Abstract
Kisspeptin (KP) signaling has been proposed as an important regulator in the mechanism of puberty. In this study, to determine the role of KP in puberty, we assessed the in vivo release pattern of KP-54 from the basal hypothalamus/stalk-median eminence in prepubertal and pubertal ovarian-intact female rhesus monkeys. We found that there was a developmental increase in mean KP-54 release, pulse frequency, and pulse amplitude, which is parallel to the developmental changes in GnRH release that we previously reported. Moreover, a nocturnal increase in KP-54 release becomes prominent after the onset of puberty. Because the pubertal increase in GnRH release occurs independent of the pubertal increase in circulating gonadal steroids, we further examined whether ovariectomy (OVX) modifies the release pattern of KP-54. Results show that OVX in pubertal monkeys enhanced mean KP-54 release and pulse amplitude but not pulse frequency, whereas OVX did not alter the release pattern of KP-54 in prepubertal monkeys. Estradiol replacement in OVX pubertal monkeys suppressed mean KP-54 release and pulse amplitude but not pulse frequency. Estradiol replacement in OVX prepubertal monkeys did not alter the KP-54 release pattern. Collectively these results suggest that the pubertal increase in KP release occurs independent of the pubertal increase in circulating estradiol. Nevertheless, the pubertal increase in KP release is not likely responsible for the initiation of the pubertal increase in GnRH release. Rather, after puberty onset, the increase in KP release contributes to further increase GnRH release during the progression of puberty.
The onset of puberty is triggered by developmental changes in the pulsatile release of GnRH from the medial basal hypothalamus (MBH). Although it is clear that pulsatile administration of GnRH triggers the onset of puberty (1) and that an increase in GnRH release, which is characterized by an increase in mean release, pulse frequency, and pulse amplitude, occurs at the time of puberty in primates (2, 3), the neurobiological mechanism triggering the pubertal increase in GnRH release remains unclear.
In the last decade, the quest for determining potential regulators of GnRH release has revealed that kisspeptin (KP) and the kisspeptin-1 receptor (KiSS1R) play a role in the mechanism of puberty onset. Specifically, several studies in humans have identified inactivating mutations in KiSS1R that result in a delay in or absence of puberty (4, 5) and activating mutations in both KiSS1 and KiSS1R that result in precocious puberty (6, 7). Moreover, a substantial number of publications show that KP is one of the most potent stimulators of GnRH neurosecretion in multiple animal species (8–12). Although a recent report in conditional knockout mice, in which the deletion of KP- or KiSS1R-expressing neurons does not compromise the timing of puberty (13), illustrated the complexity of the mechanism of puberty onset, KP-KiSS1R signaling remains an important player in the mechanism of puberty.
Recent studies in our laboratory indicate that the pubertal increase in GnRH release is, in part, due to a pubertal increase in KP-54 release as well as a developmental increase in responsiveness of GnRH neurons to KiSS1R-mediated signaling. We have shown the following: 1) KP-54 release gradually increases from the prepubertal to early pubertal and midpubertal stages along with the pubertal increase in GnRH release (14); 2) a nocturnal increase in mean GnRH and KP-54 release becomes prominent after puberty onset (2, 3, 14); 3) KP-54 release in midpubertal monkeys is pulsatile with an interpulse interval (IPI) of approximately 60 min, similar to that of GnRH in midpubertal monkeys (2, 14); 4) KP-54 and GnRH pulses coincide approximately 75% of the time in midpubertal monkeys (14); 5) there is a developmental increase in GnRH response to KiSS1R stimulation, which occurs in a dose responsive manner (15); and 6) this developmental increase in GnRH response to human KP-10 in pubertal monkeys requires the pubertal increase in circulating 17β-estradiol (E2) (15). Presently, however, it is unknown 1) whether the pulse pattern of KP-54 release undergoes developmental changes, similar to the GnRH release pattern during puberty, such as development of a nocturnal increase and changes in pulse frequency and pulse amplitude (2, 3) and 2) whether the pubertal increase in KP-54 release occurs independently from the pubertal increase in circulating E2, as does GnRH release in ovariectomized (OVX) female monkeys (3, 16). These questions are significantly important for defining the role of KP in the mechanism of puberty onset because in primates the pubertal increase in GnRH release occurs independent from the pubertal increase in gonadal steroids (17, 18). Therefore, in the present study, we conducted a series of experiments examining developmental changes in KP release patterns in female rhesus monkeys and whether these changes are dependent on the pubertal increase in ovarian steroids.
Materials and Methods
Animals
Fifteen prepubertal (13.1–21.0 months of age) and 12 pubertal (28.2–45.8 months of age) female rhesus monkeys (Macaca mulatta) were used in this study. All animals were born and raised at the Wisconsin National Primate Research Center and housed in pairs (cages 172 × 86 × 86 cm) in a room with a lighting schedule of 12 h of light and 12 h of dark, at a controlled temperature (22 C). They were fed a standard diet of Teklad Primate Chow (Harlan, Madison, WI) twice per day and water was available ad libitum. Fresh fruit or other enrichment was also provided on a daily basis. To accurately define the pubertal stage, all animals were examined daily for sex-skin development and menstrual status and received weekly blood sampling for circulating hormone measurements. The protocol was approved by the Animal Care and Use Committee, University of Wisconsin-Madison, and all experiments were conducted under the National Institutes of Health and U.S. Department of Agriculture guidelines.
Experimental design
To examine developmental changes in KP-54 release in female rhesus monkeys at the prepubertal and pubertal stages, three series of experiments using an in vivo microdialysis method were conducted. To obtain samples from a light phase (morning) and dark phase (evening), animals were housed in a room with either a morning (lights on 1000–2200 h and lights off 2200–1000 h) or evening (lights on 0300–1500 h and lights off 1500–0300 h) lighting schedule for at least 1 month before experimentation. In experiment 1, to assess developmental changes in KP-54 release in eight prepubertal (14.7 ± 0.3 months) and seven pubertal (31.0 ± 0.7 months) ovarian intact monkeys, dialysates were collected at 10-min intervals from the stalk-median eminence (S-ME)/MBH during the morning and evening for 6 h, starting 1 h before the time of lights on (morning) or lights off (evening). KP-54 in dialysates was assessed with RIA. Samples were collected from each monkey during the morning or evening or both (see Statistical analysis section for a detailed description).
In experiment 2, to determine whether developmental changes in KP-54 release are due to the pubertal rise in circulating ovarian steroids, KP-54 release was measured in nine prepubertal (19.0 ± 0.3 months) and eight pubertal (36.0 ± 1.4 months) OVX monkeys. Animals were OVX at least 1 month before experimentation. The protocol for this experiment was similar to that described for experiment 1. Because a portion of experiment 2 was conducted subsequent to experiment 1, five of the prepubertal and three of the pubertal monkeys in experiment 1 were also used in experiment 2 after OVX. Additionally, four of nine OVX prepubertal monkeys were also examined during the pubertal stage. Similar to experiment 1, samples were collected from each monkey during either the morning or evening or both (see Statistical analysis section for a detailed description).
In experiment 3, to determine whether E2 is involved in modulating the effects of OVX, 30 μg/kg of E2 was sc injected after 120 min of control sampling, while dialysates were continuously collected for an additional 360 min in prepubertal and pubertal monkeys, as previously described (16). Three new OVX prepubertal and six OVX pubertal monkeys (all from experiment 2) were used for this experiment. To limit any potential lighting (circadian rhythm) effects on KP-54 release, all animals were adapted to the morning lighting schedule as described in experiment 1, and the experiments were conducted only during the lights on phase.
