Abstract
Aldosterone has been linked to the deleterious cardiovascular effects of obesity in humans. The association of aldosterone with obesity in rodents is less well defined, particularly in models of diet-induced obesity. We hypothesized that adrenal aldosterone production and aldosterone synthase expression would be increased in rats with obesity-induced hypertension. Male Sprague Dawley rats were fed a high-fat (HF: 36% fat) or control diet from 3 wk of age, and mean arterial pressure (MAP) was measured by telemetry. MAP was increased after 4 wk of HF diet; this was 6 wk before changes in body weight. Mineralocorticoid receptor antagonism did not prevent the HF-induced increase in MAP. After 17 wk on the diets, HF rats had increased body and fat weights (abdominal and epididymal) and were insulin resistant (Homeostasis Model Assessment index: 3.53 ± 0.43 vs. 8.52 ± 1.77; control vs. HF, P < 0.05). Plasma aldosterone levels were increased in the HF rats (64.14 ± 14.96 vs. 206.25 ± 47.55 pg/ml; control vs. HF, P < 0.05). This occurred independently of plasma renin activity (4.8 ± 0.92 vs. 4.73 ± 0.66 ng/ml/h, control vs. HF). The increase in aldosterone was accompanied by a 2-fold increase in adrenal aldosterone synthase mRNA expression and zona glomerulosa hypertrophy. Rats were also studied after 8 wk of HF diet, a time when MAP, but not body weight, was increased. At this time plasma aldosterone was unchanged but plasma renin activity was increased (4.4 ± 0.5 vs. 8.1 ± 1.3 ng/ml/h; control vs. HF, P < 0.05). These studies suggest that rats fed a HF diet from weaning may be a useful model for studying obesity-associated hyperaldosteronism.
Clinical studies show a clear link between obesity and hypertension (1), with visceral obesity being a primary risk factor for developing hypertension and cardiovascular disease (2). Importantly, the National Health and Nutrition Examination Survey III suggests that a positive correlation exists between body mass index and the incidence of hypertension (3). Obesity also increases the risk of developing other conditions from the sequelae that have become known as metabolic syndrome, including insulin resistance, impaired glucose tolerance, hyperinsulinemia, and dyslipidemia (4). Recent studies suggest that hyperaldosteronism should also be included in this list of related conditions (5, 6). As the incidence of obesity increases it is important that we develop and characterize animal models that appropriately reflect the human population. There are many animal models available to facilitate the study of obesity-induced hypertension. The most physiologically relevant is to feed rats a high-fat (HF) diet. In the most commonly used variant of this model adult male rats consume an HF diet, and 50% of these rats become obese and hypertensive (7). This model closely mimics the effects of obesity in adult humans (8).
Although adult rats fed a HF diet are a useful model for studying adult onset obesity (7), they do not address the growing epidemic of obesity that begins in childhood (9). Childhood obesity is a significant risk factor for adult obesity (10), and adolescent obesity is a better predictor of risk for coronary artery disease than adult obesity (11). The incidence of obesity in children is increasing at an alarming rate. The National Health and Nutrition Examination Survey III found that the obesity rate in adolescents has tripled in the past 20 yr (12). As this population reaches adulthood it would seem prudent to attempt to predict their cardiovascular fate using appropriate animal models. We have previously developed a model of obesity in which the rats begin consuming a HF diet at weaning. Interestingly, almost 100% of the rats receiving the HF diet become obese and hypertensive adults (13). Although our previous studies show that blood pressure is increased in this model, the exact temporal profile of the development of the hypertension is unknown. Our goal for this study was 2-fold: 1) to assess the temporal development of hypertension with obesity, and 2) to measure the production of aldosterone in the HF rats. Rats were fed the HF diet for 17 wk, instead of the 10 wk as previously reported, to better model the effects of life-long obesity in an adult rat (13, 14). We hypothesized that feeding rats an HF diet from weaning would increase blood pressure, plasma aldosterone, and adrenal aldosterone synthase (CYP11B2) expression.
Materials and Methods
Animals
Male Sprague Dawley rats (3 wk of age), obtained from Harlan Sprague Hawley, Inc. (Indianapolis, IN) were maintained on a 12-h light, 12-h dark cycle, housed two per cage unless otherwise stated, and allowed access to food and water ad libitum. These studies were approved by the Institutional Animal Care and Use Committee and were carried out in accordance with the Guide for the Care and Use of Laboratory Animals (National Academy of Sciences, Institute for Laboratory Animal Research). Rats were weighed and randomized to the HF or control diet; both the HF diet (36% fat; 15.2% saturated, 20.8% unsaturated; 0.056% sodium; and 0.87% potassium; BioServe Biotechnologies, Frenchtown, NJ) and control diet (4.4% fat; 2.5% saturated, 1.9% unsaturated; 0.3% sodium; and 0.9% potassium; Harlan Sprague Dawley) was fed to the rats for 17 wk. Additional rats were fed the HF or control diet for 8 wk. Body weight was measured weekly. To test the effects of mineralocorticoid receptor (MR) antagonism, additional groups of rats were fed the HF diet + canrenoic acid (HF+CAN, 20 mg/kg/d). Canrenoic acid is the active metabolite of spironolactone (15); it was selected for use here because it is water soluble and can be administered accurately in the drinking water. Water intake was measured weekly, and the dose of canrenoic acid was adjusted appropriately. Importantly, previous studies have shown that the effects of canrenoic acid on the cardiovascular system are very similar to those of spironolactone and eplerenone (16).
Blood pressure measurement
Rats were placed on the HF or control diet at 3 wk of age. After 1 wk, mouse radiotelemetery transmitter catheters (TA11PA-C10; Data Sciences International, St. Paul, MN) were placed in the femoral artery. Because the battery life of the telemeter was not sufficient to record continuously for 17 wk, the telemeters were turned on for the same 72 h each week. Mean arterial pressure (MAP) and heart rate (HR) were recorded for 10 sec every 10 min. The average daily MAP and HR were obtained and averaged for the last 48 h of recording to provide a weekly average. These rats were cohoused with a rat that did not receive a telemeter. Separate groups of rats fed the HF and control diets were prepared with femoral arterial and venous catheters for direct measurements of blood pressure and conscious blood sampling. The catheters were tunneled sc to the nape of the neck and secured with a harness.
Blood pressure was also measured in rats fed the HF diet±canrenoic acid from 3 wk of age. At 9 wk of age rat telemeters (TA11PA-C40) were placed as described above. Blood pressure recording began 1 wk later and continued for the duration of the study. These rats were singly housed to allow for accurate dosing of the canrenoic acid. The daily blood pressures were collected for the last 7 d of the study and averaged. Blood pressure was also measured by tail-cuff (RTBP1001; Kent Scientific, Torrington, CT) in additional rats fed the control or HF diet ± canrenoic acid.
Euthanasia and tissue collection
Before euthanasia a blood sample was obtained at 0700 h from conscious rats for measurements of plasma aldosterone and corticosterone. Rats were then fasted overnight for the collection of blood for glucose and insulin measurements. The kidney, spleen, heart, and abdominal and epididymal fat pads were removed and weighed. The adrenal glands were removed, weighed, and enucleated to provide samples containing primarily zona glomerulosa (ZG) cells.
RT-PCR
RNA was extracted from the adrenal ZG samples using an RNeasy Plus Mini Kit (QIAGEN, Valencia CA). Samples containing 1 or 2 μg of RNA were reverse transcribed. Real-time PCR was performed using TaqMan primers (Applied Biosystems, Foster City, CA) for rat CYP11B2, renin, angiotensin (Ang) type 1a receptor (AT1Ra), and Ang type 1b receptor (AT1Rb). 18s RNA was used for normalization. Fold changes, from control expression, were calculated using the 2-ΔΔCt method (17).
