Abstract
GH is best known as an anterior pituitary hormone fundamental in regulating growth, differentiation, and metabolism. GH peptide and mRNA are also present in brain, in which their functions are less well known. Here we describe the distribution of GH neurons and fibers and sex differences in Gh mRNA in adult mouse brain. Cell bodies exhibiting GH immunoreactivity are distributed in many brain regions, particularly in the hypothalamus in which retrograde labeling suggests that some of these cells project to the median eminence. To determine whether Gh mRNA is sexual dimorphic, we carried out quantitative RT-PCR on microdissected brain nuclei. Ovary-intact mice had elevated Gh mRNA in the arcuate nucleus and medial preoptic area (MPOA) compared with gonad-intact males. In males, castration increased Gh mRNA in the MPOA, whereas ovariectomy decreased Gh mRNA in both regions. When gonadectomized adults of both sexes were treated with estradiol Gh mRNA increased in females but had no effect in castrated males. Tamoxifen was able to blunt the rise in Gh mRNA in response to estradiol in females. In addition, we found that estrogen receptor-α is coexpressed in GH neurons in the MPOA and arcuate nucleus. In summary, the findings reveal sexual dimorphisms in Gh gene expression in areas of the brain associated with reproduction and behavior. Interestingly, estradiol enhances Gh mRNA in females only, suggesting that multiple factors orchestrate this sexual dimorphism.
GH is a 191-amino acid peptide that is well known for its role as an endocrine hormone secreted episodically from the somatotropes of the anterior pituitary, regulating growth, differentiation, and metabolism at peripheral target sites (1). GH receptors are widely expressed in the periphery and also in the central nervous system, in which GH has a number of putative actions including regulation of neuronal growth and development, synaptic plasticity, and neuronal protection (2, 3). Furthermore, GH may influence cognition, mood, memory, and behavior, as suggested by studies in GH-deficient humans and animals (4–7). In rats, GH treatment enhances learning and memory, hippocampal N-methyl-d-aspartate receptor expression, and synaptic plasticity; and the endogenous changes in hippocampal GH expression occur after a learned memory task (8–11).
The pituitary GH secretory pattern varies at different stages of the estrous cycle, as estradiol stimulates its release (12, 13). In the rat brain, Gh mRNA levels in hippocampus are higher in adult than in prepubertal animals and higher in females than in males, particularly when females are in proestrus. After ovariectomy, hippocampal Gh mRNA is also elevated in response to estradiol. This same pattern is seen in primary neuronal cultures (11). In the rat hippocampus, Gh mRNA was higher After hippocampal-dependent learning, suggesting GH may play a role in central brain functions (14). Sex differences were examined in the transcriptomes of hypothalamus, pituitary, and cortex of male and female mice using serial analysis of gene expression (15). Interestingly, GH was expressed at dramatically higher levels in female compared with male mice in the hypothalamus and cortex but not in the pituitary (15).
The distribution of GH has been examined in the developing and adult avian brain (16–18). Although GH protein has been measured in homogenates from dissected rat and monkey brain regions (19), the precise distribution of GH protein in any mammalian brain has not been described. First, we mapped GH in the mouse brain using immunocytochemistry. Because GH immunoreactivity (GH-IR) displayed high density and intensity in nuclei of the hypothalamus, we focused our studies therein. We combined retrograde neuronal tracing with GH immunofluorescence to determine whether GH neurons extend their axon terminals to the median eminence (ME). Subsequently we tested the hypothesis that gonadal hormones, in particular estradiol, regulate Gh gene expression in several nuclei. We examined selected hypothalamic and extrahypothalamic regions from gonad-intact and gonadectomized (GDX) adults. GH mRNA was highest in ovary-intact females; thus, we compared GDX males and females treated with estradiol with untreated controls. Finally, in females only, we investigated whether the estradiol antagonist, tamoxifen, could block the effects of estradiol. We found that Gh mRNA was markedly impacted by gonadal hormone status in the medial preoptic area (MPOA) and arcuate nucleus (ARC) in a manner that was dependent on the sex. In females, estradiol increased Gh mRNA in the MPOA and ARC, which is consistent with the extensive coexpression of GH and estrogen receptor (ER)-α in both regions.
Materials and Methods
Animals
All animal procedures were conducted in accordance with the University of Virginia Animal Care and Use Committee guidelines. Male and female C57BL/6J mice used in these studies were weaned at postnatal d 20–21 and group housed until they were killed or underwent gonadectomy at between 70 and 90 d of age for males and 60 and 90 d for females. All mice were individually housed after hormone implants. Mice were maintained on a 12-h light, 12-h dark cycle (lights off at 1200 h). Animals had ad libitum access to water and food (7912; Harlan Teklad, Madison, WI).
Gonadectomy and hormone replacement
Removal of ovaries or testes and sc implantation (into the back of the neck) of a 5-mm SILASTIC tube (Dow Corning, Corp., Midland, MI; 1.98 mm inner diameter × 3.18 mm outer diameter) filled with 2 mg/ml 17β-estradiol benzoate (EB) (21) in sesame oil (25 μl), or empty (control) was conducted under isofluorane anesthesia. The EB implants yield an average plasma level of 140 pg/ml 17β-estradiol (Rissman, E.F., unpublished observations). For gonadectomies mice were anesthetized with isoflurane inhalant, one midline incision was made, and both gonads were exposed, ligated, and removed. Gut suture was used on the inner dermal layer and a single wound clip applied to suture the skin. After gonad removal, mice were administered a sc injection of 0.9% sodium chloride (for rehydration) and a topical analgesic (0.25% bupivacaine) and kept warm until awakening. For the tamoxifen study, females were implanted with a tamoxifen implant (Innovative Research of America, Sarasota, FL; 5 mg per 21 d release or 0.23 mg daily) or an empty SILASTIC implant of the same size (Dow Corning) at the time of ovariectomy, and then 5 d later all animals were reanesthetized and each received an EB implant.
