Abstract
More than 90% of ovarian cancers have been thought to arise from epithelial cells that cover the ovarian surface or, more frequently, line subserosal cysts. Recent studies suggest that histologically similar cancers can arise from the fimbriae of Fallopian tubes and from deposits of endometriosis. Different histotypes are observed that resemble epithelial cells from the normal Fallopian tube (serous), endometrium (endometrioid), cervical glands (mucinous), and vaginal rests (clear cell) and that share expression of relevant HOX genes which drive normal gynecological differentiation. Two groups of epithelial ovarian cancers have been distinguished: type I low-grade cancers that present in early stage, grow slowly, and resist conventional chemotherapy but may respond to hormonal manipulation; and type II high-grade cancers that are generally diagnosed in advanced stage and grow aggressively but respond to chemotherapy. Type I cancers have wild-type p53 and BRCA1/2, but have frequent mutations of Ras and Raf as well as expression of IGFR and activation of the phosphatidylinositol-3-kinase (PI3K) pathway. Virtually all type II cancers have mutations of p53, and almost half have mutation or dysfunction of BRCA1/2, but other mutations are rare, and oncogenesis appears to be driven by amplification of several growth-regulatory genes that activate the Ras/MAPK and PI3K pathways. Cytoreductive surgery and combination chemotherapy with platinum compounds and taxanes have improved 5-yr survival, but less than 40% of all stages can be cured. Novel therapies are being developed that target high-grade serous cancer cells with PI3Kness or BRCAness as well as the tumor vasculature. Both in silico and animal models are needed that more closely resemble type I and type II cancers to facilitate the identification of novel targets and to predict response to combinations of new agents.
Among the gynecological malignancies, ovarian cancer is the leading cause of mortality in developed countries with 225,500 new cases and 140,200 estimated deaths worldwide (1). Despite the global impact of this disease, the lifelong risk of developing ovarian cancer in the United States is one in 70, and the prevalence one in 2500, even in the postmenopausal population that is at greatest risk. Consequently, ovarian cancer is a disease that is neither common nor rare but that has an overall cure rate of less than 40% across all stages. If we are to improve outcomes for women with ovarian cancer, it will be essential to take into account the clinical, cellular, and molecular biology of the disease to move beyond current management and to personalize care.
Biology of Ovarian Cancer
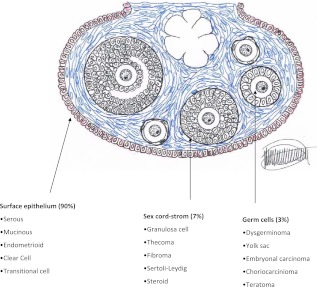
The normal ovary develops from the gonadal ridge near the mesonephros and contains three major cell types: 1) germ cells that are derived from the endoderm and that migrate to the gonadal ridge where they proliferate and differentiate into oocytes, 2) endocrine and interstitial cells that produce estrogen and progesterone, and 3) epithelial cells that are derived from the Mullerian duct and that cover the ovary and line inclusion cysts immediately beneath the ovarian surface. During normal ovulation, oocytes are released from mature follicles and enter the Fallopian tube where fertilization generally occurs. The fimbriae of the Fallopian tube cover the ruptured follicle and facilitate uptake of oocytes. Both benign and malignant tumors can arise from each of the three ovarian cell types (Fig. 1). Germ cell tumors arise most frequently in the second and third decade and account for 3–5% of ovarian cancers (2). Sex-cord-stromal tumors arise from the ovarian connective tissue, often secrete hormones, and can occur in women of all ages, comprising approximately 7% of all ovarian malignancies. Epithelial ovarian cancers generally develop after age 40 and include approximately 90% of malignant ovarian tumors. In addition to benign and malignant epithelial lesions, borderline tumors of low-malignant potential contain morphologically and molecularly partially transformed epithelial cells that do not invade underlying stroma. Approximately 10% of borderline tumors can recur after resection and prove lethal.
Fig. 1.
Different ovarian tumors originate from different cell subtypes. Prevalence of malignant components in parentheses. [Reproduced from V. W. Chen et al.: Pathology and classification of ovarian tumors. Cancer 97:2631, 2003 (89), with permission. © American Cancer Society.]
Histological subtypes of epithelial ovarian cancer
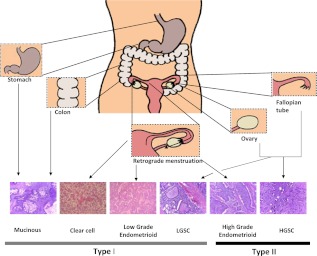
Traditionally, ovarian cancers have been thought to develop from flattened nondescript ovarian surface epithelial cells into cancers that resemble epithelium of the Fallopian tube (serous), endometrium (endometrioid), mucin-secreting endocervical glands (mucinous) and glycogen-filled vaginal rests (clear cell) (Fig 2). Ovarian cancer histotypes have been linked to expression of the HOXA9, HOXA10, and HOXA11 genes that regulate normal gynecological differentiation (3). In contrast to many other cancers, malignant transformation triggers the program of normal differentiation. Tumor histotype (4) and tumor grade or degree of differentiation (5) affect the stage at diagnosis, rate of growth, prognosis, and responsiveness to chemotherapy.
Fig. 2.
Origin and histological subtypes associated with type I and type II molecular classification. [Reproduced from S. Vaughan et al.: Rethinking ovarian cancer: recommendations for improving outcomes. Nat Rev Cancer 11:719, 2011 (14), with permission. © Nature Publishing Group.]
Pattern of spread
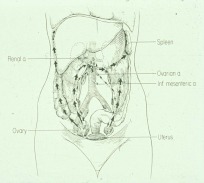
Similar to cancers that arise from other sites, epithelial ovarian cancer can spread through lymphatic and blood vessels to nodes and parenchyma of distant organs, including the liver and, because patients are surviving longer with recurrent disease, lung and brain (Fig 3). A distinctive feature of ovarian cancer is the ability to spread through the abdominal cavity, forming nodules on the surface of the parietal and visceral peritoneum including the omentum. Blockage of diaphragmatic lymphatics prevents outflow of proteinaceous fluid from the peritoneal cavity, causing the accumulation of ascites fluid in advanced disease.
Fig. 3.
Pattern of spread of epithelial ovarian cancers. Ovarian cancer cells can spread through lymphatics to nodes at the level of the renal hilum, through blood vessels to the liver, lung, and brain or over the peritoneal surface. [Reproduced from R. C. Knapp et al.: Natural history and detection of ovarian cancer. Gynecology and Obstetrics (edited by J. W. Sciarra), Harper, Row, Philadelphia, p 1 (90), with permission.]