Plasma hormone levels
Blood from the conscious blood draw was used to measure plasma aldosterone and corticosterone levels. Blood obtained by cardiac puncture at euthanasia was used to measure all other hormone levels. PRA, T3, and T4 were measured by RIA (DiaSorin, Inc., Stillwater, MN), as was aldosterone, corticosterone (Siemens, Malvern, PA), and adiponectin (Millipore Corp., Billerica, MA). For the plasma renin-activity (PRA), blood was collected into EDTA-coated tubes; the samples were kept on ice and centrifuged within 1 h of collection. The resultant plasma was stored at −20 C, and the assay was performed within 1 month of the sample collection. The manufacturers' protocol was followed verbatim. The first incubation to generate Ang I was 90 min long. Leptin and insulin were measured by ELISA (Millipore). All assays were conducted following the manufacturer's instructions. Homeostasis Model Assessment formula was used to provide a measure of insulin resistance [fasting glucose (mg/dl) * fasting insulin (μU/ml)/ 405] (18). Plasma and urinary sodium and potassium were measured (Nova Biomedical, Waltham, MA).
Vessel structure analysis
Mesenteric resistance artery (MRA) structure was assessed by pressure myography (Living Systems Instrumentation, Burlington, VT) as described previously (19, 20). Third-order MRA were mounted in a pressure myograph and kept in warm calcium free physiological salt solution (in millimolar concentration: 141.9 NaCl, 4.7 KCl, 1.7 MgSO4, 2 EDTA, 10.0 HEPES, 1.2 KH2PO4, and 5.0 glucose). The intralumenal pressure was increased from 3 to 180 mm Hg in 20-mm Hg increments. Lumen diameter, external diameter, and wall thickness were measured at each pressure after a 5-min equilibration and the wall-lumen ratio was calculated.
Adrenal histology
Adrenal glands were removed from rats fed the HF and control diets for 17 wk. The glands were postfixed in 4% paraformaldehyde before being paraffin embedded. Sections (8 μm) from the center of each gland were stained with hematoxylin and eosin. The following areas were measured in each tissue section; the whole adrenal, ZG, zona fasiculata (ZF)/zona reticularis (ZR), medulla, and cortex. For simplicity the ZF/ZR were grouped together in this analysis. Four slices were studied from each adrenal, and the results obtained were averaged to provide the values for the gland.
Data analysis and statistics
Data are presented as mean ± sem. When comparing two groups, the appropriate Student's t test was used; when comparing three groups a one-way ANOVA was used with a Bonferroni correction. Repeated-measures ANOVA was used for the assessment of body weight, and two-way ANOVA was used for the vessel structure analysis (Sigma Stat). For the long-term telemetry experiments using repeated measurements from the same animal, Proc Mixed and repeated-measures statistical analyses were performed using SAS statistical software (Version 9.1). In all cases, P values less than or equal to 0.05 were considered statistically significant.
Results
Body weight and blood pressure
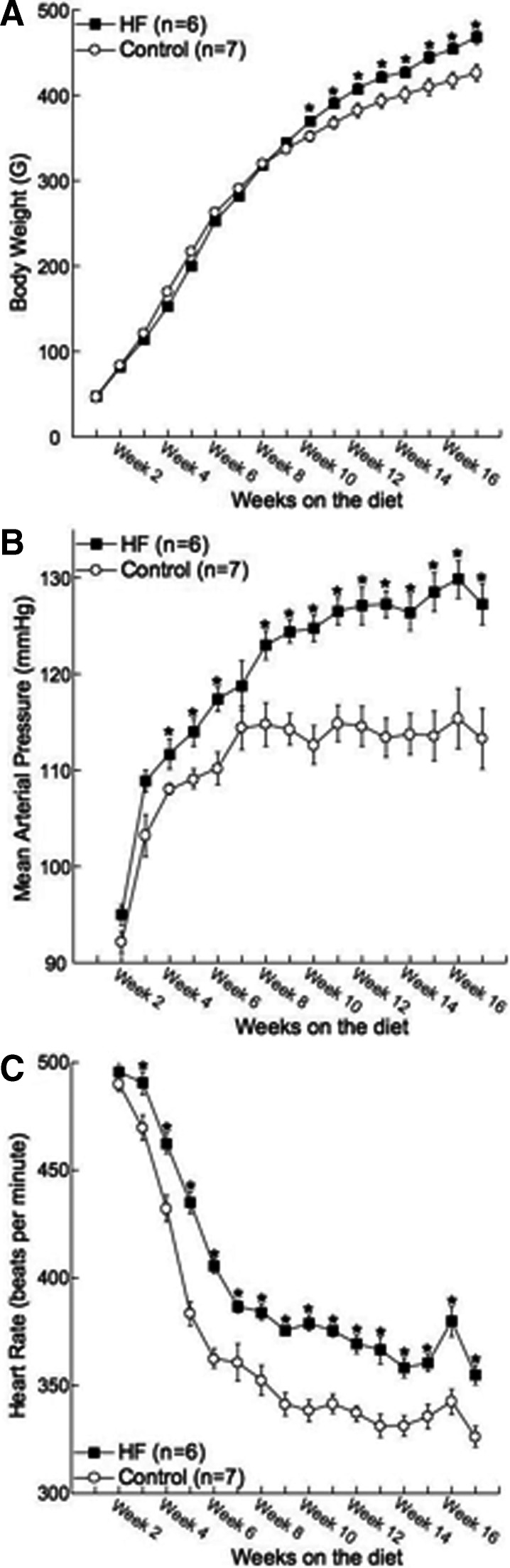
After 17 wk on the HF diet, body weight was significantly higher in the HF rats compared with control (Table 1 and Fig. 1A). Surprisingly the difference in body weight between the two groups of rats did not become apparent until the rats had been on the HF diet for 10 wk (Fig. 1A). HF rats had 4-fold more abdominal fat and approximately 2-fold more epididymal fat than the rats fed the control diet (Table 1). The heart-body weight and kidney-body weight ratios were unchanged between the two groups, suggesting that renal and cardiac hypertrophy was absent in the HF rats. The adrenal glands were significantly larger in the rats fed the HF diet; this was not only a function of the increased body weight because the adrenal-body weight ratio was also significantly increased (Table 1). MAP began to increase almost immediately after the rats were placed on the HF diet and became statistically higher than the control rats after 4 wk on the HF diet (Fig. 1B). Interestingly the differences in HR between the two groups of rats occurred even earlier, with the HR being higher in the HF rats after 3 wk on the diet (Fig. 1C). Rats were also studied after 8 wk on the diet (11-wk-old rat). Body weight was not changed at this time, although there was a significant increase in the combined abdominal and epididymal fat weight (see Table 3). Despite the normal body weight, MAP was significantly increased in the HF rats at this time point (Fig. 1, A and B).
Table 1.
Body and organ weights for rats fed the HF and control diets for 17 wk
| Control | HF | |
|---|---|---|
| Body weight (g) | 367 ± 8 | 444 ± 6a |
| Heart weight (g) | 1.22 ± 0.06 | 1.31 ± 0.02a |
| Kidney weight (g) | 2.65 ± 0.06 | 2.81 ± 0.16 |
| Adrenal weight (g) | 0.064 ± 0.007 | 0.10 ± 0.009a |
| Abdominal fat weight (g) | 1.83 ± 0.23 | 7.79 ± 0.48a |
| Epididymal fat weight (g) | 3.70 ± 0.32 | 8.90 ± 0.50a |
| Heart weight (% of body weight) | 0.33 ± 0.01 | 0.30 ± 0.003 |
| Kidney weight (% of body weight) | 0.72 ± 0.01 | 0.63 ± 0.04 |
| Adrenal weight (% of body weight) | 0.017 ± 0.001 | 0.023 ± 0.002a |
| Fat weight (% of body weight) | 1.51 ± 0.13 | 3.74 ± 0.19a |
Rats were fed the HF or control diet from 3 wk of age (n = 13 for the controls, n = 17 for the HF).
