical difference was not attained (P = 0.14). Estradiol levels were also not different (E). Data shown are mean ± sem with high calorie (n = 4) and control (n = 3). P values for these graphs represent group differences across the entire study (two way ANOVA), whereas the asterisk represents differences (P < 0.05) at individual points based on Bonferroni post hoc analyses. M, Average day of menarche (19.8 ± 1.1 months of age) of the four high-fat-diet animals. Measurements were taken from both animals of each pair on the day of the first menstruation, although only high-fat-diet animals reached menarche.
General discussion
Current worldwide trends toward obesity and early puberty onset are significant concerns in our society because children with obesity onset before normal menarche age are more likely to be obese in young adulthood (12–14), which is related to many diseases including cancers, hypertension, hypercholesterolemia, and the metabolic syndrome (15–17). In the present study, we have shown that high-calorie intake results in accelerated body weight as well as height growth curves. In addition, elevated circulating levels of leptin and IGF-I may signal to the hypothalamic-pituitary-gonadal axis, specifically GnRH neurons, and trigger the onset of puberty.
With analysis of historical data, we found that there is a secular trend toward rapid body weight growth and early menarche in animals in the WNPRC colony. Furthermore, growth acceleration of colony animals appears to be attributable to changes in feeding patterns and increased calorie intake over the 30 yr. First, the differences in growth rate for monkeys born between 1973–1975 and 1988–1990 was likely due to an increase in food intake because of competition between animals, after pairing of two animals in a double cage, which took place in 1984–1985 based on changes in U.S. Department of Agriculture regulations. Seeing empty food trays during this transition, animal caretakers began feeding greater than double amounts of chow per day. Second, growth acceleration between 1988–1990 and 2003–2005 seems to be due to major changes in feeding habits. Starting in 2002, the feeding pattern was changed from once daily to twice daily. In January 2003 the daily monkey diet was switched from a standard Purina monkey chow 5038 to a standard Harlan Teklad diet 2050, and all colony monkeys were fed with daily snacks, as a part of the implementation of a primate enrichment program. Before 1995 monkeys were fed supplemental fruits and/or fresh vegetables three to five times per week. Since 1995 they have been fed daily fresh fruits, and starting in 2002 treats that consist of higher sugar-containing foods, such as fruit roll-ups, fruit loops, marshmallows, and yogurt, were added. Nevertheless, interpretation of these historical data from colony records have a limitation, as the caloric intake by colony animals is not available, in addition to the relative inaccuracy of menarcheal data shown in Results.
Accordingly, we conducted a feeding study. Although the number of animals was small, we found a striking acceleration of both weight and height growth curves in high-calorie-diet monkeys compared with control diet monkeys, which was clearly due to a higher calorie intake. Furthermore, all high-calorie-diet monkeys exhibited a precocious increase in nipple volume, sex-skin swelling, and precocious menarche. The average age of menarche in the high-calorie-diet group was significantly younger than that in experimental controls from other studies of this laboratory. Interestingly, in this study, while the appearance of the high-calorie-diet monkeys was far from obese, their BMI, total body fat, and upper abdominal skinfolds were all consistently higher than those of control diet monkeys. Our daily observations indicated no significant difference in activity levels between the two groups, although we did not specifically measure activity levels of individual monkeys. Therefore, we suspect high-calorie-diet animals had an excess energy balance because energy expenditures of the two groups were comparable. These findings of the diet effect on physical growth differ from those described by others (18), in which no impact of a high-fat diet on body growth was observed, although the high-fat diet did accelerate the timing of sex-skin swelling and age of menarche in female rhesus monkeys. These differences may stem from many factors, including experimental designs (group housing in that study vs. pair housing in this study), the age of feeding paradigm initiation (16 months of age vs. 12 months of age), and housing conditions (outdoor housing vs. indoor housing).
An increase in circulating FSH and LH and appearance of their nocturnal increases are a sign of the pubertal increase in GnRH release in females (19, 20). In this study, we observed a nocturnal FSH increase (and similar trend in LH) and significantly higher FSH levels in high-calorie-diet monkeys but not control-diet monkeys. However, despite a precocious increase in the nipple volume and perineal sex-skin swelling in the high-calorie-diet group, we failed to detect elevated 17β-estradiol (E2) and LH levels. The exact reason for unchanged E2 and LH is unknown, but more frequent blood sampling and/or more sensitive E2 assays may be helpful in future studies.
