Abstract
Four of 391 Ni-Vanuatu women were infected with variants of human T-cell leukemia virus type 1 (HTLV-1) Melanesian subtype C. These strains had env nucleotide sequences ≈99% similar to each other and diverging from the main molecular subtypes of HTLV-1 by 6% to 9%. These strains were likely introduced during ancient human population movements in Melanesia.
Keywords: molecular epidemiology, Human retrovirus, HTLV-1, Melanesia, Vanuatu
Human T-cell leukemia virus type 1 (HTLV-1), a human oncoretrovirus, is the etiologic agent of adult T-cell leukemia and of tropical spastic paraparesis/HTLV-1–associated myelopathy. Molecular epidemiologic studies have shown HTLV-1 proviruses to be remarkably stable genetically. The low levels of genetic drift in this virus have been used as a means for monitoring viral transmission and the movement of ancient human populations (1,2). The few nucleotide substitutions observed in HTLV-1 strains are specific to the geographic origin of the patient and are unrelated to viral pathology (1,2). Four major geographic HTLV-1 subtypes have been described: subtype A, cosmopolitan (1,2); subtype B, central African; subtype C, Melanesian (3–6); and subtype D, present in central Africa, mainly in pygmies.
Previous reports have indicated that HTLV-1 is endemic in some remote or ancient populations in Melanesia (3–14). These populations include a small number of tribes from Papua New Guinea (especially the Hagahai people) (5) and some inhabitants of the Solomon Islands (7). Evidence of HTLV-1 infection has also been found in some aboriginal groups from Australia (8). Rare cases of adult T-cell leukemia and tropical spastic paraparesis/HTLV-1–associated myelopathy have also been described in these populations (9).
Genetic characterization of the few available Melanesian HTLV-1 strains has indicated that these HTLV-1 strains are the most divergent, constituting molecular subtype C (also called Melanesian subtype [3,4, 6,10]) in phylogenetic analyses. The discovery of such divergent variants has increased our understanding of the migration of HTLV-1–infected populations throughout the Pacific region. Furthermore, 1 of the calibration methods frequently used, in phylogenetic analyses, to estimate a time scale for the evolution of HTLV and simian T-cell leukemia virus (STLV) appears to coincide with the first human migrations to Melanesia and Australia 40,000–60,000 years ago (2).
We carried out a large serologic and molecular study to determine the prevalence of HTLV-1 and associated diseases in the Vanuatu Archipelago. Vanuatu, formerly known as the New Hebrides, is a Y-shaped archipelago made up of ≈80 islands. It is located in Melanesia, in the South Pacific region, northeast of Australia and south of the Solomon Islands. Vanuatu has a population of ≈200,000 inhabitants, most of whom (95%) are of Melanesian origin and are known as the Ni-Vanuatu.
ecade ago and were not based on stringent serologic criteria (11–14). No molecular characterization data are available for HTLV-1 from this area. The main goals of this study were to evaluate the situation concerning HTLV-1 infection in a remote Ni-Vanuatu population by using stringent serologic criteria for Western blotting and molecular characterization of the viruses.
The Study
In February 2002, we recruited 391 women during a clinical survey for sexually transmitted diseases in various remote rural communities of western Ambae Island in the Penama Province of the Vanuatu Archipelago. Ambae Island, also known as Aoba, has a population of ≈9,500. The women participating in this survey were offered a complete clinical examination, with Papanicolaou test analysis for all women >25 years of age. For each participant, we obtained plasma and buffy coats from 5 mL of blood obtained by venipuncture. The blood samples were rapidly transferred to Institut Pasteur de Nouvelle-Calédonie, where plasma and buffy coats were isolated, frozen, and stored (at –80°C) until HTLV screening. Informed consent was obtained from each woman participating in the field survey. This study was approved by the Ministry of Health of the Republic of Vanuatu and was supported by the Vanuatu Family Health Association, a local nongovernmental organization. Samples were taken from 391 women (mean age 36 years, range 16–82 years) with the following stratification by age: 11.2% from women 15–24 years of age, 28.4% from women 25–34 years of age, 31.2% from women 35–44 years of age, 17.4% from women 45–54 years of age, and 11.8% from women ≥55 years of age.