Significantly different from control. P < 0.05.
Fig. 1.
Feeding rats a HF diet from 3 wk of age increased body weight (A), MAP (B), and HR (C). Blood pressure and HR were measured by telemetry (n = 6 for HF and n = 7 for control; * indicates a significant difference from control).
Table 3.
Physiological profile for rats fed the HF or control diets for 8 wk
| Control | HF | |
|---|---|---|
| Body weight (g) | 308 ± 12 | 290 ± 8 |
| Combined fat weight (g) | 2.9 ± 0.2 | 4.2 ± 0.5a |
| Blood pressure (mm Hg) | 116 ± 10 | 125 ± 6a |
| Corticosterone (ng/ml) | 78.2 ± 5.7 | 74.7 ± 4.4 |
| Insulin (ng/ml) | 0.52 ± 0.11 | 0.51 ± 0.12 |
| Leptin (pg/ml) | 230.4 ± 70.4 | 276.7 ± 77.0 |
| Adiponectin (μg/ml) | 1.94 ± 0.14 | 2.52 ± 0.16a |
| Total T3 (ng/ml) | 0.47 ± 0.06 | 0.83 ± 0.12a |
| Free T4 (ng/ml) | 0.56 ± 0.01 | 0.46 ± 0.30 |
Rats began consuming the specialized diets at 3 wk of age. The combined fat weight is the sum of the abdominal and epididymal fat weights.
Significantly different from control, P < 0.05. Rats were fasted overnight before the measurement of plasma insulin (n = 5–6 for each group).
Metabolic and hormonal characteristics
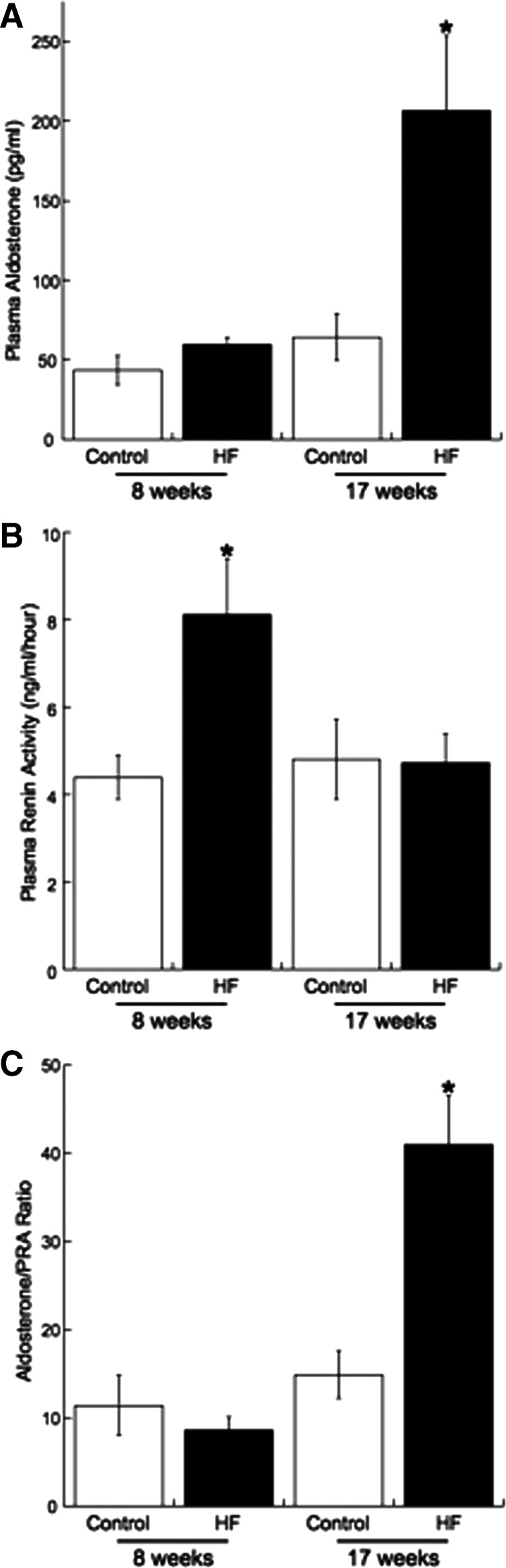
As expected, the HF rats showed signs of metabolic syndrome after 17 wk on the diet (Table 2). Fasting blood glucose and plasma insulin were significantly higher than those of control rats. Calculation of the Homeostasis Model Assessment index also suggested the rats were insulin resistant (Table 2). However, before significant body weight gain, plasma insulin was unchanged in the rats fed the HF diet for 8 wk (Table 3). Preliminary studies in a separate group of HF and control rats suggest that fasting blood glucose is also not changed after 8 wk on the HF diet (110.7 ± 9.5 vs. 116.8 ± 12.7 mg/ml; control vs. HF, n = 6 on each group). As hypothesized, there was a 4-fold increase in the plasma aldosterone levels in the rats fed the HF diet for 17 wk (Fig. 2A); at this time point PRA was unchanged (Fig. 2B). In keeping with this, the aldosterone-PRA ratio was elevated in the rats fed the HF diet for 17 wk (Fig. 2C). Despite the increase in plasma aldosterone, plasma sodium and potassium levels did not differ between the HF and control rats (Table 2). In contrast, in rats fed the HF diet for 8 wk PRA was increased (Fig. 2B), and the aldosterone-PRA ratio was unchanged (Fig. 2C). There was a trend toward an increase in the plasma aldosterone level (P = 0.062) (Fig. 2A). There was also a trend toward an increase in the plasma corticosterone levels in the rats fed the HF diet for 17 wk, but this did not reach statistical significance (Table 2); corticosterone was unchanged in the rats fed the HF diet for 8 wk (Table 3).
Table 2.
Hormone profile for control and HF rats
| Control | HF | |
|---|---|---|
| Corticosterone (ng/ml) | 65.1 ± 12.4 | 102.2 ± 29.4 |
| Insulin (ng/ml) | 0.38 ± 0.03 | 0.79 ± 0.14a |
| Blood glucose (mg/ml) | 150.7 ± 5.3 | 202.2 ± 9.5a |
| Leptin (pg/ml) | 342.3 ± 137.1 | 1483.9 ± 138.5a |
| HOMA index | 3.53 ± 0.43 | 8.52 ± 1.77a |
| Adiponectin (μg/ml) | 2.47 ± 0.16 | 3.40 ± 0.12a |
| Total T3 (ng/ml) | 0.44 ± 0.03 | 0.54 ± 0.03a |
| Free T4 (ng/ml) | 0.28 ± 0.02 | 0.32 ± 0.04 |
| Sodium (mmol/liter) | 140.2 ± 0.4 | 140.7 ± 0.5 |
| Potassium (mmol/liter) | 4.39 ± 0.07 | 4.34 ± 0.40 |
Rats were fed the HF or control diet for 17 wk from 3 wk of age. Rats were fasted overnight before measurement of blood glucose and plasma insulin. HOMA, Homeostasis Model Assessment (n = 6–8 for each group).
Significantly different from control, P < 0.05.
Fig. 2.
