Abstract
Calcitonin gene-related peptide (CGRP) is a neuropeptide with multiple neuroendocrine roles, including vasodilation, migraine, and pain. The receptor for CGRP is a G protein-coupled receptor (GPCR) that requires three proteins for function. CGRP binds to a heterodimer composed of the GPCR calcitonin-like receptor (CLR) and receptor activity-modifying protein (RAMP1), a single transmembrane protein required for pharmacological specificity and trafficking of the CLR/RAMP1 complex to the cell surface. In addition, the CLR/RAMP1 complex requires a third protein named CGRP-receptor component protein (RCP) for signaling. Previous studies have demonstrated that depletion of RCP from cells inhibits CLR signaling, and in vivo studies have demonstrated that expression of RCP correlates with CLR signaling and CGRP efficacy. It is not known whether RCP interacts directly with CLR to exert its effect. The current studies identified a direct interaction between RCP and an intracellular domain of CLR using yeast two-hybrid analysis and coimmunoprecipitation. When this interacting domain of CLR was expressed as a soluble fusion protein, it coimmunoprecipitated with RCP and inhibited signaling from endogenous CLR. Expression of this dominant-negative domain of CLR did not significantly inhibit trafficking of CLR to the cell surface, and thus RCP may not have a chaperone function for CLR. Instead, RCP may regulate CLR signaling in the cell membrane, and direct interaction between RCP and CLR is required for CLR activation. To date, RCP has been found to interact only with CLR and represents a novel neuroendocrine regulatory step in GPCR signaling.
Calcitonin gene-related peptide (CGRP) is a neuropeptide with multiple neuroendocrine functions. In the peripheral vasculature, CGRP is a potent vasodilator (1). In the cerebral vasculature, CGRP is also a vasodilator, and elevated levels of CGRP in blood are detected in patients during migraine attacks, and iv CGRP can induce migraine-like symptoms (2–4), which has led to the development of synthetic nonpeptide CGRP antagonists for the treatment of migraine (5, 6). In the central nervous system and spinal cord, CGRP mediates pain and inflammation (7–9), the development of tolerance to opiates (10, 11), and regulation of energy homeostasis (12). The receptor for CGRP is an unusual G protein-coupled receptor (GPCR) in that it requires three proteins to function: 1) a ligand-binding protein named calcitonin-like receptor (CLR) that has a seven-transmembrane structure associated with GPCR, 2) an accessory protein with a single transmembrane-spanning domain named receptor activity-modulating protein (RAMP1) that aids in trafficking of CLR to the cell surface and generates pharmacological specificity, and 3) a second accessory protein that is a peripheral membrane protein named CGRP-receptor component protein (RCP) that couples the CLR/RAMP1 complex to the cellular signaling pathway (13–16).
RCP colocalizes with CGRP in brain and spinal cord (17, 18), in coronary arteries (19), and at the neuromuscular junction (20). RCP expression is induced in spinal cord in response to application of CGRP antagonist or inflammatory stimuli, suggesting a role for RCP in increased CGRP receptor signaling in response to pain (17, 18). In the uterus, RCP expression is induced under conditions that increase CGRP efficacy during pregnancy and estrus to inhibit uterine contraction (21, 22). RCP expression also correlates with increased vascular response to CGRP in the subtotal nephrectomy-salt hypertension model. The vasculature response to CGRP was potentiated in these hypertensive rats, and this increased responsiveness correlated with increased RCP expression but not of CLR or RAMP1 (23).
Together, these in vivo studies suggest that RCP expression can modulate CLR function. Knowing the nature and site of the interaction between RCP and CLR is a first step toward determining the mechanism of RCP action and identifying novel targets for drug design to regulate CGRP receptor function. Previous cell culture studies demonstrated that loss of RCP protein correlated with loss of CLR signaling (13, 16). Signaling at other GPCR such as the β2-adrenergic receptor (β2AR) and adenosine receptor were unaffected by loss of RCP, suggesting that RCP interacted with a limited range of receptors, currently restricted to CLR. RCP is an intracellular peripheral membrane protein that coimmunoprecipitates with the CLR/RAMP complex (13). RCP does not contain any obvious functional domains that indicate how it might interact with a GPCR. It is not known whether RCP interacts directly with the CLR/RAMP1 complex or indirectly via intermediary proteins. In the present study, we identified the site of interaction of RCP with CLR and determined the effect of altered RCP-CLR interactions on CLR signaling and trafficking.
Materials and Methods
Intracellular cytoplasmic loops (ICL) and carboxyl- tail (C-tail)
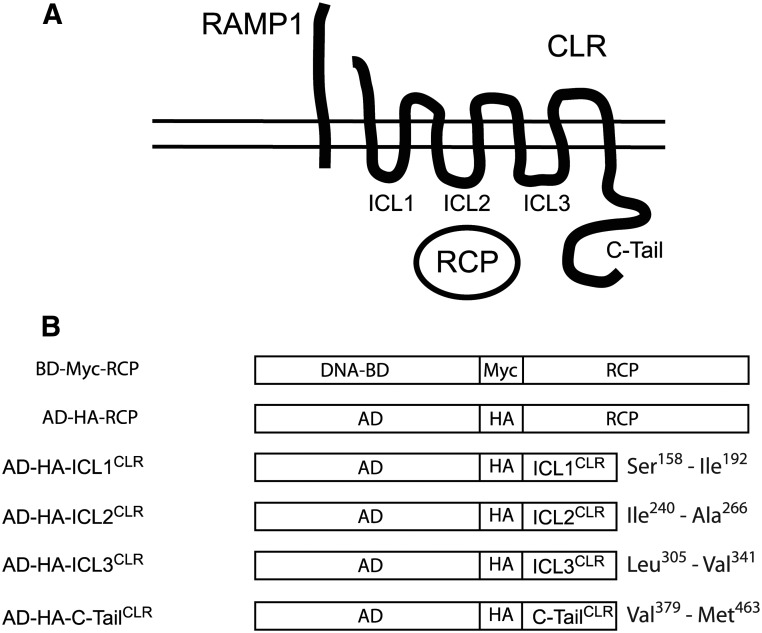
ICL and C-tail of CLR, and ICL2β2AR were delineated by first identifying hydrophobic putative transmembrane domains in a hydrophobicity plot (Lasergene Protean software; DNASTAR Inc., Madison, WI) of the mouse CLR protein (GenBank accession number NM018782) or mouse β2AR (GenBank accession number NM_007420). PCR was used to amplify the corresponding cytoplasmic domains from cDNA. Primers for PCR were designed to amplify sequences that encoded an additional 10 residues of the transmembrane domains flanking each hydrophilic cytoplasmic domain, in case the actual cytoplasmic domain varied slightly from our prediction. The amino acids bounding the domains are: ICL1CLR (S158–I192), ICL2CLR (I240–A266), ICL3CLR (L305–V341), C-tail (V379–M463), and ICL2β2AR (V129–R151).
Yeast two-hybrid screen
The ICL of CLR (three ICL plus C-tail) (Fig. 1, A and B) were cloned as fusion proteins in the yeast two-hybrid plasmid pGADT7-AD (Clontech, Mountain View, CA). The cDNA for mouse RCP (GenBank accession number AF028242) was cloned into the yeast two-hybrid plasmid pGBKT7-BD (Clontech). The cytoplasmic domains of CLR were expressed as fusion proteins and contained a hemagglutinin (HA) epitope tag. RCP was cloned similarly with a myc epitope tag (Fig. 1B).
Fig. 1.
Cytoplasmic domains of CLR. A, Functional CGRP receptor: CLR, RAMP1, and RCP. ICL and C-tail of CLR are indicated. B, Yeast two-hybrid constructs between cytoplasmic domains of CLR and GAL4 activating domain (AD) and between RCP and GAL4 DNA-binding domain (BD). Amino acids bounding the cytoplasmic domains of mouse CLR are indicated.
Amplification of all CLR cDNA was performed by PCR using the following primers: first ICL of CLR (ICL1CLR), 5′-AGC GCT AGC GAA TTC TCT CTC ATC ATA TTT TTT TAC TTC AAG AGC CT-3′ and 5′-CGC TAG CCG CTC GAG GAT GAT TGT TAC AAT CGA ATT ACA AAT AAA TGA-3′; ICL2CLR, 5′-AGC GCT AGC GAA TTC ATC GTG GTG GCT GTG TTT GCG GAG AA-3′ and 5′-CGC TAG CCG CTC GAG GGC AGG AAG CAG AGG AAA CCC CCA GC-3′); ICL3CLR, 5′-AGC GCT AGC GAA TTC CTC TTT TTC CTA TTA AAT ATT GTA CGT GTT CT-3′ and 5′-CGC TAG CCG CTC GAG TAC CAA GAT GAG AGT AGC TCT TAC G-3′; and C-TailCLR, 5′-AGC GCT AGC GAA TTC GTG GCT ACT ATT TTC TGC TTC TTT AAT-3′ and 5′-CGC TAG CCG CTC GAG CAT CAC TAG ATC ATA CGT ATT TTC TGA T-3′.