Origin of ovarian cancer
Major risk factors for ovarian cancer include advancing age, number of ovulatory cycles, and a positive family history of ovarian, breast, uterine, or colon cancer related to mutations of BRCA1, BRCA2, mismatch repair genes, or TP53 in the germ line. Risk is halved by the use of oral contraceptives for as long as 5 yr before menopause, possibly related to reduced ovulation and treatment of transforming cells with progestational agents. If understood in greater depth, oral contraceptives could provide a strategy for prevention. Approximately 15% of ovarian cancers are familial and 85% sporadic. Traditionally, ovarian cancers have been thought to arise from ovarian surface epithelial cells or, more frequently, from similar cells that line cysts immediately beneath the ovarian surface. A morphological and genetic continuum can be demonstrated between normal epithelium, dysplasia and invasive high-grade carcinoma with cortical inclusion cysts of the ovary in both BRCA mutation carriers and noncarriers (6). In recent years, it has become apparent that a fraction of ovarian cancers or primary peritoneal carcinomas can also arise from endometriosis, epithelial rests in the normal peritoneum, or the fimbriae of Fallopian tubes. Serous tubal epithelial carcinomas and tubal carcinomas with TP53 mutations have been found in up to 80% of prophylactic salpingo-oophorectomy specimens from carriers of mutant BRCA1 or BRCA2 genes (7–10) and may account for many of the 20% of sporadic cancers thought to be of primary peritoneal origin. TP53 mutations have also been found within small cysts in the ovaries, consistent with an early event in carcinogenesis that may permit transforming cells to survive telomeric crisis, leading to multiple amplicons containing genes that promote proliferation, invasion, and metastasis.
Type I and type II ovarian cancers
At a clinical, cellular, and molecular level, ovarian cancers fall into two major groups based on histological grade, molecular phenotype, and genotype (Table 1 and Fig. 2) (11–13). Type I cancers are low grade of serous, mucinous, endometrioid, or clear-cell histotype. They are often diagnosed in an early stage (I or II), grow slowly, and resist conventional chemotherapy but may respond to hormonal treatment. The more prevalent type II cancers are high grade of serous, endometrioid, or undifferentiated histotype. These cancers present at late stage (III–IV), grow aggressively, and respond to conventional chemotherapy but less often to hormonal manipulation. The distinction between type I and type II cancers provides an initial step in understanding the heterogeneity of ovarian cancers and applying this new knowledge to personalized care (13, 14).
Table 1.
Histology, precursors, and distinctive molecular features (13)
| Molecular type | Histology | Precursor | Molecular features |
|---|---|---|---|
| I | Low-grade serous carcinoma | Borderline-carcinoma sequence | KRAS and BRAF mutations |
| I | Low-grade endometrioid carcinoma | Endometriosis | Mutations CTNNB1, PTEN, and microsatellite instability |
| I | Mucinous carcinoma | Cystadenoma-borderline sequence | KRAS mutations |
| I | Clear-cell carcinoma | Endometriosis possibly | PTEN mutations and LOH, PIK3CA mutations |
| II | High-grade serous carcinoma | De novo from inclusion cysts | p53 mutations, BRCA1/2 mutations and BRCA1 methylation |
| II | High-grade endometrioid carcinoma | Epithelial inclusion cysts | p53 mutations, BRCA1/2 mutations and BRCA1 methylation |
| II | Undifferentiated, carcinosarcoma |
LOH, Loss of heterozygosity.
Molecular alterations in type I ovarian cancers
Among the type I ovarian cancers, low-grade serous carcinomas appear to grow from serous borderline tumors in 60% of cases (15). Often, these cancers exhibit papillary architecture. Low-grade serous cancers tend to have a normal karyotype and wild-type TP53 and BRCA1/2 but frequent mutations in the B-RAF (2–35%) and KRAS genes (19–54%) (16). The IGF receptor is also expressed by the majority of low-grade serous cancers. Like other type I cancers, low-grade serous tumors are resistant but not refractory to standard chemotherapy (17, 18).
Other type I tumors are uncommon and include low-grade mucinous and clear-cell histotypes that respond to conventional platinum-based chemotherapy in only 26% (19) and 15% (20) of cases, respectively. KRAS is frequently mutated in mucinous cancers (21) and in associated borderline tumors (22). Clear-cell and low-grade endometrioid carcinomas share a similar gene expression pattern, consistent with a common origin, including two genes associated with chemoresistance, ANXA4 and UGT1A1 (23). Inactivating mutations of ARID1A, a chromatin-remodeling gene, have been found in 49% of ovarian clear-cell carcinomas and 30% of endometrioid ovarian cancers (24, 25) PPP2R1A, the regulatory subunit of a serine-threonine phosphatase required for chromosome segregation, is also mutated in 7% of clear-cell ovarian cancers (24). Low-grade endometrioid cancers exhibit frequent inactivating mutations and epigenetic silencing of PTEN and activating mutations of PIK3CA that up-regulate phosphatidylinositol-3-kinase (PI3K) signaling.
Molecular alterations in type II ovarian cancers
Although low-grade type I cancers appear to be driven by activating mutations on a background of a relatively normal karyotype, high-grade type II cancers are driven by copy number abnormalities and marked genomic instability. The Cancer Genome Atlas Project, which analyzed more than 300 high-grade serous cancers, detected amplification of more than 30 growth-stimulatory genes (26). Amplification and overexpression of genes in the PI3K family occur in more than 40% of type II cancers, conferring PI3Kness, or activation of the PI3K pathway. When ovarian cancers occur in carriers of BRCA1 or BRCA2 germline mutations, they are generally type II high-grade tumors. Somatic mutations of BRCA1 and BRCA2 can occur, BRCA1 can be silenced, and upstream mutations can down-regulate BRCA function, producing BRCAness, or homologous DNA repair deficiency in more than 40% of type II ovarian cancers. Mutations of p53 were found in 96% of type II high-grade serous cancers. Judged from their sequence, most of these mutations are inactivating. Other mutations were uncommon, with NF1, RB1, and CDK12 mutated in 2–4%. Less than 1% of type II cancers had mutations of BRAF, PI3KCA, KRAS, or NRAS found in type I tumors. These genes may, however, be rare but important drivers of high-grade serous cancers. Despite the low prevalence of Rb mutations, dysfunction of the Rb pathway has been found in 67% of high-grade serous cancers (26).
Current Clinical Management of Ovarian Cancer
Over the last three decades, 5-yr survival for ovarian cancer patients has increased from 37 to 45%, related to more consistent use of cytoreductive surgery and combination chemotherapy with platinum compounds and taxanes (27). The majority of patients are diagnosed in advanced stage with multiple tumor nodules studding the parietal and visceral peritoneum in the pelvis, omentum, and diaphragm.
Surgery
Ovarian cancer is one of the few malignancies where surgeons will undertake cytoreductive operations, even if all macroscopic tumor cannot be removed. Reducing tumor burden to where no macroscopic tumor is left before chemotherapy is considered optimal cyroreduction (9). Surgery can be performed after neoadjuvant chemotherapy (10) when optimal cytoreduction is not considered feasible at initial diagnosis. Survival increases with the expertise of the surgeon (28), and optimal cytoreductive surgery is an independent prognostic factor (29). The purpose is to achieve both correct FIGO (International Federation of Gynecology and Obstetrics) staging (30) and therapeutic thorough cytoreduction.
In several retrospective series, cytoreductive surgery for recurrent disease has been associated with improved survival when all macroscopic cancer can be removed (31, 32). Two ongoing prospective trials in Europe and the United States are evaluating criteria and outcomes for secondary cytoreduction.