Plasma aldosterone (A), PRA (B), and the aldosterone- PRA ratio (C) differ temporally with the development of obesity. Aldosterone and PRA were measured by RIA (n = 5 for 8-wk control, n = 6 for 8-wk HF, and n = 7 for 17 week HF and control; * indicates a significant difference from the appropriate age-matched control).
The levels of leptin and adiponectin were both increased in the rats fed the HF diet for 17 wk (Table 2). Adiponectin levels were also increased in the rats fed the HF diet for 8 wk (Table 3). Assessments of thyroid hormone levels showed an increase in the levels of T3 without a change in T4 levels in HF rats from both durations of the diet (Tables 2 and 3).
Adrenal expression of the components of the renin angiotensin aldosterone system (RAAS)
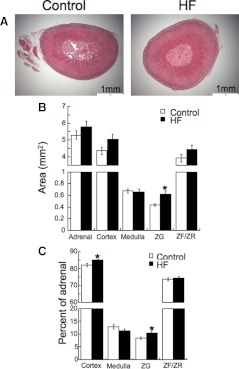
Expression of transcripts for renin, AT1Ra and AT1Rb, and CYP11B2 was examined in ZG from rats fed the control and HF diets. The relative pattern of RAAS mRNA expression in the ZG cells was similar in the rats fed the HF diet for both 8 and 17 wk. In keeping with the increase in plasma aldosterone levels, the expression of CYP11B2 was increased in the rats fed the HF diet. There were no differences in the expression of the mRNA for renin, AT1Ra, or AT1Rb (Table 4). The analysis of adrenal histology showed a marked increase in the area of the ZG in rats fed the HF diet. When expressed as a percentage of the adrenal area, the ZG was larger in the HF rats, as was the cortex as a whole (Fig. 3).
Table 4.
Fold change in mRNA expression from control ZG
| HF diet (8 wk) |
HF diet (17 wk) |
|||
|---|---|---|---|---|
| Control | HF | Control | HF | |
| CYP11B2 | 1.02 ± 0.07 | 2.34 ± 0.37a | 1.11 ± 0.18 | 1.90 ± 0.32a |
| Renin | 1.04 ± 0.10 | 0.94 ± 0.11 | 1.07 ± 0.17 | 1.18 ± 0.21 |
| AT1Ra | 1.02 ± 0.07 | 1.13 ± 0.09 | 1.03 ± 0.11 | 1.04 ± 0.14 |
| AT1Rb | 1.03 ± 0.09 | 1.19 ± 0.17 | 1.05 ± 0.12 | 1.13 ± 0.35 |
Significant difference from age-matched control, P < 0.05. All rats began the control or HF diet at 3 wk of age.
Fig. 3.
Rats fed a HF diet exhibit ZG hypertrophy. A, Representative slices of adrenal glands from rats fed the HF and control diets. B, Areas of the individual regions of the adrenal. C, Those regions expressed as a percentage of the adrenal area (n = 7 for controls and 8 for the HF; * indicates a significant difference from control).
The effects of MR blockade
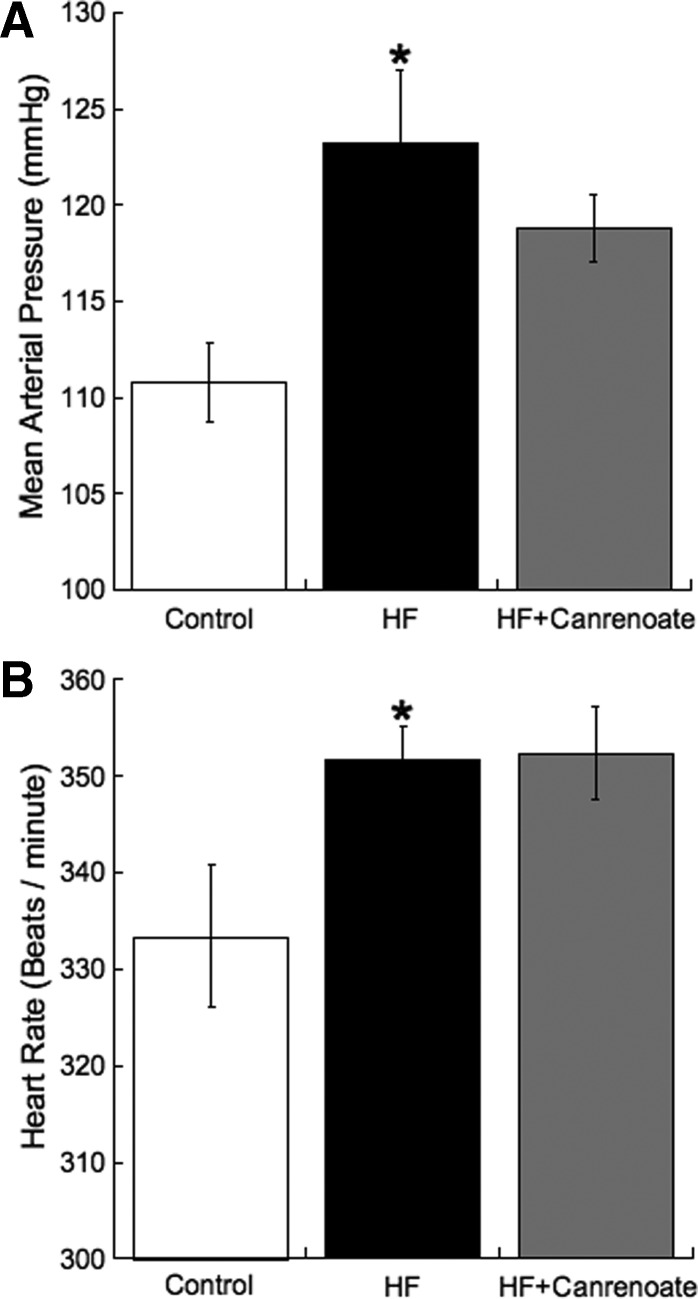
As expected, the MAP was significantly higher in the HF rats than the control. MR antagonism did not prevent the obesity-induced increase in blood pressure (Fig. 4A). The rats fed the HF diet had a higher HR than the control rats, and canrenoic acid treatment had no effect on the HR (Fig. 4B). These rats were singly housed; therefore none of the groups tested gained as much weight as they would have were they cohoused (in grams: control, 427 ± 9; HF, 464 ± 20; HF+CAN, 470 ± 15). Despite there not being a significant change in the body weight, the fat weight did differ between the groups (abdominal and epididymal fat combined in grams: control, 6.2 ± 0.3; HF, 12.8 ± 1.0*; HF+CAN, 13.2 ± 1.5*; * indicates significantly different from control, P < 0.05). The HF diet caused an increase in the fasting blood glucose (in milligrams/ml: control, 148 ± 8; HF, 180 ± 12; P < 0.05). There was a trend toward a reduction in glucose in the HF+CAN rats (in milligrams/ml: 156 ± 11). Blood pressure was also measured by tail cuff in additional control (n = 13), HF (n = 15), and HF+CAN (n = 15) rats. As expected the systolic blood pressure was increased in the HF rats compared with control (in millimeters Hg: control, 150 ± 2; HF, 162 ± 3; P < 0.05); canrenoic acid treatment had no effect on systolic blood pressure (in millimeters Hg: 165 ± 4, P < 0.01 vs. control).
Fig. 4.
MR antagonism with canrenoic acid had no effect on MAP (A) or HR (B) in rats fed the HF diet. MAP and HR were measured by telemetry, the results shown are for the last week of the study. Results were analyzed by one-way ANOVA (P = 0.03 for MAP and 0.03 for HR) (n = 7 for control, n = 6 for HF, and n = 7 for HF+CAN; * indicates a significant difference from control after Bonferroni correction).
