Abstract
Cocaine blocks uptake by neuronal plasma membrane transporters for dopamine (DAT), serotonin (SERT), and norepinephrine (NET). Cocaine reward/reinforcement has been linked to actions at DAT or to blockade of SERT. However, knockouts of neither DAT, SERT, or NET reduce cocaine reward/reinforcement, leaving substantial uncertainty about cocaine's molecular mechanisms for reward. Conceivably, the molecular bases of cocaine reward might display sufficient redundancy that either DAT or SERT might be able to mediate cocaine reward in the other's absence. To test this hypothesis, we examined double knockout mice with deletions of one or both copies of both the DAT and SERT genes. These mice display viability, weight gain, histologic features, neurochemical parameters, and baseline behavioral features that allow tests of cocaine influences. Mice with even a single wild-type DAT gene copy and no SERT copies retain cocaine reward/reinforcement, as measured by conditioned place-preference testing. However, mice with no DAT and either no or one SERT gene copy display no preference for places where they have previously received cocaine. The serotonin dependence of cocaine reward in DAT knockout mice is thus confirmed by the elimination of cocaine place preference in DAT/SERT double knockout mice. These results provide insights into the brain molecular targets necessary for cocaine reward in knockout mice that develop in their absence and suggest novel strategies for anticocaine medication development.
Cocaine is a reinforcing/rewarding abused drug that confers substantial morbidity and mortality (1). No present medication robustly blocks cocaine reward/reinforcement or substantially relieves cocaine dependence (2). Cocaine's potent actions in blocking uptake by neuronal plasma membrane transporters for dopamine (DAT), serotonin (SERT), and norepinephrine (NET) are well known (3). However, the relationships between these molecular actions and cocaine reward/reinforcement have remained obscure (4). Improved understanding of the molecular underpinnings of cocaine reward should increase understanding of brain reward mechanisms and provide better opportunities for rational design of effective anticocaine medications.
Most molecular explanations for cocaine reward/reinforcement have focused on DAT, whereas some implicate SERT. Support for DAT as a primary site for cocaine reward/reinforcement has come from structure–activity studies of DAT blockers (3, 5), effects of dopamine lesions on cocaine reward (6–8), and findings of enhanced dopamine release after cocaine administration (6). However, recent data demonstrate intact cocaine reward in each of two strains of DAT knockout mice (6, 9, 10). DAT is thus not absolutely required for cocaine reward in mice that develop in its absence. Several compounds that potently inhibit dopamine uptake, drugs that include mazindol, display only limited abuse liability in humans or animal models (11–13). Mazindol potently inhibits DAT and NET but only weakly inhibits SERT; these differences from cocaine could conceivably contribute to the distinct reward profile of mazindol (14–16). Contributions of SERT blockade to cocaine reward/reinforcement have also been suggested. Manipulations of serotonin systems that can modulate cocaine or amphetamine reward (17–21) include 5-HT1B serotonin receptor knockout (22), 5-HT1B agonists or antagonists (20, 22, 23), serotonin depletion (24), 5-HT2 antagonists (25), and 5-HT1A agonists (26). However, the SERT-selective blockers widely used for depression seldom either produce the rewarding responses characteristic of cocaine or enhance cocaine reward (27, 28). Cocaine reward is intact in SERT knockout mice (9). Indeed, the enhanced cocaine reward that can be found in SERT and NET knockout mice (9, 29) could suggest that NET- and SERT-expressing systems might contribute to the aversive features that can be associated with cocaine use (30).
Although no single monoamine transporter is absolutely necessary for cocaine reward in mice that develop in its absence, several possible roles for these transporters in cocaine reward in wild-type mice nevertheless remain (31–33). Cocaine may normally work as a “dirty drug” that produces reward through simultaneous actions at more than one transporter site. For example, DAT/SERT selectivity ratios can provide better correlations with cocaine analog reward/reinforcement than DAT affinity alone (34). Multiple molecular sites for cocaine's rewarding/reinforcing actions could provide such redundancies that no one site was absolutely necessary for cocaine reward. Such redundancies could be enhanced by compensatory mechanisms active in mice that develop with transporter absence. Thus, if cocaine altered activities in several parallel or interactive brain systems with substantial redundancy, the systems expressing the remaining transporter(s) might compensate for loss of cocaine-modulated activities in the systems that normally express the absent transporters, maintaining cocaine reward. To assess whether DAT- and SERT-expressing systems could provide such redundancy in the long-term absence of the other transporter, we have constructed double knockout mice with deletions of one or two copies of both the DAT and SERT genes. We have examined baseline features and then tested cocaine responses, including assessments of cocaine reward/reinforcement. We use cocaine-conditioned place preference as a primary measure of cocaine reward/reinforcement.