Primary chemotherapy
Six cycles of carboplatin and paclitaxel chemotherapy are considered standard adjuvant treatment for newly diagnosed ovarian cancer after cytoreductive surgery. Carboplatin is an alkylating agent that binds covalently to DNA, creating adducts that form intrachain and interchain cross-links. Paclitaxel binds noncovalently to microtubules and increases their stability, interfering with mitotic spindle formation. Both agents induce apoptosis. Chemotherapy has generally been administered iv, but three randomized phase III trials have shown a 20–25% relative risk reduction in mortality after intraperitoneal therapy for patients who have been optimally cytoreduced (33–35). Chemotherapy is generally administered every 3 wk, but weekly dose-dense administration of paclitaxel has produced improved survival in one trial from Japan (36), and a confirmatory trial has not yet been completed.
Empirical addition of three other active drugs including pegylated liposomal doxorubicin, topotecan, and gemcitabine to standard therapy failed to improve upon the progression-free or overall survival observed with paclitaxel and carboplatin alone (37). Two recent trials have added a vascular endothelial growth factor (VEGF)-binding antiangiogenic antibody, bevacizumab, to standard treatment during and for up to 15 months after chemotherapy. Improved progression-free but not overall survival was reported (91, 92).
Chemotherapy for recurrent ovarian cancer
More than 70% of patients with advanced ovarian cancer will experience disease recurrence and become candidates for second-line chemotherapy, within 12 and 18 months. Retreatment with carboplatin and paclitaxel is associated with a 20–50% response when platinum-sensitive disease recurs more than 6 months after primary chemotherapy (Table 2). Although recurrent disease is not curable, combinations of drugs can prolong survival. In platinum-sensitive disease, a combination of carboplatin with paclitaxel (38, 39), gemcitabine (40), or liposomal doxorubicin (41) is superior to single-agent carboplatin (42). Disease that recurs in less than 6 months is considered platinum resistant. In this setting, several drugs produce response rates ranging from 10–30% and increase progression-free survival such as liposomal doxorubicin (43), weekly paclitaxel (44), and topotecan (45). Other drugs have demonstrated activity in phase II clinical studies, including gemcitabine (46), bevacizumab (47, 48), docetaxel (49), and etoposide (50).
Table 2.
Recurrent populations according to interval from last platinum
| Term | Definition |
|---|---|
| Refractory | Progression while receiving last line of platinum-based therapy or within 4 wk of last platinum dose |
| Platinum resistant | Progression-free interval of less than 6 months |
| Partially platinum sensitive | Progression-free interval since last platinum of 6–12 months |
| Platinum sensitive | Progression-free interval since last platinum of 6–12 months |
Biomarkers
CA125 (MUC16) is a high-molecular-mass (1 MDa) glycosylated transmembrane mucin that is expressed by 80% of ovarian cancers (51) and is important for adhesion, motility, and invasion of ovarian cancer (52). CA125 is shed from ovarian cancers and circulates in serum where it has provided the first generally useful biomarker for monitoring the response of ovarian cancer to chemotherapy (53). Persistent elevation of CA125 after chemotherapy indicates residual disease with more than 90% accuracy. CA125 has been used routinely to detect recurrence after chemotherapy. A recent trial found that early treatment of recurrent ovarian cancer with chemotherapy based on doubling of CA125 did not prolong survival when compared with treatment 5 months later at the time of clinical or symptomatic relapse (54). Limitations of the trial included inadequate stratification for important prognostic variables, use of suboptimal thresholds for CA125, delays in treatment in one quarter of participants, and suboptimal chemotherapy in two thirds of patients (55). Consequently, only one quarter of patients were treated promptly with combinations of drugs that could improve survival. Given the limitations of chemotherapy for recurrent disease, however, it remains uncertain whether monitoring for recurrence with CA125 improves overall survival, although it does identify patients for secondary cytoreductive surgery and provides time for treatment with multiple conventional and novel drugs.
Additional applications of CA125 include its use in combination with age, ultrasound (56), or other biomarkers (57–59) to identify patients with pelvic masses who would benefit from referral to a specially trained gynecological oncologist for cytoreductive surgery. Although individual values of CA125 are not sufficiently specific for detecting early-stage disease, trials are currently underway to test the value of a rising CA125 to trigger ultrasound that would prompt surgery (60, 61). Preliminary data suggest that this strategy is sufficiently specific that only three exploratory laparotomies will be required to detect an ovarian cancer and that an increased fraction of early-stage disease can be detected.
Enhancing drug sensitivity and overcoming drug resistance
During primary chemotherapy, approximately 70% of ovarian cancers will respond to platinum alone or in combination with paclitaxel (62). In two of three major trials, the addition of paclitaxel to cisplatin or carboplatin increased disease-free and overall survival compared with platinum-based therapy alone (63, 64). Despite an additive increase in overall survival with combination chemotherapy, only 42% of previously untreated patients will respond to paclitaxel, and there is no synergy between platinum compounds and the taxanes. Consequently, more than half of patients receive the toxicity, but not the benefit of taxanes, and there is room for significant improvement. Taxanes induce apoptosis in cancer cells after increasing microtubule stability and delaying or preventing passage through the cell cycle. Recent studies suggest that knockdown of several kinases can enhance paclitaxel sensitivity of ovarian cancer cells (65). In some cases, this relates to enhancing microtubule stability, and in others, it depends upon modifying apoptotic mechanisms or centrosome function (66). Using paclitaxel in combination with specific RNA interference or specific low-molecular-weight kinase inhibitors could lead to a greater fraction of ovarian cancer patients responding to primary therapy.
After treatment with carboplatin and paclitaxel, specifically resistant cancer cells emerge. Knowledge regarding the biology of taxane and platinum resistance is beginning at the preclinical level (67–69), but strategies for reversing drug resistance have not been validated clinically.
Targeted Drugs and Antibodies to Personalize Therapy
Individual targeted agents
Improvement in outcomes might result from therapy that targets the abnormal proteins in each patient's cancer. In the Cancer Genome Atlas Research Program analysis, different fractions of high-grade type II ovarian cancers had amplification of some 22 oncogenes for which specific inhibitory drugs were already available (26). To date, however, individual targeted agents have had only a modest impact on recurrent ovarian cancer in unselected patients. With the exception of bevacizumab, eight targeted drugs, gefitinib, imatinib, sorafenib, temsirolimus, mifepristone, enzastaurine, lapatinib, and vorinostat, have produced objective response rates of less than 10% and have stabilized disease for 6 months in less than 25% of cases in phase II trials. It is clear that we must use multiple agents and seek synthetic lethality if we are to produce deep and long-lasting remissions of recurrent disease and ultimately to improve primary therapy.
BRCAness
One of the best examples of synthetic lethality to reach the clinic to date is provided by the activity of poly-ADP-ribose polymerase (PARP) inhibitors in ovarian cancers that display BRCAness, i.e. a deficiency of BRCA1/2 function (70, 71) is associated with a better overall prognosis (72) and response to platinum compounds (73). Although 10–15% of ovarian cancers have germline BRCA1/2 mutations (74–76), up to 47% of type II high-grade serous ovarian cancers have genetic or epigenetic inactivation of BRCA1/BRCA2 (77). Somatic BRCA1/2 mutations were present in 19% of unselected ovarian cancer and 23% of high-grade serous cancers (78). Another recent report described BRCA1/2 mutations or BRCA1 silencing in 33% of high-grade serous cancers (26).