The yeast two-hybrid screen was carried out using the MATCHMAKER GAL4 two-hybrid system 3 (Clontech, Inc., Palo Alto, CA), using the manufacturer's protocols. AH109 yeast were first transformed with binding domain-myc-RCP, and stable transformants were isolated. Yeast expressing binding domain-myc-RCP were subsequently transformed with activating domain-HA constructs containing the cytoplasmic domains of CLR. Double transformants were selected for growth on media lacking leucine and tryptophan. Positive interacting clones were identified by growth on plates lacking histidine in addition to lacking leucine and tryptophan, supplemented with 1 mm 3-amino-1,2,4-triazole. Positive clones were subsequently tested for α-galactosidase activity.
Construction of CLR mammalian expression constructs
ICL2CLR was amplified by PCR (5′-CGC CCG TGA CTC GAG GCC ACC ATG GTG GTG GCT GTG TTT GCG GAG AAG CAG-3′ and 5′-CGC CCG TGA GGA TCC CCG GCA GGA AGC AGA GGA AAC CCC CAG CC-3′), as was ICL2β2AR (5′-AGC GCT AGC CGT CTC GAG GCC ACC ATG GTG GAT CGC TAT GTT GCT ATC A-3′ and 5′-GC TAG CCG TAA GGA TCC CCT CGG GCC TTA TTC TTG GTC-3′). The resulting PCR amplimers were digested with XhoI and BamHI and ligated into the XhoI and BamHI sites of plasmid pEGFP-N1 (Clontech). Similar fusion constructs were made for ICL3CLR (5′-AGC GCT AGC CTC GAG GAA TGC TCT TTT TCC TAT TAA ATA TTG TAC GTG TTC T-3′ and 5′-CGC TAG CCG CTC GAG TAC CAA GAT GAG AGT AGC TCT TAC G-3′) and C-tailCLR (5′-AGC GCT AGC CTC GAG GAA TGG TGG CTA CTA TTT TCT GCT TCT TTA AT-3′ and 5′-CGC TAG CCG CTC GAG CAT CAC TAG ATC ATA CGT ATT TTC TGA T-3′), which were digested with XhoI and ligated into the XhoI site of pEGFP-N1. To facilitate scoring transfection efficiency in transient transfections, the ICL and C-tail of CLR and ICL2 of β2AR were also cloned into the plasmid mVenus-C1, a bright variant of yellow fluorescent protein (24–26). The following primer pairs were used to amplify ICL1CLR (5′-AGC GCT AGC CGT AGA TCT ATC TCT CTC ATC ATA TTT TTT TAC TTC AAG-3′ and 5′-GC TAG CCG TAA GGA TCC GAT TGT TAC AAT CGA ATT ACA AAT AA-3′), ICL2CLR (5′-AGC GCT AGC CGT AGA TCT GCT GTG TTT GCG GAG AAG CAG CAC TTG-3′ and 5′-GC TAG CCG TAA GGA TCC GGC GTG GAT GCA GGC AGG AAG CAG AGG-3′), ICL3CLR (5′-AGC GCT AGC CGT AGA TCT CTC TTT TTC CTA TTA AAT ATT GTA CGT GTT CT-3′ and 5′-GC TAG CCG TAA GGA TCC TAC CAA GAT GAG AGT AGC TCT TAC G-3′), C-tailCLR (5′-AGC GCT AGC CGT AGA TCT GTG GCT ACT ATT TTC TGC TTC TTT AAT-3′ and 5′-GC TAG CCG TAA GGA TCC CAT CAC TAG ATC ATA CGT ATT TTC TGA T-3′), and ICL2β2AR (5′-AGC GCT AGC CGT AGA TCT GTG GAT CGC TAT GTT GCT ATC A-3′ and 5′-GC TAG CCG TAA GAA TTC TCG GGC CTT ATT CTT GGT C-3′). Amplimers were digested with BglII and BamHI and ligated between the BglII and BamHI sites of pmVenus-C1 (a gift from Dr. David Piston, Vanderbilt University, Nashville, TN).
Cell culture and construction of stable mammalian cell lines
NIH3T3 cells were grown with 5% CO2 at 37 C in a humidified incubator in DMEM supplemented with 10% fetal bovine serum, 0.6% penicillin G, 1% streptomycin SO4, and 3% glutamine. NIH3T3 cells were grown to 80% confluence in a 100-mm plate and counted using a hemocytometer. A total of 2 × 106 cells were used per electroporation reaction. Cells were resuspended in 1 ml electroporation buffer [20 mm HEPES (pH 7.5), 137 mm NaCl, 5 mm KCl, 0.7 mm Na2HPO4, and 6 mm dextrose] and mixed with 20 μg pEGFP-N1 (BD Biosciences Clontech) alone or the ICL of mouse CLR (ICL2CLR-GFP, ICL3CLR-GFP, C-tailCLR-GFP) or the second ICL of mβ2AR (ICL2β2AR-GFP). The cells were mixed with DNA and electroporated in a 1.8-mm gap cuvette (BTX 485) using a BTX600 electroporator (Genetronics, Inc., San Diego, CA) using 350 V, 72 ohms, and 1500 μF. Cells were allowed to recover for 24 h in growth medium and then plated and grown in media supplemented with 1.6 mg/ml geneticin (pcDNA3, pEGFP-N1) or 4 μg/ml blasticidin (pcDNA6). The media was changed every 48 h, and after 10 d, emerging colonies were isolated and screened by Western blot analysis for proper fusion protein expression. Multiple independent clones (7–15) were isolated for each construct tested. All clones were characterized by dividing the CGRP-induced cAMP response by the cAMP response to 20 μm forskolin.
Lysate and membrane preparation
NIH3T3 cells were scraped into homogenizing buffer [15 mm HEPES (pH 8.0), 5 mm EDTA, 5 mm EGTA] with protease inhibitors (50 μg/ml lima bean trypsin inhibitor, 2 μg/ml leupeptin, 16 μg/ml benzamidine, 2 μg/ml pepstatin A, 300 μg/ml phenylmethylsulfonyl fluoride). Cells were then homogenized at high speed using a Brinkmann Polytron for 15 sec. Protein concentration was determined using the Micro BCA Assay (Pierce Biotechnology, Inc., Rockford, IL). To obtain membranes, the homogenate was centrifuged at 100,000 × g for 1 h at 4 C. The membrane pellet was resuspended in 50 mm HEPES (pH 8.0), 5 mm EDTA, 5 mm MgCl2 (50/5/5 buffer) (13, 27).
Western blot analysis
For yeast two-hybrid studies, yeast coexpressing the cloned fusion products of CLR and RCP were extracted into Y-PER buffer (Pierce Biotechnology) and analyzed by 15% SDS-PAGE, transferred to polyvinylidene difluoride membrane (Bio-Rad Laboratories, Inc., Hercules, CA), and immunoblotted with antibody 1065 raised against RCP (16–18) or with antibodies directed against the myc epitope (Covance Research Products, Inc., Denver, PA), the GAL4 binding domain (BD Biosciences Clontech), the HA epitope (monoclonal HA.11; Covance), or the activation domain (BD Biosciences Clontech).
For mammalian tissue culture studies, cells were harvested by scraping into 50 mm sodium phosphate (pH 7.4), 1% Triton X-100, and 50 mm NaCl (PTN50 buffer) and homogenized for 15 sec in a Brinkmann Polytron, and cell debris were pelleted by 3 min centrifugation at 700 × g at 4 C. The supernatant (cell lysate) was used for analysis by Western blot. Cell lysates (40 μg/lane) were resolved by 15% SDS-PAGE, transferred to polyvinylidene difluoride membranes (Bio-Rad), and immunoblotted with antibodies directed against RCP (chicken polyclonal antibody 1065) or with monoclonal antibodies that recognized GFP and Venus (BD Biosciences Clontech). Membranes were then washed with PBS plus 1% milk and 0.05% Tween 20 and incubated with appropriate secondary antibody conjugated to horseradish peroxidase (Jackson Immunoresearch Laboratories, Inc., West Grove, PA) for 30 min, at a 1:50,000 dilution. After three additional washes in PBS-T, membranes were incubated in SuperSignal West Dura Extended Duration Substrate (Pierce) for 5 min and exposed to x-ray film.
Coimmunoprecipitation of GFP fusion proteins and chimeric CLR proteins
Tissue culture cells were harvested by scraping cells into PTN50 buffer and homogenizing by Polytron, and cell debris were pelleted by 3 min centrifugation at 700 × g at 4 C. The supernatant (cell lysate) was used for an overnight immunoprecipitation at 4 C with either 2 μl anti-GFP or 2 μl anti-RCP antibody 1065. The immune complexes were captured by incubating with 80 μl protein A-agarose (Invitrogen Life Technologies, Inc., Grand Island, NY) for 1 h at 4 C. The protein A beads were collected by centrifugation for 2 min at 12,000 × g and washed once in PTN50 buffer and twice in PBS, and the immune complex was eluted by incubating beads in 50 μl sodium dodecyl sulfate sample buffer at 95 C and then analyzed by SDS-PAGE and Western blot.