Cranial pedestal implantation and guide cannula insertion
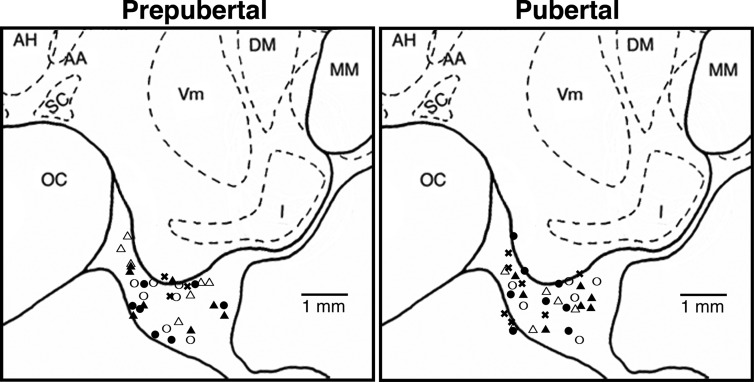
All animals were well adapted to the primate chair, experimental conditions, and researchers before experimentation. At least 1 month before microdialysis experiments, all animals were surgically implanted with a cranial pedestal under isoflurane anesthesia as previously described (19). On the day of the microdialysis experiment, the monkey was anesthetized with ketamine (10–15 mg/kg body weight) and dexmedetomidine (up to 3.0 μg/kg body weight) and then placed in a stereotaxic apparatus. A microdrive unit, which allows for three-dimensional adjustment, was secured on the cranial pedestal, and a guide cannula with an inner stylet was inserted into the skull 5 mm above the S-ME using the microdrive unit. The custom-made guide cannula (CMA, Stockholm, Sweden) consisted of a stainless steel shaft (76.0 mm in length, 0.91 mm diameter) with a removable stainless steel stylet (96.0 mm in length, 0.6 mm in diameter), which protruded 20 mm from the tip of the guide cannula. Ventriculographs were used to visualize and verify the x, y, and z coordinates of the guide cannula above the S-ME. The sites of dialysate collections in prepubertal and pubertal monkeys were comparable (Fig. 1).
Fig. 1.
Schematic diagrams of the midsagittal plane of the basal hypothalamus showing locations of the probe tips used for microdialysis experiments in the prepubertal and pubertal stages. Most of the probe tips are located in the S-ME region. However, collection of dialysates were not limited to those spots because the microdialysis probes have a semipermeable membrane with a 5-mm length. The open and closed circles indicate morning and evening sampling experiments, respectively, in ovarian intact monkeys (experiment 1), the open and closed triangles indicate morning and evening sampling experiments, respectively, in OVX monkeys (experiment 2), and the x symbols indicate OVX monkeys treated with E2 (experiment 3). The lateral position of both prepubertal and pubertal monkeys are 0.2–1.0 mm from the midline (data not shown). AH, Anterior hypothalamic nucleus; DM, dorsal medial hypothalamic nucleus; I, infundibular (arcuate) nucleus; MM, mammillary body; OC, optic chiasm; SC, suprachiasmatic nucleus; Vm, ventromedial hypothalamic nucleus. Note that there is no significant difference in the distribution pattern of probe tips between the prepubertal and pubertal stages.
Microdialysis experiment
Immediately after the guide cannula placement, the monkey was removed from the stereotaxic apparatus and placed in a primate chair. The inner stylet was then removed and a microdialysis probe (stainless steel shaft 96.0 mm in length, 0.6 mm in diameter), with a polyarylethersulfone membrane (20 kDa molecular mass cutoff, 5.0 mm in length, 0.5 mm in diameter), was inserted into the guide cannula so that the tip of the probe was located in the S-ME as previously described (20). Central nervous system perfusion fluid (147 mm NaCl, 2.7 mm KCl, 1.2 mm CaCl2, 0.85 mm MgCl2; CMA) containing bacitractin (4 U/ml) was infused through the influx tubing at 2 μl/min with a CMA/102 microdialysis pump (CMA) and either a 1- or 2.5-ml Hamilton gas syringe (Hamilton, Reno, NV). Dialysates were collected into 12 × 75 borosilicate tubes at 10-min intervals with a fraction collector (model FC203B; Gilson, Middleton, WI) and 130 μl of RIA buffer (0.3% BSA; 0.01M PO4; 0.15M NaCl; 0.1% NaN3 at pH 7.8) was added after sample collection. Samples were immediately frozen on dry ice and stored at − 80 C until assayed. Experiments were conducted for up to 12 h, during which the animal was provided monkey chow, fresh fruit, other snacks, and water, and allowed in close proximity to a partner monkey for visualization and vocalization. After the experiments, monkeys were returned to their home cage for at least 1 month before participating in a subsequent microdialysis experiment.
RIA
Samples were thawed immediately before assaying. RIA for KP-54 was conducted using the GQ2 antiserum provided by Dr. Stephen Bloom (Imperial College London, London, UK) as previously described (14, 21). Assay sensitivity was 0.05 pg/tube or 1.0 pg/ml. Intra- and interassay coefficients of variation were 10.1 and 14.3%, respectively.
Statistical analysis
KP-54 values less than 1.0 pg/ml were assigned to 1.0 pg/ml, based on the assay sensitivity. As described in the experimental design, four to seven animals were used for each experimental group. However, in some experiments, data were obtained twice in an animal at the same developmental stage. In this case we counted the results of the two trials as an independent entry because the data from each trial were obtained at least 2–5 months apart and the locations of the probe tips were not identical. In the morning experiment 1 data, none of the seven prepubertal and two of the four pubertal monkeys were examined twice (n = 7, prepubertal stage: 15.1 ± 0.4 months; n = 6, pubertal stage: 31.9 ± 0.7). In the evening experiment 1 data, one of the six prepubertal and three of the five pubertal monkeys were examined twice (n = 7, prepubertal stage: 14.3 ± 0.3 months; n = 8, pubertal stage: 30.2 ± 0.9 months). In the morning experiment 2 data, one of the six prepubertal and one of the five pubertal OVX monkeys were examined twice (n = 7, prepubertal stage: 18.9 ± 0.5 months; n = 6, pubertal stage: 34.7 ± 1.5 months). In the evening experiment 2 data, one of the seven prepubertal and one of the five pubertal OVX monkeys were examined twice (n = 8, prepubertal stage: 19.1 ± 0.4 months; n = 6, pubertal stage: 37.1 ± 2.1 months). In experiment 3 data, none of the three OVX+E2 prepubertal monkeys (n = 3, 17.5 ± 1.7 months) and one of the six OVX+E2 pubertal monkeys were examined twice (n = 7, 37.8 ± 1.6 months).
For all experiments, KP-54 pulses were identified with the PULSAR algorithm (22) as previously described (14). Parameters for pulsatile KP-54 release were calculated as follows: 1) mean KP-54 release was calculated as the mean of all KP-54 values collected at 10-min intervals for experiments 1 and 2 from the start of the lighting condition (lights on, morning; lights off, evening) and for 6 h in each trial, and for experiment 3, 120-min before E2 injection or for 6 h after E2 injection in each trial; 2) IPI, defined as the interval between peaks of KP-54 pulses, was calculated in each trial; and 3) pulse amplitude, defined as the difference between the peak and trough KP-54 values, was calculated in each trial. Subsequently, mean ± sem was calculated for each experimental group. In experiment 1, the effects of lighting (morning vs. evening) within a developmental stage and between developmental stages (prepubertal vs. pubertal) on pulse parameters of KP-54 release were analyzed with two-way ANOVA unrepeated measures followed by Bonferroni post hoc analysis (GraphPad Prism version 5.00 for Windows; GraphPad Software, San Diego, CA).
Statistical analysis in experiment 2 was similar to that described in experiment 1. Additionally, the effects of gonadal status (ovarian intact vs. OVX) on KP-54 release within and between developmental stages (experiments 1 and 2) were analyzed with two-way ANOVA and Bonferroni post hoc test. Overall significance (developmental or gonadal status, regardless of lighting condition) is described in the text, and post hoc analysis is depicted on the figures. In experiment 3, the effect of E2 on KP-54 pulse parameters before (120 min control period) and after (360 min) E2 injection or gonadal statuses (ovarian intact vs. OVX vs. OVX+E2) was examined with ANOVA and Bonferroni post hoc analysis. Differences were considered significant at P < 0.05.