Tissue collection and Immunocytochemistry
Adult male (n = 6) and female (n = 6) mice were deeply anesthetized with sodium pentobarbital (0.5 mg, ip) and perfused with heparinized saline for 10 min, followed by fixation with modified Zamboni's fixative [4% paraformaldehyde containing 15% saturated picric acid in 0.1M sodium phosphate buffer (pH7.4)] for 25 min. Brains were removed and postfixed in Zamboni's fixative for 1 h and then placed in 30% sucrose in 0.05 m Tris-buffered saline (TBS) for cryoprotection. Brains were then frozen and were cut in a cryostat at 30 μm. Coronal sections from the entire brain (including the brainstem and cerebellum) were collected in three vials and stored in antifreeze until processing. Sections were rinsed in 0.05 m TBS followed by 0.3% hydrogen peroxide and then 1% sodium borohydride. Sections were incubated overnight at room temperature in GH primary antiserum (GH rabbit antirat, 1:50K; purchased from Dr. A. F. Parlow, National Hormone and Peptide Program, Torrance, CA) in TBS carrier solution (0.25% λ-carageenan, 0.1% sodium azide, 0.5% BSA, 1.5% Triton X-100, and 3% normal goat serum) followed by incubation for 1 h in biotinylated goat antirabbit IgG (1:500; Vector Laboratories, Burlingame, CA). After rinses, sections were incubated for 1 h in avidin-biotin complex (1:1000; Vectastain Elite; Vector Laboratories) and developed with nickel-intensified diaminobenzidine (DAB) activated by 0.001% hydrogen peroxide. For negative controls, sections from one brain were incubated in carrier solution without GH antiserum, and sections from two brains were incubated with GH antiserum that had been preadsorbed with 2 mg/ml rat GH (purchased from Dr. A. F. Parlow, National Hormone and Peptide Program). This was the lowest amount of peptide that completely blocked the GH immunoreactive signal. Both control runs resulted in the absence of immunoreactivity. To aid the neuroanatomical designation of GH-IR according to the mouse brain atlas of Paxinos and Franklin (22), one third of the sections from each brain were counterstained with Nuclear Red (Sigma, St. Louis, MO).
1,1′-Dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI) retrograde tracing with GH immunofluorescence
Mice were killed and perfused as described with a shorter perfusion time with the fixative. Brains (n = 2 of each sex) were removed and postfixed for 1 h. Lightly fixed brains were removed from postfixed and carefully placed on their dorsal sides to expose the ME. Crystals of the carbocyanine compound, DiI, were applied to the ME under a dissecting microscope using a fine needle, and brains were placed back into fixative at room temperature in the dark for 3 months to allow retrograde transport of DiI (23). Next, brains were placed into 30% sucrose (TBS) for cryoprotection and were frozen on dry ice, sectioned at 30 μm into three wells of 0.05 m TBS, and processed for GH immunofluorescence. Sections were rinsed in 0.05 m TBS and then blocked for 30 min in 10% normal donkey serum (Millipore, Billerica, MA), rinsed again in TBS, and incubated overnight at room temperature in antirat GH antiserum (1:1000) in carrier solution (with 3% normal donkey serum). After rinses in TBS, the tissue was incubated in secondary antiserum Alexa-Fluor 488 donkey antirabbit (1:250; Vector Laboratories) in carrier solution for 1 h. Sections were then coverslipped using ProLong Gold antifade reagent with 4′,6′-diamino-2-phenylindole (DAPI; Invitrogen, Carlsbad, CA) (to allow fluorescent nuclear counterstaining) and stored in the dark at 4 C until visualization by confocal microscopy.
Double immunocytochemistry for GH and neuronal nuclei (Neu-N) or ERα
For double labeling, sections from four mice of each sex were processed as described above, except in place of GH antiserum, either an antimouse Neu-N antibody (1:8000; Chemicon, Temecula, CA) or a rabbit polyclonal antibody to ERα (C1355, 1:5000; Upstate Biotechnology, Lake Placid, NY) was used (20). Sections were developed with nickel-intensified DAB, rinsed in 0.05 m TBS and placed in antirat GH antiserum overnight followed by development as described above. For GH labeling, DAB without nickel intensification was used, which resulted in brown cytoplasmic staining of GH cells.
Qualitative image analysis
For qualitative analysis of GH-IR in the brain, sections from the entire brain were examined under a microscope (Olympus, Tokyo, Japan; ×60) and Metamorph imaging software (Molecular Devices, Chester, PA) was used to view and capture photomicrographs of GH-IR at different magnifications. Locations with GH-IR were defined using the landmarks of the mouse brain atlas (22) according to density (−, +, ++, or +++) and intensity (low, medium, or high) and in relation to GH-IR in the cortex, which has been previously described in the avian brain (16). For analysis of sections processed for GH immunofluorescence from DiI brains, brain regions exhibiting DiI, GH, and DAPI were visualized and captured using a confocal microscope according to the manufacturer's spectral characteristics [wavelengths: DiI (red), 555nm; GH/AlexaFluor (24), 488 nm; DAPI, 405 nm]. All areas known to display GH-IR were examined for GH/DiI colocalization. Two dimensional image stacks were constructed by scanning a series of thinner (0.112 μm) slices.
Tissue collection for gene expression studies
Animals were deeply anesthetized with isofluorane at the same time of day for each study (1600 h) and immediately decapitated. Brains were rapidly removed, frozen on dry ice, and stored at −80 C. Brains were sectioned coronally at 120 μm and mounted onto superfrost plus slides. Discrete brain nuclei were punched from frozen brain sections using a brain micropunch kit (Fisher Scientific, Pittsburgh, PA), and the appropriate diameters were chosen according to the size of nucleus and/or the proximity of surrounding nuclei (25). To ensure methodological consistency, the number of punches for each nucleus was determined in relation to a landmark (the crossing of the anterior commissure) using the coordinates of the mouse brain atlas, taking into account the proximity of other nuclei [coordinates: ARC, −1.34 to −2.70 mm; ventral pallidum (VP), 1.94 to 0.14 mm; nucleus accumbens (26), 1.94 to −0.86 mm; MPOA, 0.38 to −0.22 mm from Bregma]. Punched tissue was stored at −80 C until use.
RNA isolation, cDNA amplification, and real-time PCR
Total RNA was extracted from brain punches using an RNeasy lipid tissue minikit (Agilent, Santa Clara, CA) according to the manufacturer's protocol. The cDNA template was reverse transcribed from 50 to 200 ng RNA using an AffinityScript cDNA synthesis kit (Agilent). The PCR cycle was programmed to carry out primer annealing at 25 C for 5 min, cDNA synthesis at 42 C for 45 min, and heat inactivation at 95 C for 5 min. Real-time PCR was performed using an ABI Prism 275 7300 real-time PCR system (Applied Biosystems, Foster City, CA). GH primers (forward: 5′-TCCAGGCTCTGATGCAGGAGCT, reverse: 5′-GCTGCGCATGTTGGCGTCAA; accession number NM_008117.2) were designed using the National Center for Biotechnology Information Primerblast primer tool (Bethesda, MD) and code for a product length of 95 that spans exons 1 and 2 of the mouse Gh gene. β-Actin mRNA was measured as the endogenous control gene (forward: 5′-CCAGATCATGTTTGAGACCTTCAA, reverse: 5′-CCAGAGGCGTACAGGGATAGC). All primers were synthesized by Invitrogen. In a 25-μl total PCR volume, 5 ng cDNA was mixed with FAST SYBR Green master mix (Applied Biosystems) and 500 nm Gh or β-actin primers in duplicate for each sample. These primers were validated for equally efficient target (Gh primers, efficiency 97%) and endogenous control (β-actin primers, efficiency 96%) RNA amplifications. GH gene expression levels were quantified based on the threshold cycle for each well using the Applied Biosystems sequence detection software and normalized to β-actin as the endogenous control.