Plasma HTLV-1 antibodies were detected by enzyme-linked immunosorbent assay (ELISA) (HTLV-I+II, Abbott-Murex, Kent, United Kingdom) with Western blot (HTLV-I/II Blot 2.4, Diagnostic Biotechnology, Singapore) used for confirmation. On Western blot, plasma samples were considered HTLV-1–positive if they reacted to the 2 Gag proteins (p19 and p24) and both env-encoded glycoproteins: the HTLV-1–specific recombinant gp46-I peptide (MTA-1) and the specific HTLV-1/HTLV-2 recombinant GD 21 protein. Plasma samples were considered negative when no band were shown and indeterminate when partially reactive (15,16).
Forty-nine of the 391 plasma samples studied tested positive or borderline by ELISA, and 4 of these samples displayed full reactivity on Western blot (Figure 1). One sample also displayed a typical HTLV gag-indeterminate profile (16), and 6 displayed weak reactivity (19 or GD 21 bands). The 4 plasma samples testing positive by Western blot had higher immunofluorescence assay titers on MT2 (HTLV-1) cells than on C19 (HTLV-2) cells and high particle agglutination titers (Table 1). We carried out a second serologic survey on 64 members of the families of the 4 women seropositive for HTLV-1. This survey identified 2 more infected women; 1 was the mother of an index patient, and the other was the sister-in-law of another index patient (Table 1). These results confirm the circulation of HTLV-1 in this population.
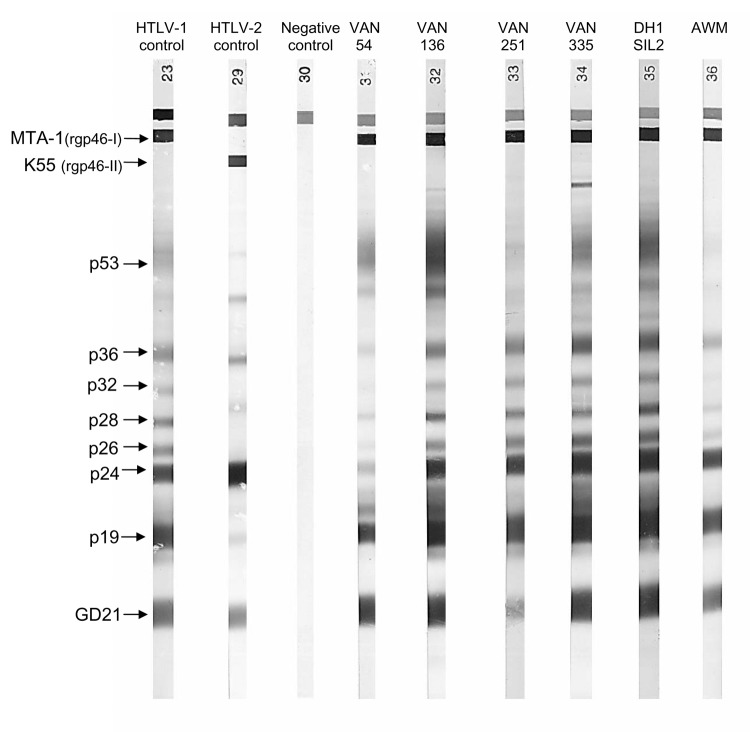
Figure 1.