Materials and Methods
Subjects.
Animals were bred from the single knockout lines previously reported under American Association of Laboratory Animal Care and National Institutes of Health guidelines (9).
Brain Neurochemistry.
Mice were killed by cervical dislocation; brains were rapidly removed and dissected on ice. Levels of monoamines, receptors, transporters, and mRNAs were assessed in regional brain samples stored at −70°C. Frontal cortex, hippocampus, striatum, brainstem, and hypothalamic samples were analyzed for monoamine neurotransmitters and their metabolites by HPLC using electrochemical detection at +0.3 V with minor modifications, as described (35), by using 10 × 4.6-mm Spherisorb 3-mm ODS-2 reversed-phase chromatography columns (Thomson Instruments, Springfield, VA) and elution with 0.1 M monochloroacetic acid/8% acetonitrile/0.5 g/liter octanesulfonic acid/0.3% triethylamine/10 mM EDTA. Monoamines were quantitated relative to the internal standard, 5-hydroxy-N-methyltryptamine, and protein determined by Lowry's method.
Receptor Binding.
To measure receptor and transporter densities and affinities, washed membranes were prepared from brain regions and incubated with [3H] ligands, and membrane-associated ligands were estimated after rapid filtration over Whatman GF/B filters using a Brandel (Bethesda, MD) apparatus. DAT, SERT, NET, dopamine D2 receptor, and serotonin 5HT1A receptor densities were analyzed by saturation binding of [3H] WIN35428 (36), [3H] paroxetine (37), [3H] nisoxetine (38), [3H] YM-09151–2 (39), and [3H] [8-hydroxy-2-(di-n-propylamino)tetralin] (8-OH DPAT) (40), respectively, using results from parallel incubations with 1 mM unlabeled 0.1 mM cocaine/0.1 mM citalopram/0.1 mM desipramine/10 μM unlabeled sulpiride/10 μM unlabeled serotonin to estimate nonspecific binding, respectively.
5-HT1B Receptor Autoradiography.
Mice were decapitated, the brains rapidly removed and frozen, and the left hemispheres sectioned sagittally (20 μm) at −20°C. Sections were thaw-mounted on gelatin-coated slides and stored at −70°C. Sections were incubated twice for 15 min in 170 mM Tris⋅HCl (pH 7.4) with 150 mM NaCl, dried, incubated for 2 h at room temperature in incubation buffer with 20 pM [125I]-cyanopindolol (CYP) (NEN Life Sciences)/20 μM isoproterenol (Research Biochemicals) to block β-adrenergic receptors, and 10 nM DPAT (Research Biochemicals) to block 5-HT1A receptors, dipped in 4°C 170 mM Tris⋅HCl (pH 7.4), washed twice in 4°C fresh wash buffer, dipped in 4°C distilled water, dried under a stream of air, and apposed to Hyperfilm-3H for 48 h, as described (41). Films were developed, and [125I]-CYP binding was quantified by using nih image Software (National Institutes of Health).
Behavioral Testing.
