Abstract
Serine racemase is a brain-enriched enzyme that synthesizes d-serine, an endogenous modulator of the glycine site of N-methyl-d-aspartate (NMDA) receptors. We now report that serine racemase catalyzes an elimination reaction toward a nonphysiological substrate that provides a powerful tool to study its neurobiological role and will be useful to develop selective enzyme inhibitors. Serine racemase catalyzes robust elimination of l-serine O-sulfate that is 500 times faster than the physiological racemization reaction, generating sulfate, ammonia, and pyruvate. This reaction provides the most simple and sensitive assay to detect the enzyme activity so far. We establish stable cell lines expressing serine racemase and show that serine racemase can also be converted into a powerful eliminase in cultured cells, while the racemization of l-serine is inhibited. Likewise, l-serine O-sulfate inhibits the synthesis of d-serine in primary astrocyte cultures. We conclude that the synthetic compound l-serine O-sulfate is a better substrate than l-serine as well as an inhibitor of d-serine synthesis. Inhibition of serine racemase provides a new strategy to selectively decrease NMDA receptor coactivation and may be useful in conditions in which overstimulation of NMDA receptors plays a pathological role.
Keywords: glutamate receptors, l-serine O-sulfate
High levels of d-serine, a d-amino acid not previously thought to occur in mammals, have recently been found in mammalian brain (1, 2). Several lines of evidence indicate that d-serine is an endogenous modulator of the N-methyl-d-aspartate (NMDA) type of glutamate receptors. NMDA receptors require coactivation at a “glycine site” at which d-serine is at least as potent as glycine (3). Extracellular levels of endogenous d-serine are comparable to glycine in prefrontal cortex, whereas in the striatum, d-serine values are more than twice the values for glycine as measured by in vivo microdialysis (4). Immunohistochemical studies show a high degree of colocalization of d-serine and NMDA receptors in the forebrain, whereas densities for glycine are more prominent in the brainstem (5, 6). d-serine occurs in protoplasmic astrocytes that ensheathe the synapses and can be released from astrocyte cultures by activation of the kainate type of glutamate receptors (5). Direct evidence that d-serine normally mediates NMDA transmission comes from experiments showing that destruction of endogenous d-serine selectively by application of d-amino acid oxidase greatly reduces NMDA receptor activity monitored both biochemically and electrophysiologically in slices and cell culture preparations (7).
Because amino acid racemases were thought to be restricted to bacteria, the origin of d-serine has been puzzling. We recently showed that d-serine is synthesized from l-serine by a pyridoxal 5′-phosphate-dependent serine racemase. We purified serine racemase from murine brain (8) and cloned mouse and human serine racemase genes (9, 10). The distribution of serine racemase was closely similar to that of endogenous d-serine with the highest concentrations in the forebrain and negligible levels in the brainstem. Both d-serine and serine racemase occur in astrocytes, in regions enriched in NMDA receptors, suggesting that serine racemase physiologically synthesizes d-serine to regulate NMDA receptor activity (9).
As an endogenous coagonist of NMDA receptors, d-serine may play a role in several pathological conditions related to NMDA receptor dysfunction. Elevations of extracellular concentrations of d-serine are observed after transient cerebral ischemia in rats (11), and drugs that block the “glycine site” of NMDA receptors prevent stroke damage (12, 13). d-serine is at least as potent as glycine in stimulating glutamate-induced activation of NMDA receptors (3, 14), and massive stimulation of NMDA receptors is implicated in neural damage following stroke (15). Thus, inhibitors of serine racemase may be useful in conditions such as stroke and neurodegenerative diseases where overstimulation of NMDA receptors plays a pathological role.
In addition to being a therapeutic target, the study of serine racemase biochemical properties and regulation are important to elucidate the neurobiological role of d-serine. However, this task has been challenging because of the difficulties involving the routine detection of small amounts of d-serine by the methods available and by the lack of an active preparation of purified recombinant enzyme. In this report we discovered a new reaction catalyzed by recombinant serine racemase that has been overexpressed and purified from mammalian cells. We found that mouse serine racemase robustly destroys l-serine O-sulfate through an elimination reaction. Degradation of l-serine O-sulfate is 500 times faster than the racemization of l-serine, demonstrating that serine racemase can function as a powerful eliminase. Additionally, detection of l-serine O-sulfate degradation products provides the most reliable and easy method to measure the activity of serine racemase so far. We also show that serine racemase can function as an eliminase in cultured cells, while d-serine synthesis is inhibited. The present findings have implications for the development of selective inhibitors acting on serine racemase to decrease NMDA receptor coactivation.