BRCA1 and BRCA2 mediate homologous recombination, which is one mechanism of DNA repair. Cancers with BRCAness are deficient in homologous repair and cannot repair DNA double strand breaks induced by platinum compounds (79). Inhibition of a second DNA repair pathway, base excision repair, by PARP inhibitors causes synthetic lethality in cancers with BRCAness. PARP inhibitors have produced response rates of more than 40% in ovarian cancers with BRCA1/2 mutations (80).
PI3Kness
Activation of PI3K signaling or PI3Kness can be produced by activating mutations of PIK3CA, inactivating mutations of PTEN, or amplification of PIK3CA, PIK3CB, PIK3R4, AKT1, AKT2, or AKT3 (81). Common copy number gains of PIK3CA, PIK3CB, and PIK3R4 in type II high-grade ovarian cancer were associated with decreased survival (82). Currently, mammalian target of rapamycin inhibitors, PI3K and AKT inhibitors are being investigated. In human ovarian cancer cell lines with PTEN deficiency, sensitivity to PARP1 inhibitors and cisplatin, but not to PARP1 inhibitors and paclitaxel, was higher than in the wild type (83). Thus, combinations of drugs that block both PI3K/AKT and PARP should be evaluated in patients with BRCAness and PI3Kness, whereas drugs that block both PI3K/AKT and MAPK kinase should be pursued in patients with abnormalities of the PI3K or Ras/MAPK pathway. The presence of activated pathways is likely to be necessary but not sufficient. Better predictive models will be required in cell cultures, animals, and in silico, to identify relevant targets and to choose optimal combinations for individual ovarian cancer patients.
Antiangiogenesis
Ovarian cancer metastases cannot grow to greater than 1 mm without blood vessel formation. Endothelial cells associated with tumor vessels depend upon proangiogenic factors for survival and can proliferate more rapidly than vessels serving normal tissues, providing targets for antiangiogenic therapy (84). Ovarian cancers produce multiple proangiogenic factors including VEGF, IL-8, and basic fibroblast growth factor. Inhibitors of proangiogenic proteins such as VEGF (bevacizumab and aflibercept), angiopoietins (AMG386), PDGF (imatinib and pazopanib), or their receptors VEGF receptor (pazopanib, sorafenib, sunitinib, and BIBF1120) are being tested in the clinic. Bevacizumab, as a single agent or in combination with daily low-dose cyclophosphamide, can produce an objective response rate of 20% in recurrent ovarian cancer and stabilize disease for 6 months in 40%. Given the extraordinary expense of bevacizumab, identifying biomarkers with high negative predictive value is an important unmet need. Putative biomarkers described include circulating endothelial cell precursors, CA125, DII4, VEGF-C, and neuropilin-1 (85). A decrease in perfusion with magnetic resonance imaging has also been evaluated, but predictive tests are not yet sufficiently precise to use routinely (86, 87). Relevant animal models will be crucial to develop multi-agent antivascular therapy and to facilitate identification of relevant predictive biomarkers.
Implications for the Development of Animal Models
Current knowledge regarding the biology and clinical management of ovarian cancer suggests that targeted agents must be used in combination to select the right drugs for the right patient at the right time. Given more than 400 anticancer drugs and antibodies in the current pharmaceutical pipeline, not all combinations can be tested in the clinic. Development of animal models that mimic the biology of human ovarian cancer will be critical for identifying new targets and useful combinations on the path to personalized therapy. The accompanying minireview considers the currently available models for ovarian cancer (88). In judging the relevance of these models, it will be important to consider the ability of primary cancers to metastasize from the ovaries not only through lymphatic and blood vessels but also to the surface of the peritoneal cavity, producing ascites. Animal models should mimic one of the two major types of ovarian cancer: low-grade type I disease driven by mutations of RAS, Raf, or members of the PI3K pathway on a background of genomic stability, wild-type TP53 and BRCA1/2, and hormonal signaling; or high-grade type II disease driven by amplification and genomic instability with mutant TP53 and dysfunctional Rb with or without BRCAness or PI3Kness. Models that produce mucinous, endometrioid, or clear-cell histotypes would also be of interest. Because mice and chickens can express CA125, animal models might also be used to identify complementary biomarkers. Whether or not animal models mimic human disease precisely, these ovarian cancers should respond to platinum compounds, taxanes, and inhibitors of Ras/MAPK, proangiogenic factors, PARP, and the PI3K pathway. Whatever the genotype and phenotype of models, validating their predictive power with agents known to be active in the clinic would be important if they are to contribute to translational research.
Acknowledgments
We thank Dr. Sergio Almenar and Dr. Carmen Illueca from the Department of Pathology of the Instituto Valenciano de Oncologia for kindly providing Fig. 1 and the images in Fig. 2, respectively.
This work was supported by funds from the M.D. Anderson SPORE in Ovarian Cancer NCI P50 CA83639, the M.D. Anderson CCSG NCI P30 CA16672, 1 R01 CA135354-01, the National Foundation for Cancer research, the Ovarian Cancer Research Fund, and philanthropic support from Golfers Against Cancer, the Tracey Jo Wilson Foundation, the Mossy Foundation, The Zarrow Foundation, and Stuart and Gaye Lynn Zarrow. I.R. was supported by the first Grupo Español de Investigación en Cáncer de Ovario (GEICO)-Jan Vermorken Grant.
Disclosure Summary: R.C.B. receives royalties for the discovery of CA125 from Fujirebio Diagnostics Inc. and serves on scientific advisory boards for Fujurebio Diagnostics Inc., Vermillion, and Illumina.
Footnotes
- PARP
- Poly-ADP-ribose polymerase
- PI3K
- phosphatidylinositol-3-kinase
- VEGF
- vascular endothelial growth factor.