In this study we also observed that circulating IGF-I levels exhibited an age-dependent increase in both groups and that in high-calorie-fed animals, IGF-I levels were consistently elevated compared with controls. In fact, at most points after the initiation of the feeding program, leptin and IGF-I, but not insulin, levels were higher in the high calorie group than those in the control group. One can speculate that the leptin increase may have influenced the IGF-I elevation because leptin directly stimulates GH at the somatotrope level or indirectly through facilitation of GHRH or suppression of somatostatin (21–25). However, because we did not see any modification of the developmental GH release pattern by high-calorie-diet feeding, it is unlikely that dietary induced IGF-I elevations are due to increased leptin action via GH secretion. The question of whether dietary-induced IGF-I elevations are due to GH-independent synthesis in the liver (26) remains unclear because we did not measure GH and IGF-I binding proteins in the circulation. Nevertheless, accelerated muscle and bone growths, as indicated by larger lean mass and crown-heal length, respectively, in high-calorie-diet animals, are likely attributable to elevated IGF-I levels.
Both IGF-I and leptin have been implicated as peripheral signals to the brain that influence GnRH neuronal activity at puberty onset. For example, circulating IGF-I increases around the time of puberty in rats, baboons, and humans (27–29), IGF-I induces stimulatory action on GnRH neurons in rodents (30), and intracerebral administration of IGF-I into sexually immature female rats (27) accelerates the timing of puberty. However, several reports indicate that IGF-I is not likely responsible for triggering the pubertal increase in GnRH release in primates: 1) the pubertal increase in E2 accelerates a pubertal increase in circulating IGF-I in humans (31); 2) Wilson (32) has shown that daily administration of IGF-I to prepubertal female monkeys advances the age of first ovulation, but not menarche; and 3) somatostatin administration to premenarcheal female monkeys, which suppressed both circulating levels of GH and IGF-I, delayed only the age at first ovulation, not menarche (33). Therefore, IGF-I appears to augment the pubertal increase in GnRH release after puberty onset.
Since their discoveries, leptin and its receptor (LepR) have been implicated in the control of reproductive function. In fact, mutations of the leptin (Ob) or LepR gene cause the absence of puberty or delayed puberty in humans and mice (34–37). Moreover, reduction of leptin by food restriction suppresses LH pulses and delays puberty onset in rats and sheep (38–41), whereas leptin injection in leptin-deficient mice and humans or food-restricted animals restores pulsatile LH secretion and puberty (37, 40, 42–46).
Prominent elevations in leptin occur across species during development. In mice, a leptin surge occurs well before puberty between postnatal day (P) 6 and P12 with a small secondary increase at P18 (47). In human girls, it is consistently shown that circulating leptin increases during the progression of puberty (21, 48–53), whereas in boys leptin levels increase at an early stage of puberty but decline about the time that circulating testosterone increases (21, 48, 52–55). Despite these reports in humans, the question of whether a spontaneous increase in circulating leptin levels gives a signal to the brain has been debatable due to conflicting data in nonhuman primates. In male rhesus monkeys, several papers consistently reported there is no leptin increase associated with puberty (56–60). In addition, continuous infusion of a low level of leptin in male juvenile monkeys does not result in a pubertal increase in LH release (61). On the other hand, Suter et al. (62) reported that an elevation of nocturnal circulating leptin levels preceded the pubertal increase in LH in male monkeys by approximately 30 d. Moreover, Wilson et al. (20) showed that a daily administration of leptin in female juvenile monkeys starting at 14 months of age increased LH levels in both the morning and evening and accelerated precocious perineal swelling and menarche. The present finding that intake of a high-calorie diet causes consistently elevated leptin and IGF-I levels, leading to early menarche with increased FSH, nipple growth, and perineal swelling, also supports the notion that leptin is a peripheral signal that facilitates the progress of puberty in nonhuman female primates. Perhaps the argument over leptin's role in pubertal onset stems from the paucity of studies in this species compounded with sex differences. Nevertheless, in this study we observed a clear association between increased serum leptin levels and dietary-induced precocious menarche.
