Abstract
The genetic diversity of the Derby serotype of Salmonella enterica in Spain was examined by pulsed-field gel electrophoresis (PFGE). Out of 24 identified PFGE profiles, a major clone was detected in 19% of strains from humans, 52% from food, and 62% from swine. This clone (clone 1) was isolated from pork products, suggesting swine as its source.
Keywords: swine, Salmonella enterica Derby, clonal spread, PFGE typing, human food
Salmonellosis, a major public health concern worldwide, is one of the most common causes of human gastroenteritis. It is caused by the ingestion of contaminated food because of a failure to control Salmonella infection in animal husbandry. Its carriers are swine, poultry, and cattle, along with eggs, milk, and fresh seafood (1). Salmonella in pork carcasses is a result of fecal contamination during slaughtering and processing. In this case, the carrier swine is the main initial source of contamination (2,3), and bacteria are usually located in the pharynx, lymph nodes, stomach, and feces (4).
During 2002, Salmonella enterica subsp. enterica serotype Derby was the second and sixth most common Salmonella serotype in clinical and nonclinical nonhuman sources, representing 24.0% and 3.7% of isolates reported to the Centers for Disease Control and Prevention and the National Veterinary Service, respectively (5). The frequency of S. Derby among isolates from animals received at the Spanish National Reference Laboratory for Salmonella and Shigella (LNRSSE) was higher than in previous years because of isolates received from swine farms. The incidence of S. Derby in Spain has remained stable in isolates of human and food origin, accounting for 0.55% and 2.5%, respectively, of Salmonella recovered from these sources from 1997 to 2003 (M.A. Echeita, unpub. data).
The persistence and clonal survival of S. Derby in swine populations and in closed environments and the role of pork and pork products as sources of outbreaks in humans have been described previously (6–8). S. Derby is one of the most common serotypes of Salmonella in swine. It accounted for 24.2% of the serotypes at 3 nurseries and 9 finishing farms of 2 commercial swine production companies in North Carolina (9) and for 37.1% at 5 swine-finishing units in Quebec, Canada (10).
To obtain epidemiologic insights into human acquisition of the S. Derby serotype compared with swine-related strains, antimicrobial susceptibility profiles were obtained and genetic characterization by pulsed-field gel electrophoresis (PFGE) was conducted in strains isolated from humans, food, and the environment. Unlike more common serotypes such as Enteritidis or Typhimurium, this serotype has not been subjected to intensive molecular epidemiologic studies.
The Study
A total of 110 S. Derby isolates collected during a 3-year period (2000–2002) were studied. Forty-seven of these isolates (LNRSSE group) were submitted by 34 Spanish laboratories and had the following origins: humans (n = 16), food (n = 23), ill animals (n = 6), and environmental sources (n = 2), as shown in Table 1. The remaining 63 isolates (swine group) were obtained from the stools and mesenteric lymph nodes of 48 swine slaughtered at 4 swine operations (A, B, C, and D) and 6 finishing units (units 1–6) (Table 2) in Spain. The swine reared on these farms shared the same feed supply and had similar genetic backgrounds.
Table 1. Characteristics of Salmonella enterica Derby serotype isolates (N = 47) from the LNRSSE Group (Spanish National Reference Laboratory for Salmonella and Shigella)*.