Materials and Methods
Materials.
Amino acids, aminooxyacetic acid (AOAA), l-homocysteic acid, l-homoserine, luminol (sodium salt), phenylmethylsulfonyl fluoride, pyridoxal 5′-phosphate, pyruvate, l-serine O-sulfate, and Tris were obtained from Sigma. d-amino acid oxidase from pig kidney (EC 1.4.3.3), horseradish peroxidase and lactate dehydrogenase were obtained from Roche Molecular Biochemicals. Glutathione-Sepharose 4B was obtained from Amersham Pharmacia. A thrombin cleavage capture kit was purchased from Novagen. Other reagents were of analytical grade.
Expression and Purification of Recombinant Serine Racemase.
Mouse full-length serine racemase was subcloned into pCMV-GST mammalian expression vector containing a cytomegalovirus (CMV) promoter and the gene for the glutathione S-transferase (GST) of Schistosoma japonicum. HEK293 cells cultured in DMEM plus 10% FBS were grown in 100-mm dishes and transiently transfected with the construct by the calcium phosphate method (16). After 48 h, the cells were harvested and lysed by sonication in media containing 50 mM Tris⋅HCl (pH 7.4), 400 mM NaCl, 0.2 mM phenylmethylsulfonyl fluoride, 2 mM EDTA, 2 mM DTT, and 15 μM pyridoxal 5′-phosphate (PLP). Then, 0.1% Triton X-100 was added and the homogenate was centrifuged at 40,000 × g for 10 min at 4°C to remove membrane and other heavy fractions. The supernatant was incubated for 6 h with glutathione-Sepharose 4B beads to bind serine racemase–GST fusion protein. The beads were washed five times with PBS supplemented with 300 mM NaCl, 1 mM EDTA, 2 mM DTT, and 15 μM PLP. To obtain purified serine racemase, the GST part of the fusion protein was cleaved out by incubation with biotinylated thrombin for 16 h at room temperature in PBS containing 15 μM PLP. Biotinylated thrombin was removed with streptavidin-agarose according to the manufacturer's instructions (Novagen) and purified serine racemase was separated from the beads containing the GST part of the fusion protein by centrifugation. Typically, 20–50 μg purified serine racemase are obtained from each transfected 100-mm dish.
Serine Racemase Activity.
Reaction media contained 50 mM Tris⋅HCl (pH 8.2), 15 μM PLP, and 10 mM l-serine. The reaction was started by addition of recombinant serine racemase (1–20 μg/ml) and stopped by addition of 5% trichloroacetic acid (TCA). The acid was subsequently extracted with diethyl ether and d-serine formed was monitored by a chemiluminescent method (8). l-serine used in the experiments was rendered free of contaminating d-serine as described (8). In experiments testing the effects of several d-amino acids on serine racemase activity, d-serine was monitored by HPLC analysis after derivatization with o-phthaldialdehyde and N-tert-butyloxycarbonyl-l-cysteine as described (17). Blanks were carried out with heat-inactivated enzyme.
Assay for l-Serine O-Sulfate Elimination.
l-serine O-sulfate levels were monitored by the same HPLC technique we used for d-amino acid determination. l-serine O-sulfate was identified as a discrete peak eluting between l-homocysteic acid and l-glutamate. Reaction media contained 50 mM Tris⋅HCl (pH 7.4), 0.5–20 mM l-serine O-sulfate, and 15 μM PLP. Pyruvate formed from l-serine O-sulfate was monitored by the decrease of NADH (0.2 mM) absorbance at 340 nm, as the pyruvate was converted to lactate in the presence of lactate dehydrogenase (1 μg/ml; ref. 18). Inclusion of PLP in the reaction media seems to be important to stabilize the enzyme. Without added PLP, serine racemase loses its activity within 30 min of incubation. Sulfate was determined as described (19). Ammonia was monitored by the oxidation of NADPH in the presence of 2-oxolutarate and glutamate dehydrogenase (20).
Stable Cell Lines Production.
HEK293 cells were cotransfected with full-length mouse serine racemase in a plasmid containing cytomegalovirus (CMV) promoter (pRK5-KS; ref. 9) together with a plasmid containing the gene for resistance to neomycin (PIRESneo from CLONTECH). Resistant clones were selected by using G418 (400 μg/ml); several independent colonies were isolated and expanded. Clones expressing the highest levels of serine racemase were selected by their ability to synthesize d-serine from added l-serine (measured from an aliquot of the conditioned culture media) and by Western blot analysis using a polyclonal antibody against serine racemase. The cells were maintained in DMEM plus 10% FBS supplemented with 200 μg/ml of G418. To serve as controls, we developed cell lines stably expressing an inactive mutant of serine racemase (Rac-K56G), in which the lysine residue predicted to bind PLP was replaced by glycine. Additional controls are HEK293 cell lines stably expressing only the gene for resistance to neomycin (Neo-vector).
d-Serine Synthesis in Cell Cultures.