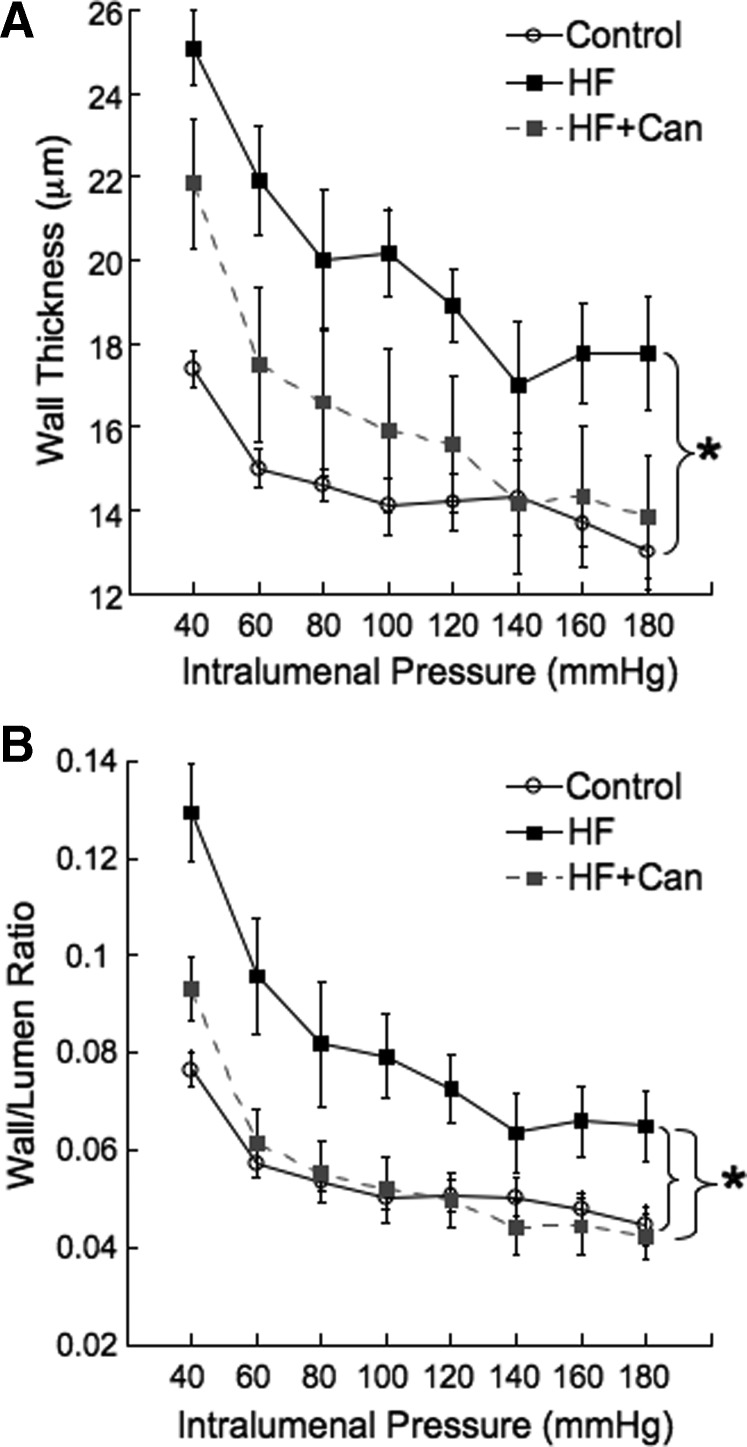
In previous studies we have shown that vessel remodeling can be prevented or reversed by MR antagonism in a blood pressure-independent manner (20, 21). Preliminary studies also showed that mesenteric blood flow is reduced in the HF rats as assessed by scanning laser Doppler (in perfusion units: control, 551 ± 40; HF, 296 ± 28, P < 0.05; n = 4 for control and 8 for HF). Therefore we assessed the effects of the HF diet on the structure of the third-order mesenteric resistance arteries. The vessel wall thickness and the wall-lumen ratio were both increased in the rats fed the HF diet, suggesting hypertrophy of the vessel wall. This vessel remodeling was prevented in the HF+CAN rats (Fig. 5).
Fig. 5.
Rats fed the HF diet exhibited MRA remodeling as evidenced by an increase in the vessel wall thickness (A) and the wall-lumen ratio (B). Canrenoic acid treatment prevented the vessel remodeling as assessed by pressure myography under zero flow and calcium-free conditions (n = 7 for control, n = 7 for HF, and n = 8 for HF+CAN; * indicates a significant difference by two-way repeated-measures ANOVA).
Discussion
There were two major findings from this study. First, MAP and HR are adversely affected by the consumption of a HF diet before body weight is increased. The second finding is that after a prolonged period on the HF diet, plasma aldosterone and adrenal CYP11B2 expression were increased and, in the more advanced stages of obesity, PRA and aldosterone become dissociated. Interestingly, the increase in aldosterone does not appear to be responsible for the hypertension observed in the fat-fed rats, but it does contribute to the vascular damage observed.
Obesity and blood pressure
We found that the temporal regulation of MAP, HR, and body weight differ dramatically. Despite consumption of the HF diet from 3 wk of age, body weight did not become significantly different until the rats had been on the diet for 10 wk, although the proportion of body fat was increased before this time point. Interestingly, for the first few weeks of treatment, the rats fed the HF diet had a lower body weight than the control rats. This finding has been highly reproducible over several groups of rats and occurs despite the calorie intake being about 10% higher in the HF rats during this time (e.g. after 8 wk on the diet the daily calorie intake in kilocalories is 69 ± 0.3 for the control rats and 76 ± 6 in the HF rats).
Few studies have used telemetry to measure blood pressure in rats with diet-induced obesity. To allow for the measurement of blood pressure throughout the entire protocol, we took the novel approach of using mouse telemeters in rats. Using this technique we observed an increase in MAP after 4 wk of administration of the HF diet and an increase in HR after only 3 wk. Importantly, human obesity has been associated with an increase in resting HR (22). Other groups have used telemetery in obesity-prone and -resistant rats fed a HF diet beginning in adulthood. The only hemodynamic parameter, which was significantly altered in the obese rats, was the HR, which was reduced less over time in the obesity-prone rats than in the control rats (23). Although that study showed no difference in the systolic blood pressure in the adult rats fed the HF diet, it is important to note that we (14, 24, 25) and others (26) have shown that blood pressure increases in rats with diet-induced obesity.
In addition to studying the rats after 17 wk on the diet, we studied a group of rats fed the HF diet for 8 wk. Our goal was to study the rats during the development, rather than the maintenance, of the hypertension, and at this time point blood pressure was still increasing rapidly. These rats had elevated MAP but no increase in body weight. However, the proportion of body fat was higher in the HF rats after 8 wk on the diet, suggesting that these rats were gaining fat weight at the expense of muscle mass in the early stages of obesity. This highlights the need to consider more than absolute body weight when conducting studies of obesity.
Our studies using canrenoic acid to antagonize the MR suggest that the elevation in blood pressure in the obese rats is not mediated by aldosterone or by MR activation. In this model blood pressure increased rapidly after the rats were placed on the HF diet. We did not measure aldosterone during the early stages of the development of hypertension; thus we cannot draw conclusions about its involvement other than to say that MR antagonism did not prevent the development of hypertension. Interestingly, although blood pressure was not completely normalized by MR antagonism, the obesity-associated changes in the MRA wall-lumen ratio were. When one considers wall thickness alone, it was increased in the HF rats compared with control, and there was a trend toward canrenoic acid reducing this. This suggests that aldosterone has detrimental affects on vasculature that may be blood pressure independent. This fits with our previous studies showing that MR antagonism has beneficial effects on the vasculature of stroke-prone spontaneously hypertensive rats that are blood pressure independent (20, 21).