A recent study indicated that mice lacking LepR in inhibitory γ-aminobutyric acid (GABA)ergic neurons in the hypothalamus exhibited hyperphagia, 10-fold increases in adipose mass, and obesity, which are similar to those seen in ob/ob mice, whereas mice lacking LepR in excitatory glutamatergic neurons exhibited only a 2-fold increase in adipose mass, with no obese phenotype compared with control mice (63). The key role for leptin action through LepR in the GABA neuronal network to control energy homeostasis has huge implications for the impact of leptin on triggering puberty onset. Previously we have proposed that limited release of GnRH before the onset of puberty is due to a tonic inhibition by GABA in female rhesus monkeys (64). Furthermore, at puberty onset a reduction in GABA inhibition allows excitatory glutamatergic input to activate GnRH neurons (10, 65, 66). With leptin effects on body size and possibly pubertal timing mediated by LepR-expressing GABAergic and glutamatergic neurons of the hypothalamus, it is tempting to speculate that the metabolic signal, leptin, plays a role in fine-tuning of pubertal timing through inhibitory and excitatory inputs to GnRH neurons (66). To further support this hypothesis, we need to determine whether a similar neurocircuitory of the LepR-expressing GABA network is responsible for leptin action in primates (human and nonhuman primates) and how the GABA network with LepR interacts with GnRH neurons. Nevertheless, evidence is already mounting to suggest that hypothalamic GABA and/or glutamate neurons with LepR play integral roles in initiating puberty.
The hypothesis that kisspeptin neurons act as a relay between energy homeostasis (i.e. leptin signaling) and reproductive function has also emerged (67, 68). Although GnRH neurons do not express LepR, kisspeptin neurons do, a study determining the impact of deleting LepR from kisspeptin neurons has been conducted. Surprisingly, deletion of LepR selectively from kisspeptin neurons did not change the normal process of puberty nor adult reproductive function in mice (69). Accordingly, these authors lesioned the ventral premammillary nucleus (PMV) in ob/ob mice, in which LepR-expressing neurons are concentrated and found that these lesions abrogate leptin-induced puberty (69). Because kisspeptin neurons are present in the arcuate nucleus, but not in the PMV, and because LepR is highly expressed in the PMV, the authors concluded that LepR-expressing neurons in the PMV, not kisspeptin neurons, appear to mediate metabolic signals to GnRH neurons. The impact of lesioning PMV of mice is particularly interesting to us because precocious puberty in human patients is often associated with lesions of this part of the hypothalamus by hematoma or tumor invasion (70–72), and we observed that lesions in the posterior hypothalamus, including PMV, advanced the timing of menarche and first ovulation in female monkeys (9). Although neuronal substrates in this part of the primate hypothalamus are currently unknown, highly concentrated LepR-expressing neurons in this region in mice warrant further investigation in nonhuman primates.
In the present study, we did not assess the effects of a high-calorie diet on the timing of first ovulation because the scope of this study was to investigate the initiation of puberty, rather than full maturational changes through puberty. The current trend of early puberty onset in humans is also based on the appearance of secondary sex characteristics, such as breast development, and age of menarche (5–8). Thus, we thought that similar parameters might be useful for comparison. Certainly, as articulated by Wilson et al. (20), it takes approximately 1 yr after menarche for first ovulation in female rhesus monkeys to occur and the mechanism of the gonadal steroid-independent GnRH increase at puberty onset in primates significantly differs from the mechanism of pubertal progression leading to first ovulation. Therefore, we will need to study whether a high-calorie diet alters the timing of first ovulation in another study.
Finally, the average weight at menarche in monkeys fed a high-calorie diet was 3.1 kg, which is very similar to that we observed in experimental control and colony monkeys during the past 30 yr (Table 1), regardless of their age. Currently we do not have sufficient data to discuss the relationship between height and age. Nevertheless, our observation enforces the validity of the critical body weight hypothesis proposed in humans 4 decades ago (4).