Representative seroreactivity pattern on Western blot that contains a recombinant GD 21 (common to human T-cell leukemia virus type 1 [HTLV-1] and HTLV-2) and 2 synthetic peptides specific for HTLV-1 (MTA-1) and HTLV-2 (K55). Lane 1, HTLV-1–positive control; lane 2, HTLV-2–positive control; lane 3, HTLV-1/2 negative control; lane 4–9, 6 plasma samples from the HTLV-1–positive women of Ambae Island displaying strong reactivity to GD 21 (faint band for VAN 251), to p19 and p24 (faint band for VAN 54), p26, p28, p32, p36 (faint bands for VAN 54 and AWM), and to MTA-1.
Table 1. Human T-cell leukemia virus type 1 (HTLV-1) antibody titers and molecular screening results for HTLV-1–seropositive women from Ambae Island, Vanuatu Archipelago*.
| Virus strain | Age (y) | PA titers | IFA titers |
WB pattern | PCR |
|||
|---|---|---|---|---|---|---|---|---|
| MT 2 | C 19 | 3´ LTR | 5´ LTR | env | ||||
| VAN 54 | 45 | 1/2,048 | 1/320 | 1/80 | HTLV-I | + | + | + |
| VAN 136 | 36 | 1/8,192 | 1/1,280 | 1/320 | HTLV-I | + | + | + |
| VAN 251 | 42 | 1/1,024 | 1/40 | <1/20 | HTLV-I | + | + | + |
| VAN 335 | 42 | 1/4,096 | 1/1,280 | 1/160 | HTLV-I | + | + | + |
| DH1SIL2 (sister-in-law of VAN 335) | 56 | 1/8,192 | 1/2,560 | 1/320 | HTLV-I | NA | NA | NA |
| AWM (mother of VAN 54) | 63 | 1/1,024 | 1/160 | 1/40 | HTLV-I | NA | NA | NA |
*PA, particle agglutination; IFA, immunofluorescence assay; WB, Western blot; PCR, polymerase chain reaction; LTR, long terminal repeat; NA, DNA not available.
High molecular-weight DNA was extracted from buffy coats from the 4 HTLV-1–seropositive women, 5 HTLV-1–seronegative persons, and 6 others with indeterminate Western blot results, by using the QIAamp DNA Blood Mini Kit (Qiagen GmbH, Hilden, Germany). The 15 DNA samples studied were subjected to polymerase chain reaction with primers specific for the human β-globin gene to check that cellular DNA was amplifiable for all samples (17). We then subjected DNA samples to 2 series of polymerase chain reaction to obtain the complete long terminal repeat (LTR) (755 bp) and a 522-bp region of the env gene as previously described (18). Fragments of the appropriate size were amplified for the 4 HTLV-1–seropositive women, whereas the other 11 samples yielded negative results. The amplified products were cloned and sequenced, and phylogenetic studies were performed as previously described (18). Both the complete LTR and the 522-bp env fragment were obtained for the 4 HTLV-1–seropositive women.
Conclusions
The gp21 gene sequences of the 4 HTLV-1 strains involved were almost identical (99.6%–99.8 % nucleotide similarity) and were very similar to those of Melanesian strains. These strains were closely related (99.4%) to certain strains from Solomon Islanders (Mel 4, 8) but were only 97.1%–98.3% similar to strains from Papua New Guinea residents (Mel 2, 7) and from Australian aborigines (MSHR-1), respectively. Finally, the sequences of these new strains diverged from those of HTLV-1 strains from the 3 other main molecular subtypes (A, B, D) by 6% to 9%.
The 4 new HTLV-1 LTR sequences were also very closely related (98%–100% nucleotide similarity). They displayed 2% nucleotide divergence from Mel 5 (from a Solomon Islander), the only available LTR from all the HTLV-1 subtype C strains. However, they also displayed up to 11% nucleotide divergence from HTLV-1 strains from other molecular subtypes.