Hormone profiles and adrenal steroidogenesis
Obesity has been linked to hyperaldosteronism (6), and weight loss reduces, not only circulating aldosterone, but also blood pressure in obese patients (27). It is important to note that the effects of obesity on aldosterone production in rodent models have not been well characterized. Most studies showing increased plasma aldosterone have been conducted in rodents with dysfunctional leptin signaling and morbid obesity; these include the db/db mice (28) and obese Zucker rats (29). Few studies have assessed plasma aldosterone levels in models in which the obesity is a function of the animals consuming a HF or high-calorie diet. One such model is the obese dog. In this model it is clear that mineralocorticoid receptor (MR) activation is involved in obesity-associated renal damage although the increase in plasma aldosterone observed in the obese dogs was modest (30).
At the end of the current study, the rats fed the HF diet had a 4-fold increase in plasma aldosterone. Interestingly, aldosterone and PRA were not associated at this time point; this is reflected by the inappropriately high aldosterone-PRA ratio. Our results suggest that hyperaldosteronism is beginning to develop in the HF rats after 8 wk on the diet at the latest. At this time point PRA and CYP11B2 expression are both increased, and there is a trend toward an increase in aldosterone. Although there was no difference in body weight between the HF and control rats after 8 wk on the HF diet, the abdominal fat weight was increased. Therefore, it is possible that this increase in adiposity is responsible for the increase in CYP11B2 expression. It is also possible that an elevated PRA is required to initiate an increase in aldosterone production but not to maintain it. A dissociation of PRA and aldosterone has been observed in human obese patients (31–33); this led people to propose that adipose tissue produces either aldosterone or a secretagogue that stimulates adrenal aldosterone production. Adipose tissue produces renin and Ang II (34); the enzymes required for aldosterone production are missing from the adipose tissue transcriptome (35). Therefore, it is more likely that cultured adipocytes produce a secretagogue (36), and several possible signaling molecules have been identified. The secretagogue may be a fatty acid metabolite; 12,13,-epoxy-9-keto-10(trans)-octadecenoic acid increases aldosterone production in cultured ZG cells, and circulating levels of 12,13,-epoxy-9-keto-10(trans)-octadecenoic acid are positively correlated with plasma aldosterone in humans (37). Recent studies suggest that Wnt-signaling molecules, released from adipocytes, increase aldosterone by increasing the production of steroidogenic acute regulatory protein (38), which is required for the transfer of cholesterol into the mitochondria (39). The adipokine complement-Clq TNF-related protein 1 (CTRP1) stimulates aldosterone production by increasing the expression of CYP11B2 (40). In keeping with the idea that the adrenal gland is receiving additional stimulation to produce aldosterone, we found marked adrenal hypertrophy in the adult rats after 17 wk on the HF diet and increased CYP11B2 expression at both time points studied. Analysis of the structure of the adrenal showed that only the ZG was altered in the HF rats. One caveat to our study is that Ang II levels were not measured directly. It is possible that PRA falls as obesity develops because the pharmacokinetics of Ang II changes to reduce Ang II metabolism or increase its production. This aspect of our model requires further investigation.
Increases in dietary potassium also stimulate aldosterone production; the potassium content was similar in both the HF and control diets (0.9%), and there was no difference in plasma potassium between the groups. This suggests that potassium cannot be the stimulation for the increase in plasma aldosterone. Moreover, other studies have shown that dietary potassium needs to reach a much higher level (4%) before it can stimulate aldosterone production (41). The sodium contents of the diets are markedly different. The control diet contained 0.3% sodium, and the HF diet contained 0.056%. Although the sodium content of the HF diet is 3-fold higher than that of diets classically used to deplete sodium (42, 43), we cannot definitively rule out the possibility that the increase in aldosterone observed in the obese rats is a response to the lower sodium diet. Were sodium the only factor affecting aldosterone in the rats fed the HF diet, we would have expected the difference between the HF and control rats to be greater at 8 wk of age.
The effects of the elevated aldosterone in this model have not been fully investigated, but aldosterone could adversely affect glucose homeostasis because there was a trend toward a reduction in fasting blood glucose in the HF+CAN rats. Recent studies have shown that aldosterone infusion increases plasma glucose levels and reduces skeletal muscle expression of glucose transporter 4, the major skeletal muscle glucose transporter (44). This reduction in glucose transporter 4 expression could potentially increase insulin resistance. Studies using ob/ob and db/db mice suggest that MR activation is important for the pathogenesis of insulin resistance. The MR antagonist eplerenone reduced the insulin resistance and adipocyte size in obese mice (45).
Glucorticoids and activation of the hypothalamic pituitary adrenal axis have been linked with obesity and metabolic syndrome (46–48). However, it is clear that the association between cortisol/corticosterone and obesity is complex and often contradictory. A major issue in the field is that measurement of the steroid alone provides too simplistic a view of the system. One has to also consider the rate of production/degradation of the steroid, and the activity of the 11β-hydroxysteroid dehydrogenase type 1 is increasingly recognized as an important determinant of the regulation of the actions of glucocorticoids (49). In our study we failed to observe a difference in the depth of the ZF/ZR in the obese rats. Corticosterone levels were not changed after 8 wk of the HF diet, and there was only a trend toward an increase after 17 wk. Although these results do not completely rule out an activation of the hypothalamic-pituitary-adrenal axis, they certainly suggest that corticosterone is not a major determinant of the pathology of obesity in this model. Our results fit with some recent clinical studies. For example, a recent study showed a negative correlation between plasma cortisol and body weight in patients ranging from 21–32 yr of age (50). Other studies have shown that cortisol is increased in Latino children (8–13 yr) with metabolic syndrome, but this increase was independent of body fat and plasma insulin (51). In terms of rodent studies, very few have been conducted using the early treatment paradigm used here. One recent study, however, used a neonatal overfeeding model to increase the body weight in rats. Corticosterone was only increased in these rats at the later time points of 16 wk of age (52). Another recent study using Zucker obese rats showed that, at 13 wk of age, corticosterone was not different between the obese and control rats (53).
The levels of many of the other hormones measured in the current study relate well to previously published human and animal studies of obesity. The one measure that did not provide the expected response was that of plasma adiponectin, which exhibited a small but significant increase in rats with both early and sustained obesity. Adiponectin is primarily produced by adipose tissue and acts in the periphery to control glucose metabolism and centrally to reduce body weight (54). Most studies suggest adiponectin is reduced in obesity and that an inverse correlation exists between adiponectin levels and insulin resistance (55). That said, there are reports in the literature showing increased circulating adiponectin in rats fed a HF diet (56). In a recent study, 6-wk-old Wistar rats were given a diet similar to the one used here for approximately 10 wk. This study showed, not only that adiponectin levels were increased in the rats fed the HF diet, but that the pattern of adiponectin secretion into the plasma changes throughout the day. The peak adiponectin production in the fat rats was earlier than observed in the lean controls. In this study the reported levels of adiponectin were about 10-fold higher than those observed here (56). It is unclear whether this is due to a strain difference or to a difference in how the plasma samples were obtained.
Conclusions
We have shown that rats fed HF diet from weaning exhibit hyperaldosteronism that is linked to an increase in the expression of CYP11B2 and ZG hypertrophy. Interestingly, the increase in circulating aldosterone is linked to PRA in the early stages of obesity, but this link appears to be broken in the sustained phase of obesity. We propose that this model of obesity may be useful to study the cardiovascular effects of obesity-related hyperaldosteronism. Our studies using MR antagonists suggest that aldosterone is not a key regulator of blood pressure in the rats with obesity-induced hypertension but that it may play a considerable role in obesity-associated changes in vascular structure.