| Specimen |
Isolation date |
Geographic origin |
PFGE profile |
Resistance profile |
GyrA mutation† |
|---|---|---|---|---|---|
| Human origin (16 strains) | |||||
| 1 | 9/4/01 | Barcelona | 19 | ||
| 2 | 3/5/02 | Lugo | 4 | T | |
| 3 | 4/25/02 | Madrid | 3 | S,Su | |
| 4 | 4/25/02 | Unknown | NT | A,S,Su,T,C,Nx | Tyr 83 (>256/0.12) |
| 5 | 5/23/02 | Orense | 10 | A,S,Su,T,C | |
| 6 | 7/27/02 | Castellón | 17 | ||
| 7 | 7/27/02 | Barcelona | 11 | A,S,G,To,Ap,Su,T,SxT | |
| 8 | 7/8/02 | Zaragoza | 11 | ||
| 9 | 8/8/02 | Barcelona | 1 | Su,T,SxT | |
| 10 | 8/20/02 | Tarragona | 23 | ||
| 11 | 8/22/02 | Alicante | 16 | S,T,Nx | Asn 87 (64/0.09) |
| 12 | 7/8/02 | Oviedo | 1 | S,Su,T | |
| 13 | 9/1/02 | Zamora | 1 | S,Su,T | |
| 14 | 9/30/02 | Huesca | 16 | T | |
| 15 | 11/28/02 | Ciudad Real | 23 | S,Su,T | |
| 16 | 11/5/02 | Caceres | 5 | A,T | |
| Food origin (23 strains) | |||||
| Unknown | 3/22/02 | Barcelona | 18 | S,Su,T,SxT | |
| Seafood | 5/1/00 | Salamanca | 14 | A,Su,T,SxT | |
| Sausage | 5/2/00 | Bilbao | 1 | Su,T | |
| Pork | 6/11/00 | Cádiz | 7 | T | |
| Pork | 6/16/00 | Granada | 23 | Nx | Phe 83 (>256/0.09) |
| Pork | 7/10/00 | Zaragoza | 1 | S,Su,T | |
| Sausage | 6/16/00 | Navarra | 1 | S,Su,T | |
| Lamb | 1/10/01 | Málaga | 1 | A,T | |
| Minced meat | 4/2/01 | Toledo | 12 | S,T | |
| Unknown | 7/4/01 | Valencia | 12 | S,T | |
| Sausage | 7/2/01 | Granada | 1 | S,Su,T | |
| Minced meat | 10/5/00 | Toledo | 13 | T | |
| Unknown | 7/18/00 | Vitoria | NT | A | |
| Corn | 12/31/01 | Bilbao | NT | ||
| Hamburger | 4/16/02 | Córdoba | 1 | Nx | Phe 83 (>256/0.1) |
| Pork | 4/19/02 | Jaén | 1 | S,Su,T | |
| Sausage | 5/28/02 | Soria | 1 | S,Su,T | |
| Pork | 6/14/02 | Oviedo | 1 | S,Su,T | |
| Pork | 6/19/02 | Oviedo | 1 | S,Su,T | |
| Pork | 9/10/02 | Madrid | 1 | T | |
| Unknown | 9/26/02 | Burgos | 1 | Su,T,SxT | |
| Sausage | 10/29/02 | Barcelona | 24 | T | |
| Pork | 9/1/02 | Lugo | 20 | T | |
| Environmental origin (2 strains) | |||||
| Groundwater | 9/3/01 | Valencia | 12 | S,T | |
| Beach | 2/29/02 | Bilbao | 1 | Su,T,SxT | |
| Ill animal origin (6 strains) | |||||
| – | – | Soria | 14 | A,T | |
| Swine | 5/22/02 | Toledo | 1 | S,SuT | |
| Swine | 3/7/02 | León | 6 | S,T | |
| Swine | 3/7/02 | León | 6 | S,T | |
| Swine | 3/6/02 | León | 6 | S,T | |
| Swine | 1/22/02 | León | 9 | Su,T,SxT | |
*PFGE, pulsed-field gel electrophoresis; Nx, nalidixic acid; Cp, ciprofloxacin; T, tetracycline; S, streptomycin; Su, sulfamethoxazole; NT, not typeable; A, ampicillin; C, chloramphenicol; G, gentamicin; To, tobramycin; Ap, apramycin; SxT, cotrimoxazole. †MIC for Nx/Cp, μg/mL.