Designs of Gh expression studies
Role of gonadal hormones
In the first study, we asked whether gonad removal affected Gh mRNA in either sex. Males and females were either gonad intact or GDX 2 wk before brain collection. Group sizes ranged from four to 10 in each group (intact females, GDX females, intact males, GDX males). We examined four nuclei: MPOA, VP, ARC, and nucleus accumbens (NAc).
Role of estradiol
In the next study, we investigated whether replacement of estradiol would alter Gh mRNA in two regions (MPOA and ARC) responsive to GDX. We used gonadectomized males and females treated with either EB or blank implants. A total of six to nine mice were used in each group; all mice were killed 1 wk after surgery.
Blockade of ER with tamoxifen
In the final experiment, females (n = 10) were ovariectomized and 1 wk later were randomly allocated (ensuring a similar mean body weight) into two groups, in which they received either a tamoxifen implant (n = 5) or an empty implant (n = 5). Five days later all mice were given an EB implant at the same dose used in the previous studies. One week later all animals were killed and the MPOA and ARC were collected as previously described.
Statistical analysis
For calculation of GH mRNA expression levels, the 2(δ δ) cycle threshold method was used (27). Gh mRNA expression in brain nuclei of randomly cycling, ovary-intact females proved to be highly variable, and thus, data were not normally distributed. Therefore, data were log transformed before statistical analysis. For Gh mRNA expression data, we used two-way ANOVA with sex and gonadal status, or sex and hormones as factors, and planned comparisons were assessed by Bonferonni's post hoc tests. Data from the tamoxifen study, with only two groups, were analyzed using a Student's t test, corrected for multiple comparisons. A P ≤ 0.05 was considered statistically significant.
Results
Neural distribution of GH-IR in mice
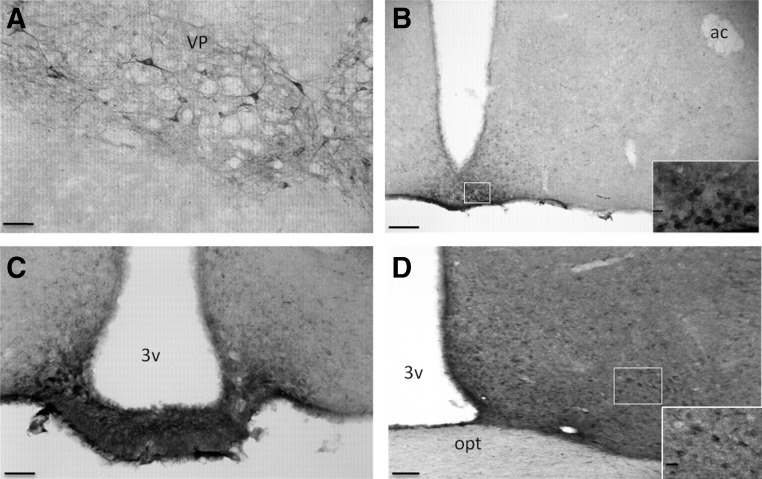
GH-IR in the mouse brain revealed specific labeling throughout the central nervous system that was limited to select populations of cells or fibers/terminals in well-defined regions (see Table 1). GH cellular staining appeared as a diffuse reaction product throughout the cytoplasm, typically not in the cell nucleus (Fig. 1A). Controls confirmed the specificity of cell staining (data not shown). In the basal ganglia, GH-IR was observed in the anterior amygdala, VP (Fig. 1A), and NAc shell. In the VP, GH neurons exhibited intense staining and were observed in bundles, with fiber tracts extending toward and around the Islands of Calleja, as visualized by nuclear red counterstaining. GH-IR was observed in many areas of the frontal cortex, and a number of discrete hypothalamic nuclei, including the nucleus circularis, dorsomedial nucleus, ME, and ARC (Fig. 1, B and C), nuclei of the MPOA (Fig. 1D), lateral hypothalamus (28), mammillary nuclei, and in terminals in the ME (Fig. 1C). In the thalamus, GH-IR was restricted to the dorsal and posterior thalamic nuclei and the periaqueductal gray (Table 2). GH-IR was found in a number of regions in the midbrain, of which the external cortex of the inferior colliculus, interpeduncular nucleus, and the nucleus of the posterior commissure displayed the highest staining intensity. In the brainstem, GH-IR was observed in the ventral cochlear nucleus, olivary nuclei, superior vestibular nucleus, raphe pallidus, reticular nucleus, and area postrema (Table 3). Double-labeled GH and Neu-N was noted in the vast majority of GH cells, which suggested that GH is produced primarily in neurons (data not shown).
Table 1.
Distribution of GH in the telencephalon
| Brain structure | GH cell density | GH cell intensity | GH fiber density |
|---|---|---|---|
| Telencephalon | |||
| Olfactory bulb | |||
| GH cellsa | + | H | – |
| Cortical regions | |||
| Prelimbic cortex | ++ | M | – |
| Orbital cortex | + | M | – |
| Insular cortex | + | M | – |
| Cingulate cortex | ++ | M | – |
| Primary motor cortex | +++ | M | – |
| Primary somatosensory cortex | ++ | M | – |
| Tenia tecta | ++ | H | – |
| Lateral septum | + | M | – |
| Septal regions | |||
| Medial septum | ++ | M | – |
| Nucleus of the diagonal band | |||
| Vertical | ++ | M | – |
| Horizontal | +++ | H | +++ |
| Amygdaloid nuclei | – | ||
| Basal ganglia | |||
| Anterior amygdaloid area | ++ | H | ++ |
| Medial amygdaloid nucleus | + | M | – |
| Globus pallidus | ++ | L | – |
| Nucleus accumbens (shell) | +++ | H | – |
| Ventral pallidum | +++ | H | +++ |
| Islands of Calleja | – | − | +++ |
| Pyramidal cell layer | + | L | – |
| Hippocampus | |||
| Dentate gyrus | + | L | – |
L, Low; M, medium; H, high; −, not present.
GH cells: a diffuse population of neurons that were observed in the forebrain, lateral to the anterior olfactory nucleus and piriform cortex. This GH-IR-displaying region was designated as GH cells in our table because they did not fall into a predefined brain nucleus according to a published report (22).
Fig. 1.
Representative photomicrographs of GH-IR staining in VP (A), ARC (B), ME (C), and MPOA (D). Scale bar (A, C, and D), 50 μm, scale bar (B), 100 μm and in box insert, scale bar, 20 μm. ac, Anterior commissure; opt, optic chiasm; 3v, third ventricle.
Table 2.