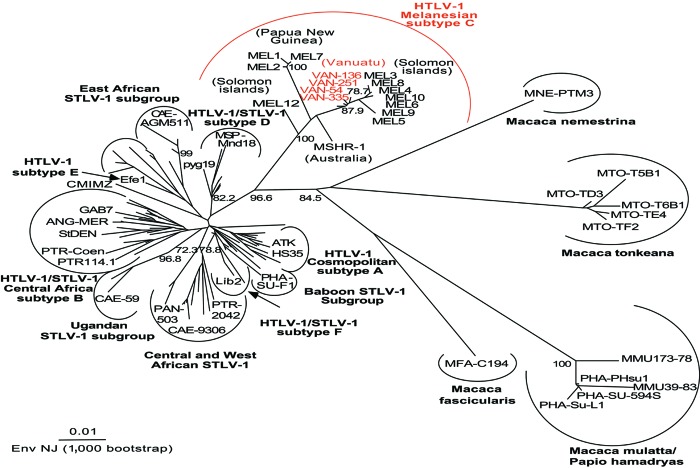
Phylogenetic analyses were performed on all the available env and LTR HTLV-1 sequences from Melanesia, and on several representatives of HTLV-1 and STLV-1 strains from the various subtypes/subgroups as described (18), by the neighbor-joining (NJ) method. Similar tree topologies were obtained for both genomic regions (Figure 2 and Figure A1). Analyses of these trees confirmed that the 4 novel Vanuatu HTLV-1 strains were closely related to all available HTLV-1 subtype C strains (Table 2). Indeed, in the env analysis, which included 71 HTLV-1 strains (including 12 Melanesian strains and 1 from an Australian aborigine, Table 2) and 55 STLV-1 strains, the 4 new HTLV-1 strains clustered with subtype C (Figure 2). This subtype only includes strains from Australia, Papua New Guinea, the Solomon Islands, and Vanuatu. Within this clade are at least 2 subgroups, strongly supported phylogenetically: 1 comprises the Vanuatu strains and most of the strains from the Solomon Islands (bootstrap values of 88%), and the other comprises the 3 isolates from Papua New Guinea (the Hagahai population), with a bootstrap value of 100%. Two other unique and divergent strains, the only strain available from an Australian aborigine (MSHR-1) and the other from a Solomon Islander (Mel-12), may represent prototypes of 2 other clades within the Melanesian subtype C.
Figure 2.
Unrooted phylogenetic tree generated by the neighbor-joining method by using the 522-bp fragment of the env gene. Distance matrices were generated with the DNADIST program, using the Kimura 2-parameter method and 5.65 as the transition/transversion ratio. Bootstrap analysis was carried out with 1,000 datasets. The values on the branches indicate frequencies of occurrence for 1,000 trees. The 4 new human T-cell leukemia virus type 1 (HTLV-1) sequences (VAN 54, VAN 136, VAN 251, VAN 335; GenBank accession nos. AY549879, AY549880, AY549881, AY549882) were analyzed with 126 HTLV-1/simian T-cell leukemia virus type 1 (STLV-1) sequences available from the GenBank database. The branch lengths are proportional to the evolutionary distance between the taxa.
Table 2. Epidemiologic data and GenBank accession numbers of the human T-cell leukemia virus type 1 (HTLV-1) strains of the Melanesian subtype C.