Second messenger assays
Assays for cAMP production were carried out as described previously (13, 27). Briefly, cells were aliquoted into 24-well plates and incubated for 24 h in complete serum-free medium (DMEM supplemented with 2% BSA, 0.02% transferrin, and 0.05% insulin) supplemented with 2 μCi/ml [3H]adenine (Amersham Pharmacia Biotech, Piscataway, NJ). The cells were then incubated with the phosphodiesterase inhibitor isobutylmethylxanthine (1 mm) for 20 min at 37 C and treated with various concentrations of agonists plus isobutylmethylxanthine for 30 min. The reactions were terminated by applying cold 5% trichloroacetic acid and assayed for cAMP production by sequential chromatography through Dowex and alumina columns (13, 27).
Cell surface ELISA
COS1 cells were plated into 12-well plates and transiently transfected with cDNA encoding RAMP1 tagged on the extracellular NH2 terminus with the myc epitope (a gift from Dr. Deborah Hay, University of Auckland, Auckland, New Zealand) and CLR tagged on the NH2 terminus with the HA epitope (a gift from Dr. David Poyner, Aston University, Birmingham, UK) (28), and the four intracellular domains of CLR expressed as fusion proteins with Venus (as described above) or with Venus alone. Cells were transfected with a constant amount of total DNA (1.6 μg/well), with a ratio of 1:1:2 CLR to RAMP1 to ICL construct. Forty-eight hours after transfection, expression of the fluorescent fusion proteins was confirmed by microscopy, and cells were washed with PBS and then incubated in blocking buffer (PBS/5% normal horse serum) for 60 min at room temperature with shaking. Blocking buffer was then removed and replaced with fresh blocking buffer supplemented with primary antibody directed against myc (Calbiochem, La Jolla, CA; 1:200 dilution) or HA (Sigma Chemical Co., St. Louis, MO; 1:2000 dilution) and incubated for 90 min at room temperature with shaking. Primary antibody was then removed and cells washed twice with PBS and incubated for 60 min with donkey antimouse secondary antibody diluted 1:2000 in blocking buffer. Cells were then washed three times with PBS and incubated with triethylamine substrate (Sigma) until a color change was observed. Reactions were stopped by addition of 2 m sulfuric acid, and absorbance at 450 nm determined with a PerkinElmer (Waltham, MA) Victor plate reader.
Data analysis
Data were analyzed using Prism (GraphPad Software, San Diego, CA). Data for cAMP response were normalized to maximal response observed in untransfected cells. Data were fit by nonlinear regression with a sigmoidal dose-response curve and are expressed as mean ± sem. The pEC50 and Emax (−log of EC50 and maximal agonist-induced response) were obtained from dose-response curves, and differences between untransfected cells and cells transfected with ICL fusion proteins were detected by one-way ANOVA, using ANOVA followed by Tukey's multiple-comparison test. Data from cell-surface ELISA were normalized to signal from cells transfected with receptor alone, and differences between cells transfected with receptor alone and cells transfected with receptor and ICL fusion proteins were detected by one-way ANOVA, using ANOVA followed by Tukey's multiple-comparison test.
Results
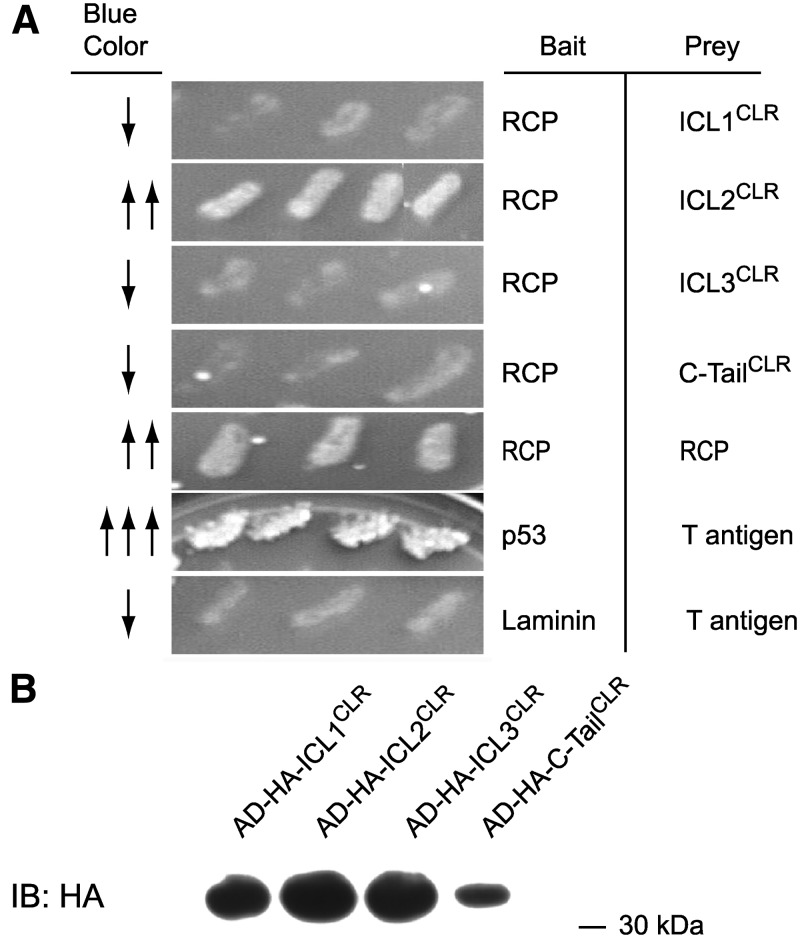
Previous experiments in our lab indicated that RCP was an intracellular peripheral membrane protein (13). This suggested that if RCP interacted directly with CLR, then it should contact the intracellular domains of CLR. To test this CLR-RCP interaction directly, the predicted intracellular domains of CLR were cloned as fusion proteins for analysis in a yeast two-hybrid assay and tested for interaction with RCP. Yeast were cotransformed with the RCP plasmid, and each of the four cytoplasmic domains of CLR, and growth on His-deficient media was recorded. Growth was observed only in yeast cotransformed with RCP and the second ICL of CLR (ICL2CLR) (Fig. 2A). This was not due to lack of expression of the CLR fusion constructs, because Western blot of yeast lysate with antibodies against the HA tag of the fusion protein detected approximately equal expression of each of the four cytoplasmic domains (Fig. 2B). Growth on His-deficient media was also observed when RCP was cloned into both the bait and the prey plasmids, suggesting that RCP can interact with itself. This agrees with previous studies where we observed dimer-sized bands of RCP on Western blot (13, 16). The interaction between RCP and ICL2CLR was specific, because growth on His-deficient media was not observed when RCP was transformed into yeast either by itself or when cotransformed with p53 or simian virus 40 T antigen or with the second cytoplasmic domain of the β2AR (data not shown), a GPCR that we have previously shown does not require RCP for function (13, 16).
Fig. 2.
RCP interacts directly with the second ICL of CLR in yeast two-hybrid assay. A, RCP and the indicated CLR domains were cotransformed into yeast and plated on selective media. Growth was observed only when RCP was expressed with either ICL2CLR or with itself. Positive control for growth is p53 and T antigen; negative control is laminin and T antigen. B, Yeast cotransformed with RCP and with CLR cytoplasmic domains were lysed and expression of HA-tagged cytoplasmic domains confirmed by Western blot with anti-HA antibody. Blue color indicates alpha-galactosidase activity. IB, Immunoblot.