Distribution of GH in the diencephalon and mesencephalon
| Brain structure | GH density | GH intensity | GH fiber density |
|---|---|---|---|
| Diencephalon | |||
| hypothalamus | |||
| Nucleus circularis | +++ | M | – |
| Anteroventral periventricular area | ++ | H | – |
| Medial preoptic nucleus | ++ | H | – |
| Magnocellular preoptic nucleus | ++ | H | ++ |
| Suprachiasmatic nucleus | ++ | M | – |
| Lateral preoptic nucleus | ++ | M | – |
| Ventrolateral preoptic nucleus | ++ | M | – |
| Dorsomedial nucleus | ++ | H | – |
| Arcuate nucleus | +++ | H | ++ |
| Median eminence | – | – | +++ |
| Ventromedial nucleus | + | M | – |
| Premammillary nucleus | ++ | M | – |
| Supramammillary nucleus | ++ | M | – |
| Lateral hypothalamic area | +++ | H | +++ |
| Posterior hypothalamic nucleus | ++ | M | – |
| Thalamus | |||
| Anterodorsal thalamic nucleus | ++ | H | ++ |
| Laterodorsal thalamic nucleus | ++ | H | ++ |
| Lateroposterior thalamic nucleus | ++ | H | – |
| Periaqueductal grey (dorsomedial) | ++ | M | – |
| Mesencephalon | |||
| Median raphe nucleus | ++ | M | – |
| Dorsal raphe nucleus | ++ | M | – |
| Interfascicular nucleus | ++ | M | – |
| Parabigeminal nucleus | +++ | L | – |
| Edinger-Westphall nucleus | + | M | – |
| Substantia nigra | +++ | M | – |
| Interpeduncular nucleus | +++ | H | +++ |
| Nucleus of the posterior commissure | +++ | H | – |
| External cortex of inferior colliculus | +++ | H | – |
L, Low; M, medium; H, high; −, not present.
Table 3.
Distribution of GH in the rhombencephalon and cerebellum
| Brain structure | GH density | GH intensity | GH fiber density |
|---|---|---|---|
| Rhombencephalon | |||
| Principal sensory trigeminal nucleus | + | M | +++ |
| Pontine nuclei | ++ | M | +++ |
| Parabrachial nucleus | ++ | M | ++ |
| Prepositus nucleus | + | H | + |
| Peripyramidal nucleus | +++ | H | ++ |
| Locus coeruleus | ++ | M | – |
| Inferior olivary nucleus | + | L | – |
| Ventral cochlear nucleus | +++ | H | + |
| Nucleus of the trapezoid body | ++ | L | – |
| Lateral lemniscus (ventral nucleus) | ++ | M | ++ |
| Paralemniscal nucleus | ++ | M | ++ |
| Medioventral preolivary nucleus | +++ | H | +++ |
| Superior paraolivary nucleus | + | H | + |
| Lateral superior olive | ++ | H | +++ |
| Superior vestibular nucleus | ++ | H | +++ |
| Gigantocellular nucleus (α-part) | ++ | M | ++ |
| Raphe magnus | – | – | ++ |
| Raphe pallidus | +++ | H | – |
| Area postrema | ++ | H | ++ |
| Facial nucleus (7n) | + | H | – |
| Spinal 5 nucleus | ++ | M | +++ |
| Intermediate reticular nucleus | +++ | H | ++ |
| Cerebellum | |||
| Purkinje cells | ++ | M | – |
L, Low; M, medium; H, high; −, not present.
Some GH neurons project to the ME and many cells are dual labeled with ERα
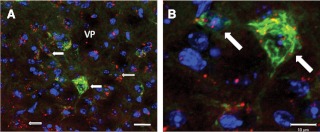
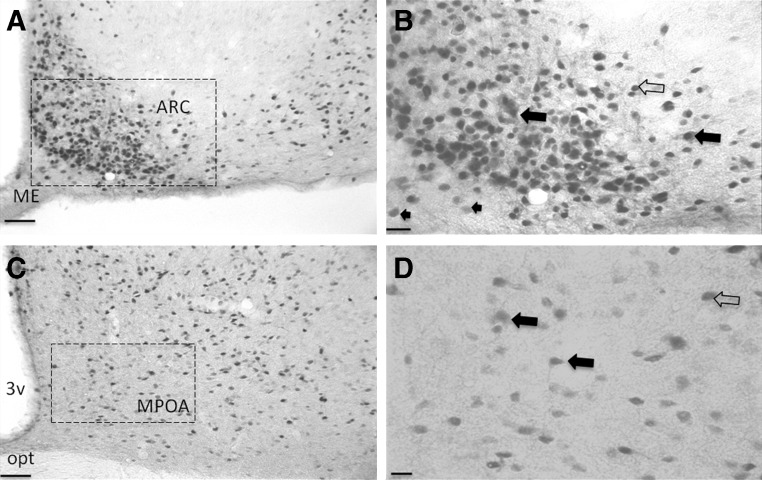
In the paraventricular nucleus, a region containing neurosecretory cells that project to the ME, intense DiI labeling was observed, which suggested that our protocol allowed enough time for DiI retrograde diffusion into cell bodies. DiI was found in many GH-positive neurons in the MPOA, lateral hypothalamus, and in VP (Fig. 2, A and B), suggesting that a subpopulation of GH neurons in these regions project to the ME, which may have physiological significance in the functions of GH neurons. In addition, many cells in the MPOA and ARC coexpressed GH and ERα (Fig. 3).
Fig. 2.
Confocal image of GH neurons in the VP. GH-IR was labeled green, and DAPI, a neuronal nucleus marker, was labeled blue, whereas DiI fluoresces red. Filled arrows indicate colocalization of GH, DAPI, and DiI, whereas open arrows indicate colocalization of DiI and DAPI only. A, GH, DAPI, and DiI colocalization in neurons within the VP (scale bar, 20 μm). B, High-magnification image of the white box in A showing a ±15 μm diameter GH neuron exhibiting DiI and DAPI colocalization (scale bar, 10 μm).
Fig. 3.
Sections dual labeled for ERα and GH. A, ARC showing GH cytoplasmic label (brown) and ERα in the nucleus (black) (scale bar, 50 μm). B, Magnified version of the area within the black box in A: closed arrows indicate colocalization between GH and ERα, whereas open arrows indicate cells labeled for ERα only, and arrowheads indicate cell labeled for GH only (scale bar, 20 μm). C, MPOA showing GH and ERα labeling (scale bar, 50 μm). D, Magnified version of the area within the black box in C showing colocalization between ERα and GH cells of the MPOA (scale bar, 10 μm). opt, Optic chiasm.