To measure synthesis of d-serine in cell cultures, HEK293 stable cell lines were cultured in 25-mm dishes with media supplemented with 10 mM l-serine to maximize racemization activity. d-serine synthesized by the cells was measured 36 h after adding l-serine. The cells were washed twice with cold PBS, followed by addition of 5% trichloroacetic acid (TCA) to extract free amino acids. The suspension was centrifuged at 20,000 × g for 5 min and the supernatant was analyzed for d-serine by HPLC after removal of TCA by two extractions with water-saturated diethyl ether.
Immunocytochemistry.
To facilitate cell attachment, chamber glass slides were coated with 0.2% gelatin for 1 h, followed by a 10-min treatment with 0.5% glutaraldehyde, which was subsequently rinsed with sterile water. For immunocytochemistry, HEK293 stable cell lines were cultured in the chamber slides, fixed, and permeabilized with methanol 100% for 20 min at −20°C. After blocking with 4% normal goat serum in PBS, the slides were incubated with affinity-purified anti-serine racemase (0.5 μg/ml) for 12 h at 4°C. Secondary antibody consisted of a Cy3-conjugated anti-rabbit used at 1 μg/ml (Jackson ImmunoResearch). Images were captured on a Nikon fluorescence microscope.
Primary Astrocyte Cultures.
Primary astrocyte cultures were prepared from cerebral cortex as described (21). Cells were cultured in glass chamber slides coated with poly d-lysine in DMEM plus 10% FBS for 2 weeks before use. Synthesis of d-serine was monitored after supplementation of culture media with 10 mM l-serine and processed as described above for stable cell lines expressing serine racemase.
Results
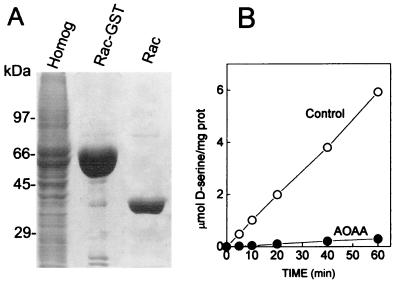
To study the properties of serine racemase, we purified the recombinant fusion protein from mammalian cells (Fig. 1A). Subsequent cleavage of fusion protein with biotinylated thrombin generates pure and active serine racemase (Fig. 1). The rate of racemization measured by the conversion of l- to d-serine is identical to that previously observed for the native serine racemase purified from rat brain (8). Aminooxyacetic acid, an inhibitor of pyridoxal 5′-phosphate-dependent enzymes, abolishes the activity (Fig. 1B).
Figure 1.
Purification of active recombinant serine racemase from mammalian cells. Mouse serine racemase-GST fusion protein was expressed in HEK293 cells and purified to homogeneity. (A) SDS/PAGE (12%) stained with Coomassie brilliant blue. Lane 1, 40 μg transfected HEK293 cell protein extract; lane 2, 30 μg mouse serine racemase-GST fusion protein purified with glutathione-Sepharose beads; lane 3, 5 μg purified serine racemase after GST portion was removed by cleavage with thrombin. (B) Serine racemase activity was measured at 37°C in media containing 50 mM Tris⋅HCl (pH 8.2), 10 μg/ml recombinant serine racemase, 15 μM PLP, and 10 mM l-serine. Recombinant serine racemase exhibited robust racemase activity (○), which was totally inhibited by 1 mM AOAA (●). The data are representative of six different purifications.
To verify the specificity and stereoselectivity of serine racemase, we tested the effects of a wide variety of amino acids and substrate analogues (Fig. 2). Among the amino acids tested, l-lysine and l- and d-cysteine significantly inhibit serine racemization. These amino acids are known to strongly interact with PLP. Among d-amino acids, only d-cysteine inhibits racemase activity, demonstrating the high specificity of the enzyme. l-serine derivatives O-phospho-l-serine and l-homoserine have no effect, whereas l-serine O-sulfate strongly inhibits serine racemization (Fig. 2A).
Figure 2.