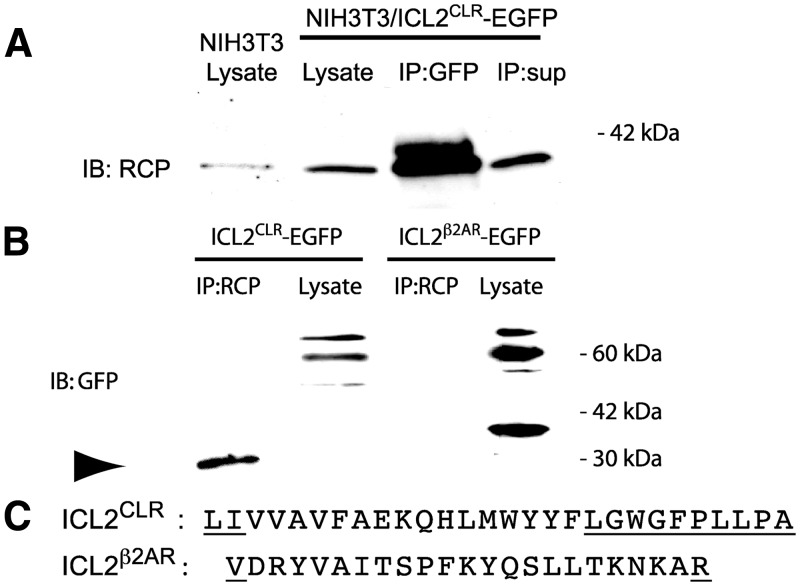
The interaction observed between RCP and ICL2CLR in the yeast two-hybrid assay was next tested in cell culture by coimmunoprecipitation. To ensure expression of a stable protein, the 28-amino-acid ICL2CLR was expressed as a fusion protein with enhanced green fluorescent protein (EGFP), which also provided an epitope for immunoprecipitation and Western blot detection. NIH3T3 cells endogenously express RCP, CLR, and RAMP1 (13) and were stably transfected with cDNA encoding an ICL2CLR-EGFP fusion protein. Cell lysates were prepared and immunoprecipitated with antibodies against GFP, and the immunoprecipitates were analyzed by Western blot with anti-RCP antibody (Fig. 3A). RCP was efficiently coimmunoprecipitated with ICL2CLR-EGFP, detected as a 42-kDa band on the Western blot, consistent with previous coimmunoprecipitation results between RCP and intact CLR (13, 16). A second band of higher apparent molecular weight was also detected in the RCP blot. We have identified three conserved sites for phosphorylation in RCP, and the higher-molecular-weight RCP band may be due to conformational changes associated with phosphorylation. We consistently observed elevated levels of RCP expression in cells transfected with ICL2CLR when analyzed by Western blot (Fig. 3A, lanes 1 and 2) and by fluorescence microscopy (data not shown).
Fig. 3.
RCP coimmunoprecipitates with the second ICL of CLR. A, NIH3T3 cells expressing ICL2CLR-EGFP were lysed and immunoprecipitated with anti-GFP antibody. Immunoprecipitate (IP) was analyzed by Western blot with anti-RCP antibody. Lysate from untransfected NIH3T3 cells was loaded in first lane. For Western blots, 30 μg lysate was loaded per lane. For immunoprecipitation, 500 μg lysate was precipitated with anti-GFP antibody, and the immune complex was collected and loaded into a single lane. Supernatant from immunoprecipitation was loaded as a single lane. B, NIH3T3 cells expressing either ICL2CLR-EGFP or ICL2β2AR-EGFP were lysed and immunoprecipitated with anti-RCP antibody. The immunoprecipitate was analyzed by Western blot with anti-GFP antibody, as above. RCP monomer is indicated by arrowhead. C, Comparison of sequence of ICL2CLR and ICL2β2AR used for expression studies. Residues in putative flanking transmembrane domain are underlined. IP, Immunoprecipitation; IB, immunoblot.
Similar interactions were detected for the reciprocal experiment, when lysate was first immunoprecipitated with anti-RCP antibody, and the immunoprecipitate was analyzed by Western blot with anti-GFP antibody (Fig. 3B). The predicted 30-kDa EGFP band was observed, corresponding to a monomer of EGFP fused with the approximately 3-kDa (27 amino acids) ICL2CLR. However, the predominant species observed in lysate was approximately 60 kDa, possibly due to dimerization of GFP (29). In contrast, no interaction between RCP and the second ICL of the β2AR (ICL2β2AR) was observed by coimmunoprecipitation in similar stable NIH3T3 cell lines. Both the monomer and dimer of the ICL2β2AR-EGFP fusion protein were detected in the lysate (Fig. 3, B and C).
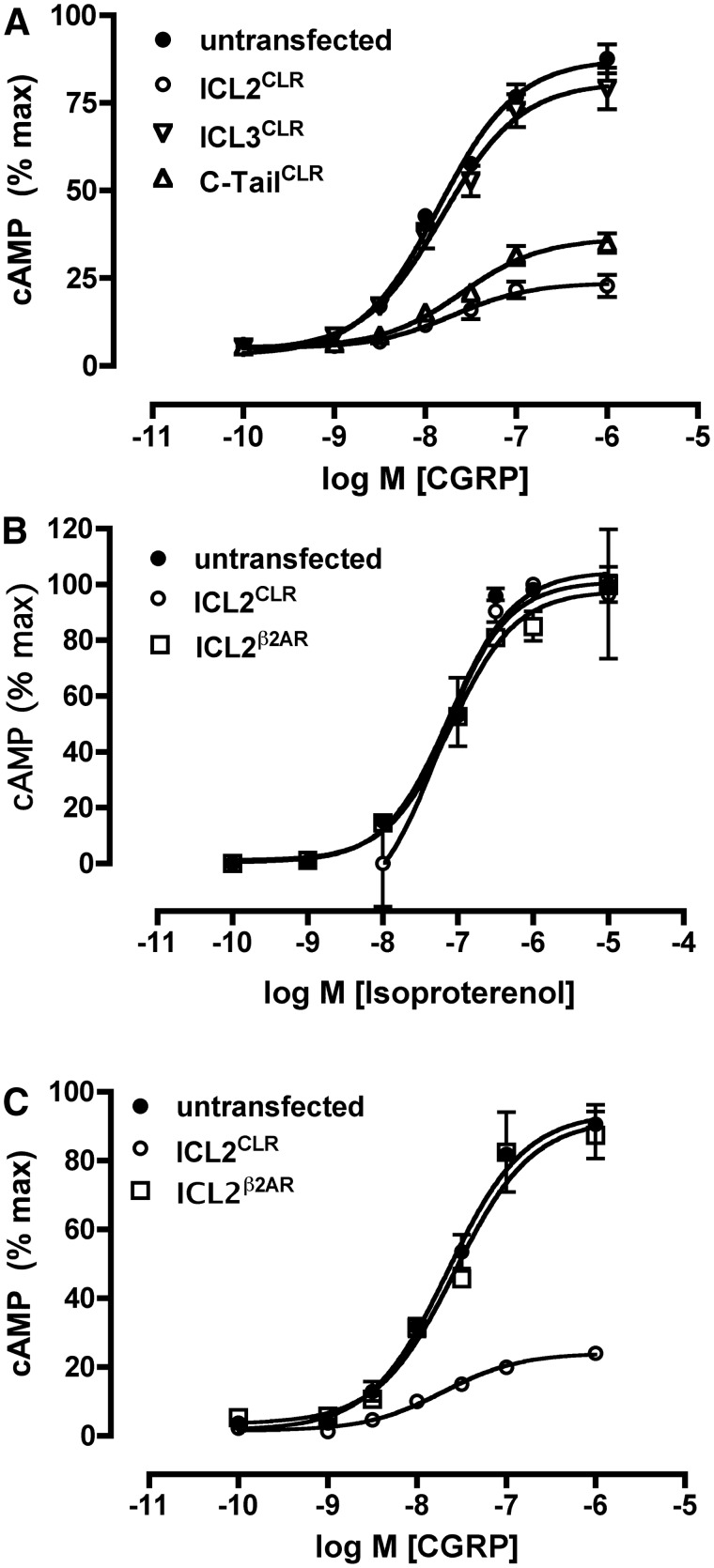
Based on the interaction between RCP and ICL2CLR in the two-hybrid assay in yeast and by coimmunoprecipitation in cell culture, we predicted that ICL2CLR transfected into cell culture would bind to endogenous RCP and sequester RCP from the CLR/RAMP1 complex. Previous work indicated that depletion of RCP from the CLR/RAMP1 complex inhibited CLR signaling (13, 16), and we thus predicted that expression of ICL2CLR would act as a dominant-negative to inhibit CLR signaling. Expression of ICL2CLR in stable cell lines proved to be a potent inhibitor of CLR signaling (Fig. 4A), suppressing the Emax for CLR signaling to 26% of control but not significantly altering the EC50 for CGRP (Table 1). This suggests that ICL2CLR expression affected the ability of CLR to signal but not the potency of CGRP for CLR. The inhibitory effect of ICL2CLR on CLR signaling was not a general function of overexpressing the ICL of CLR, because expression of the third cytoplasmic loop (ICL3CLR) did not inhibit CGRP-mediated signaling (Fig. 4A). However, expression of the carboxy terminus of CLR (C-tailCLR) was inhibitory on receptor signaling. Expression of C-tailCLR suppressed the maximal cAMP response in response to CGRP to 41% of control, similar to the value obtained for ICL2CLR (27% of control) (Table 1). The inhibitory effects of ICL2CLR and C-tailCLR on CLR signaling were not statistically different (P > 0.05). The EC50 of CGRP at CLR was not significantly affected by expression of C-tailCLR compared with control, suggesting that expression of C-tailCLR did not alter the potency of CGRP for CLR. Expression of C-tailCLR also inhibited signaling at a second GPCR, the β2AR (data not shown), which we have previously shown is not dependent on RCP for signaling (13), suggesting a more pleiotropic effect of C-tailCLR than ICL2CLR on GPCR signaling.
Fig. 4.