| Country of origin | Age (y) | Sex | Birth | Residence | Clinical status | Virus name | env GenBank accession no. | LTR GenBank accession no. |
|---|---|---|---|---|---|---|---|---|
| Vanuatu | 45 | F | Ambae | Filakalaka | AC | HTLV-1 VAN 54 | AY549879 | AY549875 |
| 36 | F | Ambae | Ndui Ndui | AC | HTLV-1 VAN 136 | AY549880 | AY549876 | |
| 42 | F | Ambae | Vinangwangwe | AC | HTLV-1 VAN 251 | AY549881 | AY549877 | |
| 42 | F | Ambae | Lolobinanungwa | AC | HTLV-1 VAN 335 | AY549882 | AY549878 | |
| Papua New Guinea | 21 | M | Madang | Madang | AC | HTLV-1 MEL 1 | L02533 | NA |
| 60 | F | Madang | Madang | AC | HTLV-1 MEL 2 | M94197 | NA | |
| 31 | M | Madang | Madang | AC | HTLV-1 MEL 7 | U11576 | NA | |
| Solomon Islands | 39 | F | New Georgia | Guadalcanal | AC | HTLV-1 MEL 3 | M94198 | NA |
| 60 | F | Guadalcanal | Guadalcanal | AC | HTLV-1 MEL 4 | M94199 | NA | |
| 58 | M | Guadalcanal | Guadalcanal | AC | HTLV-1 MEL 5 | M94200 | L02534 | |
| 38 | M | Guadalcanal | Guadalcanal | TSP/HAM | HTLV-1 MEL 6 | M93099 | NA | |
| 49 | M | New Georgia | Guadalcanal | AC | HTLV-1 MEL 8 | U11578 | NA | |
| 75 | M | Rendova | Guadalcanal | AC | HTLV-1 MEL 9 | U11580 | NA | |
| 13 | F | Guadalcanal | Guadalcanal | AC | HTLV-1 MEL 10 | U11566 | NA | |
| 42 | F | Guadalcanal | Guadalcanal | AC | HTLV-1 MEL 11 | U11568 | NA | |
| 60 | F | Guadalcanal | Guadalcanal | AC | HTLV-1 MEL 12 | U11570 | NA | |
| Australia | NA | NA | NA | NA | AC | HTLV-1 MSHR-1 | M92818 | NA |
*LTR, long terminal repeat; F, female; M, male; AC, Asymptomatic carrier; TSP/HAM, tropical spastic paraparesis/HTLV-1–associated myelopathy; NA, not available.
In conclusion, we report, for the first time, the presence of HTLV-1 infection in a Ni-Vanuatu population living in remote villages. We also demonstrate that the viruses infecting these Ni-Vanuatu persons are novel HTLV-1 molecular variants belonging to the Melanesian divergent C subtype. This finding suggests that these viruses were introduced into Vanuatu by ancient migrations of Melanesian populations. The first people to reach Santa Cruz, Banks, Vanuatu, and the Loyalties Islands ≈3,600 years ago seem to have been Austronesian speakers (19). Epidemiologic and clinical surveys are under way in this area to determine the extent of such retroviral infection and associated neurologic and hematologic diseases. In addition, studies of viral and mitochondrial/nuclear DNA are being conducted and should provide insight into the migrations of the first settlers and the origin, evolution, and modes of dissemination of such retroviruses.
Acknowledgments
We thank Myriam Abel, Maturine Tary, Rose Bahor, Yvanna Taga, and Rachel Wells for their continual support and interest in this work; Blandine Boulekone, Hélène Walter, and Woreka Mera for field work; Sylviane Bassot, Françoise Charavay, and Fréderic Touzain for excellent assistance during serologic testing of the samples; and Renaud Mahieux for critically reviewing this manuscript.
This study received financial support from the Institut Pasteur, the Institut Pasteur de Nouvelle-Calédonie, and the Regional Office for the Western Pacific of the World Health Organization (WHO-WPRO). Laurent Meertens was supported by a fellowship from the Caisse Nationale d'Assurance Maladie (CANAM) and the Pasteur-Weizmann Foundation.
Biography
Mr. Cassar is a PhD student whose primary research interests are the clinical and molecular epidemiology and physiopathology of dengue viruses. He is currently working on the epidemiology of HTLV-1 in Melanesian populations.