Gonadectomy alters GH gene expression in select brain nuclei
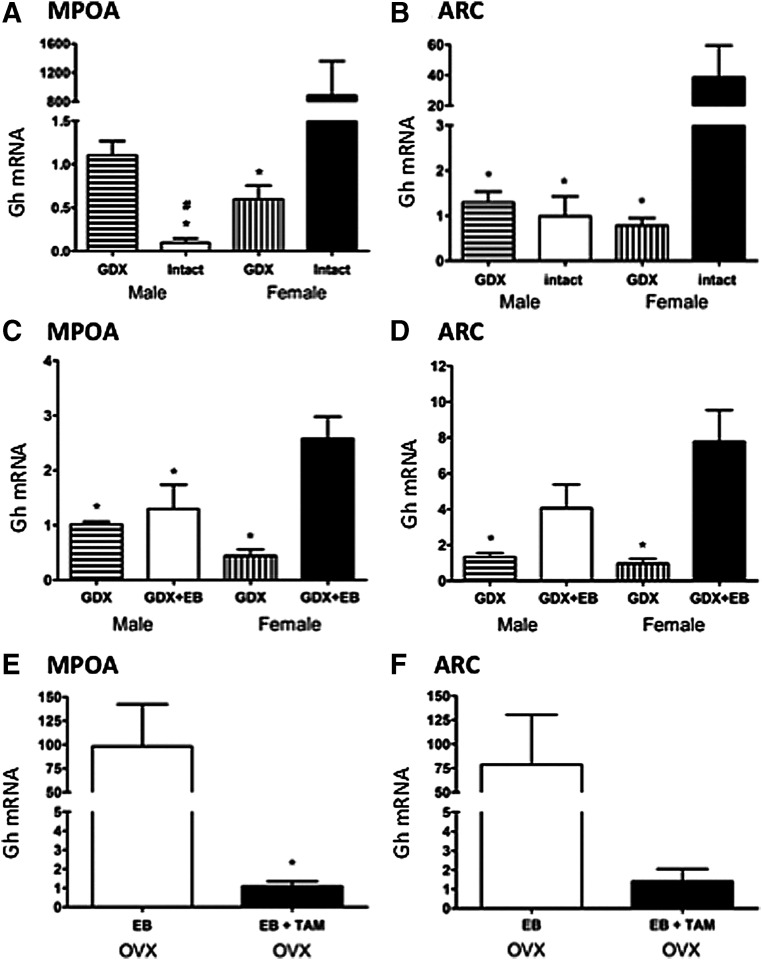
Confirming the appearance of more GH-IR in females we noted that expression of Gh mRNA in ovary-intact, randomly cycling females was much higher than in other groups in all areas examined. In the MPOA, expression of Gh mRNA was significantly influenced by sex [F(1, 26) = 125, P < 0.001] and gonadal status [F(1, 26) = 31.92, P < 0.001], and an interaction between these two factors was observed [F(1, 26) = 179.07, P < 0.001, Fig. 4A]. Expression of GH mRNA in the MPOA of gonad-intact females was significantly higher as compared with all three other groups (P < 0.05, Fig. 4A). Conversely, expression of Gh mRNA was significantly higher in GDX than in gonad-intact males (P < 0.05, Fig. 4A). In the ARC, expression of Gh mRNA was significantly influenced by gonadal status [F(1, 30) = 18.29, P < 0.001], and an interaction between sex and gonadal status was observed [F(1, 30) = 5.49, P < 0.03]. Expression of Gh mRNA in the ARC of ovary-intact females was nearly 40 times higher than in ovariectomized (OVX) females and different from all other groups (P < 0.05, Fig. 4B). Expression of Gh mRNA in the NAc showed a similar pattern as in the MPOA (higher in intact females than OVX females and lower in intact males than GDX males), but these differences did not reach statistical significance (Table 4). In the VP, expression of Gh mRNA was significantly influenced by gonadal status [F(1, 22) = 22.31, P < 0.001], and gonad-intact mice had higher Gh expression than GDX mice. There was no interaction between sex and gonadal status for the VP. Because the regulation of GH in the ARC and MPOA was most robustly affected by sex and gonadal status, these nuclei were further investigated in remaining studies.
Fig. 4.
Influence of sex and gonadal hormones on Gh mRNA expression (mean ± sem) in the MPOA and ARC. Gh mRNA expression MPOA (A, C, and E) and ARC (B, D, and F). In A and B, GDX and gonad males and females were compared. In C and D, GDX males and females implanted with EB (21) or empty implants for 7 d were examined. In E and F, we examined the combined effects of the ER antagonist tamoxifen (38) and EB on Gh mRNA in females only. *, Significantly different from ovary-intact females (A and B), GDX + EB females (C and D), and OVX + EB (E) P < 0.05, #, Significantly different from GDX males (P < 0.05).
Table 4.
GH mRNA expression in the NAc and VP of gonad-intact and GDX male and female mice
| Brain area | Group | GH mRNA relative to GDX males (mean ± sem) |
|---|---|---|
| NAc | GDX males | 1.12 ± 0.23 |
| Intact males | 0.27 ± 0.12a | |
| GDX females | 2.68 ± 0.8 | |
| Intact females | 5.13 ± 2.22 | |
| VP | GDX males | 1.4 ± 0.4 |
| Intact males | 62.7 ± 26.5a | |
| GDX females | 3.22 ± 1.63 | |
| Intact females | 42.0 ± 26.9 |
Significantly different from GDX males.
Estradiol increases GH gene expression in female ARC and MPOA
Here we found high levels of Gh mRNA in both the MPOA and ARC after GDX females were treated with EB. In the MPOA, Gh mRNA was significantly influenced by estradiol [F(1, 30) = 19.01, P < 0.001], and there was a significant interaction between sex and estradiol [F(1, 30) = 11.08, P < 0.003]. Expression of Gh mRNA in the MPOA of OVX females treated with EB was significantly higher than all other groups (P < 0.05, Fig. 4C). In the ARC, expression of GH mRNA was significantly increased by estradiol [F(1, 31) = 19.89, P < 0.001]. We also noted a trend toward an interaction between sex and estradiol [F(1, 31) = 3.54, P = 0.07]. Expression of Gh mRNA in the ARC of GDX females given EB was around 8 times higher than that of untreated GDX males and females (P < 0.05, Fig. 4D). In both the ARC and MPOA, there were no significant differences in Gh mRNA expression between GDX males given EB and untreated male mice.
Blockade of ER blocks the EB increase in GH mRNA
Because EB significantly increased Gh mRNA only in female brains, we investigated whether this effect could be blocked by pretreating OVX females with tamoxifen 5 d before estradiol treatment. In the MPOA, Gh mRNA was significantly lower in females treated with tamoxifen and EB compared with females treated with EB only (P < 0.01, Fig. 4E). In the ARC, we observed a trend in the same direction (Fig. 4F).