Expression of the second ICL of CLR inhibits CGRP receptor signaling in NIH3T3 cells. A, CGRP-mediated cAMP response in NIH3T3 cells stably expressing ICL2CLR, ICL3CLR, or C-tailCLR. B, Isoproterenol-mediated cAMP response in NIH3T3 cells stably expressing either ICL2CLR or ICL2β2AR. C, CGRP-mediated cAMP response in NIH3T3 cells expressing either ICL2CLR or ICL2β2AR. All data are presented as cAMP response normalized to maximal response obtained in untransfected NIH3T3 cells. All experiments were done in triplicate and repeated seven to eight times.
Table 1.
Effect of CLR ICL expression on endogenous CLR and β2AR cAMP-mediated signaling
| Figure | Construct | Ligand | pEC50 | Emax (%) |
|---|---|---|---|---|
| 4A | Untransfected | CGRP | 7.84 ± 0.07 | 100 ± 2 |
| 4A | ICL2CLR | CGRP | 7.70 ± 0.17 | 27 ± 2b |
| 4A | ICL3CLR | CGRP | 7.79 ± 0.08 | 92 ± 2 |
| 4A | C-tailCLR | CGRP | 7.58 ± 0.1 | 41 ± 2a |
| 4B | Untransfected | Isoproterenol | 7.11 ± 0.03 | 104 ± 3 |
| 4B | ICL2CLR | Isoproterenol | 7.24 ± 0.20 | 101 ± 1 |
| 4B | ICL2β2AR | Isoproterenol | 7.09 ± 0.04 | 98 ± 2 |
| 4C | Untransfected | CGRP | 7.66 ± 0.03 | 100 ± 2 |
| 4C | ICL2CLR | CGRP | 7.71 ± 0.05 | 26 ± 1b |
| 4C | ICL2β2AR | CGRP | 7.58 ± 0.05 | 98 ± 2 |
Values are mean ± sem of seven to eight experiments carried out in triplicate. Emax is the maximal cAMP response normalized to the maximal response obtained in control (untransfected) NIH3T3 cells. Significant differences from control were detected by one-way ANOVA (followed by Tukey's test).
P < 0.01.
P < 0.001.
To determine whether the inhibitory effect of ICL2CLR expression was specific for CLR or was more widespread as for the inhibitory effect of C-tailCLR expression, signaling at the β2AR was monitored in NIH3T3 cells expressing ICL2CLR. As shown in Fig. 4B, there was no significant inhibition of β2AR signaling, in either EC50 or Emax in NIH3T3 cells expressing ICL2CLR (Table 1). We next investigated the possibility that the inhibitory effects of ICL2 expression were specific to the GPCR from which they were derived. In these experiments, ICL2β2AR was expressed in NIH3T3 cell lines, and β2AR signaling was monitored. No inhibitory effects were observed on signaling at either β2AR or CLR by expression of ICL2β2AR (Fig. 4, B and C), suggesting that the inhibitory effect of ICL2CLR was specific for CLR and not a general characteristic of expression of the second ICL of GPCR.
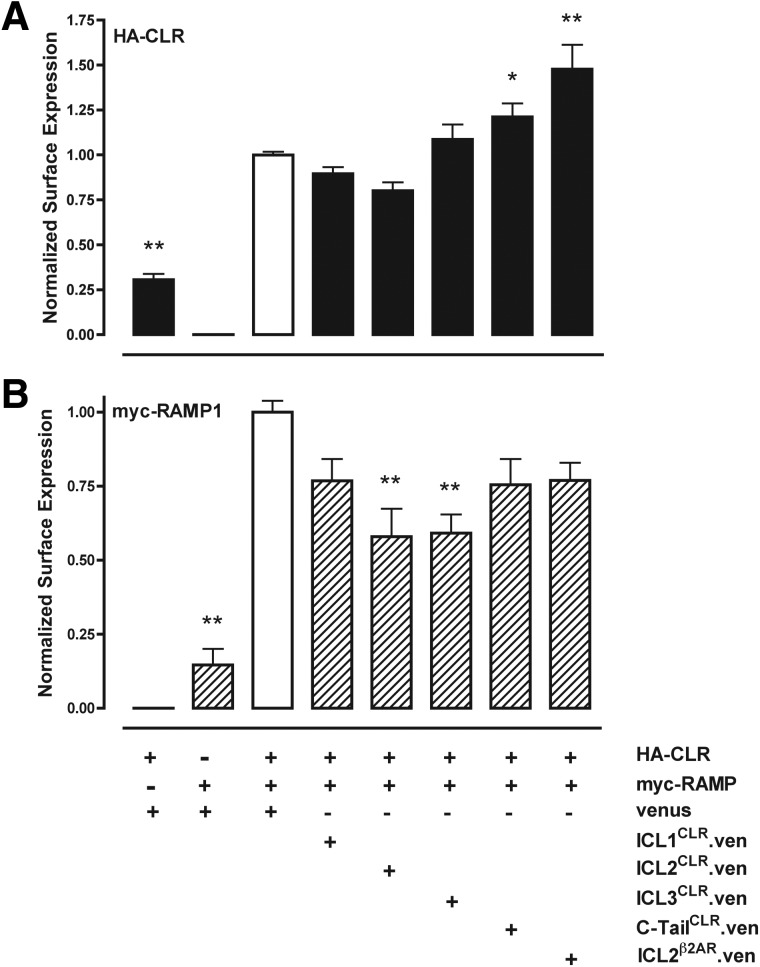
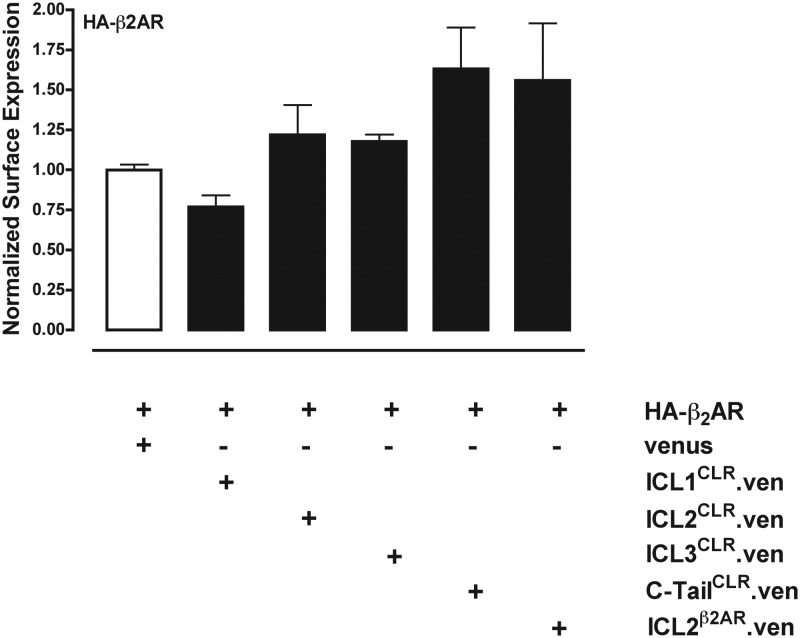
Previous binding studies indicated that depletion of RCP did not decrease CGRP binding sites in total cell membranes (13). However, those previous studies could not distinguish between receptors located on the cell surface membrane or within intracellular membranes. We therefore next used cell-surface ELISA against CLR (epitope tagged with NH2-terminal HA) and RAMP1 (epitope-tagged with NH2-terminal myc) to determine whether exogenous expression of the four intracellular domains of CLR could affect trafficking of CLR or RAMP1 to the cell surface in COS1 cells, which lack endogenous CLR or RAMP1 (13). Expression of HA-CLR and myc-RAMP1 was monitored by cell-surface ELISA using monoclonal antibodies against either the myc or HA tag, and expression of fusion proteins between ICL and the fluorescent protein Venus was monitored by Western blot (data not shown). Similar to previous reports (15, 30), neither CLR nor RAMP1 was efficiently trafficked to the cell surface when expressed in COS cells alone (Fig. 5, A and B, first two columns). Coexpression of ICL2CLR decreased the amount of CLR detected on the cell surface by 20%, but this was not statistically significant (Table 1 and Fig. 5A). Coexpression of ICL1CLR and ICL3CLR similarly did not inhibit cell-surface CLR to a significant extent. In contrast, coexpression of C-tailCLR increased surface CLR by 21% (Table 1), and coexpression of the second ICL of the β2AR (ICL2β2AR) increased surface of expression of CLR by 47% over control. In parallel experiments, RAMP1 surface expression was inhibited 40% by coexpression with either ICL2CLR or ICL3CLR (Table 1 and Fig. 5B), and small decreases not found to be statistically significant were observed for coexpression of ICL1CLR, C-tailCLR, or ICL2β2AR. Experiments with HA-tagged β2AR demonstrated that coexpression of ICL2CLR did not inhibit surface expression of β2AR and instead resulted in 22% increase, although this was not found to be statistically significant (Table 2 and Fig. 6). Similarly, coexpression of C-tailCLR or ICL2β2AR resulted in increased β2AR detected on the cell surface, but the differences were not statistically significant.
Fig. 5.