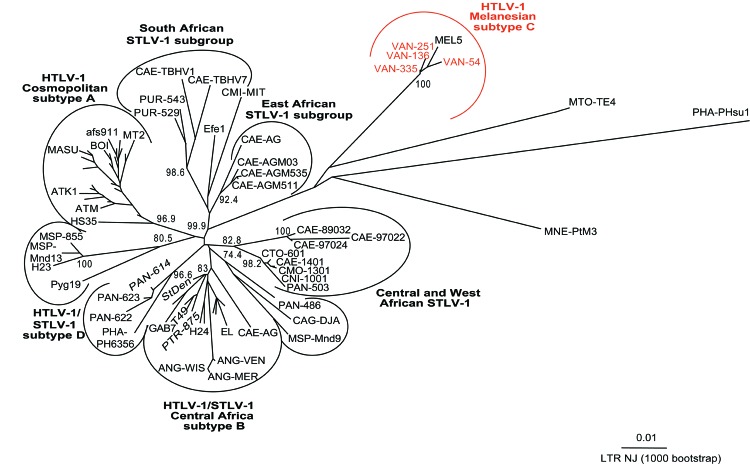
Figure A1.
Unrooted phylogenetic tree generated by the neighbor-joining method by using the complete fragment of the long terminal repeat (LTR) (755 bp). Distance matrices were generated with the DNADIST program, using the Kimura 2-parameter method and 5.17 as the transition/transversion ratio. Bootstrap analysis was carried out with 1,000 datasets. The values on the branches indicate frequencies of occurrence for 1,000 trees. The 4 new human T-cell leukemia virus type 1 (HTLV-1) sequences (VAN 54, VAN 136, VAN 251, VAN 335; GenBank Accession nos. AY549875, AY549876, AY549877, AY549878) were analyzed with 75 HTLV-1/STLV-1 sequences available from the GenBank database. The branch lengths are proportional to the evolutionary distance between the taxa.
Footnotes
Suggested citation for this article: Cassar O, Capuano C, Meertens L, Chungue E, Gessain A. Human T-cell leukemia virus type 1 molecular variants, Vanuatu, Melanesia. Emerg Infect Dis [serial on the Internet]. 2005 May [date cited]. http://dx.doi.org/10.3201/eid1105.041015
References
- 1.Gessain A, Gallo RC, Franchini G. Low degree of human T-cell leukemia/lymphoma virus type I genetic drift in vivo as a means of monitoring viral transmission and movement of ancient human populations. J Virol. 1992;66:2288–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slattery JP, Franchini G, Gessain A. Genomic evolution, patterns of global dissemination, and interspecies transmission of human and simian T-cell leukemia/lymphotropic viruses. Genome Res. 1999;9:525–40. [PubMed] [Google Scholar]
- 3.Gessain A, Boeri E, Yanagihara R, Gallo RC, Franchini G. Complete nucleotide sequence of a highly divergent human T-cell leukemia (lymphotropic) virus type I (HTLV-I) variant from Melanesia: genetic and phylogenetic relationship to HTLV-I strains from other geographical regions. J Virol. 1993;67:1015–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gessain A, Yanagihara R, Franchini G, Garruto RM, Jenkins CL, Ajdukiewicz AB, et al. Highly divergent molecular variants of human T-lymphotropic virus type I from isolated populations in Papua New Guinea and the Solomon Islands. Proc Natl Acad Sci U S A. 1991;88:7694–8. 10.1073/pnas.88.17.7694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saksena NK, Sherman MP, Yanagihara R, Dube DK, Poiesz BJ. LTR sequence and phylogenetic analyses of a newly discovered variant of HTLV-I isolated from the Hagahai of Papua New Guinea. Virology. 1992;189:1–9. 10.1016/0042-6822(92)90675-F [DOI] [PubMed] [Google Scholar]
- 6.Yanagihara R. Geographic-specific genotypes or topotypes of human T-cell lymphotropic virus type I as markers for early and recent migrations of human populations. Adv Virus Res. 1994;43:147–86. 10.1016/S0065-3527(08)60048-2 [DOI] [PubMed] [Google Scholar]
- 7.Yanagihara R, Ajdukiewicz AB, Garruto RM, Sharlow ER, Wu XY, Alemaena O, et al. Human T-lymphotropic virus type I infection in the Solomon Islands. Am J Trop Med Hyg. 1991;44:122–30. [DOI] [PubMed] [Google Scholar]