Acknowledgments
This work was supported by Grant 7-07-RA28 from the American Diabetes Association (to A.M.D.), Grant 0840122N from the American Heart Association (to A.M.D.), and Grants from the National Institutes of Health (Grant HL087927 to C.A.N. and Grant DK43140 to W.E.R.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Ang
- Angiotensin
- AT1Ra
- angiotensin type 1a receptor
- CYP11B2
- aldosterone synthase
- HF
- high fat
- HF+CAN
- HF diet + canrenoic acid
- HR
- heart rate
- MAP
- mean arterial pressure
- MR
- mineralocorticoid receptor
- MRA
- mesenteric resistance artery
- PRA
- plasma renin activity
- ZF
- zona fasiculata
- ZG
- zona glomerulosa
- ZR
- zona reticularis.
References
- 1. Hall JE. 2003. The kidney, hypertension, and obesity. Hypertension 41:625–633 [DOI] [PubMed] [Google Scholar]
- 2. Sironi AM, Gastaldelli A, Mari A, Ciociaro D, Positano V, Postano V, Buzzigoli E, Ghione S, Turchi S, Lombardi M, Ferrannini E. 2004. Visceral fat in hypertension: influence on insulin resistance and β-cell function. Hypertension 44:127–133 [DOI] [PubMed] [Google Scholar]
- 3. Malik S, Wong ND, Franklin SS, Kamath TV, L'Italien GJ, Pio JR, Williams GR. 2004. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation 110:1245–1250 [DOI] [PubMed] [Google Scholar]
- 4. Reaven GM. 1988. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 37:1595–1607 [DOI] [PubMed] [Google Scholar]
- 5. Nagase M, Fujita T. 2009. Mineralocorticoid receptor activation in obesity hypertension. Hypertens Res 32:649–657 [DOI] [PubMed] [Google Scholar]
- 6. Sowers JR, Whaley-Connell A, Epstein M. 2009. Narrative review: the emerging clinical implications of the role of aldosterone in the metabolic syndrome and resistant hypertension. Ann Intern Med 150:776–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Levin BE, Triscari J, Sullivan AC. 1983. Relationship between sympathetic activity and diet-induced obesity in two rat strains. Am J Physiol 245:R364–R371 [DOI] [PubMed] [Google Scholar]
- 8. Dobrian AD, Davies MJ, Prewitt RL, Lauterio TJ. 2000. Development of hypertension in a rat model of diet-induced obesity. Hypertension 35:1009–1015 [DOI] [PubMed] [Google Scholar]
- 9. Rocchini AP. 2002. Childhood obesity and a diabetes epidemic. N Engl J Med 346:854–855 [DOI] [PubMed] [Google Scholar]
- 10. Kvaavik E, Tell GS, Klepp KI. 2003. Predictors and tracking of body mass index from adolescence into adulthood: follow-up of 18 to 20 years in the Oslo Youth Study. Arch Pediatr Adolesc Med 157:1212–1218 [DOI] [PubMed] [Google Scholar]
- 11. Must A, Jacques PF, Dallal GE, Bajema CJ, Dietz WH. 1992. Long-term morbidity and mortality of overweight adolescents. A follow-up of the Harvard Growth Study of 1922 to 1935. N Engl J Med 327:1350–1355 [DOI] [PubMed] [Google Scholar]
- 12. Ogden CL, Carroll MD, Flegal KM. 2008. High body mass index for age among US children and adolescents, 2003–2006. JAMA 299:2401–2405 [DOI] [PubMed] [Google Scholar]
- 13. Smith AD, Brands MW, Wang MH, Dorrance AM. 2006. Obesity-induced hypertension develops in young rats independently of the renin-angiotensin-aldosterone system. Exp Biol Med (Maywood) 231:282–287 [DOI] [PubMed] [Google Scholar]
- 14. Deutsch C, Portik-Dobos V, Smith AD, Ergul A, Dorrance AM. 2009. Diet-induced obesity causes cerebral vessel remodeling and increases the damage caused by ischemic stroke. Microvasc Res 78:100–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Karim A. 1978. Spironolactone: disposition, metabolism, pharmacodynamics, and bioavailability. Drug Metab Rev 8:151–188 [DOI] [PubMed] [Google Scholar]
- 16. Young M, Funder J. 2003. Mineralocorticoid action and sodium-hydrogen exchange: studies in experimental cardiac fibrosis. Endocrinology 144:3848–3851 [DOI] [PubMed] [Google Scholar]
- 17. Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Δ Δ C(T)) Method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 18. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. 1985. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419 [DOI] [PubMed] [Google Scholar]
- 19. Pires PW, Deutsch C, McClain JL, Rogers CT, Dorrance AM. 2010. Tempol, a super oxide dismutase mimetic, prevents cerebral vessel remodeling in hypertensive rats. Microvasc Res 80:445–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rigsby CS, Ergul A, Portik Dobos V, Pollock DM, Dorrance AM. 2011. Effects of spironolactone on cerebral vessel structure in rats with sustained hypertension. Am J Hypertens 24:708–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rigsby CS, Pollock DM, Dorrance AM. 2007. Spironolactone improves structure and increases tone in the cerebral vasculature of male spontaneously hypertensive stroke-prone rats. Microvasc Res 73:198–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kaltman AJ, Goldring RM. 1976. Role of circulatory congestion in the cardiorespiratory failure of obesity. Am J Med 60:645–653 [DOI] [PubMed] [Google Scholar]
- 23. Carroll JF, Zenebe WJ, Strange TB. 2006. Cardiovascular function in a rat model of diet-induced obesity. Hypertension 48:65–72 [DOI] [PubMed] [Google Scholar]
- 24. Smith AD, Dorrance AM. 2006. Arachidonic acid induces augmented vasoconstriction via cyclooxygenase 1 in the aorta from rats fed a high-fat diet. Prostaglandins Leukot Essent Fatty Acids 75:43–49 [DOI] [PubMed] [Google Scholar]
- 25. Zhou Y, Lin S, Chang HH, Du J, Dong Z, Dorrance AM, Brands MW, Wang MH. 2005. Gender differences of renal CYP-derived eicosanoid synthesis in rats fed a high-fat diet. Am J Hypertens 18:530–537 [DOI] [PubMed] [Google Scholar]
- 26. Dobrian AD, Schriver SD, Khraibi AA, Prewitt RL. 2004. Pioglitazone prevents hypertension and reduces oxidative stress in diet-induced obesity. Hypertension 43:48–56 [DOI] [PubMed] [Google Scholar]
- 27. Tuck ML, Sowers J, Dornfeld L, Kledzik G, Maxwell M. 1981. The effect of weight reduction on blood pressure, plasma renin activity, and plasma aldosterone levels in obese patients. N Engl J Med 304:930–933 [DOI] [PubMed] [Google Scholar]
- 28. Guo C, Ricchiuti V, Lian BQ, Yao TM, Coutinho P, Romero JR, Li J, Williams GH, Adler GK. 2008. Mineralocorticoid receptor blockade reverses obesity-related changes in expression of adiponectin, peroxisome proliferator-activated receptor-gamma, and proinflammatory adipokines. Circulation 117:2253–2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ndisang JF, Lane N, Jadhav A. 2009. The heme oxygenase system abates hyperglycemia in Zucker diabetic fatty rats by potentiating insulin-sensitizing pathways. Endocrinology 150:2098–2108 [DOI] [PubMed] [Google Scholar]