In conclusion, we found that intake of a high-calorie diet during juvenile development significantly advances the onset of puberty. Serum leptin levels were stably elevated by high-calorie intake before precocious menarche. We speculate that leptin gives a signal to the GnRH neurosecretory system through GABA neurons. The stable, persistent elevations would provide an underlying excitable background for the activity of the GnRH neuronal system leading to puberty onset. This means that leptin may be an initial contributor to facilitate puberty onset and/or progress of puberty, rather than triggering puberty. Although this study indicates that feeding a high-calorie diet induces weight gain and precocious puberty in juvenile monkeys, the fundamental question still remains: what triggers puberty onset? Given current worldwide trends toward obesity and early puberty onset and that early onset obesity is related to development of cancers, hypertension, hypercholesterolemia, and the metabolic syndrome, these data suggest the importance of close monitoring of juvenile feeding behaviors as an important intervention to reduce the prevalence of precocious development and adult metabolic diseases.
Supplementary Material
Acknowledgments
We greatly acknowledge Mr. Scott Baum for his great technical assistance.
This work was supported by National Institutes of Health Grants R01HD11355 and American Recovery and Reinvestment Act award (to E.T.) and was possible to perform by the National Institutes of Health support (Grants P51RR000167, RR15459, and RR020141 to the Wisconsin National Primate Research Center).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMI
- Body mass index
- DXA
- dual-energy x-ray absorptiometry
- E2
- 17β-estradiol
- GABA
- γ-amino butyric acid
- LepR
- leptin receptor
- P
- postnatal day
- PMV
- ventral premammillary nucleus
- WNPRC
- Wisconsin National Primate Research Center.
References
- 1. Styne DM, Grumbach MM. 2011. Puberty: ontogeny, neuroendocrinology, physiology, and disorders. In: Melmed S, Polonsky KS, Larsen PR, Kronenberg HM, eds. Williams textbook of endocrinology. 12th ed Chap 25 Philadelphia: Saunders Elsevier [Google Scholar]
- 2. Wyshak G, Frisch RE. 1982. Evidence for a secular trend in age of menarche. N Engl J Med 306:1033–1035 [DOI] [PubMed] [Google Scholar]
- 3. Parent AS, Teilmann G, Juul A, Skakkebaek NE, Toppari J, Bourguignon JP. 2003. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocr Rev 24:668–693 [DOI] [PubMed] [Google Scholar]
- 4. Frisch RE, Revelle R. 1971. Height and weight at menarche and a hypothesis of menarche. Arch Dis Child 46:695–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Biro FM, McMahon RP, Striegel-Moore R, Crawford PB, Obarzanek E, Morrison JA, Barton BA, Falkner F. 2001. Impact of timing of pubertal maturation on growth in black and white female adolescents: the National Heart, Lung, and Blood Institute Growth and Health Study. J Pediatr 138:636–643 [DOI] [PubMed] [Google Scholar]
- 6. Demerath EW, Li J, Sun SS, Chumlea WC, Remsberg KE, Czerwinski SA, Towne B, Siervogel RM. 2004. Fifty-year trends in serial body mass index during adolescence in girls: the Fels Longitudinal Study. Am J Clin Nutr 80:441–446 [DOI] [PubMed] [Google Scholar]
- 7. Harris MA, Prior JC, Koehoorn M. 2008. Age at menarche in the Canadian population: secular trends and relationship to adulthood BMI. J Adolesc Health 43:548–554 [DOI] [PubMed] [Google Scholar]
- 8. Bau AM, Ernert A, Schenk L, Wiegand S, Martus P, Grüters A, Krude H. 2009. Is there a further acceleration in the age at onset of menarche? A cross-sectional study in 1840 school children focusing on age and bodyweight at the onset of menarche. Eur J Endocrinol 160:107–113 [DOI] [PubMed] [Google Scholar]