Effect of intracellular cytoplasmic domains of CLR on CLR trafficking to the cell surface. COS1 cells were transiently transfected with myc-tagged RAMP1 and HA-tagged CLR, and the intracellular cytoplasmic domains of either CLR or the β2AR are expressed as fusion proteins with Venus (ven). A, Cell surface ELISA with anti-HA antibody to detect CLR. B, Cell surface ELISA with anti-myc antibody to detect RAMP1. All data have been normalized to control cells transfected with HA-CLR and myc-RAMP1 and Venus (white bar). Data are pooled from six experiments carried out in triplicate. Values significantly different from control (*, P < 0.05; **, P < 0.01) were determined by one-way ANOVA (followed by Tukey's test).
Table 2.
Effect of CLR ICL on cell-surface expression of CLR and β2AR signaling
| Receptor | ICL | Antibody | Surface expression (%) |
|---|---|---|---|
| HA-CLR + RAMP1 | None | Anti-HA | 100 ± 2 |
| HA-CLR | None | Anti-HA | 31 ± 6b |
| HA-CLR + myc-RAMP1 | ICL1CLR | Anti-HA | 90 ± 4 |
| HA-CLR + myc-RAMP1 | ICL2CLR | Anti-HA | 80 ± 5 |
| HA-CLR + myc-RAMP1 | ICL3CLR | Anti-HA | 108 ± 8 |
| HA-CLR + myc-RAMP1 | C-tailCLR | Anti-HA | 121 ± 7a |
| HA-CLR + myc-RAMP1 | ICL2β2AR | Anti-HA | 147 ± 2b |
| HA-CLR + myc-RAMP1 | None | Anti-myc | 100 ± 4 |
| myc-RAMP1 | None | Anti-myc | 15 ± 5b |
| HA-CLR + RAMP1 | ICL1CLR | Anti-myc | 76 ± 7 |
| HA-CLR + myc-RAMP1 | ICL2CLR | Anti-myc | 58 ± 9b |
| HA-CLR + myc-RAMP1 | ICL3CLR | Anti-myc | 59 ± 6b |
| HA-CLR + myc-RAMP1 | C-tailCLR | Anti-myc | 75 ± 9 |
| HA-CLR + myc-RAMP1 | ICL2β2AR | Anti-myc | 77 ± 6 |
| HA-β2AR | None | Anti-HA | 100 ± 3 |
| HA-β2AR | ICL1CLR | Anti-HA | 77 ± 7 |
| HA-β2AR | ICL2CLR | Anti-HA | 122 ± 19 |
| HA-β2AR | ICL3CLR | Anti-HA | 118 ± 4 |
| HA-β2AR | C-tailCLR | Anti-HA | 163 ± 26 |
| HA-β2AR | ICL2β2AR | Anti-HA | 156 ± 36 |
Values are mean ± sem of four to five experiments carried out in triplicate. Surface expression was determined by ELISA for either HA (CLR and β2AR) or myc (RAMP1) epitope in transfected COS1 cells. Significant differences from control (HA-CLR + RAMP1 alone or β2AR alone) were detected by one-way ANOVA (followed by Tukey's test).
P < 0.05.
P < 0.01.
Fig. 6.
Expression of intracellular cytoplasmic domains of CLR on β2AR trafficking to the cell surface. COS1 cells were transiently transfected with HA-tagged β2AR and the ICL constructs described for Fig. 5. Cell-surface ELISA was done with anti-HA antibody to detect cell-surface HA-β2AR. All data have been normalized to control cells transfected with HA-β2AR and Venus (white bar). Data are pooled from four experiments carried out in triplicate. No significant differences between experimental and control were found when analyzed by one-way ANOVA (followed by Tukey's test).
An alternate role for RCP in CGRP receptor function could be to couple CLR to signaling pathways. In this case, loss of RCP interaction, which results in loss of cAMP-mediated signaling, might facilitate signaling by CLR through alternate pathways, such as MAPK. CGRP has been reported to signal through MAPK via the phospho-ERK (pERK) pathway (31, 32), and we investigated this possibility. NIH3T3 were transfected with ICL2CLR, and pERK production was monitored by Western blot in response to CGRP. Intensity of pERK was normalized to ERK, and the increase of normalized pERK at 5′ was calculated. A 2-fold induction in pERK was detected in control cells in response to CGRP. In cells transfected with ICL2CLR, the pERK induction was 2.3 ± 0.24-fold (Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). This difference was not statistically significant.
Discussion
RCP is an important component of the CLR signaling complex. Previous studies demonstrated that depletion of RCP by antisense inhibited CLR signaling (13, 16), and in the current studies, we have discovered that RCP exerts its effect by direct interaction with the second ICL of CLR (ICL2CLR). Interaction between RCP and ICL2CLR was discovered by yeast two-hybrid assay and was confirmed by coimmunoprecipitation in cell culture. Interestingly, when ICL2CLR was expressed as a soluble protein, it coimmunoprecipitated with RCP and inhibited CLR signaling, possibly by sequestering RCP away from CLR. Thus, uncoupling CLR from RCP, either by competition for binding with ICL2CLR or by depletion with antisense, is an effective method to inhibit CLR signal transduction. Expression of ICL2CLR significantly suppressed the maximal CGRP-induced cAMP levels to 27% of control, without affecting the potency of CGRP for CLR, because the EC50 for cAMP production after CGRP incubation was unchanged from control. These data suggest that a decreased number of functional CGRP receptors were present on the cell surface when ICL2CLR was expressed. However, when we carried out a cell-surface ELISA we did not detect a significant decrease in surface CLR when ICL2CLR was expressed and found only a modest decrease (60% of control) for surface RAMP1. Because a functional CGRP receptor requires both RAMP1 and CLR, it is tempting to attribute the loss of CLR signaling observed with expression of ICL2CLR to this diminished cell-surface RAMP1. However, a similar loss of RAMP1 was observed for coexpression of ICL3CLR, which did not inhibit CLR signaling, so the decreased surface RAMP1 may not be significant for CLR function. For example, it is possible that there was a sufficient reserve of RAMP1 on the cell surface, such that the loss of 40% of RAMP1 did not outstrip surface CLR, thus having minimal effect on CLR function. CLR is a family B class GPCR, and transmembrane domain 4 (TM4) of another family B receptor, the secretin receptor, has been implicated in formation of high-affinity receptor homodimers (33). Because our ICL2CLR construct contained 10 amino acids of flanking TM4 residues, we cannot exclude the possibility that some of the inhibitory effects of ICL2CLR expression on CLR signaling may be caused by interaction between TM4 residues in our construct and TM4 of CLR. Future experiments subdividing our ICL2CLR construct should address this possibility.
Our data suggest that the primary role for RCP in CLR function is to enable signal transduction. Inhibiting CLR-RCP interactions had a major effect on signaling with a minor effect on cell-surface expression. Previous studies have found that CLR and RAMP1 are required for efficient trafficking of both molecules to the cell surface (15, 30, 34). Our finding that there is little effect on cell-surface CLR when RCP is inhibited suggests that RCP exerts its effect on CLR after the receptor has been transported to the cell surface and may be required for stabilizing the CLR/RAMP1 interaction once the pair has reached the cell surface or for coupling CLR/RAMP1 with signaling proteins, either directly or by sorting CLR/RAMP1 into discrete membrane domains such as lipid rafts (35–37). A role for RCP in CLR/RAMP1 trafficking may be possible if most of the cell-surface CLR was nonfunctional, in which case RCP may facilitate trafficking of the small percentage of CLR that was functional. In this case, inhibition of RCP would have a minimal effect on total surface CLR but would inhibit surface expression of functional CLR, which would result in the significantly reduced CLR signaling that we observed. An alternate role for RCP in CGRP receptor function could be to couple CLR to different signaling pathways. In this case, loss of RCP interaction, which results in loss of cAMP-mediated signaling, might enhance signaling by CLR through alternate pathways, such as MAPK. However, we did not detect a change in ERK phosphorylation in cells transfected with ICL2CLR, so uncoupling CLR from RCP did not result in an increase in MAPK activity in NIH3T3 cells.
The inhibitory effect of ICL2CLR on signaling appears to be specific for CLR because signaling at the β2AR, a GPCR that is not dependent on RCP for signaling (13), was not inhibited by expression of ICL2CLR. Expression of ICL2 of β2AR did not inhibit signaling at either CLR or β2AR, so the inhibitory effect of ICL2CLR on signaling does not appear to be a general effect of expressing the ICL2 of GPCR. Expression of ICL2β2AR did increase cell surface expression of CLR by 47%. Expression of ICL2β2AR did not result in increased CGRP-mediated signaling at CLR, and RAMP1 was not increased on the cell surface in these experiments, so this most likely represents an accumulation of nonfunctional CLR on the cell surface. The mechanism of increased surface CLR is not clear, but expression of ICL2β2AR also increased cell surface expression of β2AR without an increase in β2AR signaling; thus, the effect may more broadly affect GPCR.