References
- 1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. 2011. Global cancer statistics. CA Cancer J Clin 61:69–90 [DOI] [PubMed] [Google Scholar]
- 2. Smith HO, Berwick M, Verschraegen CF, Wiggins C, Lansing L, Muller CY, Qualls CR. 2006. Incidence and survival rates for female malignant germ cell tumors. Obstet Gynecol 107:1075–1085 [DOI] [PubMed] [Google Scholar]
- 3. Cheng W, Liu J, Yoshida H, Rosen D, Naora H. 2005. Lineage infidelity of epithelial ovarian cancers is controlled by HOX genes that specify regional identity in the reproductive tract. Nat Med 11:531–537 [DOI] [PubMed] [Google Scholar]
- 4. Soslow RA. 2008. Histologic subtypes of ovarian carcinoma: an overview. Int J Gynecol Pathol 27:161–174 [DOI] [PubMed] [Google Scholar]
- 5. Silverberg SG. 2000. Histopathologic grading of ovarian carcinoma: a review and proposal. Int J Gynecol Pathol 19:7–15 [DOI] [PubMed] [Google Scholar]
- 6. Pothuri B, Leitao MM, Levine DA, Viale A, Olshen AB, Arroyo C, Bogomolniy F, Olvera N, Lin O, Soslow RA, Robson ME, Offit K, Barakat RR, Boyd J. 2010. Genetic analysis of the early natural history of epithelial ovarian carcinoma. PLoS One 5:e10358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee Y, Miron A, Drapkin R, Nucci MR, Medeiros F, Saleemuddin A, Garber J, Birch C, Mou H, Gordon RW, Cramer DW, McKeon FD, Crum CP. 2007. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J Pathol 211:26–35 [DOI] [PubMed] [Google Scholar]
- 8. Piek JM, van Diest PJ, Zweemer RP, Jansen JW, Poort-Keesom RJ, Menko FH, Gille JJ, Jongsma AP, Pals G, Kenemans P, Verheijen RH. 2001. Dysplastic changes in prophylactically removed Fallopian tubes of women predisposed to developing ovarian cancer. J Pathol 195:451–456 [DOI] [PubMed] [Google Scholar]
- 9. Stuart GC, Kitchener H, Bacon M, duBois A, Friedlander M, Ledermann J, Marth C, Thigpen T, Trimble E. 2011. Gynecologic Cancer InterGroup (GCIG) consensus statement on clinical trials in ovarian cancer: report from the Fourth Ovarian Cancer Consensus Conference. Int J Gynecol Cancer 21:750–755 [DOI] [PubMed] [Google Scholar]
- 10. Vergote I, Tropé CG, Amant F, Kristensen GB, Ehlen T, Johnson N, Verheijen RH, van der Burg ME, Lacave AJ, Panici PB, Kenter GG, Casado A, Mendiola C, Coens C, Verleye L, Stuart GC, Pecorelli S, Reed NS. 2010. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med 363:943–953 [DOI] [PubMed] [Google Scholar]
- 11. Landen CN, Jr, Birrer MJ, Sood AK. 2008. Early events in the pathogenesis of epithelial ovarian cancer. J Clin Oncol 26:995–1005 [DOI] [PubMed] [Google Scholar]
- 12. Shih IeM, Kurman RJ. 2004. Ovarian tumorigenesis: a proposed model based on morphological and molecular genetic analysis. Am J Pathol 164:1511–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bast RC, Jr, Hennessy B, Mills GB. 2009. The biology of ovarian cancer: new opportunities for translation. Nat Rev Cancer 9:415–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vaughan S, Coward JI, Bast RC, Jr, Berchuck A, Berek JS, Brenton JD, Coukos G, Crum CC, Drapkin R, Etemadmoghadam D, Friedlander M, Gabra H, Kaye SB, Lord CJ, Lengyel E, Levine DA, McNeish IA, Menon U, Mills GB, Nephew KP, Oza AM, Sood AK, Stronach EA, Walczak H, Bowtell DD, Balkwill FR. 2011. Rethinking ovarian cancer: recommendations for improving outcomes. Nat Rev Cancer 11:719–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Malpica A, Deavers MT, Lu K, Bodurka DC, Atkinson EN, Gershenson DM, Silva EG. 2004. Grading ovarian serous carcinoma using a two-tier system. Am J Surg Pathol 28:496–504 [DOI] [PubMed] [Google Scholar]
- 16. Hsu CY, Bristow R, Cha MS, Wang BG, Ho CL, Kurman RJ, Wang TL, Shih IeM. 2004. Characterization of active mitogen-activated protein kinase in ovarian serous carcinomas. Clin Cancer Res 10:6432–6436 [DOI] [PubMed] [Google Scholar]
- 17. Schmeler KM, Sun CC, Bodurka DC, Deavers MT, Malpica A, Coleman RL, Ramirez PT, Gershenson DM. 2008. Neoadjuvant chemotherapy for low-grade serous carcinoma of the ovary or peritoneum. Gynecol Oncol 108:510–514 [DOI] [PubMed] [Google Scholar]
- 18. Gershenson DM, Sun CC, Bodurka D, Coleman RL, Lu KH, Sood AK, Deavers M, Malpica AL, Kavanagh JJ. 2009. Recurrent low-grade serous ovarian carcinoma is relatively chemoresistant. Gynecol Oncol 114:48–52 [DOI] [PubMed] [Google Scholar]
- 19. Hess V, A'Hern R, Nasiri N, King DM, Blake PR, Barton DP, Shepherd JH, Ind T, Bridges J, Harrington K, Kaye SB, Gore ME. 2004. Mucinous epithelial ovarian cancer: a separate entity requiring specific treatment. J Clin Oncol 22:1040–1044 [DOI] [PubMed] [Google Scholar]
- 20. Itamochi H, Kigawa J, Sugiyama T, Kikuchi Y, Suzuki M, Terakawa N. 2002. Low proliferation activity may be associated with chemo-resistance in clear cell carcinoma of the ovary. Obstet Gynecol 100:281–287 [DOI] [PubMed] [Google Scholar]
- 21. Pieretti M, Hopenhayn-Rich C, Khattar NH, Cao Y, Huang B, Tucker TC. 2002. Heterogeneity of ovarian cancer: relationships among histological group, stage of disease, tumor markers, patient characteristics, and survival. Cancer Invest 20:11–23 [DOI] [PubMed] [Google Scholar]
- 22. Garrett AP, Lee KR, Colitti CR, Muto MG, Berkowitz RS, Mok SC. 2001. k-ras mutation may be an early event in mucinous ovarian tumorigenesis. Int J Gynecol Pathol 20:244–251 [DOI] [PubMed] [Google Scholar]
- 23. Zorn KK, Bonome T, Gangi L, Chandramouli GV, Awtrey CS, Gardner GJ, Barrett JC, Boyd J, Birrer MJ. 2005. Gene expression profiles of serous, endometrioid, and clear cell subtypes of ovarian and endometrial cancer. Clin Cancer Res 11:6422–6430 [DOI] [PubMed] [Google Scholar]
- 24. Jones S, Wang TL, Shih IeM, Mao TL, Nakayama K, Roden R, Glas R, Slamon D, Diaz LA, Jr, Vogelstein B, Kinzler KW, Velculescu VE, Papadopoulos N. 2010. Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear cell carcinoma. Science 330:228–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wiegand KC, Shah SP, Al-Agha OM, Zhao Y, Tse K, Zeng T, Senz J, McConechy MK, Anglesio MS, Kalloger SE, Yang W, Heravi-Moussavi A, Giuliany R, Chow C, Fee J, Zayed A, Prentice L, Melnyk N, Turashvili G, Delaney AD, Madore J, Yip S, McPherson AW, Ha G, Bell L, et al. 2010. ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med 363:1532–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cancer Genome Atlas Research Network 2011. Integrated genomic analyses of ovarian carcinoma. Nature 474:609–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. 2009. Cancer Statistics 2009. CA Cancer J Clin 59:225–249 [DOI] [PubMed] [Google Scholar]