- 9. Terasawa E, Noonan JJ, Nass TE, Loose MD. 1984. Posterior hypothalamic lesions advance the onset of puberty in the female rhesus monkey. Endocrinology 115:2241–2250 [DOI] [PubMed] [Google Scholar]
- 10. Windsor-Engnell BM, Kasuya E, Mizuno M, Keen KL, Terasawa E. 2007. An increase in in vivo release of LHRH and precocious puberty by posterior hypothalamic lesions in female rhesus monkeys (Macaca mulatta). Am J Physiol Endocrinol Metab 292:E1000–E1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Keen KL, Burich AJ, Mitsushima D, Kasuya E, Terasawa E. 1999. Effects of pulsatile infusion of the GABAA receptor blocker bicuculline on the onset of puberty in female rhesus monkeys. Endocrinology 140:5257–5266 [DOI] [PubMed] [Google Scholar]
- 12. van Lenthe FJ, Kemper CG, van Mechelen W. 1996. Rapid maturation in adolescence results in greater obesity in adulthood: the Amsterdam Growth and Health Study. Am J Clin Nutr 64:18–24 [DOI] [PubMed] [Google Scholar]
- 13. Wang Y. 2002. Is obesity associated with early sexual maturation? A comparison of the association in American boys versus girls. Pediatrics 110:903–910 [DOI] [PubMed] [Google Scholar]
- 14. Thompson DR, Obarzanek E, Franko DL, Barton BA, Morrison J, Biro FM, Daniels SR, Striegel-Moore RH. 2007. Childhood overweight and cardiovascular disease risk factors: the National Heart, Lung, and Blood Institute Growth and Health Study. J Pediatr 150:18–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hamilton AS, Mack TM. 2003. Puberty and genetic susceptibility to breast cancer in a case-control study in twins. N Engl J Med 348:2313–2322 [DOI] [PubMed] [Google Scholar]
- 16. Wardle J, Brodersen NH, Cole TJ, Jarvis MJ, Boniface DR. 2006. Development of adiposity in adolescence: five year longitudinal study of an ethnically and socioeconomically diverse sample of young people in Britain. BMJ 332:1130–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jasik CB, Lustig RH. 2008. Adolescent obesity and puberty: the “perfect storm.” Ann NY Acad Sci 1135:265–279 [DOI] [PubMed] [Google Scholar]
- 18. Schwartz SM, Wilson ME, Walker ML, Collins DC. 1988. Dietary influences on growth and sexual maturation in premenarchial rhesus monkeys. Horm Behav 22:231–251 [DOI] [PubMed] [Google Scholar]
- 19. Terasawa E, Nass TE, Yeoman RR, Loose MD, Schultz NJ. 1983. Hypo-thalamic control of puberty in the female rhesus macaque. In: Norman RL, ed. Neuroendocrine aspects of reproduction. New York: Academic Press; 149–182 [Google Scholar]
- 20. Wilson ME, Fisher J, Chikazawa K, Yoda R, Legendre A, Mook D, Gould KG. 2003. Leptin administration increases nocturnal concentrations of luteinizing hormone and growth hormone in juvenile female rhesus monkeys. J Clin Endocrinol Metab 88:4874–4883 [DOI] [PubMed] [Google Scholar]
- 21. Blum WF, Englaro P, Hanitsch S, Juul A, Hertel NT, Müller J, Skakkebaek NE, Heiman ML, Birkett M, Attanasio AM, Kiess W, Rascher W. 1997. Plasma leptin levels in healthy children and adolescents: dependence on body mass index, body fat mass, gender, pubertal stage, and testosterone. J Clin Endocrinol Metab 82:2904–2910 [DOI] [PubMed] [Google Scholar]
- 22. Quintela M, Señaris R, Heiman ML, Casanueva FF, Dieguez C. 1997. Leptin inhibits in vitro hypothalamic somatostatin secretion and somatostatin mRNA levels. Endocrinology 138:5641–5644 [DOI] [PubMed] [Google Scholar]
- 23. Gómez JM, Maravall FJ, Gómez N, Navarro MA, Casamitjana R, Soler J. 2003. Interactions between serum leptin, the insulin-like growth factor-I system, and sex, age, anthropometric and body composition variables in a healthy population randomly selected. J Clin Endocrinol (Oxf) 58:213–219 [DOI] [PubMed] [Google Scholar]