Substrate specificity of serine racemase. Racemase activity was assayed at 37°C in media containing 50 mM Tris⋅HCl (pH 8.2), 5 mM l-serine, 10 μg/ml purified enzyme, 15 μM PLP, and 10 mM of test compounds. After 1 h, the reaction was terminated by the addition of 5% trichloroacetic acid (TCA), and the d-serine produced was monitored by HPLC. The values are mean ± SEM of three different experiments with three enzyme preparations. *, Different from control at P < 0.01. O-P-L-ser, O-phospho-l-serine; L-homo, l-homoserine; L-ser O-sulf, l-serine O-sulfate.
l-serine O-sulfate is thought to be a close analogue of glutamate because the additional negative charge of the sulfate group mimics the carboxyl residue of glutamate (22, 23). Because l-serine O-sulfate has not been shown to interact with enzymes of serine metabolism, we sought to clarify the mechanism of its interaction with serine racemase. Inhibition by l-serine O-sulfate is observed in the millimolar range (Fig. 3A). Kinetic analysis reveals a noncompetitive inhibition, as l-serine O-sulfate does not change the apparent Km for l-serine (5.6 ± 0.5 mM), implying that l-serine O-sulfate is not able to directly displace l-serine from the catalytic site (Fig. 3).
Figure 3.
Inhibition of serine racemase by l-serine O-sulfate. (A) Enzyme activity was monitored at 37°C in media containing 50 mM Tris⋅HCl (pH 8.2), 10 mM l-serine, 10 μg/ml purified enzyme, 15 μM PLP, and different concentrations of l-serine O-sulfate (●). (B) Reaction medium and conditions were as described in A, except that l-serine concentration was varied from 1 to 20 mM either in the absence (○) or in the presence (●) of 8 mM l-serine O-sulfate. Inhibition exhibited a noncompetitive kinetics (Inset). The values are mean ± SEM of three different experiments with three enzyme preparations.
To examine whether serine racemase was able to racemize
l-serine O-sulfate, we carried out HPLC analysis
of the reaction medium. Although we did not detect any formation of
d-serine O-sulfate, we observed a
significant decrease of l-serine
O-sulfate content (Fig.
4A).
l-serine O-sulfate disappearance can
be followed over long periods of time, indicating a continuous
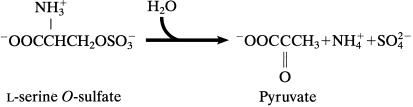
degradation by serine racemase (Fig. 4B). By analogy with
the sulfatases group of enzymes, l-serine
O-sulfate degradation should yield
l-serine and inorganic SO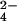 .
However, we did not detect any l-serine as a
reaction product. Instead, we detected the formation of large amounts
of pyruvate, along with stoichiometric amounts of sulfate (Fig.
5A) and ammonia (data not
shown). The amounts of product formed correspond exactly to the amount
of l-serine O-sulfate consumed (Fig.
5A). The overall reaction of l-serine
O-sulfate degradation is represented in Scheme
S1.
.
However, we did not detect any l-serine as a
reaction product. Instead, we detected the formation of large amounts
of pyruvate, along with stoichiometric amounts of sulfate (Fig.
5A) and ammonia (data not
shown). The amounts of product formed correspond exactly to the amount
of l-serine O-sulfate consumed (Fig.
5A). The overall reaction of l-serine
O-sulfate degradation is represented in Scheme
S1.
Figure 4.
Destruction of l-serine O-sulfate by serine racemase. (A) Serine racemase (10 μg/ml) was incubated for 2 h with 10 mM l-serine O-sulfate in media containing 50 mM Tris⋅HCl (pH 8.2) and 15 μM PLP. The HPLC traces show the content of l-serine O-sulfate before and after incubation with the enzyme. l-homocysteic acid (2.5 nmol) was used as an internal standard. (B) Time course of l-serine O-sulfate consumption. Conditions were the same as in A. The values are representative of three different experiments with different enzyme preparations.
Figure 5.
Elimination reaction catalyzed by serine racemase. (A) Serine racemase catalyzes stoichiometric production of pyruvate and ammonia, which corresponds to the amount of serine racemase consumption. The reaction media contained 50 mM Tris⋅HCl (pH 7.4), 10 mM l-serine O-sulfate, and 15 μM PLP. Reaction was started by addition of 10 μg/ml recombinant serine racemase. The values correspond to the amount of l-serine O-sulfate consumed and products formed after 30 min of reaction. (B) Production of pyruvate is inhibited by l-serine and aminooxyacetic acid. The conditions were the same as in A, except that reaction media contained 0.2 mM NADH, 1 μg/ml lactate dehydrogenase, and recombinant serine racemase (2 μg/ml final concentration). Pyruvate production was continuously monitored by the decrease of absorbance at 340 nm. Control (○), 20 mM l-serine (●), or 1 mM AOAA (▴). (C) Noncompetitive inhibition of pyruvate production by l-serine. The reaction was as in A, except that media contained either no (○) or 5 mM (●) l-serine. (Inset) A double-reciprocal plot of the substrate curves. The values are representative of three to five different experiments with different enzyme preparations.