- 30. de Paula RB, da Silva AA, Hall JE. 2004. Aldosterone antagonism attenuates obesity-induced hypertension and glomerular hyperfiltration. Hypertension 43:41–47 [DOI] [PubMed] [Google Scholar]
- 31. Rocchini AP, Key J, Bondie D, Chico R, Moorehead C, Katch V, Martin M. 1989. The effect of weight loss on the sensitivity of blood pressure to sodium in obese adolescents. N Engl J Med 321:580–585 [DOI] [PubMed] [Google Scholar]
- 32. Egan BM, Stepniakowski K, Goodfriend TL. 1994. Renin and aldosterone are higher and the hyperinsulinemic effect of salt restriction greater in subjects with risk factors clustering. Am J Hypertens 7:886–893 [DOI] [PubMed] [Google Scholar]
- 33. Licata G, Scaglione R, Ganguzza A, Corrao S, Donatelli M, Parrinello G, Dichiara MA, Merlino G, Cecala MG. 1994. Central obesity and hypertension. Relationship between fasting serum insulin, plasma renin activity, and diastolic blood pressure in young obese subjects. Am J Hypertens 7:314–320 [DOI] [PubMed] [Google Scholar]
- 34. Thatcher S, Yiannikouris F, Gupte M, Cassis L. 2009. The adipose renin-angiotensin system: role in cardiovascular disease. Mol Cell Endocrinol 302:111–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. MacKenzie SM, Huda SS, Sattar N, Fraser R, Connell JM, Davies E. 2008. Depot-specific steroidogenic gene transcription in human adipose tissue. Clin Endocrinol (Oxf) 69:848–854 [DOI] [PubMed] [Google Scholar]
- 36. Ehrhart-Bornstein M, Lamounier-Zepter V, Schraven A, Langenbach J, Willenberg HS, Barthel A, Hauner H, McCann SM, Scherbaum WA, Bornstein SR. 2003. Human adipocytes secrete mineralocorticoid-releasing factors. Proc Natl Acad Sci USA 100:14211–14216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Goodfriend TL, Ball DL, Egan BM, Campbell WB, Nithipatikom K. 2004. Epoxy-keto derivative of linoleic acid stimulates aldosterone secretion. Hypertension 43:358–363 [DOI] [PubMed] [Google Scholar]
- 38. Schinner S, Willenberg HS, Krause D, Schott M, Lamounier-Zepter V, Krug AW, Ehrhart-Bornstein M, Bornstein SR, Scherbaum WA. 2007. Adipocyte-derived products induce the transcription of the StAR promoter and stimulate aldosterone and cortisol secretion from adrenocortical cells through the Wnt-signaling pathway. Int J Obes (Lond) 31:864–870 [DOI] [PubMed] [Google Scholar]
- 39. Stocco DM, Wang X, Jo Y, Manna PR. 2005. Multiple signaling pathways regulating steroidogenesis and steroidogenic acute regulatory protein expression: more complicated than we thought. Mol Endocrinol 19:2647–2659 [DOI] [PubMed] [Google Scholar]
- 40. Jeon JH, Kim KY, Kim JH, Baek A, Cho H, Lee YH, Kim JW, Kim D, Han SH, Lim JS, Kim KI, Yoon do Y, Kim SH, Oh GT, Kim E, Yang Y. 2008. A novel adipokine CTRP1 stimulates aldosterone production. FASEB J 22:1502–1511 [DOI] [PubMed] [Google Scholar]
- 41. Manger WM, Simchon S, Stier CT, Jr, Loscalzo J, Jan KM, Jan R, Haddy F. 2003. Protective effects of dietary potassium chloride on hemodynamics of Dahl salt-sensitive rats in response to chronic administration of sodium chloride. J Hypertens 21:2305–2313 [DOI] [PubMed] [Google Scholar]
- 42. Osmond JM, Mintz JD, Stepp DW. 2010. Preventing increased blood pressure in the obese Zucker rat improves severity of stroke. Am J Physiol Heart Circ Physiol 299:H55–H61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tran PV, Georgieff MK, Engeland WC. 2010. Sodium depletion increases sympathetic neurite outgrowth and expression of a novel TMEM35 gene-derived protein (TUF1) in the rat adrenal zona glomerulosa. Endocrinology 151:4852–4860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Selvaraj J, Muthusamy T, Srinivasan C, Balasubramanian K. 2009. Impact of excess aldosterone on glucose homeostasis in adult male rat. Clin Chim Acta 407:51–57 [DOI] [PubMed] [Google Scholar]
- 45. Hirata A, Maeda N, Hiuge A, Hibuse T, Fujita K, Okada T, Kihara S, Funahashi T, Shimomura I. 2009. Blockade of mineralocorticoid receptor reverses adipocyte dysfunction and insulin resistance in obese mice. Cardiovasc Res 84:164–172 [DOI] [PubMed] [Google Scholar]
- 46. Walker BR. 2006. Cortisol–cause and cure for metabolic syndrome? Diabet Med 23:1281–1288 [DOI] [PubMed] [Google Scholar]
- 47. Anagnostis P, Athyros VG, Tziomalos K, Karagiannis A, Mikhailidis DP. 2009. Clinical review: the pathogenetic role of cortisol in the metabolic syndrome: a hypothesis. J Clin Endocrinol Metab 94:2692–2701 [DOI] [PubMed] [Google Scholar]
- 48. Duclos M, Marquez Pereira P, Barat P, Gatta B, Roger P. 2005. Increased cortisol bioavailability, abdominal obesity, and the metabolic syndrome in obese women. Obes Res 13:1157–1166 [DOI] [PubMed] [Google Scholar]
- 49. Seckl JR, Walker BR. 2001. Minireview: 11beta-hydroxysteroid dehydrogenase type 1—a tissue-specific amplifier of glucocorticoid action. Endocrinology 142:1371–1376 [DOI] [PubMed] [Google Scholar]
- 50. Praveen EP, Sahoo JP, Kulshreshtha B, Khurana ML, Gupta N, Dwivedi SN, Kumar G, Ammini AC. 2011. Morning cortisol is lower in obese individuals with normal glucose tolerance. Diabetes Metab Syndr Obes 4:347–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Weigensberg MJ, Toledo-Corral CM, Goran MI. 2008. Association between the metabolic syndrome and serum cortisol in overweight Latino youth. J Clin Endocrinol Metab 93:1372–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hou M, Liu Y, Zhu L, Sun B, Guo M, Burén J, Li X. 2011. Neonatal overfeeding induced by small litter rearing causes altered glucocorticoid metabolism in rats. PLoS One 6:e25726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. De Ceuninck F, Rolin JO, Caliez A, Baschet L, Ktorza A. 2011. Metabolic imbalance of the insulin-like growth factor-I axis in Zucker diabetic fatty rats. Metabolism 60:1575–1583 [DOI] [PubMed] [Google Scholar]
- 54. Qi Y, Takahashi N, Hileman SM, Patel HR, Berg AH, Pajvani UB, Scherer PE, Ahima RS. 2004. Adiponectin acts in the brain to decrease body weight. Nat Med 10:524–529 [DOI] [PubMed] [Google Scholar]
- 55. Nishida M, Funahashi T, Shimomura I. 2007. Pathophysiological significance of adiponectin. Med Mol Morphol 40:55–67 [DOI] [PubMed] [Google Scholar]
- 56. Cano P, Cardinali DP, Rios-Lugo MJ, Fernandez-Mateos MP, Reyes Toso CF, Esquifino AI. 2009. Effect of a high-fat diet on 24-hour pattern of circulating adipocytokines in rats. Obesity (Silver Spring) 17:1866–1871 [DOI] [PubMed] [Google Scholar]