- 24. Vuagnat BA, Pierroz DD, Lalaoui M, Englaro P, Pralong FP, Blum WF, Aubert ML. 1998. Evidence for a leptin-neuropeptide Y axis for the regulation of growth hormone secretion in the rat. Neuroendocrinology 67:291–300 [DOI] [PubMed] [Google Scholar]
- 25. Grégoire Nyomba BL, Johnson M, Berard L, Murphy LJ. 1999. Relationship between serum leptin and the insulin-like growth factor-I system in humans. Metabolism 48:840–844 [DOI] [PubMed] [Google Scholar]
- 26. Veldhuis JD, Roemmich JN, Richmond EJ, Bowers CY. 2006. Somatotropic and gonadotropic axes linkages in infancy, childhood, and the puberty-adult transition. Endocr Rev 27:101–140 [DOI] [PubMed] [Google Scholar]
- 27. Hiney JK, Srivastava V, Nyberg CL, Ojeda SR, Dees WL. 1996. Insulin-like growth factor I of peripheral origin acts centrally to accelerate the initiation of female puberty. Endocrinology 137:3717–3728 [DOI] [PubMed] [Google Scholar]
- 28. Copeland KC, Kuehl TJ, Castracane VD. 1982. Pubertal endocrinology of the baboon: elevated somatomedin-C/insulin-like growth factor I at puberty. J Clin Endocrinol Metab 55:1198–1201 [DOI] [PubMed] [Google Scholar]
- 29. Rosenfield RI, Furlanetto R, Bock D. 1983. Relationship of somatomedin-C concentrations to pubertal changes. J Pediatr 103:723–728 [DOI] [PubMed] [Google Scholar]
- 30. Daftary SS, Gore AC. 2004. The hypothalamic insulin-like growth factor-1 receptor and its relationship to gonadotropin-releasing hormones neurones during postnatal development. J Neuroendocrinol 16:160–169 [DOI] [PubMed] [Google Scholar]
- 31. Cuttler L, Van Vliet G, Conte FA, Kaplan SL, Grumbach MM. 1985. Somatomedin-C levels in children and adolescents with gonadal dysgenesis: differences from age-matched normal females and effect of chronic estrogen replacement therapy. J Clin Endocrinol Metab 60:1087–1092 [DOI] [PubMed] [Google Scholar]
- 32. Wilson ME. 1998. Premature elevation in serum insulin-like growth factor-I advances first ovulation in rhesus monkeys. J Endocrinol 158:247–257 [DOI] [PubMed] [Google Scholar]
- 33. Wilson ME, Tanner JM. 1994. Somatostatin analog treatment slows growth and the tempo of reproductive maturation in female rhesus monkeys. J Clin Endocrinol Metab 79:495–501 [DOI] [PubMed] [Google Scholar]
- 34. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. 1994. Positional cloning of the mouse obese gene and its human homologue. Nature [Erratum (1995) 374:479] 372:425–432 [DOI] [PubMed] [Google Scholar]
- 35. Strobel A, Issad T, Camoin L, Ozata M, Strosberg AD. 1998. A leptin missense mutation associated with hypogonadism and morbid obesity. Nat Genet 18:213–215 [DOI] [PubMed] [Google Scholar]
- 36. Ozata M, Ozdemir IC, Licinio J. 1999. Human leptin deficiency caused by a missense mutation: multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. J Clin Endocrinol Metab [Erratum (2000) 85:416] 84:3686–3695 [DOI] [PubMed] [Google Scholar]
- 37. Farooqi IS, Wangensteen T, Collins S, Kimber W, Matarese G, Keogh JM, Lank E, Bottomley B, Lopez-Fernandez J, Ferraz-Amaro I, Dattani MT, Ercan O, Myhre AG, Retterstol L, Stanhope R, Edge JA, McKenzie S, Lessan N, Ghodsi M, De Rosa V, Perna F, Fontana S, Barroso I, Undlien DE, O'Rahilly S. 2007. Clinical and molecular genetic spectrum of congenital deficiency of the leptin receptor. N Engl J Med 356:237–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cheung CC, Thornton JE, Kuijper JL, Weigle DS, Clifton DK, Steiner RA. 1997. Leptin is a metabolic gate for the onset of puberty in the female rat. Endocrinology 138:855–858 [DOI] [PubMed] [Google Scholar]
- 39. Nagatani S, Guthikonda P, Thompson RC, Tsukamura H, Maeda KI, Foster DL. 1998. Evidence for GnRH regulation by leptin: leptin administration prevents reduced pulsatile LH secretion during fasting. Neuroendocrinology 67:370–376 [DOI] [PubMed] [Google Scholar]