Table 2. Salmonella enterica* Derby strains (N = 63) from the swine group.
| Swine operations/ finishing units | No. of PFGE profiles/resistance patterns | No. of PFGE profiles (no. of swine) | Resistance phenotype (no. of swine) |
|---|---|---|---|
| A-1 (6 strains) | 1/2 | 1 (5) | S,G,To,Su,T,C (1) |
| A,S,G,To,Nt,Su,T,Nx,C (4)† | |||
| A-2 (3 strains) | 1/1 | 1 (3) | A,S,G,Su,T,C (3) |
| B-3 (16 strains) | 2/7 | 1 (4) | (1)‡ |
| S,Su,T (2) | |||
| S,Su,T,C (1) | |||
| 8 (11) | (1)§ | ||
| (1)‡ | |||
| S,Su,T (8) | |||
| A,S,G,To (1) | |||
| C-4 (1 strain) | 1/1 | 1 (1) | S,Su,T (1) |
| D-5 (32 strains) | 3/3 | 1 (17) | S,Su,T (17) |
| 2 (2) | S,Su,T (2) | ||
| 21 (1) | A,S,Su,T,SxT (1) | ||
| D-6 (5 strains) | 2/4 | 22 (2) | A,S,G,T,Ap,Su,SxT,C (1) |
| A,S,G,To,Ak,Su,T,SxT,C (1) | |||
| 15 (2) | T (1) | ||
| A,S,G,To,T (1) |
*Identified in 48 swine slaughtered at 4 swine operations (A–D) and 6 finishing units (1-6) in Spain. PFGE, pulsed-field gel electrophoresis; S, streptomycin; G, gentamicin; To, tobramycin; Su, sulfamethoxazole; T, tetracycline; C, chloramphenicol; A, ampicillin; Nt, netilmicin; SxT, cotrimoxazole. †GyrA mutation (MIC nalidixic acid/ciprofloxacin): Hys 87 = 48/0.09 μg/mL. ‡Susceptible to all antimicrobial agents tested except spectinomycin. §Susceptible to spectinomycin.
The isolates were screened for antimicrobial resistance by the disk diffusion method (Clinical and Laboratory Standards Institute, Wayne, PA, USA). All isolates were susceptible to amoxicillin-clavulanate, cephalothin, cefuroxime, cefotaxime, ceftriaxone, cefoxitin, imipenem, ciprofloxacin, and enrofloxacin. All strains but 1 were resistant to spectinomycin and had variable resistances to the remaining antimicrobial agents tested (Tables 1 and 2).
A total of 70% and 85.7% of the isolates in the LNRSSE and swine groups were resistant to tetracycline, 36.6% and 85.7% to streptomycin, 33.3% and 71.4% to sulfonamides, 23.3% and 50% to ampicillin and ticarcillin, 16.6% and 42.8% to cotrimoxazole, and 13.3% and 7.1% to nalidixic acid, respectively (Tables 1 and 2). Phenotypes in both groups were most commonly resistant to tetracycline and sulfamethoxazole/tetracycline. Multiple drug resistance (resistance to ≥4 drugs), was observed in 23.3% of LNRSSE isolates and 64.3% of swine isolates.
In addition, the MICs for nalidixic acid and ciprofloxacin were determined in 7 nalidixic acid–resistant strains by the E test (AB Biodisk Dalvagen, Solma, Sweden). The results of these tests are shown in Tables 1 and 2. GyrA, GyrB, ParC, and ParE point mutations in these isolates were genetically characterized (11). The results showed amino acid substitutions only in GyrA: Ser83→ Phe or Tyr and Asp87 → Asn or His (GenBank accession no. AY858891) (Tables 1 and 2).