Expression of C-tailCLR did inhibit signaling at CLR. However, C-tailCLR also inhibited signaling at β2AR, so the effect of C-tailCLR may represent a more generic effect on GPCR function. The C-tail of CLR has been shown to be important for coupling the receptor to G proteins, cell-surface expression, and receptor internalization (38–40). In previous studies, deletion of residues that comprise a consensus GαS-binding motif in the C-tail of CLR inhibited CLR signaling via cAMP, and the receptor was uncoupled from GαS without affecting receptor internalization (38). In light of these previous studies, our finding that overexpression of C-tailCLR inhibited signaling of endogenous CLR suggests that exogenous C-tailCLR may be competing with the C-tail of endogenous CLR to bind factors required for coupling with GαS, thereby inhibiting CGRP-mediated cAMP production. In contrast, expression of C-tailCLR did not inhibit cell-surface expression of CLR and caused a 20% elevation in cell-surface CLR. Elevated levels of receptor may have been due to inhibited internalization of endogenous CLR. Internalization of GPCR is preceded by phosphorylation of the C-tail by GPCR kinases and subsequent binding by β-arrestin (41). Our results overexpressing C-tailCLR could be explained by the production of a soluble target for G protein-coupled receptor kinase and β-arrestin, resulting in a slower rate of phosphorylation and of subsequent binding of CLR by β-arrestin, resulting in slower rates of internalization and therefore increased levels of surface receptor. In support of this model, an increase in surface β2AR was also observed with C-tailCLR expression, suggesting that the carboxy terminus of CLR was binding and sequestering proteins that could either inhibit GPCR trafficking to the cell surface or enhance receptor internalization, and when these proteins were bound with soluble C-tailCLR, the full-length CLR or β2AR spent more time on the cell surface. This effect was limited to GPCR in our experiments because cell-surface expression of RAMP1 was not similarly increased with expression of C-tailCLR.
Our data suggest that direct interaction between RCP and CLR is required for CLR signaling in the peripheral membrane of the cell. Previous studies on CLR have suggested that the second intracellular loop was important for receptor function (28). In those studies, scanning alanine mutagenesis on the second intracellular loop of CLR identified mutations that blocked signaling but not trafficking and identified mutations that blocked both signaling and trafficking. Future experiments correlating the ability of RCP to bind to the ICL2CLR mutants from Conner et al. (28) should help determine whether RCP is involved in CLR trafficking as well as signaling. The requirement for ICL2 of CLR for signaling is in contrast to the receptor for vasoactive intestinal peptide and pituitary adenylate cyclase-activating peptide (VPAC1) receptor, which is also a family B GPCR (42). Scanning alanine mutagenesis determined that none of the residues in ICL2VPAC1 were required for signaling or expression of the VPAC1 receptor (43). Thus, the involvement of specific residues of ICL2 in CLR for signaling and trafficking described by Conner et al. (28) appear to reflect a more complex milieu of proteins required for CLR function. Our studies suggest that one such interacting protein is RCP.
The ability of ICL2CLR to bind RCP, thereby inhibiting CLR function, makes it a target for designing therapeutic agents. These could include small-molecule inhibitors, or ICL2CLR itself could be synthesized as a pepducin, which is a small protein coupled to a hydrophobic group that allows the protein to penetrate the peripheral membrane of a mammalian cell and become localized to the intracellular face of the membrane, where it can interact with ICL of GPCR. Pepducins have been designed using ICL of GPCR and can activate or inhibit GPCR in cell culture and in animal studies (44–46). Using this model system, membrane-associated ICL2CLR could be a potent inhibitor of CLR signaling, because it should bind membrane-associated RCP and inhibit CLR signaling. To date, RCP has been found to interact only with CLR, and the RCP-CLR interaction represents a novel neuroendocrine regulatory step in GPCR signaling.
Supplementary Material
Acknowledgments
Thanks to D. Byun for excellent technical assistance and to P. Hinkle and J. Levenkron for helpful discussions.
This work was funded in part by NIH DK52328 and a grant from the Schmitt Program for Integrative Brain Research (to I.M.D.). S.C.E. was funded by a predoctoral fellowship from the American Heart Association.
Disclosure Summary: The authors have no financial or professional conflicts of interest with the described studies.
Footnotes
- β2AR
- β2-Adrenergic receptor
- CGRP
- calcitonin gene-related peptide
- CLR
- calcitonin-like receptor
- C-tail
- carboxyl-tail
- EGFP
- enhanced green fluorescent protein
- GPCR
- G protein-coupled receptor
- HA
- hemagglutinin
- ICL
- intracellular cytoplasmic loop
- pERK
- phospho-ERK
- RAMP1
- receptor activity-modulating protein
- RCP
- CGRP-receptor component protein
- TM4
- transmembrane domain 4
- VPAC1
- receptor for vasoactive intestinal peptide and pituitary adenylate cyclase-activating peptide.
References
- 1. Brain SD, Grant AD. 2004. Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol Rev 84:903–934 [DOI] [PubMed] [Google Scholar]
- 2. Goadsby PJ, Edvinsson L. 1993. The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurol 33:48–56 [DOI] [PubMed] [Google Scholar]
- 3. Goadsby PJ, Edvinsson L, Ekman R. 1990. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol 28:183–187 [DOI] [PubMed] [Google Scholar]
- 4. Lassen LH, Haderslev PA, Jacobsen VB, Iversen HK, Sperling B, Olesen J. 2002. CGRP may play a causative role in migraine. Cephalalgia 22:54–61 [DOI] [PubMed] [Google Scholar]
- 5. Edvinsson L. 2005. Clinical data on the CGRP antagonist BIBN4096BS for treatment of migraine attacks. CNS Drug Rev 11:69–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Salvatore CA, Hershey JC, Corcoran HA, Fay JF, Johnston VK, Moore EL, Mosser SD, Burgey CS, Paone DV, Shaw AW, Graham SL, Vacca JP, Williams TM, Koblan KS, Kane SA. 2008. Pharmacological characterization of MK-0974 [N-[(3R,6S)-6-(2,3-difluorophenyl)-2-oxo-1-(2,2,2-trifluoroethyl)azepan-3- yl]-4-(2-oxo-2,3-dihydro-1H-imidazo[4,5-b]pyridin-1-yl)piperidine-1-carbox amide], a potent and orally active calcitonin gene-related peptide receptor antagonist for the treatment of migraine. J Pharmacol Exp Ther 324:416–421 [DOI] [PubMed] [Google Scholar]
- 7. Han JS, Adwanikar H, Li Z, Ji G, Neugebauer V. 2010. Facilitation of synaptic transmission and pain responses by CGRP in the amygdala of normal rats. Mol Pain 6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mogil JS, Miermeister F, Seifert F, Strasburg K, Zimmermann K, Reinold H, Austin JS, Bernardini N, Chesler EJ, Hofmann HA, Hordo C, Messlinger K, Nemmani KV, Rankin AL, Ritchie J, Siegling A, Smith SB, Sotocinal S, Vater A, Lehto SG, Klussmann S, Quirion R, Michaelis M, Devor M, Reeh PW. 2005. Variable sensitivity to noxious heat is mediated by differential expression of the CGRP gene. Proc Natl Acad Sci USA 102:12938–12943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Salmon AM, Damaj MI, Marubio LM, Epping-Jordan MP, Merlo-Pich E, Changeux JP. 2001. Altered neuroadaptation in opiate dependence and neurogenic inflammatory nociception in αCGRP-deficient mice. Nat Neurosci 4:357–358 [DOI] [PubMed] [Google Scholar]
- 10. Menard DP, van Rossum D, Kar S, St Pierre S, Sutak M, Jhamandas K, Quirion R. 1996. A calcitonin gene-related peptide receptor antagonist prevents the development of tolerance to spinal morphine analgesia. J Neurosci 16:2342–2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang Z, Ma W, Chabot JG, Quirion R. 2009. Cell-type specific activation of p38 and ERK mediates calcitonin gene-related peptide involvement in tolerance to morphine-induced analgesia. FASEB J 23:2576–2586 [DOI] [PubMed] [Google Scholar]
- 12. Walker CS, Li X, Whiting L, Glyn-Jones S, Zhang S, Hickey AJ, Sewell MA, Ruggiero K, Phillips AR, Kraegen EW, Hay DL, Cooper GJ, Loomes KM. 2010. Mice lacking the neuropeptide α-calcitonin gene-related peptide are protected against diet-induced obesity. Endocrinology 151:4257–4269 [DOI] [PubMed] [Google Scholar]