Results
Developmental changes in KP-54 release patterns (experiment 1)
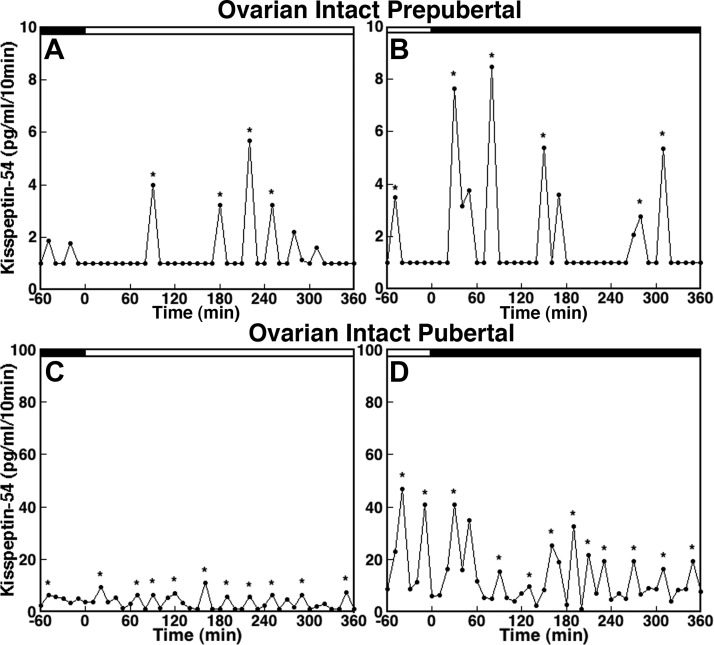
Examination of the in vivo KP-54 release patterns in individual prepubertal monkeys during the morning (Fig. 2A) and evening (Fig. 2B) showed that KP-54 release was pulsatile, with pulses consisting of a long IPI, low pulse amplitude, and no clear differences between the morning and evening values. KP-54 release in pubertal monkeys during the morning (Fig. 2C) and evening (Fig. 2D) was also pulsatile. However, in pubertal monkeys the IPI was shorter and the pulse amplitude was larger than those in prepubertal monkeys. Pulse amplitude during the evening in pubertal monkeys (Fig. 2D) was also larger than that during the morning (Fig. 2C).
Fig. 2.
KP-54 in vivo from the S-ME/MBH of ovarian intact prepubertal (A and B) and pubertal (C and D) monkeys. Samplings during the morning (A and C) and during the evening (B and D) are shown by open bars and closed bars, respectively, at the top of each graph. KP-54 release occurs in a pulsatile manner in both prepubertal (morning in A and evening in B) and pubertal (morning in C and evening in D) monkeys. Note that the scale of the y-axis in C and D (pubertal monkeys) is 10-fold higher than that in A and B (prepubertal monkeys). The IPI and the pulse amplitude in prepubertal monkeys are longer and lower, respectively, compared with those in pubertal monkeys. Asterisks indicate peaks as determined by PULSAR.
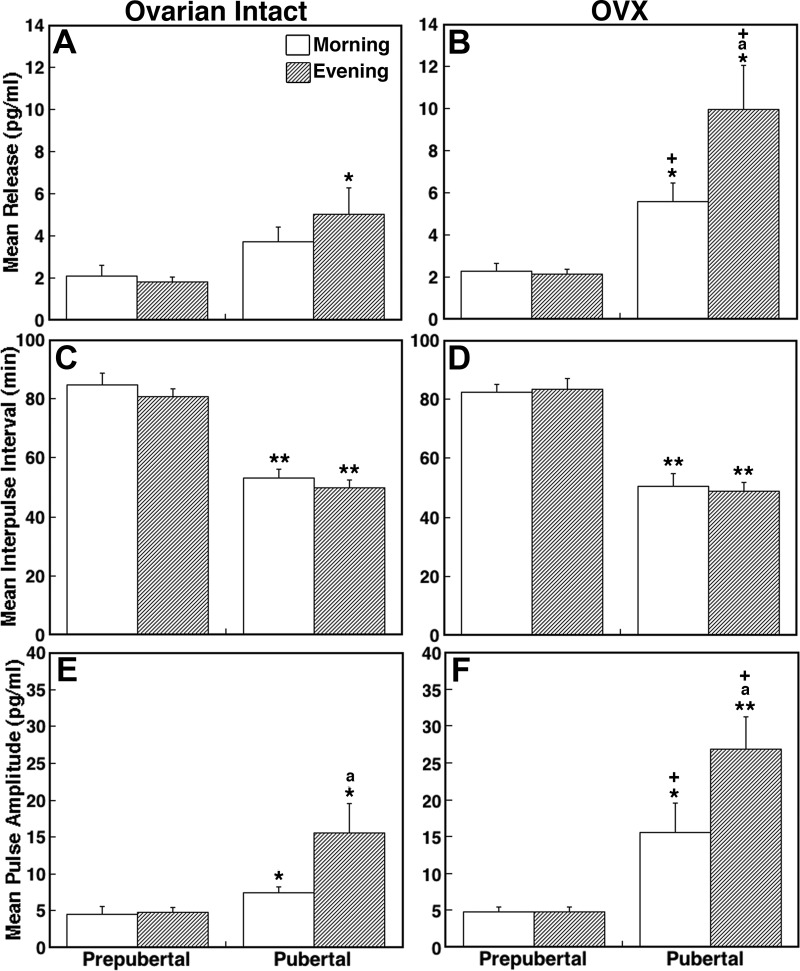
Consistent with our previous observation (14), there was a developmental increase in mean KP-54 release (overall significance: P < 0.01, Fig. 3A). Post hoc analysis indicated that whereas mean evening release in pubertal monkeys was significantly larger than that during the corresponding time period in prepubertal monkeys (P < 0.05, Table 1), mean morning release only had a trend toward being larger in pubertal monkeys compared with prepubertal monkeys (P = 0.08). There was no difference in mean KP-54 release between morning and evening in prepubertal monkeys (Fig. 3A).
Fig. 3.
Developmental changes in KP-54 release in ovarian-intact (A, C, and E) and OVX (B, D, and F) female monkeys. Comparison of the mean release (top panels), IPI (middle panels), and pulse amplitude (bottom panels) are shown. Data were obtained during the morning (open bars) or evening (shaded bars) sessions in prepubertal and pubertal monkeys. Overall mean KP-54 release and pulse amplitude in ovarian intact pubertal monkeys were significantly (P < 0.01 and P < 0.05, respectively) greater than those in ovarian intact prepubertal monkeys. Similarly, the IPI in ovarian intact pubertal monkeys was significantly (P < 0.01) shorter than that in ovarian intact prepubertal monkeys. Overall mean KP-54 release and pulse amplitude in OVX pubertal monkeys were significantly (P < 0.01 for both) greater than those in OVX prepubertal monkeys. Similarly, the IPI in OVX pubertal monkeys was significantly (P < 0.01) shorter than that in OVX prepubertal monkeys. Comparison between OVX and ovarian-intact monkeys indicated that mean KP-54 and pulse amplitude in OVX pubertal monkeys were significantly (P < 0.01 for both) greater than those in ovarian intact pubertal monkeys. *, P < 0.05 and **, P < 0.01 vs. prepubertal; a, P < 0.05 vs. morning; +, P < 0.05 vs. ovarian-intact monkeys. The number of prepubertal animals: ovarian intact morning, n = 7, and evening, n = 7; OVX: morning, n = 7, and evening, n = 8. The number of pubertal animals: ovarian-intact morning, n = 6, and evening, n = 8; OVX morning, n = 6, and evening, n = 6. Data are mean ± sem.
Table 1.