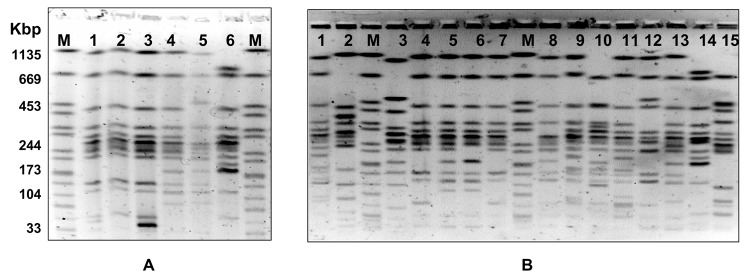
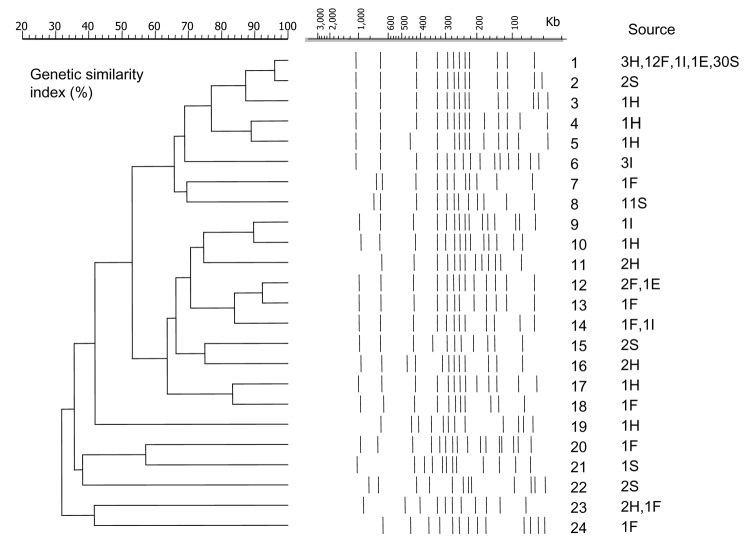
To define clusters, PFGE was carried out according to the Salm-gene protocol (12) by using the CHEF-DR II System (Bio-Rad Laboratories, Hercules, CA, USA). Fingerprints were compared with GelCompar (Applied Maths, Kortrijk, Belgium). Pattern clustering was performed by using the unweighted pair group method with an arithmetic mean and the Dice coefficient (13) with a tolerance of 0.8%. Each PFGE profile or clone, which differed by at least 1 band from a previously recognized type, was considered a distinct profile. Twenty-four PFGE profiles were observed among the 110 strains (Figure 1). A total of 10, 9, 2, 4, and 6 clones were detected in strains from human, food, environmental, sick animal, and slaughtered swine sources, respectively (Tables 1 and 2). Twelve clones displayed closely related profiles, and paired PFGE profiles differed from each other by 1 band (1-2, 2-3, 4-5, 9-10, 12-13, and 12-14), 2 bands (1-3 and 13-14), or 3 bands (16-18) (Figure 2).
Figure 1.
Twenty-one pulsed-field gel electrophoresis profiles identified in Derby strains of Salmonella enterica. A) profiles 3, 1, 2, 4, 5, and 8 (lanes 1–6). B) profiles 20, 21, 23, 14, 12, 14, 13, 14, 15, 9, 11, 10, 16, 17, 7, and 19 (lanes 1–15). The control strain (Braenderup serotype) used as a molecular marker in shown in lane M.
Figure 2.
Twenty-four pulsed-field gel electrophoresis profiles and similarity dendrogram of 110 genomic DNAs of Derby strains of Salmonella enterica isolated from human stools (H), food (F), ill animals (I), environmental sources (E), and slaughtered swine (S). The number refers to the profile number. The number of strains and their corresponding sources are shown. A DNA molecular weight marker derived from the Braenderup serotype was used as a control.
Eleven PFGE profiles (10.0% of the strains) were unique. Conversely, PFGE profile 1 accounted for 52.7% of all isolates studied: 3 from humans, 12 from food, 1 from the environment, 1 from an ill swine, and 42 from 30 slaughtered swine (Tables 1 and 2). PFGE profile 8 represented 10.0% of the characterized strains isolated from 11 swine slaughtered at operation B finishing unit B3. No other PFGE profile accounted for >3% of the strains. In the 63 strains obtained from 48 swine slaughtered, 6 PFGE profiles were observed: 1 (62.5%), 2 (4.2%), 8 (22.9%), 15 (4.2%), 21 (2.8%), and 22 (4.2%).