Behavioral tests were performed as described (9). Locomotor activity was assessed as total distance traveled, calculated from measurement of beam breaks in Optovarimex activity monitors (Columbus Instruments, Columbus, OH), to which the mice had not been previously exposed, under dim light sound-attenuated conditions (9). After 3 h of habituation, mice were injected with cocaine HCl (10 mg/kg s.c.). Reward/reinforcement was assessed by conditioned place preference testing using a two-compartment Plexiglas chamber, one with a wire mesh floor and one with corncob bedding on a smooth Plexiglas floor, as described (9). During conditioning sessions, mice were restricted to single compartments for 20 min after injection with cocaine (10 or 20 mg/kg s.c.) or saline, returned to their home cages for 4 h, and then subjected to another 20-min conditioning trial. Injections were counterbalanced. Cocaine withdrawal has not produced aversive effects in this same apparatus at 4 h after these cocaine doses. A single conditioned place preference assessment session followed the last conditioning session by 24 h. In these sessions, mice had access to both compartments, and the proportion of the 20-min session spent on each side was recorded. Results were compared with the proportion of time spent on that side in preconditioning sessions (9). Statistical comparisons used the Statistical Package for Social Science, t tests, and ANOVAs, followed by Scheffe post hoc analyses. Data are presented as mean ± SEM. Conditioning studies were conducted blind to genotype, often resulting in uneven numbers of mice in each group.
Results
Features of Combined DAT and SERT Knockout Mice.
(i) Viability.
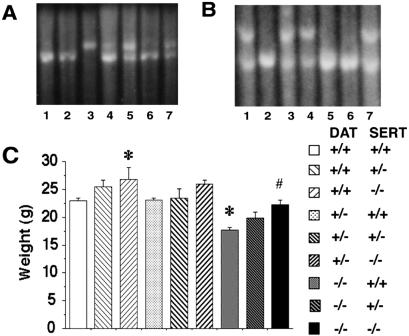
Matings between DAT and SERT knockout mice produce mice with all possible genotypes at the DAT and SERT loci in ratios close to those expected (Fig. 1A) (9, 42, 43). Gross and histologic examinations reveal that both DAT and DAT/SERT knockout mice display reduced pituitary sizes and abnormal central anterior pituitaries with fewer acidophilic cells, as previously reported in DAT knockout mice (44). DAT−/− SERT+/+ mice demonstrate slower and smaller postweaning weight gains, as previously reported (9, 42). These are partially complemented by SERT knockout (Fig. 1B). Eight-week-old DAT−/− homozygous knockouts are larger in the absence of SERT than in the presence of one or two copies of the wild-type SERT gene. No other gross or microscopic pathology in the double knockouts not seen in single knockouts has been identified (D. Huso, I.S., and G.R.U., unpublished data).
Figure 1.
Disruption of the DAT and SERT genes and effects on weights at 2 months. (A) Southern analysis of hybridization of the 3′ SERT genomic probe to KpnI-digested DNA extracted from tail tips of seven mice displaying wild-type (+/+, lane 3), heterozygote (+/−, lanes 4, 5, 7), and homozygote (−/−, lanes 1, 2, 6) patterns. The presence of an 8.1-kb fragment indicates a homozygous mutant genotype, whereas wild-type fragments are 9.6 kb32. (B) Southern analysis of hybridization of the 5′ DAT genomic probe to EcoRI-digested DNA extracted from tail tips of seven mice displaying wild-type (+/+, lane 7), heterozygote (+/−, lanes 2, 3, and 6), and homozygote (−/−, lanes 1, 4, and 5) patterns. The presence of a 5.3-kb fragment indicates the homologous recombinant genotype, whereas wild-type fragments are 6.0 kb9. (C) Weights of mice of each genotype at 8 weeks of age. Mean (± SEM; n, 5–30) values for weights were compared by using ANOVA followed by Scheffe post hoc analyses; *, significantly different from wild type; #, significantly different from DAT−/− SERT+/+; P < 0.05 for each.
(ii) Monoamine transporter and receptor binding.