Comparison of kisspeptin-54 pulse parameters assessed by in vivo measurements from ovarian intact (INT), OVX, and E2-injected OVX prepubertal and pubertal monkeys
| Developmental stage | Gonadal status | Lighting condition | n | Mean Release (pg/ml) | IPI (min) | Pulse amplitude (pg/ml) |
|---|---|---|---|---|---|---|
| Prepubertal | INT | Morning | 7 | 2.10 ± 0.50 | 84.6 ± 4.2 | 4.58 ± 1.07 |
| Evening | 7 | 1.81 ± 0.25 | 80.6 ± 2.7 | 4.81 ± 0.70 | ||
| OVX | Morning | 7 | 2.30 ± 0.37 | 82.4 ± 2.6 | 4.95 ± 0.70 | |
| Evening | 8 | 2.12 ± 0.25 | 83.5 ± 3.6 | 4.82 ± 0.65 | ||
| OVX+E2 | Morning | 3 | 2.41 ± 0.16 | 79.3 ± 3.7 | 4.81 ± 0.25 | |
| Pubertal | INT | Morning | 6 | 3.74 ± 0.69 | 53.1 ± 2.9a | 7.45 ± 0.78b |
| Evening | 8 | 5.02 ± 1.28b | 49.9 ± 2.7a | 15.61 ± 3.97b,c | ||
| OVX | Morning | 6 | 5.59 ± 0.88b,d | 50.4 ± 4.5a | 16.51 ± 2.73b,d | |
| Evening | 6 | 9.91 ± 2.10b,c,d | 49.0 ± 2.8a | 26.83 ± 4.41a,c,d | ||
| OVX+E2 | Morning | 7 | 4.51 ± 0.20b,e | 53.5 ± 6.0a | 11.11 ± 0.69b,e |
OVX+E2 indicates KP-54 release after E2 injection; data before E2 injection was not included because it is similar to OVX alone. Values are the mean ± sem.
P < 0.01 vs. prepubertal.
P < 0.05 vs. prepubertal.
P < 0.05 vs. morning.
P < 0.05 vs. INT.
P < 0.05 vs. before E2 injection.
There was also a developmental change in the IPI of KP-54 release (overall significance: P < 0.01, Fig. 3C). Post hoc analysis indicated that the IPI in pubertal monkeys during both morning and evening were shorter than those during the corresponding time periods in the prepubertal monkeys (P < 0.01 for both, Table 1), whereas there was no difference in the IPI between morning and evening in either developmental stage (Fig. 3C). Similarly, there was a developmental change in the pulse amplitude of KP-54 release (overall significance: P < 0.05, Fig. 3E). Post hoc analysis indicated that the pulse amplitude during both the morning and evening in pubertal monkeys was significantly larger than those in the prepubertal monkeys (P < 0.05 for both, Table 1). Whereas there was no difference in the pulse amplitude between the morning and evening in prepubertal monkeys, the pulse amplitude in pubertal monkeys during the evening was significantly larger than that in the morning (P < 0.05, Fig. 3E).
Effects of OVX on KP-54 release patterns (experiment 2)
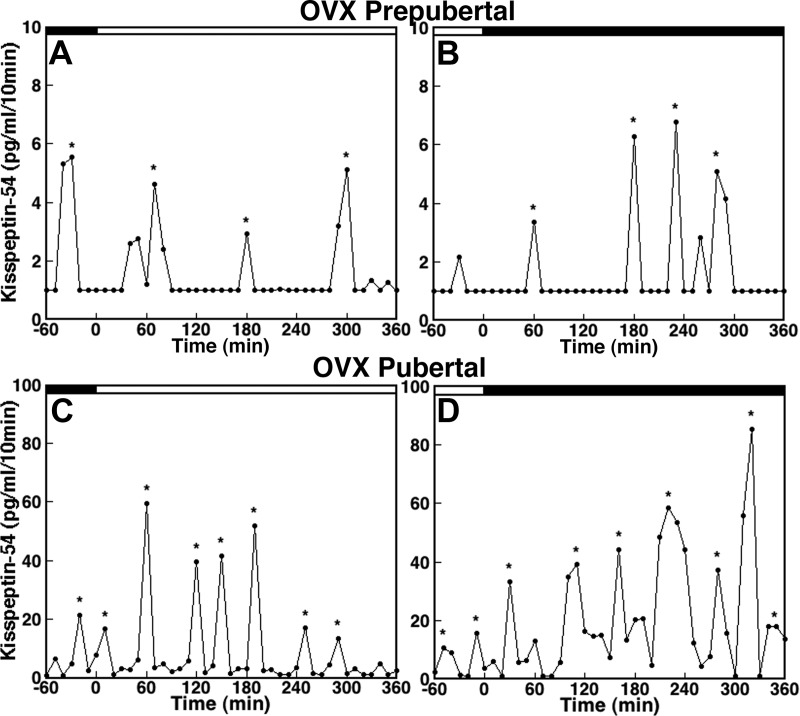
Because the level of circulating ovarian steroid hormones differs before and after puberty onset, we next examined whether ovarian steroid feedback plays a role in the developmental changes in KP-54 release observed in experiment 1. The KP-54 release pattern in OVX prepubertal monkeys during the morning (Fig. 4A) and evening (Fig. 4B) were very similar to those observed in their ovarian intact counter parts. The IPI in OVX prepubertal monkeys was long and pulse amplitude was low, regardless of time of day. In contrast, KP-54 release in OVX pubertal monkeys during the morning (Fig. 4C) and evening (Fig. 4D) showed an elevated amplitude, with a shorter IPI when compared with those in prepubertal OVX monkeys. Furthermore, pulse amplitude in the OVX pubertal monkeys appeared higher than those in the ovarian intact pubertal monkeys (Fig. 4, C and D, vs. Fig. 2, C and D).
Fig. 4.
KP-54 release in vivo from the S-ME/MBH in OVX prepubertal (A and B) and pubertal (C and D) monkeys. Samplings during the morning and evening are shown by open and closed bars, respectively, at the top of each graph. Note that the scale of the y-axis in C and D (pubertal monkeys) is 10-fold higher than that in A and B (prepubertal monkeys). KP-54 release in both prepubertal and pubertal monkeys occurs in a pulsatile manner. The IPI in OVX prepubertal monkeys is longer and the pulse amplitude is lower compared with OVX pubertal monkeys. Asterisks indicate peaks as determined by PULSAR.
Data analysis indicated that there was a developmental increase in mean KP-54 release (overall significance: P < 0.01, Fig. 3B). Post hoc analysis further indicated that mean KP-54 release in OVX pubertal monkeys during the morning and evening was significantly larger than OVX prepubertal monkeys during the respective lighting conditions (P < 0.05 for both). Moreover, there was a significant (P < 0.05) increase in mean KP-54 release during the evening compared with KP-54 release during the morning in OVX pubertal monkeys, whereas there was no morning-evening difference in mean KP-54 release in prepubertal monkeys (Table 1). Importantly, KP-54 levels in four OVX pubertal monkeys (either morning or evening), in which OVX was conducted during the prepubertal stage, had comparable levels with those OVX during the pubertal stage and had much higher KP-54 levels when compared with KP-54 measurements during the prepubertal stage.
The comparison of mean KP-54 release with ovarian-intact monkeys indicated that there was no OVX effect in prepubertal monkeys, whereas OVX significantly increased the mean KP-54 release in pubertal monkeys (overall significance: P < 0.01, Fig. 3, A and B). Post hoc analysis further suggested that mean KP-54 in both the morning and the evening OVX pubertal monkeys was significantly larger than those in the ovarian-intact counterparts (P < 0.05 for both, Table 1).
Statistical analysis indicated that a developmental change in the IPI was observed in OVX monkeys (overall significance: P < 0.01, Fig. 3D). Post hoc analysis further indicated that the IPI in pubertal monkeys during both the morning and evening were significantly shorter than those in OVX prepubertal monkeys during the respective lighting condition (P < 0.01 for both). However, the IPI between the morning and evening was not different for either developmental stage, nor was it different between the ovarian intact and OVX monkeys for either developmental stage (Fig. 3, C and D).
There was a developmental increase in the pulse amplitude in OVX monkeys (overall significance: P < 0.01, Fig. 3F). Post hoc analysis further indicated that the pulse amplitude in OVX pubertal monkeys during both the morning and evening was significantly greater than those in the OVX prepubertal counterparts (P < 0.05 for morning and P < 0.01 for evening). Although there was no morning-evening difference in the pulse amplitude in OVX prepubertal monkeys, pulse amplitude in OVX pubertal monkeys during the evening was significantly greater than that during the morning (P < 0.05, Table 1).