Scheme 1.
Remarkably, we found the initial rate of l-serine O-sulfate degradation by serine racemase to be about 500 times faster than the rate of normal racemization (compare Figs. 5B and 1B; note that the time and specific activity scales are different). Degradation of l-serine O-sulfate depends on PLP, because AOAA abolishes both pyruvate (Fig. 5B) and sulfate production (data not shown). l-serine, the physiological substrate, inhibits the production of pyruvate (Fig. 5B). Half-maximal inhibition is observed at about 5 mM l-serine, which matches the Km of serine racemase for l-serine. Inhibition by l-serine exhibits a noncompetitive kinetics, indicating that l-serine cannot directly displace l-serine O-sulfate molecule from the catalytic site (Fig. 5C).
Further evidence that the type of interaction of l-serine O-sulfate with the enzyme differs from that of l-serine comes from the pH-dependence we observed. Pyruvate production peaks around pH 7.0–7.5, contrasting with racemization activity, which displays optimal pH around 8.5 (Fig. 6). Note that the specific activities of elimination and racemization reactions differ by two orders of magnitude.
Figure 6.
pH-dependence of elimination and racemization reactions. (A) Elimination reaction was assayed for pyruvate formation at 37°C in media containing 50 mM Tris⋅HCl, 10 mM l-serine O-sulfate, 2 μg/ml recombinant enzyme, and 15 μM PLP (○). (B) Racemization of l-serine was assayed at 37°C in media containing 50 mM Tris⋅HCl, 10 mM l-serine, 10 μg/ml recombinant enzyme, and 15 μM PLP (●). The experiment was replicated three times, using different preparations with similar results.
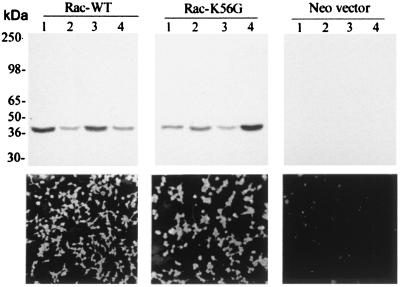
We wondered whether l-serine O-sulfate could inhibit the racemase in vivo. Accordingly, we established HEK293 stable cell lines expressing wild-type serine racemase (Rac-WT), catalytically inactive serine racemase mutant (Rac-K56G) constructed by replacing the lysine predicted to bind PLP with glycine, and a control cell line expressing only the neomycin resistance vector (Neo-vector) (Fig. 7). Immunocytochemistry revealed that more than 98% of the cells homogeneously expressed the serine racemase constructs (Fig. 7 Lower). To verify whether the stable cell lines were suitable models for experiments of d-serine synthesis, we measured several intracellular amino acids in those cells. d-serine is not detectable in control cell lines, while Rac-WT cells accumulate high levels of intracellular free d-serine (70 nmol d-serine/mg protein; Fig. 8B). Intracellular levels of other amino acid, such as l-aspartate, l-glutamate, l-asparagine, l-serine, l-glutamine, l-threonine, glycine, and l-alanine are unchanged, suggesting that accumulation of intracellular d-serine does not appear to change significantly the metabolism of other amino acids (data not shown).
Figure 7.
Stable cell lines expressing serine racemase. (Upper) Western blot analysis of different stable cell lines expressing Rac-WT, Rac-K56G, or neomycin resistance gene alone (Neo-vector). Each lane contains 20 μg of HEK293 cell extract. The blots were probed with a polyclonal antibody against serine racemase at 1:1000 dilution, revealing a single 37-kDa band corresponding to serine racemase. (Lower) Immunocytochemistry of HEK293 cells showing the expression of serine racemase in Rac-WT (cell line of lane 1), Rac-K56G (cell line of lane 4), and Neo-vector (cell line of lane 4). The experiment was replicated three times with similar results.
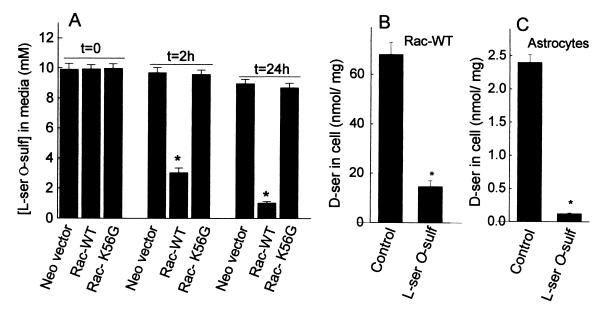
Figure 8.