- 28. Giede KC, Kieser K, Dodge J, Rosen B. 2005. Who should operate on patients with ovarian cancer? An evidence-based review. Gynecol Oncol 99:447–461 [DOI] [PubMed] [Google Scholar]
- 29. Colombo N, Pecorelli S. 2003. What have we learned from ICON1 and ACTION? Int J Gynecol Cancer 13(Suppl 2):140–143 [DOI] [PubMed] [Google Scholar]
- 30. Odicino F, Pecorelli S, Zigliani L, Creasman WT. 2008. History of the FIGO cancer staging system. Int J Gynaecol Obstet 101:205–210 [DOI] [PubMed] [Google Scholar]
- 31. Harter P, Hahmann M, Lueck HJ, Poelcher M, Wimberger P, Ortmann O, Canzler U, Richter B, Wagner U, Hasenburg A, Burges A, Loibl S, Meier W, Huober J, Fink D, Schroeder W, Muenstedt K, Schmalfeldt B, Emons G, du Bois A. 2009. Surgery for recurrent ovarian cancer: role of peritoneal carcinomatosis: exploratory analysis of the DESKTOP I Trial about risk factors, surgical implications, and prognostic value of peritoneal carcinomatosis. Ann Surg Oncol 16:1324–1330 [DOI] [PubMed] [Google Scholar]
- 32. Chi DS, McCaughty K, Diaz JP, Huh J, Schwabenbauer S, Hummer AJ, Venkatraman ES, Aghajanian C, Sonoda Y, Abu-Rustum NR, Barakat RR. 2006. Guidelines and selection criteria for secondary cytoreductive surgery in patients with recurrent, platinum-sensitive epithelial ovarian carcinoma. Cancer 106:1933–1939 [DOI] [PubMed] [Google Scholar]
- 33. Markman M, Bundy BN, Alberts DS, Fowler JM, Clark-Pearson DL, Carson LF, Wadler S, Sickel J. 2001. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol 19:1001–1007 [DOI] [PubMed] [Google Scholar]
- 34. Alberts DS, Liu PY, Hannigan EV, O'Toole R, Williams SD, Young JA, Franklin EW, Clarke-Pearson DL, Malviya VK, DuBeshter B. 1996. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med 335:1950–1955 [DOI] [PubMed] [Google Scholar]
- 35. Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, Copeland LJ, Walker JL, Burger RA; Gyncologic Oncology Group 2006. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med 354:34–43 [DOI] [PubMed] [Google Scholar]
- 36. Katsumata N, Yasuda M, Takahashi F, Isonishi S, Jobo T, Aoki D, Tsuda H, Sugiyama T, Kodama S, Kimura E, Ochiai K, Noda K. 2009. Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open-label, randomised controlled trial. Lancet 374:1331–1338 [DOI] [PubMed] [Google Scholar]
- 37. Bookman MA, Brady MF, McGuire WP, Harper PG, Alberts DS, Friedlander M, Colombo N, Fowler JM, Argenta PA, De Geest K, Mutch DG, Burger RA, Swart AM, Trimble EL, Accario-Winslow C, Roth LM. 2009. Evaluation of new platinum-based treatment regimens in advanced-stage ovarian cancer: a Phase III Trial of the Gynecologic Cancer Intergroup. J Clin Oncol 27:1419–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. González-Martín AJ, Calvo E, Bover I, Rubio MJ, Arcusa A, Casado A, Ojeda B, Balañá C, Martínez E, Herrero A, Pardo B, Adrover E, Rifá J, Godes MJ, Moyano A, Cervantes A. 2005. Randomized phase II trial of carboplatin versus paclitaxel and carboplatin in platinum-sensitive recurrent advanced ovarian carcinoma: a GEICO (Grupo Espanol de Investigacion en Cancer de Ovario) study. Ann Oncol 16:749–755 [DOI] [PubMed] [Google Scholar]
- 39. Parmar MK, Ledermann JA, Colombo N, du Bois A, Delaloye JF, Kristensen GB, Wheeler S, Swart AM, Qian W, Torri V, Floriani I, Jayson G, Lamont A, Tropé C. 2003. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 trial. Lancet 361:2099–2106 [DOI] [PubMed] [Google Scholar]
- 40. Pfisterer J, Plante M, Vergote I, du Bois A, Hirte H, Lacave AJ, Wagner U, Stähle A, Stuart G, Kimmig R, Olbricht S, Le T, Emerich J, Kuhn W, Bentley J, Jackisch C, Lück HJ, Rochon J, Zimmermann AH, Eisenhauer E. 2006. Gemcitabine plus carboplatin compared with carboplatin in patients with platinum-sensitive recurrent ovarian cancer: an intergroup trial of the AGO-OVAR, the NCIC CTG, and the EORTC GCG. J Clin Oncol 24:4699–4707 [DOI] [PubMed] [Google Scholar]
- 41. Pujade-Lauraine E, Wagner U, Aavall-Lundqvist E, Gebski V, Heywood M, Vasey PA, Volgger B, Vergote I, Pignata S, Ferrero A, Sehouli J, Lortholary A, Kristensen G, Jackisch C, Joly F, Brown C, Le Fur N, du Bois A. 2010. Pegylated liposomal Doxorubicin and Carboplatin compared with Paclitaxel and Carboplatin for patients with platinum-sensitive ovarian cancer in late relapse. J Clin Oncol 28:3323–3329 [DOI] [PubMed] [Google Scholar]
- 42. Bast RC, Jr, Markman M. 2010. A new standard combination for recurrent ovarian cancer? Nat Rev Clin Oncol 7:559–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gordon AN, Tonda M, Sun S, Rackoff W; Doxil Study 30–49 Investigators 2004. Long-term survival advantage for women treated with pegylated liposomal doxorubicin compared with topotecan in a phase 3 randomized study of recurrent and refractory epithelial ovarian cancer. Gynecol Oncol 95:1–8 [DOI] [PubMed] [Google Scholar]
- 44. Gynecologic Oncology Group; Markman M, Blessing J, Rubin SC, Connor J, Hanjani P, Waggoner S. 2006. Phase II trial of weekly paclitaxel (80 mg/m2) in platinum and paclitaxel-resistant ovarian and primary peritoneal cancers: a Gynecologic Oncology Group study. Gynecol Oncol 101:436–440 [DOI] [PubMed] [Google Scholar]
- 45. ten Bokkel Huinink W, Gore M, Carmichael J, Gordon A, Malfetano J, Hudson I, Broom C, Scarabelli C, Davidson N, Spanczynski M, Bolis G, Malmström H, Coleman R, Fields SC, Heron JF. 1997. Topotecan versus paclitaxel for the treatment of recurrent epithelial ovarian cancer. J Clin Oncol 15:2183–2193 [DOI] [PubMed] [Google Scholar]
- 46. Friedlander M, Millward MJ, Bell D, Bugat R, Harnett P, Moreno JA, Campbell L, Varette C, Ripoche V, Kayitalire L. 1998. A phase II study of gemcitabine in platinum pre-treated patients with advanced epithelial ovarian cancer. Ann Oncol 9:1343–1345 [DOI] [PubMed] [Google Scholar]
- 47. Burger RA, Sill MW, Monk BJ, Greer BE, Sorosky JI. 2007. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a Gynecologic Oncology Group Study. J Clin Oncol 25:5165–5171 [DOI] [PubMed] [Google Scholar]