Comparison between OVX pubertal and ovarian-intact pubertal monkeys revealed that OVX significantly increased the pulse amplitude (overall significance: P < 0.01, Fig. 3, E and F). Post hoc analysis further indicated that pulse amplitude during both the morning and evening in OVX pubertal monkeys were significantly (P < 0.05 for both) greater than those in the ovarian intact pubertal counterparts. In contrast, there were no significant differences in pulse amplitude between the OVX and ovarian-intact prepubertal monkeys (Table 1).
The role of E2 in KP-54 release (experiment 3)
To determine whether replacement of E2 can reverse the OVX effects observed in experiment 2, we examined the effect of E2 injection on KP-54 release in OVX prepubertal and pubertal monkeys. Before E2, the KP-54 release patterns in both OVX prepubertal and pubertal monkeys in experiment 3 (Fig. 5, A and B) were very similar to those in experiment 2 (Fig. 4, A and C), and all pulse parameters of KP-54 release in both developmental groups (Fig. 6, A–C) were comparable with those in their OVX counterparts in experiment 2 (Fig. 3, B, D, and F). However, the KP-54 release patterns and pulse parameters in OVX pubertal monkeys in this study were different from those in ovarian intact pubertal monkeys in experiment 1 (Figs. 2C, 3A, 3C, and 3E).
Fig. 5.
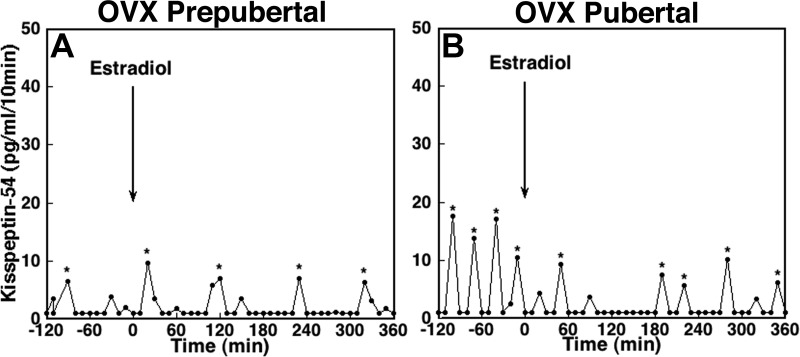
The effects of E2 on KP-54 release in an OVX prepubertal (A) and pubertal (B) monkey. After a 120-min control sampling, 30 μg/kg estradiol benzoate was sc injected at time 0, as indicated by the arrow. E2 suppressed KP-54 release in a pubertal, but not a prepubertal, monkey. Asterisks indicate peaks as determined by PULSAR.
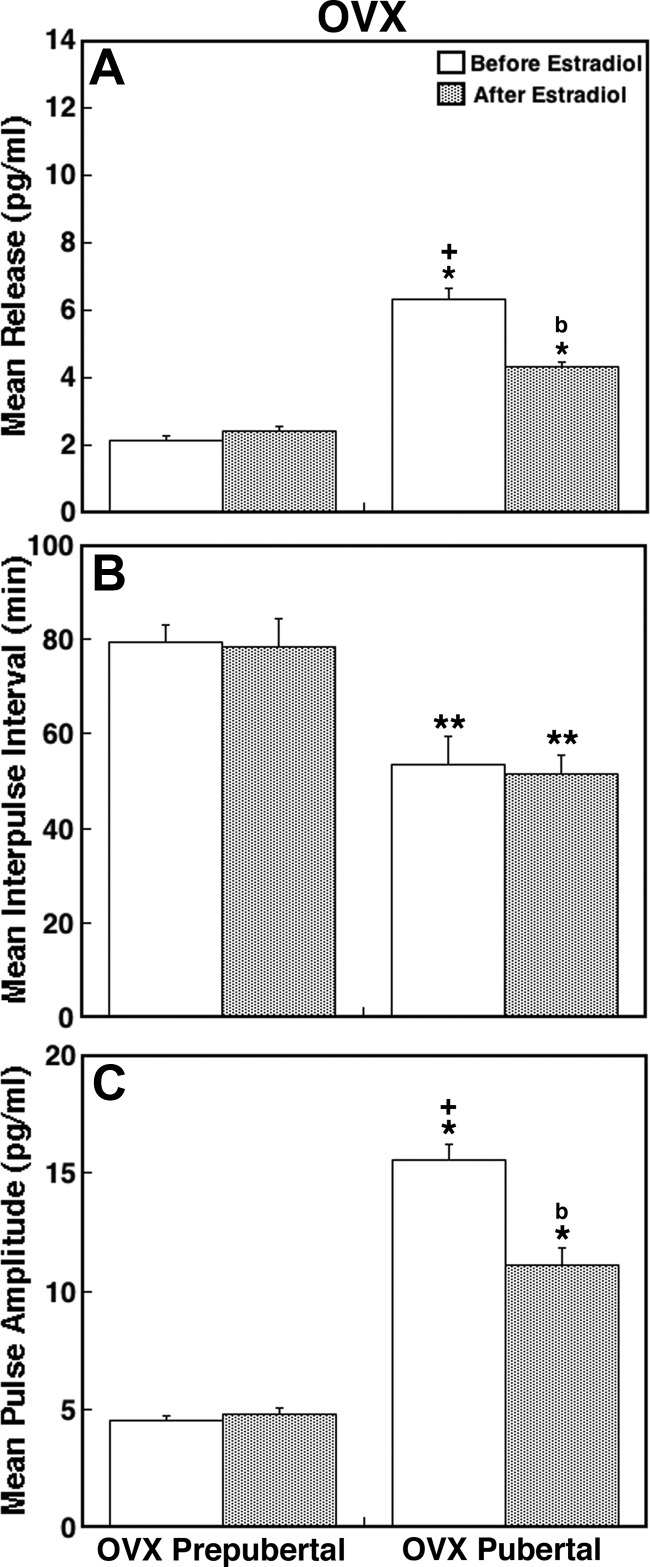
Fig. 6.
Analysis of KP-54 pulse parameters (A, mean release; B, IPI; and C, pulse amplitude) in OVX prepubertal and pubertal monkeys injected with E2. Note that before E2 injection, the mean KP-54 release, IPI, and pulse amplitude in OVX prepubertal or pubertal monkeys were similar to that in OVX prepubertal or pubertal monkeys shown in Fig. 3, B, D, and F, respectively. E2 injection clearly suppressed mean KP-54 release and pulse amplitude in OVX pubertal monkeys, whereas E2 caused no significant changes in OVX prepubertal monkeys.*, P < 0.05 and **, P < 0.01 vs. prepubertal; b, P < 0.05 vs. before E2 injection; +, P < 0.05 vs. ovarian-intact monkeys. Data obtained before (open bars) and after (shaded bars) E2 injection in prepubertal (n = 3) and pubertal (n = 7) monkeys are shown. Data are mean ± sem.
E2 administration in OVX prepubertal monkeys did not cause any significant change in KP-54 release (Fig. 5A). In contrast, E2 injection in OVX pubertal monkeys clearly suppressed KP-54 release (Fig. 5B). Statistical analysis indicated that mean KP-54 release after E2 injection in OVX pubertal monkeys was significantly lower than those before E2 (P < 0.05, Fig. 6A), whereas there was no difference before or after E2 injection in OVX prepubertal monkeys (Fig. 6A). Similarly, the pulse amplitude in OVX pubertal, but not prepubertal, monkeys was significantly suppressed by the E2 treatment (P < 0.05, Fig. 6C). The IPI in both groups was not altered by E2 (Fig. 6B).
Comparison of the data between pubertal OVX+E2 and OVX groups indicate that E2 significantly suppressed mean KP-54 release and pulse amplitude (P < 0.05 for both, Fig. 6, A and C, vs. Fig. 3, B and F), but not IPI. In contrast, comparison of the data between pubertal OVX+E2 and ovarian intact (morning) monkeys indicated that mean release, pulse amplitude, and IPI in OVX+E2 pubertal monkeys after E2 (Fig. 6, A–C) were all comparable with those in their ovarian-intact counter parts (Fig. 3, A, C, and E).