Consumption of l-serine O-sulfate in stable cell lines expressing serine racemase and inhibition of d-serine synthesis. (A) l-serine O-sulfate (10 mM) was added to cell culture media and aliquots were collected immediately (t = 0), 2 h after, and 24 h after. Levels of l-serine O-sulfate were analyzed by HPLC. A stable cell line expressing Rac-WT consumed a large fraction of l-serine O-sulfate added to culture media. Control cell line expressing only the neomycin resistance vector (Neo-vector) or a catalytically inactive serine racemase mutant (Rac-K56G) did not degrade any l-serine O-sulfate. (B) l-serine O-sulfate inhibits d-serine synthesis in Rac-WT cells. Accumulation of d-serine was significantly inhibited by inclusion of 10 mM l-serine O-sulfate in the culture media. (C) l-serine O-sulfate inhibits d-serine synthesis in primary cultures of astrocytes. The conditions were the same as in B. The values represent the mean ± SEM of three experiments with three different cultures. *, Different from control at P < 0.01.
l-Serine O-sulfate added to culture media permeates the cells and is robustly consumed by those expressing Rac-WT, but not by Rac-K56G or control cell line (Neo-vector) (Fig. 8A). As much as 7 mM l-serine O-sulfate added to culture media is degraded after 2 h in Rac-WT cells (Fig. 8A). Intracellular levels of l-serine O-sulfate are also depleted in Rac-WT cells, but not in the Rac-K56G or control cell line (data not shown).
To verify whether l-serine O-sulfate inhibits serine racemase in culture cells, we treated Rac-WT cells and primary astrocyte cultures with l-serine O-sulfate and monitored intracellular d-serine levels by HPLC. Accordingly, synthesis of d-serine is inhibited to a large extent by incubation of Rac-WT cells or primary astrocyte cultures with l-serine O-sulfate, indicating that serine racemase activity can be converted into an eliminase in vivo (Fig. 8 B and C).
Conceivably, l-serine O-sulfate could inhibit serine racemization by decreasing intracellular levels of l-serine or by simply killing the cells. However, intracellular levels of l-serine are unchanged in cells treated with l-serine O-sulfate, and microscopic examination of the cells does not reveal any obvious abnormalities, suggesting that inhibition of serine racemization is due to a specific effect on serine racemase activity (data not shown).
Discussion
Serine racemase was the first racemase cloned from eukaryotes and is highly enriched in mammalian brain, where it synthesizes d-serine to regulate NMDA receptor activity (9, 10). Overactivation of the NMDA receptor is known to play a key role in cell death that takes place in stroke and neurodegenerative diseases (15). Thus, drugs that block serine racemase will be useful to treat such pathological conditions, providing a new strategy to decrease NMDA receptor coactivation by d-serine.
In the present report, we developed a method to produce substantial amounts of recombinant serine racemase and demonstrated an alternative reaction catalyzed by the enzyme. The degradation of l-serine O-sulfate is two to three orders of magnitude faster than the physiological racemization of l-serine. PLP-dependent enzymes have been shown to catalyze alternative reactions, but they are always several orders of magnitude slower than the physiological reaction (24). Degradation of l-serine O-sulfate provides the most reliable and easy method to measure serine racemase activity so far. Because of its sensitivity and simplicity, it is an ideal method for high-throughput screening of drugs.
It has been shown that serine O-sulfate irreversibly inactivates other types of enzymes involved in glutamate metabolism, such as aspartate aminotransferase (AAT; refs. 23 and 25). The reactions involved in inactivation of AAT by l-serine O-sulfate consist of the release of sulfate (β-elimination) and production of aminoacrylate, an unstable intermediate that either hydrolyzes to pyruvate and ammonia or reacts irreversibly to inactivate AAT. Thus, l-serine O-sulfate is considered a suicidal substrate that completely inactivates AAT in less than 1 min because of the reaction of aminoacrylate intermediate with the enzyme (23, 25).
The robust degradation of l-serine O-sulfate by serine racemase suggests that the β-elimination of sulfate and subsequent hydrolysis of aminoacrylate to ammonia and pyruvate are thermodynamically favored. Furthermore, unlike AAT, serine racemase is very resistant to inactivation by aminoacrylate, as shown by the stable degradation of l-serine O-sulfate over long periods of time. Thus, in our experimental conditions, l-serine O-sulfate acts as a better substrate than l-serine, as well as an inhibitor of racemization. Although theoretical considerations would have predicted a competitive kinetics, the inhibition pattern of d-serine synthesis by l-serine O-sulfate reveals a noncompetitive mechanism. Additional studies addressing the structural attributes of the catalytic site of the enzyme will be required to explain those findings.