Densities of DAT and SERT expression are reduced in gene-dose-dependent fashion. DAT+/− mice show similar reductions in DAT expression on either wild-type or knockout SERT−/− backgrounds (Table 1). SERT expression also follows the number of intact copies of the SERT gene on DAT+/+ and on DAT−/− backgrounds. There is thus little evidence for any substantial crosstalk in determining striatal or frontal cortical levels of expression of these two transporters. Receptor autoradiographic analyses also fail to document any striking regional differences in knockout effects on transporter expression (data not shown). Each region assessed follows similar genotype-dependent patterns of expression. We have characterized several of the many dopamine and serotonin receptors that could be assessed in these mice. We have characterized striatal expression of the DRD2 dopamine receptor subtype (45–48) because of its high level of expression in this region and previous reports of reduced striatal expression in DAT−/− mice (42). DRD2 expression was not further reduced in the DAT−/− SERT−/− double knockout mice. Binding to one of the most abundant serotonin receptors, the 5-HT1A, was most reproducible and previously documented in the hippocampus (Table 1). Hippocampal 5-HT1A binding, assessed by using 8-OH DPAT, was reduced in SERT+/− and −/− mice. DAT knockout returned these binding levels toward wild-type values. Half-wild-type levels of DAT exacerbated the hippocampal 5-HT1A-binding changes induced by SERT deletions (Table 1). Autoradiographic analyses of 5HT1B receptor densities revealed statistically significant differences from wild-type values only in ventral medial midbrain in double knockout DAT−/− SERT−/− mice. SERT−/− mice with either a single copy of DAT or DAT−/− mice with a single SERT gene copy displayed smaller 15% reductions in ventral midbrain that did not reach statistical significance (Table 2). No other region sampled displayed significant alterations in 5HT1B-binding levels. NET levels, assessed by using [3H] nisoxetine binding, also provided only a trend toward down-regulation in DAT−/− and SERT−/− mice. Mice with the combined DAT−/− SERT−/− knockouts displayed more than 60% reductions of nisoxetine Bmax values. Indeed, this difference and a trend in ventral midbrain 5HT1B receptor densities are the only changes in the combined DAT−/− SERT−/− knockouts that convincingly distinguish them from the mice that retain a single wild-type copy of one of these genes.
Table 1.
Dopamine and serotonin transporter and receptor alterations in single- and multiple-knockout mice
| Genotype | DAT | SERT | NET | DRD2 | 5-HT1A |
|---|---|---|---|---|---|
| DAT+/+ SERT+/− | 90 ± 16.5 | 56 ± 5.0 | 39 ± 12 | 119 ± 26.8 | 73 ± 9.6 |
| DAT+/+ SERT−/− | 77 ± 13.6 | ND* | 76 ± 18 | 80 ± 10.1 | 81 ± 9.5 |
| DAT+/− SERT+/+ | 47 ± 5.5* | 113 ± 11.4 | 56 ± 19 | 68 ± 13.5 | 104 ± 29.1 |
| DAT+/− SERT+/− | 49 ± 10.5* | 47 ± 0.6* | 85 ± 42 | 73 ± 6.6 | 50 ± 11.2 |
| DAT+/− SERT−/− | 34 ± 4.1* | ND* | 87 ± 42 | 71 ± 11.9 | 38 ± 7.5* |
| DAT−/− SERT+/+ | ND* | 121 ± 19.1 | 70 ± 10 | 50 ± 8.3* | 75 ± 16.4 |
| DAT−/− SERT+/− | ND* | 43 ± 5.2* | 81 ± 13 | 47 ± 9.6* | 119 ± 20.6 |
| DAT−/− SERT−/− | ND* | ND* | 33 ± 12 | 67 ± 8.3 | 74 ± 21 |
DAT, SERT, NET, DRD2, and 5HT1A receptor densities were assessed in brain regions dissected from mice of each genotype: DAT binding by using [3H] CFT, DRD2 binding by using [3H]-YM09151-2, SERT binding by using [3H] paroxetine, NET binding by using [3H] nisoxetine, and 5HT1A binding by using [3H] 8 OH-DPAT. Each Bmax value was normalized to wild-type values. Data were analyzed by one-way ANOVA with Bonferroni post hoc comparisons (
, significantly different from wild-type littermates). ANOVAs revealed significant main effects of group for SERT, DAT, DRD2, and 5-HT1A binding.
Table 2.