Discussion
In the present study, we found the following: 1) there was a developmental increase in mean KP-54 release, pulse frequency (IPI per 60 min), and pulse amplitude in ovarian-intact female monkeys; 2) OVX in prepubertal monkeys did not cause any changes in the KP-54 release pattern, whereas OVX in pubertal monkeys increased mean KP-54 release and pulse amplitude but not pulse frequency; and 3) replacement of E2 in OVX pubertal, but not prepubertal, monkeys suppressed mean KP-54 release and pulse amplitude but not pulse frequency. These results indicate that the release pattern of KP-54 undergoes developmental changes.
An increase in pulsatile GnRH release triggers the onset of puberty. Our previous study indicated that this pubertal increase in GnRH release is, in part, due to an increase in responsiveness of GnRH neurons to human KP-10 via KiSS1R (15). If the pubertal increase in GnRH release is also due to a developmental increase in KP-54 release, we expect that parallel developmental changes in KP-54 and GnRH release would occur. Indeed, the results of the present study indicate that mean KP-54 release increased during pubertal development, consistent with that observed in our previous study (14), and this increase in mean KP-54 release is parallel to the developmental increase in mean GnRH release (2, 3). Moreover, the frequency of KP-54 release increased at puberty, i.e. the IPI of KP-54 release was shortened from approximately 80 min during the prepubertal stage to approximately 50 min during the pubertal stage, which is identical to the developmental change in IPI of GnRH release previously observed (2, 3). In addition, a developmental increase in the pulse amplitude of KP-54 release mirrored developmental changes in GnRH pulse amplitude (2, 3). These analogous changes in release of KP-54 and GnRH strongly suggest that KP-54 pulses may drive GnRH pulses during the progression of puberty.
Ideally, serial measurement of KP-54 and GnRH within the same samples collected at 10-min intervals would prove this possibility. In fact, in a previous study, we found that there was approximately a 75% correlation between KP-54 and GnRH pulses in midpubertal monkeys (14). However, in the present study, we were unable to examine the correlation between KP-54 and GnRH pulses in prepubertal monkeys because levels of both KP-54 and GnRH within the same 10-min samples were too low for dual detection. It is important to note that despite the methodological difference between the studies, i.e. GnRH release was measured by a push-pull perfusion method, whereas KP-54 release was assessed by a microdialysis method, there are parallel developmental changes in GnRH release (2, 3) and KP-54 release in this study. Although perifusates collected with the push-pull perfusion method were exclusively from the S-ME in which neuroterminals are concentrated, dialysates with the microdialysis method could contain samples from not only the S-ME but also the MBH, in which cell bodies and fibers are present. Therefore, it is possible that the KP-54 measured in this study were from both the S-ME and MBH. Nonetheless, these parallel changes in KP-54 and GnRH release provide strong evidence for the regulation of pulsatile GnRH release by pulsatile KP-54 release.
Circulating gonadal steroid levels rise after puberty onset, and in primates developmental changes in the GnRH pulse pattern are independent from the feedback influence of gonadal steroids (3, 23). If the pubertal increase in GnRH release is due to a developmental increase in KP-54 release, then we expect that developmental changes in KP-54 release would occur independent from circulating gonadal steroid hormones. Indeed, the results of the present study indicate that OVX in pubertal monkeys increased mean KP-54 release and pulse amplitude, whereas OVX in prepubertal monkeys did not alter any of the KP-54 pulse parameters. Moreover, the pulse frequency of KP-54 release in OVX pubertal monkeys was significantly shorter than that in OVX prepubertal monkeys despite the absence of the ovary. Importantly, the developmental increase in KP-54 release was also seen in a few OVX prepubertal monkeys, which were subsequently examined during the pubertal stage, indicating that the pubertal increase in KP-54 release is independent of ovarian feedback. Again, these developmental differences in the KP-54 response to OVX are identical with those observed in the GnRH response to OVX in female monkeys (3).
The effects of OVX can be attributed to the removal of the ovarian steroid estrogen as well as peptides, such as inhibin and growth factors. However, the results of the present study showing that injection of E2 in OVX pubertal monkeys fully suppressed the OVX-induced elevation in mean KP-54 release and pulse amplitude suggest that the effects of OVX on the increased release of KP-54 in pubertal monkeys are primarily due to the absence of circulating E2. Similar to our previous report showing that E2 does not alter the pulse pattern of GnRH release in OVX prepubertal monkeys (16), we found that the KP-54 release pattern in OVX prepubertal monkeys was not modified by E2. Collectively these parallel changes in the release patterns of KP-54 and GnRH during the pubertal process further support the hypothesis that an increase in pulsatile KP-54 release is a contributor to the pubertal increase in pulsatile GnRH release, but it is not likely responsible for puberty onset.
It has been known for several decades that a nocturnal increase in LH and GnRH becomes prominent during puberty in humans and monkeys (2, 24–26), and this nocturnal increase is further enhanced by the absence of gonadal steroid feedback (3, 27–29). Similar to that seen with GnRH release, in this and a previous study (14), we observed a nocturnal increase in KP-54 release in pubertal monkeys, which is further enhanced after OVX. However, unlike our observation in the previous study (14), in the present study, no nocturnal increase in KP-54 release was seen in prepubertal monkeys, regardless of the presence or absence of the ovary. The reason for this difference is unclear, but it is likely due to the methodological difference in neuropeptide collection. Nonetheless, our results indicate that the nocturnal increase in KP-54 release may contribute to the nocturnal increase in GnRH release during puberty.
Numerous studies in rodents have demonstrated that GnRH release is modulated by circadian input from the suprachiasmatic nucleus (SCN) (30, 31). A recent study further suggested that there is a circadian rhythm in KP mRNA expression in the anteroventral periventricular nucleus (AVPV) of female mice, which is dependent on circulating E2 (32). Moreover, vasopressin neurons from the SCN directly innervate KP neurons in the AVPV of mice (33). Whether a similar anatomical feature is present in the primate brain or whether input from the SCN increases at the time of puberty needs to be investigated.
Despite the parallel ovarian steroid-independent changes in KP-54 release and GnRH release across puberty onset, we do not believe that KP plays a role in triggering puberty. Previously we and others have proposed the hypothesis in primates that before puberty onset, a predominantly inhibitory neuronal input suppresses GnRH release and reduction in this central inhibition triggers the pubertal increase in GnRH release. The GnRH neurosecretory system is active during the neonatal period but becomes dormant during the juvenile period until the time of puberty, despite the absence of the gonads (17, 18, 34). We have postulated that γ-aminobutyric acid (GABA) neurons are the neural substrates for this central inhibition in female monkeys (17), although Plant and Witchel (18) have proposed that neuropeptide Y neurons are responsible in male monkeys. We have further found that reduction in GABA tone in the MBH leads to a relative increase in glutamate tone (35, 36). Therefore, it is possible that the absence of OVX effects on KP-54 release in prepubertal monkeys may be due to the tonic GABA inhibition over KP neurons. A subsequent reduction in this tonic inhibition at puberty would facilitate the initiation of increased GnRH release, leading to E2 secretion from the ovary. Elevation of E2 in circulation would subsequently increase the activity of KP neurons as well as the responsiveness of GnRH neurons to KP input (15). Although it is possible that the pubertal increase in KP-54 precedes the pubertal increase in GnRH release, our preliminary data in prepubertal monkeys indicate that the GABAA antagonist, bicuculline, potently stimulates GnRH release, and this increase is blocked by a KP antagonist (Kurian J.R. and E. Terasawa, unpublished observation), suggesting that a reduction in tonic GABA tone needs to be removed before KP-54 increases at puberty. Therefore, KP-54 does not likely play a critical role in triggering the onset of puberty in female rhesus monkeys.