- 40. Finn PD, Cunningham MJ, Pau KY, Spies HG, Clifton DK, Steiner RA. 1998. The stimulatory effect of leptin on the neuroendocrine reproductive axis of the monkey. Endocrinology 139:4652–4662 [DOI] [PubMed] [Google Scholar]
- 41. Foster DL, Nagatani S. 1999. Physiological perspectives on leptin as a regulator of reproduction: role in timing puberty. Biol Reprod 60:205–215 [DOI] [PubMed] [Google Scholar]
- 42. Barash IA, Cheung CC, Weigle DS, Ren H, Kabigting EB, Kuijper JL, Clifton DK, Steiner RA. 1996. Leptin is a metabolic signal to the reproductive system. Endocrinology 137:3144–3147 [DOI] [PubMed] [Google Scholar]
- 43. Ahima RS, Dushay J, Flier SN, Prabakaran D, Flier JS. 1997. Leptin accelerates the onset of puberty in normal female mice. J Clin Invest 99:391–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chehab FF, Lim ME, Lu R. 1996. Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat Genet 12:318–320 [DOI] [PubMed] [Google Scholar]
- 45. Gruaz NM, Lalaoui M, Pierroz DD, Englaro P, Sizonenko PC, Blum WF, Aubert ML. 1998. Chronic administration of leptin into the lateral ventricle induces sexual maturation in severely food-restricted female rats. J Neuroendocrinol 10:627–633 [DOI] [PubMed] [Google Scholar]
- 46. Nagatani S, Zeng Y, Keisler DH, Foster DL, Jaffe CA. 2000. Leptin regulates pulsatile luteinizing hormone and growth hormone secretion in the sheep. Endocrinology 141:3965–3975 [DOI] [PubMed] [Google Scholar]
- 47. Ahima RS, Prabakaran D, Flier JS. 1998. Postnatal leptin surge and regulation of circadian rhythm of leptin by feeding. Implications for energy homeostasis and neuroendocrine function. J Clin Invest 101:1020–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mantzoros CS, Flier JS, Rogol AD. 1997. A longitudinal assessment of hormonal and physical alterations during normal puberty in boys. V. Rising leptin levels may signal the onset of puberty. J Clin Endocrinol Metab 82:1066–1070 [DOI] [PubMed] [Google Scholar]
- 49. Matkovic V, Ilich JZ, Skugor M, Badenhop NE, Goel P, Clairmont A, Klisovic D, Nahhas RW, Landoll JD. 1997. Leptin is inversely related to age at menarche in human females. J Clin Endocrinol Metab 82:3239–3245 [DOI] [PubMed] [Google Scholar]
- 50. Carlsson B, Ankarberg C, Rosberg S, Norjavaara E, Albertsson-Wikland K, Carlsson LM. 1997. Serum leptin concentrations in relation to pubertal development. Arch Dis Child 77:396–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Palmert MR, Radovick S, Boepple PA. 1998. Leptin levels in children with central precocious puberty. J Clin Endocrinol Metab 83:2260–2265 [DOI] [PubMed] [Google Scholar]
- 52. Horlick MB, Rosenbaum M, Nicolson M, Levine LS, Fedun B, Wang J, Pierson RN, Jr, Leibel RL. 2000. Effect of puberty on the relationship between circulating leptin and body composition. J Clin Endocrinol Metab 85:2509–2518 [DOI] [PubMed] [Google Scholar]
- 53. Ankarberg-Lindgren C, Dahlgren J, Carlsson B, Rosberg S, Carlsson L, Wikland KA, Norjavaara E. 2001. Leptin levels show diurnal variation throughout puberty in healthy children, and follow a gender-specific pattern. Eur J Endocrinol 145:43–51 [DOI] [PubMed] [Google Scholar]
- 54. Garcia-Mayor RV, Andrade MA, Rios M, Lage M, Dieguez C, Casanueva FF. 1997. Serum leptin levels in normal children: relationship to age, gender, body mass index, pituitary-gonadal hormones, and pubertal stage. J Clin Endocrinol Metab 82:2849–2855 [DOI] [PubMed] [Google Scholar]
- 55. Ahmed ML, Ong KK, Watts AP, Morrell DJ, Preece MA, Dunger DB. 2001. Elevated leptin levels are associated with excess gains in fat mass in girls, but not boys, with type 1 diabetes: longitudinal study during adolescence. J Clin Endocrinol Metab 86:1188–1193 [DOI] [PubMed] [Google Scholar]
- 56. Plant TM, Durrant AR. 1997. Circulating leptin does not appear to provide a signal for triggering the initiation of puberty in the male rhesus monkey (Macaca mulatta). Endocrinology 138:4505–4508 [DOI] [PubMed] [Google Scholar]