- 48. Cannistra SA, Matulonis UA, Penson RT, Hambleton J, Dupont J, Mackey H, Douglas J, Burger RA, Armstrong D, Wenham R, McGuire W. 2007. Phase II study of bevacizumab in patients with platinum-resistant ovarian cancer or peritoneal serous cancer. J Clin Oncol 25:5180–5186 [DOI] [PubMed] [Google Scholar]
- 49. Berkenblit A, Seiden MV, Matulonis UA, Penson RT, Krasner CN, Roche M, Mezzetti L, Atkinson T, Cannistra SA. 2004. A phase II trial of weekly docetaxel in patients with platinum-resistant epithelial ovarian, primary peritoneal serous cancer, or fallopian tube cancer. Gynecol Oncol 95:624–631 [DOI] [PubMed] [Google Scholar]
- 50. Rose PG, Blessing JA, Mayer AR, Homesley HD. 1998. Prolonged oral etoposide as second-line therapy for platinum-resistant and platinum-sensitive ovarian carcinoma: a Gynecologic Oncology Group study. J Clin Oncol 16:405–410 [DOI] [PubMed] [Google Scholar]
- 51. Yin BW, Lloyd KO. 2001. Molecular cloning of the CA125 ovarian cancer antigen: identification as a new mucin, MUC16. J Biol Chem 276:27371–27375 [DOI] [PubMed] [Google Scholar]
- 52. Bast RC, Jr, Spriggs DR. 2011. More than a biomarker: CA125 may contribute to ovarian cancer pathogenesis. Gynecol Oncol 121:429–430 [DOI] [PubMed] [Google Scholar]
- 53. Bast RC, Jr, Klug TL, St John E, Jenison E, Niloff JM, Lazarus H, Berkowitz RS, Leavitt T, Griffiths CT, Parker L, Zurawski VR, Jr, Knapp RC. 1983. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N Engl J Med 309:883–887 [DOI] [PubMed] [Google Scholar]
- 54. Rustin GJ, van der Burg ME, Griffin CL, Guthrie D, Lamont A, Jayson GC, Kristensen G, Mediola C, Coens C, Qian W, Parmar MK, Swart AM. 2010. Early versus delayed treatment of relapsed ovarian cancer (MRC OV05/EORTC 55955): a randomised trial. Lancet 376:1155–1163 [DOI] [PubMed] [Google Scholar]
- 55. Bast RC., Jr 2010. Commentary: CA125 and the detection of recurrent ovarian cancer: A reasonably accurate biomarker for a difficult disease. Cancer 116:2850–2853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jacobs I, Oram D, Fairbanks J, Turner J, Frost C, Grudzinskas JG. 1990. A risk of malignancy index incorporating CA 125, ultrasound and menopausal status for the accurate preoperative diagnosis of ovarian cancer. Br J Obstet Gynaecol 97:922–929 [DOI] [PubMed] [Google Scholar]
- 57. Ueland FR, Desimone CP, Seamon LG, Miller RA, Goodrich S, Podzielinski I, Sokoll L, Smith A, van Nagell JR, Jr, Zhang Z. 2011. Effectiveness of a multivariate index assay in the preoperative assessment of ovarian tumors. Obstet Gynecol 117:1289–1297 [DOI] [PubMed] [Google Scholar]
- 58. Moore RG, Jabre-Raughley M, Brown AK, Robison KM, Miller MC, Allard WJ, Kurman RJ, Bast RC, Skates SJ. 2010. Comparison of a novel multiple marker assay vs the Risk of Malignancy Index for the prediction of epithelial ovarian cancer in patients with a pelvic mass. Am J Obstet Gynecol 203:228.e1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Moore RG, Miller MC, Disilvestro P, Landrum LM, Gajewski W, Ball JJ, Skates SJ. 2011. Evaluation of the diagnostic accuracy of the risk of ovarian malignancy algorithm in women with a pelvic mass. Obstet Gynecol 118:280–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Menon U, Gentry-Maharaj A, Hallett R, Ryan A, Burnell M, Sharma A, Lewis S, Davies S, Philpott S, Lopes A, Godfrey K, Oram D, Herod J, Williamson K, Seif MW, Scott I, Mould T, Woolas R, Murdoch J, Dobbs S, Amso NN, Leeson S, Cruickshank D, McGuire A, Campbell S, Fallowfield L, Singh N, Dawnay A, Skates SJ, Parmar M, Jacobs I. 2009. Sensitivity and specificity of multimodal and ultrasound screening for ovarian cancer, and stage distribution of detected cancers: results of the prevalence screen of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). Lancet Oncol 10:327–340 [DOI] [PubMed] [Google Scholar]
- 61. Lu KH, Skates S, Bevers TB, Newland W, Moore RG, Leeds L, Harris S, Adeyinka OW, Fritsche HA, Bast RC. 2010. A prospective U.S. ovarian cancer screening study using the risk of ovarian cancer algorithm (ROCA). J Clin Oncol 28:15s (Abstract 5003) [Google Scholar]
- 62. Muggia FM, Braly PS, Brady MF, Sutton G, Niemann TH, Lentz SL, Alvarez RD, Kucera PR, Small JM. 2000. Phase III randomized study of cisplatin versus paclitaxel versus cisplatin and paclitaxel in patients with suboptimal stage III or IV ovarian cancer: a gynecologic oncology group study. J Clin Oncol 18:106–115 [DOI] [PubMed] [Google Scholar]
- 63. McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, Clarke-Pearson DL, Davidson M. 1996. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med 334:1–6 [DOI] [PubMed] [Google Scholar]
- 64. Piccart MJ, Bertelsen K, Stuart G, Cassidy J, Mangioni C, Simonsen E, James K, Kaye S, Vergote I, Blom R, Grimshaw R, Atkinson R, Swenerton K, Trope C, Nardi M, Kaern J, Tumolo S, Timmers P, Roy JA, Lhoas F, Lidvall B, Bacon M, Birt A, Andersen J, Zee B, Paul J, Pecorelli S, Baron B, McGuire W. 2003. Long-term follow-up confirms a survival advantage of the paclitaxel-cisplatin regimen over the cyclophosphamide-cisplatin combination in advanced ovarian cancer. Int J Gynecol Cancer 13:144–148 [DOI] [PubMed] [Google Scholar]
- 65. Ahmed AA, Wang X, Lu Z, Goldsmith J, Le XF, Grandjean G, Bartholomeusz G, Broom B, Bast RC., Jr 2011. Modulating microtubule stability enhances the cytotoxic response of cancer cells to paclitaxel. Cancer Res 71:5806–5817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ahmed AA, Lu Z, Jennings NB, Etemadmoghadam D, Capalbo L, Jacamo RO, Barbosa-Morais N, Le XF, Australian Ovarian Cancer Study Group, Vivas-Mejia P, Lopez-Berestein G, Grandjean G, Bartholomeusz G, Liao W, Andreeff M, Bowtell D, Glover DM, Sood AK, Bast RC., Jr 2010. SIK2 is a centrosome kinase required for bipolar spindle formation that provides a potential target for therapy in ovarian cancer. Cancer Cell 18:109–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Selvakumaran M, Pisarcik DA, Bao R, Yeung AT, Hamilton TC. 2003. Enhanced cisplatin cytotoxicity by disturbing the nucleotide excision repair pathway in ovarian cancer cell lines. Cancer Res 63:1311–1316 [PubMed] [Google Scholar]
- 68. Reed E, Yu JJ, Davies A, Gannon J, Armentrout SL. 2003. Clear cell tumors have higher mRNA levels of ERCC1 and XPB than other histological types of epithelial ovarian cancer. Clin Cancer Res 9:5299–5305 [PubMed] [Google Scholar]