The corresponding values of genetic similarity ranged from 32% to 96% according to the dendrogram constructed with the different PFGE profiles (Figure 2). Typeability was ≈96.3% because autodigestion occurred in 4 strains. The discriminatory ability calculated by using the Simpson index was 0.9931 for the LNRSSE group, which was selected without a geographic link for any isolates.
Conclusions
The study was conducted because of the S. Derby isolate, detected through Salmonella surveillance programs, emerged on several swine farms in Spain. We investigated the genetic diversity of S. Derby isolates in these herds by using PFGE to obtain information on their genetic backgrounds.
The predominant clone (clone 1) was identified in 42 strains isolated from 30 animals at 4 swine operations and 5 finishing units. It appeared as a single clone in 3 swine operation finishing units (A-1, A-2, and C-4), or in combination with other PFGE profiles (8 for B-3; 2 and 21 for D-5).
When the persistence of a clone was detected, we tracked its spread from food products to humans by testing S. Derby strains isolated from humans, food, the environment, and ill animals in different areas of Spain. Clone 1 was identified in 18.7% of human strains, 52.2% of food strains, as well as in a strain collected on a beach and in another from an ill animal. Moreover, a closely related clone (clone 2) was detected with clone 1 in the swine operation finishing unit D4. This finding might be the result of these clones sharing a common ancestor. The dissemination of clone 1 may be more widespread because LNRSSE group isolates were selected on the basis of no epidemiologic links and were not associated with national or local food outbreaks.
Because resistance of Salmonella to antimicrobial agents is a worldwide problem, drug susceptibility was also examined. Resistance to amoxicillin-clavulanate, cephalosporins, imipenem, and fluoroquinolones was not detected. However, resistance to tetracycline was high (≈75%), which is similar to that recorded in a study of 304 S. Derby swine isolates (9), but differs from that obtained in human and food isolates (49%) (14). In addition, susceptible isolates from the swine group showed increased resistance to other drugs when compared with those in the LNRSSE group. No relationship was observed between the time of isolation of a specific clone and increased resistance.
A novel GyrA mutation (Hys 87) was identified in 4 swine strains of clone 1 that showed resistance to nalidixic acid and low-level resistance to ciprofloxacin. These strains, which also showed resistance to other antimicrobial agents (ampicillin, streptomycin, gentamicin, tobramycin, netilmicin, tetracycline, and sulfamethoxazole), may be attributed to multiple resistance factors present on transmissible genetic elements (15).
In summary, this study detected a clone of S. Derby in pork-derived products and subsequently in humans. Our results emphasize the need for safe food-handling practices to limit the intake of raw or undercooked pork, and procedures to avoid colonization of swine herds with Salmonella. They also support establishing guidelines to reduce foodborne pathogens on swine farms and in slaughterhouses. Moreover, because of the emergence of bacterial resistance to antimicrobial agents as a result of their extensive use in animal husbandry, measures such as modification of doses of antimicrobial agents, control of Salmonella infections in primary production facilities, and effective epidemiologic surveillance must be implemented to determine potential routes and spread of infection.
Acknowledgments
We thank the laboratories in Spain for sending strains to LNRSSE.
This work was supported by a grant from the Ministerio de Ciencia y Tecnología (AGL 2002-04161-02-01). Sylvia Valdezate and Ana Vidal were supported by fellowships from Instituto de Salud Carlos III (02/0008) and the Junta de Castilla y León, respectively.
Biography
Dr. Valdezate is a microbiologist in the bacteriology department at the Instituto de Salud Carlos III in Majadahonda, Spain. Her research focuses on the transmission and dynamics of nosocomial and foodborne infectious diseases by using molecular typing methods.