A substantial number of reported studies supports a role for KP and KiSS1R in the mechanism of puberty. Specifically, several studies in humans have identified inactivating mutations in KiSS1R that result in a delay in or absence of puberty (4, 5), whereas activating mutations in both KiSS1 and KiSS1R result in precocious puberty (6, 7). Mice lacking KiSS1R exhibit small testes in males and a delay in vaginal opening and an absence of follicular maturation in females (4, 37, 38), and KiSS1 knockout mice exhibit no puberty or delayed puberty (38, 39). Moreover, physiological studies also show that administration of KP advances the timing of vaginal opening (40, 41), whereas administration of KP antagonist delays the age at vaginal opening in female rats (42). If KP and/or KiSS1R signaling play a key role in puberty onset, deletion of KP neurons would delay the timing of puberty. Surprisingly, however, deletion of estrogen receptor-α in KP neurons advanced the timing of puberty (43). Moreover, neither deletion of KP nor KiSS1R-expressing neurons before P20 compromises the timing of puberty onset, presumably due to a compensatory mechanism (13).
Although these molecular genetic approaches indicate the complexity of the mechanisms controlling the GnRH neuronal system and the exact role of KP neurons in the mechanism of puberty, the results of the present study along recent reports in mice and sheep (44, 45) suggest that the KP neuronal system is a player for the mechanism of pubertal progression. There is, however, a significant species difference. In rodents, and perhaps sheep, E2 appears to inhibit GnRH release via KP neurons in the arcuate nucleus before puberty onset, whereas after puberty onset it stimulates GnRH release via KP neurons in the AVPV (43–45). In primates, before puberty onset, involvement of KP-KiSS1R signaling to GnRH neurons is limited, such that KP can stimulate GnRH neurons through KiSS1R, but E2 modifies neither KiSS1R function (15) nor KP release as observed in this study. In contrast, after puberty onset KP-KiSS1R signaling in GnRH release is profoundly influenced by E2, such that E2inhibits GnRH release via KP neurons, as seen in the present study, despite its stimulatory action on KiSS1R function (15). Taken together, we conclude that KP-KiSS1R signaling contributes to the increase in GnRH release during the progression of puberty, but it may not play a role in triggering the onset of puberty.
Acknowledgments
The authors express their sincere appreciation to Mr. Frederick Wegner for his great assistance with KP-54 RIA and to Mr. Nicholas Shiel for his technical assistance. This study was not possible without assistance by the veterinarians and veterinary technicians at the Wisconsin National Primate Research Center.
This work was supported by National Institutes of Health Grants R01HD11355 and R01HD15433 (to E.T.) and T32HD041921 (to K.A.G.) and was possible to perform by National Institutes of Health support Grants P51RR000167, RR15459, and RR020141 (to the Wisconsin National Primate Research Center).
Disclosure Summary: The authors have no conflicts to disclose.
Footnotes
- AVPV
- Anteroventral periventricular nucleus
- E2
- 17β-estradiol
- GABA
- γ-aminobutyric acid
- IPI
- interpulse interval
- KiSS1R
- kisspeptin-1 receptor
- KP
- kisspeptin
- MBH
- medial basal hypothalamus
- OVX
- ovariectomized
- SCN
- suprachiasmatic nucleus
- S-ME
- stalk-median eminence.
References
- 1. Wildt L, Häusler A, Marshall G, Hutchison JS, Plant TM, Belchetz PE, Knobil E. 1981. Frequency and amplitude of gonadotropin-releasing hormone stimulation and gonadotropin secretion in the rhesus monkey. Endocrinology 109:376–385 [DOI] [PubMed] [Google Scholar]
- 2. Watanabe G, Terasawa E. 1989. In vivo release of luteinizing hormone releasing hormone increases with puberty in the female rhesus monkey. Endocrinology 125:92–99 [DOI] [PubMed] [Google Scholar]
- 3. Chongthammakun S, Claypool LE, Terasawa E. 1993. Ovariectomy increases in vivo luteinizing hormone-releasing hormone release in pubertal, but not prepubertal female rhesus monkeys. J Neuroendocrinol 5:41–50 [DOI] [PubMed] [Google Scholar]
- 4. Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zhan D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. 2003. The GPR54 gene as a regulator of puberty. N Engl J Med 349:1614–1627 [DOI] [PubMed] [Google Scholar]
- 5. de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. 2003. Hypogonadotropic hypogonadism due to loss of function of the kiss1-derived peptide receptor GPR54. Proc Natl Acad Sci USA 100:10972–10976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Silveira LG, Noel SD, Silvera-Neto AP, Abreu AP, Brito VN, Santos MG, Bianco SD, Kuohung W, Xu S, Gryngarten M, Escobar ME, Arnhold IJ, Mendonca BB, Kaiser UB, Latronico AC. 2010. Mutations of the KISS1 gene in disorders of puberty. J Clin Endocrinol Metab 95:2276–2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Teles MG, Bianco SD, Brito VN, Trarbach EB, Kuohung W, Xu S, Seminara SB, Mendonca BB, Kaiser UB, Latronico AC. 2008. A GPR54-activating mutation in a patient with central precocious puberty. N Engl J Med 358:709–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Seminara SB, Dipietro MJ, Ramaswamy S, Crowley WF, Jr, Plant TM. 2006. Continuous human metastin 45–54 infusion desensitizes G protein-coupled receptor 54-induced gonadotropin-releasing hormone release monitored indirectly in the juvenile male Rhesus monkey (Macaca mulatta): a finding with therapeutic implications. Endocrinology 147:2122–2126 [DOI] [PubMed] [Google Scholar]
- 9. Caraty A, Smith JT, Lomet D, Ben Saïd S, Morrissey A, Cognie J, Doughton B, Baril G, Briant C, Clarke IJ. 2007. Kisspeptin synchronizes preovulatory surges in cyclical ewes and causes ovulation in seasonally acyclic ewes. Endocrinology 148:5258–5267 [DOI] [PubMed] [Google Scholar]
- 10. Roa J, Vigo E, García-Galiano D, Castellano JM, Navarro VM, Pineda R, Diéguez C, Aguilar E, Pinilla L, Tena-Sempere M. 2008. Desensitization of gonadotropin responses to kisspeptin in the female rat: analyses of LH and FSH secretion at different developmental and metabolic states. Am J Physiol Endocrinol Metab 294:E1088–E1096 [DOI] [PubMed] [Google Scholar]
- 11. Plant TM, Ramaswamy S. 2009. Kisspeptin and the regulation of the hypothalamic-pituitary-gonadal axis in the rhesus monkey (Macaca mulatta). Peptides 30:67–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Oakley AE, Clifton DK, Steiner RA. 2009. Kisspeptin signaling in the brain. Endocr Rev 30:713–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mayer C, Boehm U. 2011. Female reproductive maturation in the absence of kisspeptin/GPR54 signaling. Nature Neurosci 14:704–710 [DOI] [PubMed] [Google Scholar]
- 14. Keen KL, Wegner FH, Bloom SR, Ghatei MA, Terasawa E. 2008. An increase in kisspeptin-54 release occurs with the pubertal increase in luteinizing hormone-releasing hormone-1 release in the stalk-median eminence of female rhesus monkeys in vivo. Endocrinology 149:4151–4157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guerriero KA, Keen KL, Millar RP, Terasawa E. 2012. Developmental changes in GnRH release in response to kisspeptin agonist and antagonist in female rhesus monkeys (Macaca mulatta): implication to the mechanism of puberty. Endocrinology 153:825–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chongthammakun S, Terasawa E. 1993. Negative feedback effects of estrogen on luteinizing hormone-releasing hormone release occur in pubertal, but not prepubertal, ovariectomized female rhesus monkeys. Endocrinology 132:735–743 [DOI] [PubMed] [Google Scholar]