- 69. Agarwal R, Kaye SB. 2003. Ovarian cancer: strategies for overcoming resistance to chemotherapy. Nat Rev Cancer 3:502–516 [DOI] [PubMed] [Google Scholar]
- 70. Turner N, Tutt A, Ashworth A. 2004. Hallmarks of 'BRCAness' in sporadic cancers. Nat Rev Cancer 4:814–819 [DOI] [PubMed] [Google Scholar]
- 71. Bast RC, Jr, Mills GB. 2010. Personalizing therapy for ovarian cancer: BRCAness and beyond. J Clin Oncol 28:3545–3548 [DOI] [PubMed] [Google Scholar]
- 72. Chetrit A, Hirsh-Yechezkel G, Ben-David Y, Lubin F, Friedman E, Sadetzki S. 2008. Effect of BRCA1/2 mutations on long-term survival of patients with invasive ovarian cancer: the national Israeli study of ovarian cancer. J Clin Oncol 26:20–25 [DOI] [PubMed] [Google Scholar]
- 73. Tan DS, Rothermundt C, Thomas K, Bancroft E, Eeles R, Shanley S, Ardern-Jones A, Norman A, Kaye SB, Gore ME. 2008. “BRCAness” syndrome in ovarian cancer: a case-control study describing the clinical features and outcome of patients with epithelial ovarian cancer associated with BRCA1 and BRCA2 mutations. J Clin Oncol 26:5530–5536 [DOI] [PubMed] [Google Scholar]
- 74. Malander S, Rambech E, Kristoffersson U, Halvarsson B, Ridderheim M, Borg A, Nilbert M. 2006. The contribution of the hereditary nonpolyposis colorectal cancer syndrome to the development of ovarian cancer. Gynecol Oncol 101:238–243 [DOI] [PubMed] [Google Scholar]
- 75. Risch HA, McLaughlin JR, Cole DE, Rosen B, Bradley L, Fan I, Tang J, Li S, Zhang S, Shaw PA, Narod SA. 2006. Population BRCA1 and BRCA2 mutation frequencies and cancer penetrances: a kin-cohort study in Ontario, Canada. J Natl Cancer Inst 98:1694–1706 [DOI] [PubMed] [Google Scholar]
- 76. Pal T, Permuth-Wey J, Betts JA, Krischer JP, Fiorica J, Arango H, LaPolla J, Hoffman M, Martino MA, Wakeley K, Wilbanks G, Nicosia S, Cantor A, Sutphen R. 2005. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer 104:2807–2816 [DOI] [PubMed] [Google Scholar]
- 77. Press JZ, De Luca A, Boyd N, Young S, Troussard A, Ridge Y, Kaurah P, Kalloger SE, Blood KA, Smith M, Spellman PT, Wang Y, Miller DM, Horsman D, Faham M, Gilks CB, Gray J, Huntsman DG. 2008. Ovarian carcinomas with genetic and epigenetic BRCA1 loss have distinct molecular abnormalities. BMC Cancer 8:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Hennessy BT, Timms KM, Carey MS, Gutin A, Meyer LA, Flake DD, 2nd, Abkevich V, Potter J, Pruss D, Glenn P, Li Y, Li J, Gonzalez-Angulo AM, McCune KS, Markman M, Broaddus RR, Lanchbury JS, Lu KH, Mills GB. 2010. Somatic mutations in BRCA1 and BRCA2 could expand the number of patients that benefit from poly (ADP ribose) polymerase inhibitors in ovarian cancer. J Clin Oncol 28:3570–3576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bhattacharyya A, Ear US, Koller BH, Weichselbaum RR, Bishop DK. 2000. The breast cancer susceptibility gene BRCA1 is required for subnuclear assembly of Rad51 and survival following treatment with the DNA cross-linking agent cisplatin. J Biol Chem 275:23899–239903 [DOI] [PubMed] [Google Scholar]
- 80. Audeh MW, Carmichael J, Penson RT, Friedlander M, Powell B, Bell-McGuinn KM, Scott C, Weitzel JN, Oaknin A, Loman N, Lu K, Schmutzler RK, Matulonis U, Wickens M, Tutt A. 2010. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet 376:245–251 [DOI] [PubMed] [Google Scholar]
- 81. Bast RC, Jr, Mills GB. 2012. Dissecting “PI3Kness”: the complexity of personalized therapy for ovarian cancer. Cancer Discovery 2:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Huang J, Zhang L, Greshock J, Colligon TA, Wang Y, Ward R, Katsaros D, Lassus H, Butzow R, Godwin AK, Testa JR, Nathanson KL, Gimotty PA, Coukos G, Weber BL, Degenhardt Y. 2011. Frequent genetic abnormalities of the PI3K/AKT pathway in primary ovarian cancer predict patient outcome. Genes Chromosomes Cancer 50:606–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Mendes-Pereira AM, Martin SA, Brough R, McCarthy A, Taylor JR, Kim JS, Waldman T, Lord CJ, Ashworth A. 2009. Synthetic lethal targeting of PTEN mutant cells with PARP inhibitors. EMBO Mol Med 1:315–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Folkman J. 1995. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1:27–31 [DOI] [PubMed] [Google Scholar]
- 85. Jubb AM, Harris AL. 2010. Biomarkers to predict the clinical efficacy of bevacizumab in cancer. Lancet Oncol 11:1172–1183 [DOI] [PubMed] [Google Scholar]
- 86. Mehta S, Hughes NP, Buffa FM, Li SP, Adams RF, Adwani A, Taylor NJ, Levitt NC, Padhani AR, Makris A, Harris AL. 2011. Assessing early therapeutic response to bevacizumab in primary breast cancer using magnetic resonance imaging and gene expression profiles. J Natl Cancer Inst Monogr 2011:71–74 [DOI] [PubMed] [Google Scholar]
- 87. Hirashima Y, Yamada Y, Tateishi U, Kato K, Miyake M, Horita Y, Akiyoshi K, Takashima A, Okita N, Takahari D. 21 July 2011. Pharmacokinetic parameters from 3-Tesla DCE-MRI as surrogate biomarkers of antitumor effects of bevacizumab plus FOLFIRI in colorectal cancer with liver metastasis. Int J Cancer 10.1002/ijc.26282 [DOI] [PubMed] [Google Scholar]
- 88. Richards JS. 2012. Animal models and mechanisms of ovarian cancer development. Endocrinology 153:1585–1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Chen VW, Ruiz B, Killeen JL, Coté TR, Wu XC, Correa CN. 2003. Pathology and classification of ovarian tumors. Cancer 97:2631–2642 [DOI] [PubMed] [Google Scholar]
- 90. Knapp RC, Berkowitz RS, Leavitt T, Jr, Bast RC., Jr 1988. Natural history and detection of ovarian cancer. In: Sciarra JW, ed. Gynecology and obstetrics. Philadelphia: Harper, Row; 1–16 [Google Scholar]
- 91. Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G, Carey MS, Beale P, Cervantes A, Kurzeder C, du Bois A, Sehouli J, Kimmig R, Stähle A, Collinson F, Essapen S, Gourley C, Lortholary A, Selle F, Mirza MR, Leminen A, Plante M, Stark D, Qian W, Parmar MK, Oza AM; ICON7 Investigators 2011. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med:365(26):2484-96; 2012 Erratum in: N Engl J Med:366(3):284 [DOI] [PubMed] [Google Scholar]
- 92. Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, Mannel RS, Homesley HD, Fowler J, Greer BE, Boente M, Birrer MJ, Liang SX; Gynecologic Oncology Group 2011. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med:365(26):2473-83 [DOI] [PubMed] [Google Scholar]