Regional 5-HT1B receptor densities in DAT/SERT knockout mice
| DAT+/−/SERT+/− | DAT−/−/SERT+/− | DAT+/−/SERT−/− | DAT−/−/SERT−/− | |
|---|---|---|---|---|
| Frontal cortex—M | 147.3 ± 14.5 | 125.4 ± 17.4 | 89.4 ± 9.1 | 108.7 ± 16.0 |
| Frontal cortex—L | 118.5 ± 8.8 | 129.0 ± 12.6 | 114.9 ± 21.3 | 114.4 ± 16.6 |
| Subiculum—M | 107.1 ± 20.3 | 94.1 ± 14.2 | 108.5 ± 8.6 | 95.2 ± 5.6 |
| Subiculum—L | 105.8 ± 23.0 | 81.7 ± 10.0 | 121.6 ± 12.8 | 96.6 ± 2.4 |
| Striatum—M | 131.9 ± 12.3 | 130.1 ± 19.1 | 105.1 ± 8.8 | 129.2 ± 19.4 |
| Striatum—L | 121.5 ± 20.1 | 145.4 ± 14.3 | 117.3 ± 6.5 | 144.1 ± 21.0 |
| Nucleus accumbens | 111.6 ± 0.9 | 118.2 ± 18.6 | 93.8 ± 13.1 | 107.3 ± 20.0 |
| Lateral globus pallidus | 103.5 ± 8.4 | 96.3 ± 4.5 | 77.1 ± 12.0 | 102.6 ± 9.8 |
| Medial globus pallidus | 88.8 ± 9.2 | 90.9 ± 11.4 | 70.2 ± 9.9 | 99.6 ± 11.5 |
| Ventral pallidum—M | 138.9 ± 22.9 | 121.2 ± 11.4 | 91.1 ± 14.9 | 123.3 ± 14.5 |
| Ventral pallidum—L | 130.4 ± 21.0 | 107.1 ± 11.7 | 93.5 ± 19.5 | 111.7 ± 15.6 |
| Substantia nigra—M | 93.9 ± 13.1 | 85.8 ± 2.9 | 86.9 ± 5.0 | **63.0 ± 3.7 |
| Substantia nigra—L | 82.9 ± 11.7 | 82.2 ± 4.5 | 67.4 ± 18.2 | 79.6 ± 10.3 |
5-HT1B receptor densities were determined by autoradiography. Results are expressed as mean ± SEM (nCi/mg of protein) percentages of the following control group values: frontal cortex—M, 1.00 ± 0.1; frontal cortex—L, 1.0 ± 0.2; subiculum—M, 2.6 ± 0.1; subiculum—L, 2.8 ± 0.3; striatum—M, 1.4 ± 0.1; striatum—L, 1.5 ± 0.3; nucleus accumbens, 2.3 ± 0.1; lateral globus pallidus, 5.0 ± 0.4; medial globus pallidus, 4.1 ± 0.5; ventral pallidum—M, 4.3 ± 0.3; ventral pallidum—L, 4.2 ± 0.4; substantia nigra—M, 6.3 ± 0.3; substantia nigra—L, 7.0 ± 0.4. L, lateral portion of brain region; M, medial portion of brain region. Data were analyzed by one-way ANOVA with Bonferroni post hoc comparisons (
P < 0.01 vs. wild type).
(iii) Monoamine neurochemistry.
We focused neurochemical analyses on the heavily monoamine innervated striatum and frontal cortex. DAT−/− mice display marked alterations in striatal dopamine and its metabolites homovanillic acid (HVA) and 3,4-dihydroxyphenylacetic acid (DOPAC) [Table 3, previously noted (42)]. SERT−/− mice display reduced levels of serotonin and its metabolite 5-HIAA in frontal cortex [Table 3, previously noted (43)]. DAT−/− mice display modest 15–35% reductions in frontal cortical norepinephrine content (Table 3). Adding SERT deletion to DAT knockouts enhances DOPAC alterations (Bonferroni post hoc comparisons; Table 3) but fails to further alter serotonin or norepinephrine in any dramatic fashion.
Table 3.