- 17. Terasawa E, Fernandez DL. 2001. Neurobiological mechanisms of the onset of puberty in primates. Endocr Rev 22:111–151 [DOI] [PubMed] [Google Scholar]
- 18. Plant TM, Witchel SF. 2006. Puberty in nonhuman primates and humans. In: Neill JD, ed. Physiology of reproduction. Boston: Elsevier; 2177–2230 [Google Scholar]
- 19. Terasawa E. 1994. In vivo measurement of pulsatile release of neuropeptides and neurotransmitters in rhesus monkeys using push-pull perifusion. In: Conn PM, Levine JE, eds. Methods in neurosciences. San Diego: Academic Press; 184–202 [Google Scholar]
- 20. Frost SI, Keen KL, Levine JE, Terasawa E. 2008. Microdialysis methods for in vivo neuropeptide measurement in the stalk-median eminence in the rhesus monkey. J Neurosci Methods 168:26–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dhillo WS, Chaudhri OB, Thompson EL, Murphy KG, Patterson M, Ramachandran R, Nijher GK, Amber V, Kokkinos A, Donaldson M, Ghatei MA, Bloom SR. 2007. Kisspeptin-54 stimulates gonadotropin release most potently during the preovulatory phase of the menstrual cycle in women. J Clin Endocrinol Metab 92:3958–3966 [DOI] [PubMed] [Google Scholar]
- 22. Merriam GR, Wachter KW. 1982. Algorithms for the study of episodic hormone secretion. Am J Physiol 243:E310–E318 [DOI] [PubMed] [Google Scholar]
- 23. Plant TM. 1985. A study of the role of the postnatal testes in determining the ontogeny of gonadotropin secretion in the male rhesus monkey (Macaca mulatta). Endocrinology 116:1341–1350 [DOI] [PubMed] [Google Scholar]
- 24. Boyar RM, Finkelstein JW, Roffwarg H, Kapen S, Weitzman E, Hellman L. 1972. Synchronization of augmented luteinizing hormone secretion with sleep during puberty. N Engl J Med 287:582–586 [DOI] [PubMed] [Google Scholar]
- 25. Judd HL, Parker DC, Siler TM, Yen SS. 1974. The nocturnal rise of plasma testosterone in pubertal boys. J Clin Endocrinol Metab 38:710–713 [DOI] [PubMed] [Google Scholar]
- 26. Terasawa E, Nass TE, Yeoman RR, Loose MD, Schultz NJ. 1983. Hypothalamic control of puberty in female rhesus macaque. In: Norman RL, ed. Neuroendocrine aspects of reproduction. New York: Academic Press; 149–182 [Google Scholar]
- 27. Boyar RM, Finkelstein JW, Roffwarg H, Kapen S, Weitzman D, Hellman L. 1973. Twenty-four-hour luteinizing hormone and follicle-stimulating hormone secretory patterns in gonadal dysgenesis. J Clin Endocrinol Metab 37:521–525 [DOI] [PubMed] [Google Scholar]
- 28. Wu FC, Butler GE, Kelnar CJ, Stirling HF, Huhtaniemi I. 1991. Patterns of pulsatile luteinizing and follicle stimulating hormone secretion in prepubertal (midchildhood) boys and girls and patients with idiopathic hypogonadotrophic hypogonadism (Kallmann's syndrome): a study using an ultrasensitive time-resolved immunofluorometric assay. J Clin Endocrinol Metab 72:1229–1237 [DOI] [PubMed] [Google Scholar]
- 29. Schultz NJ, Terasawa E. 1988. Posterior hypothalamic lesions advance the time of the pubertal changes in luteinizing hormone release in ovariectomized female rhesus monkeys. Endocrinology 123:445–455 [DOI] [PubMed] [Google Scholar]
- 30. Chappell PE. 2005. Clocks and the black box: circadian influences on gonadotropin-releasing hormone secretion. J Neuroendocrinol 17:119–130 [DOI] [PubMed] [Google Scholar]
- 31. Christian CA, Moenter SM. 2010. The neurobiology of preovulatory and estradiol-induced gonadotropin-releasing hormone surges. Endocr Rev 31:544–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Robertson JL, Clifton DK, de la Iglasia HO, Steiner RA, Kauffman AS. 2009. Circadian regulation of Kiss1 neurons: implications for timing of preovulatory GnRH/LH surge. Endocrinology 150:3664–3671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vida B, Deli L, Hrabovszky E, Kalamatianos T, Caraty A, Coen CW, Liposits Z, Kalló I. 2010. Evidence for suprachiasmatic vasopression neruones innervating kisspeptin neurons in the rostral periventricular area of the mouse brain: regulation by oestrogen. J Neuroendocrinol 22:1032–1039 [DOI] [PubMed] [Google Scholar]
- 34. Grumbach MM. 2002. The neuroendocrinology of human puberty revisited. Horm Res 57:2–14 [DOI] [PubMed] [Google Scholar]
- 35. Terasawa E, Luchansky LL, Kasuya E, Nyberg CL. 1999. An increase in glutamate release follows a decrease in γ aminobutyric acid and the pubertal increase in luteinizing hormone releasing hormone release in the female rhesus monkey. J Neuroendocrinol 11:275–282 [DOI] [PubMed] [Google Scholar]
- 36. Keen KL, Burich AJ, Mitsushima D, Kasuya E, Terasawa E. 1999. Effects of pulsatile infusion of the GABA(A) receptor blocker bicuculline on the onset of puberty in female rhesus monkeys. Endocrinology 140:5257–5266 [DOI] [PubMed] [Google Scholar]
- 37. Funes S, Hedrick JA, Vassileva G, Markowitz L, Abbondanzo S, Golovko A, Yang S, Monsma FJ, Gustafson EL. 2003. The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem Biophys Res Commun 312:1357–1363 [DOI] [PubMed] [Google Scholar]
- 38. Lapatto R, Pallais JC, Zhang D, Chan YM, Mahan A, Cerrato F, Le WW, Hoffman GE, Seminara SB. 2007. Kiss1−/− mice exhibit more variable hypogonadism that Gpr54−/− mice. Endocrinology 148:4927–4936 [DOI] [PubMed] [Google Scholar]
- 39. d'Angelmont de Tassigny X, Fagg LA, Dixon JP, Day K, Leitch HG, Hendrick AG, Zhan D, Franceschini I, Caraty A, Carlton MB, Aparicio SA, Colledge WH. 2007. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci USA 104:10714–10719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Matsui H, Takatsu Y, Kumano S, Matsumoto H, Ohtaki T. 2004. Peripheral administration of metastin induces marked gonadotropin release and ovulation in the rat. Biochem Biophys Res Commun 320:383–388 [DOI] [PubMed] [Google Scholar]
- 41. Navarro VM, Fernández-Fernández R, Castellano JM, Roa J, Mayen A, Barreiro ML, Gaytan F, Aguilar E, Pinilla L, Dieguez C, Tena-Sempere M. 2004. Advanced vaginal opening and precocious activation of the reproductive axis by KiSS-1 peptide, the endogenous ligand of GPR54. J Physiol 561:379–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pineda R, Garcia-Galiano D, Rosweir A, Romero M, Sanchez-Garrido MA, Ruiz-Pino F, Morgan K, Pinilla L, Millar RP, Tena-Sempere M. 2010. Critical roles of kisspeptins in female puberty and preovulatory gonadotropin surges as revealed by a novel antagonist. Endocrinology 151:722–730 [DOI] [PubMed] [Google Scholar]
- 43. Mayer C, Acosta-Martinez M, Dubois SL, Wolfe A, Radovick S, Boehm U, Levine JE. 2010. Timing and completion of puberty in female mice depend on estrogen receptor α-signaling in kisspeptin neurons. Proc Natl Acad Sci USA 107:22693–22698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Clarkson J, Boon WC, Simpson ER, Herbison AE. 2009. Postnatal development of an estradiol-kisspeptin positive feedback mechanism implicated in puberty onset. Endocrinology 150:3214–3220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Redmond JS, Baez-Sandoval GM, Spell KM, Spencer TE, Lents CA, Williams GL, Amstalden M. 2011. Developmental changes in hypothalamic Kiss1 expression during activation of the pulsatile release of luteinising hormone in maturing ewe lambs. J Neuroendocrinol 23:815–822 [DOI] [PubMed] [Google Scholar]