Discussion
Here we describe the general distribution of GH-IR in the mouse brain, sex differences in Gh mRNA, and the ability of estradiol to enhance expression particularly in females. The role of GH as a pituitary hormone regulating growth and differentiation at its target sites has been well characterized. However, the physiological role(s) of GH-producing neurons has remained largely unexplored. We found GH protein widely distributed throughout the brain, within neuronal cell bodies, and within fibers, suggesting it may be important for neural functioning. This is in line with evidence that GH may be involved in several neuronal processes including; neurogenesis, synaptic plasticity, and neural protection (2, 3, 29, 30). Indeed, GH has been found in the developing human and chick brains, in which in the latter it plays a role in the growth of neuronal axons in the neural retina (31, 32). In the adult avian brain, GH distribution was examined in the forebrain and was found to be predominantly expressed in cells and fibers in the hypothalamus and hippocampus (33). In accord with these results, we observed similar intense staining of neurons in the ARC (infundibular nucleus in the avian and human brains) and ME in the mouse brain, although we observed only light intensity staining in the dentate gyrus of the hippocampus of mice, which is in contrast to the intense staining in polydendritic cells as described in the hippocampus of avian and rat brains (11, 33). In contrast, adult male rat brain probed with Gh cDNA labeled with 32P or 3H, produced widespread signal in hippocampus, cortex, and caudate putamen, yet the signal was absent in the amygdala and hypothalamus (34).
We focused our experiments in the MPOA and ARC because of the appearance of qualitative sex differences in staining intensity. This observation was quantified at the level of Gh mRNA in which we found more expression in gonad-intact females and in males. The high degree of variability in gonad-intact females and the fact that removal of the ovaries in females reduced Gh mRNA levels led us to further investigate direct effects of estradiol on this sexual dimorphism. In both the ARC and MPOA, estradiol resulted in increased GH expression in females but not in males. Treatment of females with tamoxifen before estradiol blocked the ability of estradiol to enhance Gh gene expression in the MPOA, with a trend toward an effect in the ARC. This suggests the effect of estradiol on GH is dependent on activation of the estrogen receptors. Thus, our data are in agreement with the findings of Donahue et al. (11), who observed enhanced GH expression in the hippocampus of females on estradiol and that primary cortical neuronal cell lines significantly up-regulated GH mRNA in response to estradiol treatment. We observed ERα in the nuclei of most GH neurons in the ARC and MPOA, which implies that GH neurons may be directly influenced by estradiol via ERα. We did not assess ERβ; thus, whether estradiol influences GH expression in other areas of the brain through this receptor subtype remains to be determined. Our findings confirm a serial analysis of gene expression study that reported GH gene expression was dramatically higher in the hypothalamus and cortex of randomly cycling female mice compared with males (15).
There is substantial evidence for sexually dimorphic GH release from the pituitary. Pulses are larger, more regular, and episodic in males and smaller, more frequent, and more irregular in females (28). This leads to sex differences in downstream signaling pathways in target tissues, for instance, driving sex-specific gene expression in the liver (35). It is possible that sex differences in GH expression in the brain may be somehow related to sexual dimorphism in episodic GH release from the pituitary. It is possible that GH neurons project to the ME and contribute somehow to pituitary GH release. The fact that GH is found in hypophyseal portal blood in humans, and trace amounts of circulating GH are found in hypophysectomized rodents also suggests that small amounts of GH may be secreted at the level of the ME into the periphery (36, 37). There is evidence of physiological cross talk between GH synthesized in the brain and in the pituitary. Overexpression of GH in either the cerebral cortex or hypothalamus causes dwarfism due to reduced GH synthesis in the pituitary and reduced IGF-1 signaling in the liver. Furthermore, acute injection of GH into discrete hypothalamic areas reduces the release of GH by the pituitary (38). Interestingly, GH overexpression results in reduced amplitude of GH pulses and reduced growth when targeted to vasopressin neurons (39) and in dwarfism when targeted to GHRH neurons (26).
This negative feedback circuit also appears to work in the opposite direction because a central injection of a GH receptor antagonist enhances GH pulse amplitude and a central administration of a GH receptor mRNA antisense increases GH pulsatility and decreases somatostatin expression (40, 41). Because GH receptors are expressed by neuropeptide Y (NPY) and somatostatin neurons in the ARC and anteroventral paraventricular nucleus, respectively, GH was postulated to directly influence its own release via short loop negative feedback in the hypothalamus (40). Whether GH neurons influence the release of GH and/or other neuroendocrine factors by the pituitary is intriguing, in fish, GH receptors are found in the pituitary (42), but there are no direct reports of this in mice (43). Our finding that subpopulations of GH neurons in the brain project to the ME could support the hypothesis that GH may be released into the hypophyseal portal system to influence somatotropes at the level of the anterior pituitary.
One clue on the mechanisms that underlie the neural Gh sex differences is the fact that only females were responsive to differences in estradiol. We did not quantify the number of ERα and GH dual-labeled cells, but it is possible that there is a sex difference and that males have fewer of these neurons than females. Of interest, Gh expression in the male MPOA increased after gonadectomy. It would therefore be pertinent to investigate the role of androgens in regulating GH gene expression in the male. Another source of variation between the sexes is sex chromosome complement (44), and we are presently asking whether this factor may explain the dichotomous effects of gonad removed on Gh mRNA in males (XY) vs. females (XX).
One sexually dimorphic behavior that GH could be involved in is food intake (21). Intracerebroventricular injections of GH increase food intake and GH administration to GH-deficient patients also results in increased appetite (45, 46). Conversely, GH-deficient patients are more obese than normal subjects, and GH administration has also been associated with decreases in fat mass. These data suggest that the role of GH in energy balance and metabolism is complex. Interestingly, overexpression of GH in glial cells of the mouse brain results in hyperphagia and obesity in mice (46). GH receptors have also been observed on NPY neurons in the ARC, and administration of GH to hypophysectomized rats normalizes NPY mRNA levels (47, 48). Whether GH in the ARC is colocalized in NPY neurons, possibly playing an autoregulatory role, or in neurons that express proopiomelanocortin will require further investigation. It is also possible that GH neurons are involved in mediating the downstream signaling effects of orexigenic neuropeptides from the periphery, such as ghrelin, in the ARC (1, 49).
In summary, we observed moderate to high intensity and density of GH protein in nuclei exhibiting sexual dimorphism in Gh mRNA levels, suggesting that changes in GH gene expression may reflect important functional changes in the production of GH protein within these nuclei. Furthermore, because GH treatment in humans and animals has been associated with increases in well-being, libido, enhancement of mood, and changes in appetite, brain nuclei that mediate these behaviors are of interest. In the hypothalamus, the MPOA and ARC are well known to mediate sexual behavior, GnRH, and appetite (50). The fact that Gh mRNA is responsive to environmental stimuli, such as stress and learning, and that GH mRNA responds to gonadal status and sex suggests that Gh may regulate behaviors and/or physiological processes that are sexually dimorphic.
Acknowledgments
This work was supported by National Institutes of Health Grant R01 MH057759.
Disclosure Summary: The authors do not have any conflicts of interest.