- 13. Evans BN, Rosenblatt MI, Mnayer LO, Oliver KR, Dickerson IM. 2000. CGRP-RCP, a novel protein required for signal transduction at calcitonin gene-related peptide and adrenomedullin receptors. J Biol Chem 275:31438–31443 [DOI] [PubMed] [Google Scholar]
- 14. Luebke AE, Dahl GP, Roos BA, Dickerson IM. 1996. Identification of a protein that confers calcitonin gene-related peptide responsiveness to oocytes by using a cystic fibrosis transmembrane conductance regulator assay. Proc Natl Acad Sci USA 93:3455–3460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, Solari R, Lee MG, Foord SM. 1998. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature 393:333–339 [DOI] [PubMed] [Google Scholar]
- 16. Prado MA, Evans-Bain B, Oliver KR, Dickerson IM. 2001. The role of the CGRP-receptor component protein (RCP) in adrenomedullin receptor signal transduction. Peptides 22:1773–1781 [DOI] [PubMed] [Google Scholar]
- 17. Ma W, Chabot JG, Powell KJ, Jhamandas K, Dickerson IM, Quirion R. 2003. Localization and modulation of calcitonin gene-related peptide-receptor component protein-immunoreactive cells in the rat central and peripheral nervous systems. Neuroscience 120:677–694 [DOI] [PubMed] [Google Scholar]
- 18. Pokabla MJ, Dickerson IM, Papka RE. 2002. Calcitonin gene-related peptide-receptor component protein expression in the uterine cervix, lumbosacral spinal cord, and dorsal root ganglia. Peptides 23:507–514 [DOI] [PubMed] [Google Scholar]
- 19. Hasbak P, Saetrum Opgaard O, Eskesen K, Schifter S, Arendrup H, Longmore J, Edvinsson L. 2003. Investigation of CGRP receptors and peptide pharmacology in human coronary arteries. Characterization with a nonpeptide antagonist. J Pharmacol Exp Ther 304:326–333 [DOI] [PubMed] [Google Scholar]
- 20. Rossi SG, Dickerson IM, Rotundo RL. 2003. Localization of the calcitonin gene-related peptide receptor complex at the vertebrate neuromuscular junction and its role in regulating acetylcholinesterase expression. J Biol Chem 278:24994–25000 [DOI] [PubMed] [Google Scholar]
- 21. Naghashpour M, Dahl G. 2000. Sensitivity of myometrium to CGRP varies during mouse estrous cycle and in response to progesterone. Am J Physiol Cell Physiol 278:C561–C569 [DOI] [PubMed] [Google Scholar]
- 22. Naghashpour M, Rosenblatt MI, Dickerson IM, Dahl GP. 1997. Inhibitory effect of calcitonin gene-related peptide on myometrial contractility is diminished at parturition. Endocrinology 138:4207–4214 [DOI] [PubMed] [Google Scholar]
- 23. Supowit SC, Katki KA, Hein TW, Gupta P, Kuo L, Dickerson IM, Dipette DJ. 2011. Vascular reactivity to calcitonin gene-related peptide is enhanced in subtotal nephrectomy-salt induced hypertension. Am J Physiol Heart Circ Physiol 301:H683–H688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Elsliger MA, Wachter RM, Hanson GT, Kallio K, Remington SJ. 1999. Structural and spectral response of green fluorescent protein variants to changes in pH. Biochemistry 38:5296–5301 [DOI] [PubMed] [Google Scholar]
- 25. Nagai T, Ibata K, Park ES, Kubota M, Mikoshiba K, Miyawaki A. 2002. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat Biotechnol 20:87–90 [DOI] [PubMed] [Google Scholar]
- 26. Ormö M, Cubitt AB, Kallio K, Gross LA, Tsien RY, Remington SJ. 1996. Crystal structure of the Aequorea victoria green fluorescent protein. Science 273:1392–1395 [DOI] [PubMed] [Google Scholar]
- 27. Sarkar A, Dickerson IM. 1997. Cloning, characterization, and expression of a calcitonin receptor from guinea pig brain. J Neurochem 69:455–464 [DOI] [PubMed] [Google Scholar]
- 28. Conner AC, Simms J, Howitt SG, Wheatley M, Poyner DR. 2006. The second intracellular loop of the calcitonin gene-related peptide receptor provides molecular determinants for signal transduction and cell surface expression. J Biol Chem 281:1644–1651 [DOI] [PubMed] [Google Scholar]
- 29. Tsien RY. 1998. The green fluorescent protein. Annu Rev Biochem 67:509–544 [DOI] [PubMed] [Google Scholar]
- 30. Christopoulos A, Christopoulos G, Morfis M, Udawela M, Laburthe M, Couvineau A, Kuwasako K, Tilakaratne N, Sexton PM. 2003. Novel receptor partners and function of receptor activity-modifying proteins. J Biol Chem 278:3293–3297 [DOI] [PubMed] [Google Scholar]
- 31. Schaeffer C, Vandroux D, Thomassin L, Athias P, Rochette L, Connat JL. 2003. Calcitonin gene-related peptide partly protects cultured smooth muscle cells from apoptosis induced by an oxidative stress via activation of ERK1/2 MAPK. Biochim Biophys Acta 1643:65–73 [DOI] [PubMed] [Google Scholar]
- 32. Yu XJ, Li CY, Wang KY, Dai HY. 2006. Calcitonin gene-related peptide regulates the expression of vascular endothelial growth factor in human HaCaT keratinocytes by activation of ERK1/2 MAPK. Regul Pept 137:134–139 [DOI] [PubMed] [Google Scholar]
- 33. Gao F, Harikumar KG, Dong M, Lam PC, Sexton PM, Christopoulos A, Bordner A, Abagyan R, Miller LJ. 2009. Functional importance of a structurally distinct homodimeric complex of the family B G protein-coupled secretin receptor. Mol Pharmacol 76:264–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kuwasako K, Shimekake Y, Masuda M, Nakahara K, Yoshida T, Kitaura M, Kitamura K, Eto T, Sakata T. 2000. Visualization of the calcitonin receptor-like receptor and its receptor activity-modifying proteins during internalization and recycling. J Biol Chem 275:29602–29609 [DOI] [PubMed] [Google Scholar]
- 35. Ilegems E, Iwatsuki K, Kokrashvili Z, Benard O, Ninomiya Y, Margolskee RF. 2010. REEP2 enhances sweet receptor function by recruitment to lipid rafts. J Neurosci 30:13774–13783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Itoh K, Sakakibara M, Yamasaki S, Takeuchi A, Arase H, Miyazaki M, Nakajima N, Okada M, Saito T. 2002. Cutting edge: negative regulation of immune synapse formation by anchoring lipid raft to cytoskeleton through Cbp-EBP50-ERM assembly. J Immunol 168:541–544 [DOI] [PubMed] [Google Scholar]
- 37. Simons K, Toomre D. 2000. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol 1:31–39 [DOI] [PubMed] [Google Scholar]
- 38. Kuwasako K, Kitamura K, Nagata S, Hikosaka T, Kato J. 2010. Function of the cytoplasmic tail of human calcitonin receptor-like receptor in complex with receptor activity-modifying protein 2. Biochem Biophys Res Commun 392:380–385 [DOI] [PubMed] [Google Scholar]
- 39. Kuwasako K, Kitamura K, Nagata S, Hikosaka T, Kato J. 2011. Structure-function analysis of helix 8 of human calcitonin receptor-like receptor within the adrenomedullin 1 receptor. Peptides 32:144–149 [DOI] [PubMed] [Google Scholar]
- 40. Conner M, Hicks MR, Dafforn T, Knowles TJ, Ludwig C, Staddon S, Overduin M, Günther UL, Thome J, Wheatley M, Poyner DR, Conner AC. 2008. Functional and biophysical analysis of the C-terminus of the CGRP-receptor; a family B GPCR. Biochemistry 47:8434–8444 [DOI] [PubMed] [Google Scholar]
- 41. Gurevich VV, Gurevich EV. 2004. The molecular acrobatics of arrestin activation. Trends Pharmacol Sci 25:105–111 [DOI] [PubMed] [Google Scholar]
- 42. Laburthe M, Couvineau A, Marie JC. 2002. VPAC receptors for VIP and PACAP. Receptors Channels 8:137–153 [PubMed] [Google Scholar]
- 43. Couvineau A, Lacapere JJ, Tan YV, Rouyer-Fessard C, Nicole P, Laburthe M. 2003. Identification of cytoplasmic domains of hVPAC1 receptor required for activation of adenylyl cyclase. Crucial role of two charged amino acids strictly conserved in class II G protein-coupled receptors. J Biol Chem 278:24759–24766 [DOI] [PubMed] [Google Scholar]
- 44. Covic L, Misra M, Badar J, Singh C, Kuliopulos A. 2002. Pepducin-based intervention of thrombin-receptor signaling and systemic platelet activation. Nat Med 8:1161–1165 [DOI] [PubMed] [Google Scholar]
- 45. Covic L, Gresser AL, Talavera J, Swift S, Kuliopulos A. 2002. Activation and inhibition of G protein-coupled receptors by cell-penetrating membrane-tethered peptides. Proc Natl Acad Sci USA 99:643–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Edwards RJ, Moran N, Devocelle M, Kiernan A, Meade G, Signac W, Foy M, Park SD, Dunne E, Kenny D, Shields DC. 2007. Bioinformatic discovery of novel bioactive peptides. Nat Chem Biol 3:108–112 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.