The degradation of l-serine O-sulfate in stable cell lines expressing serine racemase suggests that serine racemase can either function as racemase or eliminase, depending on the type of substrate it reacts with. Conceivably, serine racemase may play other roles in addition to being the enzyme that physiologically synthesizes d-serine. For instance, previous studies have detected high levels of serine racemase in hepatic tissue of rats, which normally do not accumulate significant levels of d-serine in their livers (9). However, liver expresses d-amino acid oxidase, an enzyme that destroys d-serine (26). Although it may be possible that serine racemase catalyzes elimination reaction of other substrates, data in the literature and in the present study fully support the notion that serine racemase physiologically works as a racemase, rather than an eliminase. Serine racemase has been biochemically purified from brain by its ability to directly convert l-serine to d-serine, and its properties, such as the alkaline pH optimum and Km values for l-serine, resemble those of bacterial racemases (8). Serine racemase displays 30% sequence homology with l-serine dehydratase, but we do not detect elimination reaction toward l-serine. The enzyme colocalizes with d-serine in astrocytes, and inhibition of serine racemase in primary astrocyte cultures decreases cellular d-serine (ref. 9 and Fig. 8B). Moreover, expression of serine racemase in cultured cells leads to a selective accumulation of intracellular d-serine, without changing the levels of any other amino acid analyzed. In contrast to l-serine, l-serine O-sulfate is an artificial substrate not found in animal tissues, and we did not detect elimination reaction toward any other l-serine analog we tested. Although the rate of elimination is higher than the racemization, the affinity for l-serine O-sulfate is in the millimolar range and it is unlikely that physiological l-serine analogs would occur in such high concentrations. Nonetheless, we examined the existence of alternative serine racemase substrates in brain by preparing concentrated extracts of soluble amino acids and small molecules, obtained by removing the protein fraction by acid-precipitation. Serine racemase activity was not affected by the soluble fractions we prepared, indicating that alternative substrates for the racemase are not present at significant amounts (data not shown).
NMDA receptor activation has been implicated in a wide variety of cellular processes, including survival of cerebellar granule cells in culture, migration of cerebellar granule cells during development, and glutamate neurotoxicity (3, 15, 27). Changing endogenous d-serine levels in culture systems will be important to establish the role of d-serine in NMDA-dependent processes. In the present report, we demonstrated pharmacological inhibition of d-serine synthesis in astrocytes, which physiologically express high levels of serine racemase. Thus, inhibition of serine racemase by compounds such as l-serine O-sulfate will be valuable in studying the neurobiological role of d-serine and will provide a strategy to selectively decrease NMDA receptor coactivation.
Acknowledgments
We thank to Simone Leao and Paulo Henrique dos Santos for technical assistance, Raquel Cazes for the administration of the grant, and Dr. Randal Reed (Johns Hopkins University, Baltimore) for kindly providing pCMV-GST vector. This work was supported by a Research Grant from the Theodore and Vada Stanley Foundation (to S.E. and H.W.).