Footnotes
- ARC
- Arcuate nucleus
- DAB
- diaminobenzidine
- DAPI
- 4′,6′-diamino-2-phenylindole
- DiI
- 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate
- EB
- 17β-estradiol benzoate
- ER
- estrogen receptor
- GDX
- gonadectomized
- GH-IR
- GH immunoreactivity
- ME
- median eminence
- MPOA
- medial preoptic area
- NAc
- nucleus accumbens
- Neu-N
- neuronal nuclei
- NPY
- neuropeptide Y
- OVX
- ovariectomized
- TBS
- Tris-buffered saline
- VP
- ventral pallidum.
References
- 1. Chen HY, Trumbauer ME, Chen AS, Weingarth DT, Adams JR, Frazier EG, Shen Z, Marsh DJ, Feighner SD, Guan XM, Ye Z, Nargund RP, Smith RG, Van der Ploeg LH, Howard AD, MacNeil DJ, Qian S. 2004. Orexigenic action of peripheral ghrelin is mediated by neuropeptide Y and agouti-related protein. Endocrinology 145:2607–2612 [DOI] [PubMed] [Google Scholar]
- 2. Harvey S. 2010. Extrapituitary growth hormone. Endocrine 38:335–359 [DOI] [PubMed] [Google Scholar]
- 3. Sun LY, Al-Regaiey K, Masternak MM, Wang J, Bartke A. 2005. Local expression of GH and IGF-1 in the hippocampus of GH-deficient long-lived mice. Neurobiol Aging 26:929–937 [DOI] [PubMed] [Google Scholar]
- 4. Sartorio A, Conti A, Molinari E, Riva G, Morabito F, Faglia G. 1996. Growth, growth hormone and cognitive functions. Horm Res 45:23–29 [DOI] [PubMed] [Google Scholar]
- 5. McGauley G, Cuneo R, Salomon F, Sönksen PH. 1996. Growth hormone deficiency and quality of life. Horm Res 45:34–37 [DOI] [PubMed] [Google Scholar]
- 6. Nieves-Martinez E, Sonntag WE, Wilson A, Donahue A, Molina DP, Brunso-Bechtold J, Nicolle MM. 2010. Early-onset GH deficiency results in spatial memory impairment in mid-life and is prevented by GH supplementation. J Endocrinol 204:31–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wass JA, Reddy R. 2010. Growth hormone and memory. J Endocrinol 207:125–126 [DOI] [PubMed] [Google Scholar]
- 8. Li RC, Guo SZ, Raccurt M, Moudilou E, Morel G, Brittian KR, Gozal D. 2011. Exogenous growth hormone attenuates cognitive deficits induced by intermittent hypoxia in rats. Neuroscience 196:237–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schneider-Rivas S, Rivas-Arancibia S, Vázquez-Pereyra F, Vázquez-Sandoval R, Borgonio-Pérez G. 1995. Modulation of long-term memory and extinction responses induced by growth hormone (GH) and growth hormone releasing hormone (GHRH) in rats. Life Sci 56:PL433–PL441 [DOI] [PubMed] [Google Scholar]
- 10. Le Grevès M, Steensland P, Le Grevès P, Nyberg F. 2002. Growth hormone induces age-dependent alteration in the expression of hippocampal growth hormone receptor and N-methyl-d-aspartate receptor subunits gene transcripts in male rats. Proc Natl Acad Sci USA 99:7119–7123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Donahue CP, Kosik KS, Shors TJ. 2006. Growth hormone is produced within the hippocampus where it responds to age, sex, and stress. Proc Natl Acad Sci USA 103:6031–6036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. MacLeod JN, Pampori NA, Shapiro BH. 1991. Sex differences in the ultradian pattern of plasma growth hormone concentrations in mice. J Endocrinol 131:395–399 [DOI] [PubMed] [Google Scholar]
- 13. Hull KL, Harvey S. 2001. Growth hormone: roles in female reproduction. J Endocrinol 168:1–23 [DOI] [PubMed] [Google Scholar]
- 14. Donahue CP, Jensen RV, Ochiishi T, Eisenstein I, Zhao M, Shors T, Kosik KS. 2002. Transcriptional profiling reveals regulated genes in the hippocampus during memory formation. Hippocampus 12:821–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nishida Y, Yoshioka M, St-Amand J. 2005. Sexually dimorphic gene expression in the hypothalamus, pituitary gland, and cortex. Genomics 85:679–687 [DOI] [PubMed] [Google Scholar]
- 16. Render CL, Hull KL, Harvey S. 1995. Neural expression of the pituitary GH gene. J Endocrinol 147:413–422 [DOI] [PubMed] [Google Scholar]
- 17. Alba-Betancourt C, Arámburo C, Avila-Mendoza J, Ahumada-Solórzano SM, Carranza M, Rodríguez-Méndez AJ, Harvey S, Luna M. 2011. Expression, cellular distribution, and heterogeneity of growth hormone in the chicken cerebellum during development. Gen Comp Endocrinol 170:528–540 [DOI] [PubMed] [Google Scholar]
- 18. Lechan RM, Molitch ME, Jackson IM. 1983. Distribution of immunoreactive human growth hormone-like material and thyrotropin-releasing hormone in the rat central nervous system: evidence for their coexistence in the same neurons. Endocrinology 112:877–884 [DOI] [PubMed] [Google Scholar]
- 19. Hojvat S, Baker G, Kirsteins L, Lawrence AM. 1982. Growth hormone (GH) immunoreactivity in the rodent and primate CNS: distribution, characterization and presence posthypophysectomy. Brain Res 239:543–557 [DOI] [PubMed] [Google Scholar]
- 20. Friend KE, Resnick EM, Ang LW, Shupnik MA. 1997. Specific modulation of estrogen receptor mRNA isoforms in rat pituitary throughout the estrous cycle and in response to steroid hormones. Mol Cell Endocrinol 131:147–155 [DOI] [PubMed] [Google Scholar]
- 21. Symonds ME, Sebert SP, Budge H. 2009. The impact of diet during early life and its contribution to later disease: critical checkpoints in development and their long-term consequences for metabolic health. Proc Nutr Soc 68:416–421 [DOI] [PubMed] [Google Scholar]
- 22. Franklin KBJ, Paxinos G. 2001. The mouse brain in stereotaxic coordinates. 2nd ed San Diego: Academic Press [Google Scholar]
- 23. Buchanan KL, Yellon SM. 1993. Developmental study of GnRH neuronal projections to the medial basal hypothalamus of the male Djungarian hamster. J Comp Neurol 333:236–245 [DOI] [PubMed] [Google Scholar]
- 24. Greenfield A, Carrel L, Pennisi D, Philippe C, Quaderi N, Siggers P, Steiner K, Tam PP, Monaco AP, Willard HF, Koopman P. 1998. The UTX gene escapes X inactivation in mice and humans. Hum Mol Genet 7:737–742 [DOI] [PubMed] [Google Scholar]
- 25. Palkovits M. 1973. Isolated removal of hypothalamic or other brain nuclei of the rat. Brain Res 59:449–450 [DOI] [PubMed] [Google Scholar]