- 57. Urbanski HF, Pau KY. 1998. A biphasic developmental pattern of circulating leptin in the male rhesus macaque (Macaca mulatta). Endocrinology 139:2284–2286 [DOI] [PubMed] [Google Scholar]
- 58. Mann DR, Akinbami MA, Gould KG, Castracane VD. 2000. A longitudinal study of leptin during development in the male rhesus monkey: the effect of body composition and season on circulating leptin levels. Biol Reprod 62:285–291 [DOI] [PubMed] [Google Scholar]
- 59. Plant TM. 2001. Leptin, growth hormone, and the onset of primate puberty. J Clin Endocrinol Metab 86:458–460 [DOI] [PubMed] [Google Scholar]
- 60. Mann DR, Bhat GK, Ramaswamy S, Stah CD, Plant TM. 2007. Regulation of circulating leptin and its soluble receptor during pubertal development in the male rhesus monkey (Macaca mulatta). Endocrine 31:125–129 [DOI] [PubMed] [Google Scholar]
- 61. Barker-Gibb ML, Sahu A, Pohl CR, Plant TM. 2002. Elevating circulating leptin in prepubertal male rhesus monkeys (Macaca mulatta) does not elicit precocious gonadotropin-releasing hormone release, assessed indirectly. J Clin Endocrinol Metab 87:4976–4983 [DOI] [PubMed] [Google Scholar]
- 62. Suter KJ, Pohl CR, Wilson ME. 2000. Circulating concentrations of nocturnal leptin, growth hormone, and insulin-like growth factor-I increase before the onset of puberty in agonadal male monkeys: potential signals for the initiation of puberty. J Clin Endocrinol Metab 85:808–814 [DOI] [PubMed] [Google Scholar]
- 63. Vong L, Ye C, Yang Z, Choi B, Chua S, Jr, Lowell BB. 2011. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron 71:142–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mitsushima D, Hei DL, Terasawa E. 1994. GABA is an inhibitory neurotransmitter restricting the release of luteinizing hormone-releasing hormone before the onset of puberty. Proc Natl Acad Sci USA 91:395–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Terasawa E, Luchansky LL, Kasuya E, Nyberg CL. 1999. An increase in glutamate release follows a decrease in gamma-aminobutyric acid (GABA) and the pubertal increase in LHRH release in female rhesus monkeys. J Neuroendocrinol 11:275–282 [DOI] [PubMed] [Google Scholar]
- 66. Terasawa E, Fernandez DL. 2001. Neurobiological mechanisms of the onset of puberty in primates. Endocr Rev 22:111–151 [DOI] [PubMed] [Google Scholar]
- 67. Roa J, Tena-Sempere M. 2010. Energy balance and puberty onset: emerging role of central mTOR signaling. Trends Endocrinol Metab 21:519–528 [DOI] [PubMed] [Google Scholar]
- 68. George JT, Millar RP, Anderson RA. 2010. Hypothesis: kisspeptin mediates male hypogonadism in obesity and type 2 diabetes. Neuroendocrinology 91:302–307 [DOI] [PubMed] [Google Scholar]
- 69. Donato J, Jr, Cravo RM, Frazão R, Gautron L, Scott MM, Lachey J, Castro IA, Margatho LO, Lee S, Lee C, Richardson JA, Friedman J, Chua S, Jr, Coppari R, Zigman JM, Elmquist JK, Elias CF. 2011. Leptin's effect on puberty in mice is relayed by the ventral premammillary nucleus and does not require signaling in Kiss1 neurons. J Clin Invest 121:355–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Morley TP. 1954. Hypothalamic tumor and precocious puberty. J Clin Endocrinol Metab 14:1. [DOI] [PubMed] [Google Scholar]
- 71. Byrnes ND, Cloutier MD, Hayles AB. 1974. The central nervous system and precocious puberty. In: Grumbach MM, Grave GD, Mayer FE, eds. The control of the onset of puberty. New York: Wiley and Sons; 213 [Google Scholar]
- 72. Siegel-Witchel S. 1995. CNS lesions, neurologic disorders, and puberty in man. In: Plant TM, Lee PA, eds. The neurobiology of puberty. Bristol, UK: Journal of Endocrinology Ltd.; 229–239 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.