Abbreviations
- AAT
aspartate aminotransferase
- AOAA
aminooxyacetic acid
- GST
glutathione S-transferase
- NMDA
N-methyl-d-aspartate
- PLP
pyridoxal 5′-phosphate
- Rac-WT
wild-type serine racemase
- Rac-K56G
catalytically inactive serine racemase mutant
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Hashimoto A, Nishikawa T, Hayashi T, Fujii N, Harada K, Oka T, Takahashi K. FEBS Lett. 1992;296:33–36. doi: 10.1016/0014-5793(92)80397-y. [DOI] [PubMed] [Google Scholar]
- 2.Hashimoto A, Nishikawa T, Oka T, Takahashi K. J Neurochem. 1993;60:783–786. doi: 10.1111/j.1471-4159.1993.tb03219.x. [DOI] [PubMed] [Google Scholar]
- 3.Danysz W, Parsons A C G. Pharmacol Rev. 1998;50:597–664. [PubMed] [Google Scholar]
- 4.Hashimoto A, Oka T, Nishikawa T. Neuroscience. 1995;66:635–643. doi: 10.1016/0306-4522(94)00597-x. [DOI] [PubMed] [Google Scholar]
- 5.Schell M J, Molliver M E, Snyder S H. Proc Natl Acad Sci USA. 1995;92:3948–3952. doi: 10.1073/pnas.92.9.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schell M J, Brady R O, Jr, Molliver M E, Snyder S H. J Neurosci. 1997;17:1604–1615. doi: 10.1523/JNEUROSCI.17-05-01604.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mothet J P, Parent A T, Wolosker H, Brady R O, Jr, Linden D J, Ferris C D, Rogawski M A, Snyder S H. Proc Natl Acad Sci USA. 2000;97:4926–4931. doi: 10.1073/pnas.97.9.4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolosker H, Sheth K N, Takahashi M, Mothet J P, Brady R O, Jr, Ferris C D, Snyder S H. Proc Natl Acad Sci USA. 1999;96:721–725. doi: 10.1073/pnas.96.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolosker H, Blackshaw S, Snyder S H. Proc Natl Acad Sci USA. 1999;96:13409–13414. doi: 10.1073/pnas.96.23.13409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Miranda J, Santoro A, Engelender S, Wolosker H. Gene. 2000;256:183–188. doi: 10.1016/s0378-1119(00)00356-5. [DOI] [PubMed] [Google Scholar]
- 11.Lo E H, Pierce A R, Matsumoto K, Kano T, Evans C J, Newcomb R. Neuroscience. 1998;83:449–458. doi: 10.1016/s0306-4522(97)00434-x. [DOI] [PubMed] [Google Scholar]
- 12.Gill R, Hargreaves R J, Kemp J A. J Cereb Blood Flow Metab. 1995;15:197–204. doi: 10.1038/jcbfm.1995.25. [DOI] [PubMed] [Google Scholar]
- 13.Warner D S, Martin H, Ludwig P, McAllister A, Keana J F, Weber E. J Cereb Blood Flow Metab. 1995;15:188–196. doi: 10.1038/jcbfm.1995.24. [DOI] [PubMed] [Google Scholar]
- 14.Matsui T, Sekiguchi M, Hashimoto A, Tomita U, Nishikawa T, Wada K. J Neurochem. 1995;65:454–458. doi: 10.1046/j.1471-4159.1995.65010454.x. [DOI] [PubMed] [Google Scholar]
- 15.Choi D W, Rothman S M. Annu Rev Neurosci. 1990;13:171–182. doi: 10.1146/annurev.ne.13.030190.001131. [DOI] [PubMed] [Google Scholar]
- 16.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning—A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 17.Hashimoto A, Nishikawa T, Oka T, Takahashi K, Hayashi T. J Chromatogr. 1992;582:41–48. doi: 10.1016/0378-4347(92)80300-f. [DOI] [PubMed] [Google Scholar]
- 18.Eisenthal R, Danson M J. Enzyme Assays: A Practical Approach. New York: Oxford Univ. Press; 1993. pp. 76–78. [Google Scholar]
- 19.Dodgson K S. Biochem J. 1961;78:312–319. doi: 10.1042/bj0780312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colon A D, Plaitakis A, Perakis A, Berl S, Clarke D D. J Neurochem. 1986;46:1811–1819. doi: 10.1111/j.1471-4159.1986.tb08500.x. [DOI] [PubMed] [Google Scholar]
- 21.Kettenmann H, Ransom B R. Neuroglia. New York: Oxford Univ. Press; 1995. [Google Scholar]
- 22.Kingston A E, Lowndes J, Evans N, Clark B, Tomlinson R, Burnett J P, Mayne N G, Cockerham S L, Lodge D. Neuropharmacology. 1998;37:277–287. doi: 10.1016/s0028-3908(98)00018-5. [DOI] [PubMed] [Google Scholar]
- 23.Ueno H, Likos J J, Metzler D E. Biochemistry. 1982;21:4387–4393. doi: 10.1021/bi00261a030. [DOI] [PubMed] [Google Scholar]
- 24.Mehta P K, Christen P. Adv Enzymol Relat Areas Mol Biol. 2000;74:129–184. doi: 10.1002/9780470123201.ch4. [DOI] [PubMed] [Google Scholar]
- 25.Birolo L, Sandmeier E, Christen P, John R A. Eur J Biochem. 1995;232:859–864. [PubMed] [Google Scholar]
- 26.D'Aniello A, D'Onofrio G, Pischetola M, D'Aniello G, Vetere A, Petrucelli L, Fisher G H. J Biol Chem. 1993;268:26941–26949. [PubMed] [Google Scholar]
- 27.Komuro H, Rakic P. Science. 1993;260:95–97. doi: 10.1126/science.8096653. [DOI] [PubMed] [Google Scholar]