Monoamine tissue levels in DAT/SERT knockout mice
| Genotype | DA | DOPAC | HVA | 5-HT | 5-HIAA | NE |
|---|---|---|---|---|---|---|
| DAT +/+/SERT+/− | ||||||
| Frontal cortex | 107.0 ± 11.0 | ND | ND | 96.7 ± 6.0 | 93.4 ± 6.0 | 85.3 ± 5.2 |
| Striatum | 96.2 ± 4.1 | 90.7 ± 12.0 | 100.2 ± 5.7 | 112.1 ± 7.8 | 82.5 ± 4.2 | ND |
| Hippocampus | ND | ND | ND | 88.9 ± 4.9 | 79.2 ± 3.5 | 93.3 ± 2.3 |
| Hypothalamus | 78.1 ± 7.0 | 96.8 ± 4.4 | 97.4 ± 9.0 | 107.9 ± 6.1 | 87.6 ± 5.4 | 98.5 ± 7.8 |
| Brain stem | ND | ND | ND | 98.8 ± 8.6 | 88.7 ± 8.9 | 98.8 ± 9.4 |
| DAT +/+/SERT −/− | ||||||
| Frontal cortex | 102.6 ± 11.0 | ND | ND | 34.9 ± 1.6* | 47.6 ± 2.6* | 87.8 ± 3.8 |
| Striatum | 97.7 ± 4.1 | 78.4 ± 4.2 | 91.5 ± 3.5 | 40.5 ± 2.6* | 64.0 ± 3.9* | ND |
| Hippocampus | ND | ND | ND | 28.1 ± 2.0* | 51.6 ± 2.6* | 105.2 ± 7.3 |
| Hypothalamus | 98.9 ± 14.0 | 97.7 ± 9.3 | — | 37.3 ± 4.4* | 49.1 ± 3.0* | 94.5 ± 5.8 |
| Brain stem | ND | ND | ND | 24.1 ± 1.4* | 71.6 ± 2.4* | 107.8 ± 6.5 |
| DAT +/−/SERT +/+ | ||||||
| Frontal cortex | 66.3 ± 5.4 | ND | ND | 105.3 ± 4.7 | 81.9 ± 4.7 | 101.7 ± 3.6 |
| Striatum | 96.8 ± 6.0 | 73.2 ± 7.7 | 93.1 ± 5.5 | 88.0 ± 3.3 | 81.3 ± 4.3 | ND |
| Hippocampus | ND | ND | ND | 91.9 ± 2.4 | 87.1 ± 4.0 | 98.4 ± 2.8 |
| Hypothalamus | 101.8 ± 6.7 | 98.4 ± 9.0 | 108.7 ± 8.3 | 98.9 ± 5.3 | 97.4 ± 4.7 | 92.9 ± 3.8 |
| Brain stem | ND | ND | ND | 100.5 ± 4.8 | 92.8 ± 4.7 | 99.3 ± 4.8 |
| DAT +/−/SERT +/− | ||||||
| Frontal cortex | 89.5 ± 10.0 | ND | ND | 87.8 ± 9.9 | 71.9 ± 8.0 | 87.4 ± 4.9 |
| Striatum | 94.7 ± 2.8 | 105.4 ± 9.5 | 104.1 ± 4.6 | 83.4 ± 7.8 | 75.3 ± 5.2 | ND |
| Hippocampus | ND | ND | ND | 75.2 ± 7.6 | 72.0 ± 2.1 | 86.3 ± 5.8 |
| Hypothalamus | 62.6 ± 5.3* | 67.2 ± 3.1 | 76.4 ± 2.9 | 95.4 ± 8.8 | 76.5 ± 6.9 | 92.0 ± 3.8 |
| Brain stem | ND | ND | ND | 81.8 ± 9.7 | 80.0 ± 8.5 | 84.7 ± 2.1 |
| DAT +/−/SERT −/− | ||||||
| Frontal cortex | 102.9 ± 12.0 | ND | ND | 42.8 ± 1.5* | 39.6 ± 1.2* | 91.3 ± 4.4 |
| Striatum | 93.2 ± 2.0 | 103.3 ± 7.0 | 93.1 ± 2.6 | 28.1 ± 1.7* | 56.0 ± 2.5* | ND |
| Hippocampus | ND | ND | ND | 22.5 ± 1.2* | 46.4 ± 2.5* | 88.3 ± 6.5 |