- 26. Flavell DM, Wells T, Wells SE, Carmignac DF, Thomas GB, Robinson IC. 1996. Dominant dwarfism in transgenic rats by targeting human growth hormone (GH) expression to hypothalamic GH-releasing factor neurons. EMBO J 15:3871–3879 [PMC free article] [PubMed] [Google Scholar]
- 27. Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2[−ΔΔC(T)] method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 28. Xu J, Bekaert AJ, Dupont J, Rouve S, Annesi-Maesano I, De Magalhaes Filho CD, Kappeler L, Holzenberger M. 2011. Exploring endocrine GH pattern in mice using rank plot analysis and random blood samples. J Endocrinol 208:119–129 [DOI] [PubMed] [Google Scholar]
- 29. Khodr CE, Clark S, Bokov AF, Richardson A, Strong R, Hurley DL, Phelps CJ. 2010. Early postnatal administration of growth hormone increases tuberoinfundibular dopaminergic neuron numbers in Ames dwarf mice. Endocrinology 151:3277–3285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Scheepens A, Sirimanne ES, Breier BH, Clark RG, Gluckman PD, Williams CE. 2001. Growth hormone as a neuronal rescue factor during recovery from CNS injury. Neuroscience 104:677–687 [DOI] [PubMed] [Google Scholar]
- 31. Costa A, Zoppetti G, Benedetto C, Bertino E, Marozio L, Fabris C, Arisio R, Giraudi GF, Testori O, Ariano M, et al. 1993. Immunolike growth hormone substance in tissues from human embryos/fetuses and adults. J Endocrinol Invest 16:625–633 [DOI] [PubMed] [Google Scholar]
- 32. Baudet ML, Rattray D, Martin BT, Harvey S. 2009. Growth hormone promotes axon growth in the developing nervous system. Endocrinology 150:2758–2766 [DOI] [PubMed] [Google Scholar]
- 33. Ramesh R, Kuenzel WJ, Buntin JD, Proudman JA. 2000. Identification of growth-hormone- and prolactin-containing neurons within the avian brain. Cell Tissue Res 299:371–383 [DOI] [PubMed] [Google Scholar]
- 34. Gossard F, Dihl F, Pelletier G, Dubois PM, Morel G. 1987. In situ hybridization to rat brain and pituitary gland of growth hormone cDNA. Neurosci Lett 79:251–256 [DOI] [PubMed] [Google Scholar]
- 35. Wauthier V, Sugathan A, Meyer RD, Dombkowski AA, Waxman DJ. 2010. Intrinsic sex differences in the early growth hormone responsiveness of sex-specific genes in mouse liver. Mol Endocrinol 24:667–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Paradisi R, Frank G, Magrini O, Capelli M, Venturoli S, Porcu E, Flamigni C. 1993. Adeno-pituitary hormones in human hypothalamic hypophysial blood. J Clin Endocrinol Metab 77:523–527 [DOI] [PubMed] [Google Scholar]
- 37. Harvey S, Hull K. 2003. Neural growth hormone: an update. J Mol Neurosci 20:1–14 [DOI] [PubMed] [Google Scholar]
- 38. Minami S, Suzuki N, Sugihara H, Tamura H, Emoto N, Wakabayashi I. 1997. Microinjection of rat GH but not human IGF-I into a defined area of the hypothalamus inhibits endogenous GH secretion in rats. J Endocrinol 153:283–290 [DOI] [PubMed] [Google Scholar]
- 39. Wells SE, Flavell DM, Bisset GW, Houston PA, Christian H, Fairhall KM, Robinson IC. 2003. Transgenesis and neuroendocrine physiology: a transgenic rat model expressing growth hormone in vasopressin neurones. J Physiol 551:323–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nass R, Toogood AA, Hellmann P, Bissonette E, Gaylinn B, Clark R, Thorner MO. 2000. Intracerebroventricular administration of the rat growth hormone (GH) receptor antagonist G118R stimulates GH secretion: evidence for the existence of short loop negative feedback of GH. J Neuroendocrinol 12:1194–1199 [DOI] [PubMed] [Google Scholar]
- 41. Pellegrini E, Bluet-Pajot MT, Mounier F, Bennett P, Kordon C, Epelbaum J. 1996. Central administration of a growth hormone (GH) receptor mRNA antisense increases GH pulsatility and decreases hypothalamic somatostatin expression in rats. J Neurosci 16:8140–8148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wong AO, Zhou H, Jiang Y, Ko WK. 2006. Feedback regulation of growth hormone synthesis and secretion in fish and the emerging concept of intrapituitary feedback loop. Comp Biochem Physiol A Mol Integr Physiol 144:284–305 [DOI] [PubMed] [Google Scholar]
- 43. Asa SL, Coschigano KT, Bellush L, Kopchick JJ, Ezzat S. 2000. Evidence for growth hormone (GH) autoregulation in pituitary somatotrophs in GH antagonist-transgenic mice and GH receptor-deficient mice. Am J Pathol 156:1009–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Abel JL, Rissman EF. 2011. Location, location, location: genetic regulation of neural sex differences. Rev Endocr Metab Disord, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Snel YE, Brummer RJ, Doerga ME, Zelissen PM, Koppeschaar HP. 1995. Energy and macronutrient intake in growth hormone-deficient adults: the effect of growth hormone replacement. Eur J Clin Nutr 49:492–500 [PubMed] [Google Scholar]
- 46. Bohlooly-Y M, Bohlooly M, Olsson B, Bruder CE, Lindén D, Sjögren K, Bjursell M, Egecioglu E, Svensson L, Brodin P, Waterton JC, Isaksson OG, Sundler F, Ahrén B, Ohlsson C, Oscarsson J, Törnell J. 2005. Growth hormone overexpression in the central nervous system results in hyperphagia-induced obesity associated with insulin resistance and dyslipidemia. Diabetes 54:51–62 [DOI] [PubMed] [Google Scholar]
- 47. Kamegai J, Minami S, Sugihara H, Hasegawa O, Higuchi H, Wakabayashi I. 1996. Growth hormone receptor gene is expressed in neuropeptide Y neurons in hypothalamic arcuate nucleus of rats. Endocrinology 137:2109–2112 [DOI] [PubMed] [Google Scholar]
- 48. Chan YY, Steiner RA, Clifton DK. 1996. Regulation of hypothalamic neuropeptide-Y neurons by growth hormone in the rat. Endocrinology 137:1319–1325 [DOI] [PubMed] [Google Scholar]
- 49. Wang L, Saint-Pierre DH, Taché Y. 2002. Peripheral ghrelin selectively increases Fos expression in neuropeptide Y-synthesizing neurons in mouse hypothalamic arcuate nucleus. Neurosci Lett 325:47–51 [DOI] [PubMed] [Google Scholar]
- 50. Bray GA. 1997. Obesity and reproduction. Hum Reprod 12(Suppl 1):26–32 [DOI] [PubMed] [Google Scholar]