Footnotes
Suggested citation for this article: Valdezate S, Vidal A, Herrera-León S, Pozo J, Rubio P, Usera MA, et al. Salmonella Derby clonal spread from pork. Emerg Infect Dis [serial on the Internet]. 2005 May [date cited]. http://dx.doi.org/10.3201/eid1105.0410142
References
- 1.Gomez TM, Motarjemi Y, Miyagawa S, Kaferstein FK, Stohr K. Foodborne salmonellosis. World Health Stat Q. 1997;50:81–9. [PubMed] [Google Scholar]
- 2.Berends BR, van Knapen F, Mossel DA, Burt SA, Snijders JM. Identification and quantification of risk factors regarding Salmonella spp. on pork carcasses. Int J Food Microbiol. 1997;36:199–206. 10.1016/S0168-1605(97)01267-1 [DOI] [PubMed] [Google Scholar]
- 3.Korsak N, Daube G, Ghafir Y, Chahed A, Jolly S, Vindevogel H. An efficient sampling technique used to detect 4 foodborne pathogens on pork and beef carcasses in nine Belgian abattoirs. J Food Prot. 1998;61:535–41. [DOI] [PubMed] [Google Scholar]
- 4.Mogstad LL. Salmonella spp. in the stomach contents of slaughtered pigs. A possible factor of contamination in the plucks and fresh meat. Dansk Veterinaertidskrift. 1995;78:554–7. [Google Scholar]
- 5.Centers for Disease Control and Prevention. Salmonella surveillance summary 2002. Atlanta: 2003. [Google Scholar]
- 6.Giovannacci I, Oueguiner S, Ragimbeau C, Salvat G, Vendeuvre JL, Carlier V, et al. Tracing of Salmonella spp. in 2 pork slaughter and cutting plants using serotyping and macrorestriction genotyping. J Appl Microbiol. 2001;90:131–47. 10.1046/j.1365-2672.2001.01228.x [DOI] [PubMed] [Google Scholar]
- 7.Watabe M, Rao JR, Stewart TA, Xu J, Millar BC, Xia L, et al. Prevalence of bacterial faecal pathogens in separated and unseparated stored pig slurry. Lett Appl Microbiol. 2003;36:208–12. 10.1046/j.1472-765X.2003.01293.x [DOI] [PubMed] [Google Scholar]
- 8.Maguire HC, Codd AA, Mackay VE, Rowe B, Mitchell E. Outbreak of human salmonellosis traced to a local pig farm. Epidemiol Infect. 1993;110:239–46. 10.1017/S0950268800068151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grebeyenes WA, Davies PR, Morrow WE, Funk JA, Altier C. Antimicrobial resistance of Salmonella isolates from swine. J Clin Microbiol. 2000;38:4633–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Letellier A, Messier S, Pare J, Menard J, Quessy S. Distribution of Salmonella in swine herds in Quebec. Vet Microbiol. 1999;67:299–306. 10.1016/S0378-1135(99)00049-8 [DOI] [PubMed] [Google Scholar]
- 11.Giraud E, Brisabois A, Martel JL, Chaslus-Dancla E. Comparative studies of mutations in animal isolates and experimental in vitro- and in vivo-selected mutants of Salmonella spp. suggest a counterselection of highly fluoroquinolone-resistant strains in the field. Antimicrob Agents Chemother. 1999;43:2131–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peters TM, Maguire C, Threlfall EJ, Fisher IST, Guill N, Gatto AJ. The Salm-gene project - a European collaboration for DNA fingerprinting for food-related salmonellosis. Eurosurveillance. 2003;8:46–50. [DOI] [PubMed] [Google Scholar]
- 13.Nei M, Li WH. Mathematical model for subtyping genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci U S A. 1979;76:5269–73. 10.1073/pnas.76.10.5269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ling JM, Chan EW, Lam AW, Cheng AF. Mutations in topoisomerase genes of fluoroquinolone-resistant salmonellae in Hong Kong. Antimicrob Agents Chemother. 2003;47:3567–73. 10.1128/AAC.47.11.3567-3573.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piddock LJ. Fluoroquinolone resistance in Salmonella serovars isolated from humans and food animals. FEMS Microbiol Rev. 2002;26:3–16. [DOI] [PubMed] [Google Scholar]