- 8.Bastian I, Gardner J, Webb D, Gardner I. Isolation of a human T-lymphotropic virus type I strain from Australian aboriginals. J Virol. 1993;67:843–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seaton RA, Wembri JP, Nwokolo NC. Clinical associations with human T-cell lymphotropic virus type-I in Papua New Guinea. Med J Aust. 1996;165:403–6. [PubMed] [Google Scholar]
- 10.Nerurkar VR, Song KJ, Bastian IB, Garin B, Franchini G, Yanagihara R. Genotyping of human T cell lymphotropic virus type I using Australo-Melanesian topotype-specific oligonucleotide primer-based polymerase chain reaction: insights into viral evolution and dissemination. J Infect Dis. 1994;170:1353–60. 10.1093/infdis/170.6.1353 [DOI] [PubMed] [Google Scholar]
- 11.Asher DM, Goudsmit J, Pomeroy KL, Garruto RM, Bakker M, Ono SG, et al. Antibodies to HTLV-I in populations of the southwestern Pacific. J Med Virol. 1988;26:339–51. 10.1002/jmv.1890260402 [DOI] [PubMed] [Google Scholar]
- 12.Brindle RJ, Eglin RP, Parsons AJ, Hill AV, Selkon JB. HTLV-1, HIV-1, hepatitis B and hepatitis delta in the Pacific and South-East Asia: a serological survey. Epidemiol Infect. 1988;100:153–6. 10.1017/S095026880006564X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nicholson SR, Efandis T, Dimitrakakis M, Karopoulos A, Lee H, Gust ID. HTLV-I infection in selected populations in Australia and the western Pacific region. Med J Aust. 1992;156:878–80. [PubMed] [Google Scholar]
- 14.Zhao LG, Yanagihara R, Mora C, Garruto RM, Wong TW, Gajdusek DC. Prevalence of human T-cell lymphotropic virus type I infection in Singapore: a preliminary report. Asia Pac J Public Health. 1991;5:236–8. 10.1177/101053959100500308 [DOI] [PubMed] [Google Scholar]
- 15.Gessain A, Mahieux R, De Thé G. HTLV-I "indeterminate" Western blot patterns observed in sera from tropical regions: the situation revisited. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;9:316–9. [PubMed] [Google Scholar]
- 16.Mauclere P, Le Hesran JY, Mahieux R, Salla R, Mfoupouendoun J, Abada ET, et al. Demographic, ethnic, and geographic differences between human T cell lymphotropic virus (HTLV) type I-seropositive carriers and persons with HTLV-I Gag-indeterminate Western blots in Central Africa. J Infect Dis. 1997;176:505–40. 10.1086/514071 [DOI] [PubMed] [Google Scholar]
- 17.Mahieux R, Horal P, Mauclere P, Mercereau-Puijalon O, Guillotte M, Meertens L, et al. Human T-cell lymphotropic virus type 1 gag indeterminate Western blot patterns in Central Africa: relationship to Plasmodium falciparum infection. J Clin Microbiol. 2000;38:4049–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meertens L, Rigoulet J, Mauclere P, Van Beveren M, Chen GM, Diop O, et al. Molecular and phylogenetic analyses of 16 novel simian T cell leukemia virus type 1 from Africa: close relationship of STLV-1 from Allenopithecus nigroviridis to HTLV-1 subtype B strains. Virology. 2001;287:275–85. 10.1006/viro.2001.1018 [DOI] [PubMed] [Google Scholar]
- 19.Cavalli-Sforza LL, Menozzi P, Piazza A. Australia, New Guinea, and the Pacific Islands. In: The history and geography of human genes. Princeton (NJ): Princeton University Press; 1994. p. 343–71. [Google Scholar]