| Hypothalamus | 62.2 ± 6.2* | 64.6 ± 5.3 | 68.0 ± 4.3* | 18.8 ± 0.6* | 47.6 ± 2.0* | 91.1 ± 4.0 |
| Brain stem | ND | ND | ND | 24.2 ± 1.7* | 59.5 ± 1.8* | 94.4 ± 3.9 |
| DAT −/−/SERT +/+ | ||||||
| Frontal cortex | 56.8 ± 4.1* | ND | ND | 95.9 ± 5.0 | 95.9 ± 5.7 | 82.6 ± 5.0 |
| Striatum | 4.7 ± 0.2* | 80.1 ± 6.7 | 320.3 ± 19.0* | 75.6 ± 4.6 | 102.4 ± 3.9 | ND |
| Hippocampus | ND | ND | ND | 89.7 ± 4.4 | 91.3 ± 2.6 | 92.3 ± 4.1 |
| Hypothalamus | 76.6 ± 7.0 | 124.9 ± 13 | 184.7 ± 8.3* | 107.3 ± 6.0 | 97.7 ± 5.4 | 116.9 ± 5.1 |
| Brain stem | ND | ND | ND | 113.8 ± 5.6 | 113.9 ± 4.4 | 114.0 ± 4.3 |
| DAT −/−/SERT +/− | ||||||
| Frontal cortex | 77.7 ± 4.7 | ND | ND | 101.0 ± 4.2 | 95.1 ± 5.7 | 68.7 ± 2.4 |
| Striatum | 2.7 ± 0.2* | 50.7 ± 5.2* | 274.3 ± 17.0* | 61.6 ± 2.7 | 103.8 ± 4.3 | ND |
| Hippocampus | ND | ND | ND | 88.1 ± 4.8 | 88.9 ± 5.0 | 80.5 ± 2.4 |
| Hypothalamus | 57.2 ± 6.2* | 117.8 ± 16 | 199.2 ± 24.0* | 95.8 ± 8.5 | 105.1 ± 6.4 | 95.8 ± 5.4 |
| Brain stem | ND | ND | ND | 96.0 ± 3.9 | 103.8 ± 5.7 | 92.9 ± 3.1 |
| DAT −/−/SERT −/− | ||||||
| Frontal cortex | 70.8 ± 3.1 | ND | ND | 45.8 ± 4.7* | 53.6 ± 2.5* | 81.0 ± 2.9 |
| Striatum | 1.7 ± 0.19* | 44.7 ± 4.4* | 318.0 ± 25.0* | 25.7 ± 2.9* | 65.7 ± 3.7* | ND |
| Hippocampus | ND | ND | ND | 32.0 ± 4.4* | 43.4 ± 2.9* | 81.7 ± 6.4 |
| Hypothalamus | 53.0 ± 6.2* | 123.9 ± 9.0 | 207.6 ± 21.0* | 32.2 ± 4.1* | 59.7 ± 2.0* | 114.6 ± 3.5 |
| Brain stem | ND | ND | ND | 29.5 ± 3.8* | 67.5 ± 3.2* | 97.6 ± 3.2 |
DA, DOPAC, HVA, 5-HT, 5-HIAA, and NE concentrations in brain regions dissected from DAT (first genotype indicators) and SERT (second genotype indicators) knockout mice. DA, DOPAC, HVA, 5-HT, 5-HIAA, and NE were measured by HPLC—electrochemical detection. Results represent the mean ± SEM (% of wild-type means). Means for wild-type mice are as follows (ng/mg of protein): frontal cortex–DA, 0.4 ± 0.1; 5-HT, 7.8 ± 0.3; 5-HIAA, 2.2 ± 0.1; NE, 4.2 ± 0.2; striatum—DA, 116.7 ± 3.7; DOPAC, 13.6 ± 0.9; HVA, 11.7 ± 0.4; 5-HT, 4.0 ± 0.2; 5-HIAA, 3.0 ± 0.1; hippocampus—5-HT, 7.1 ± 0.2; 5-HIAA, 4.8 ± 0.2; NE, 4.9 ± 0.1; hypothalamus—DA, 5.5 ± 0.3; DOPAC, 1.7 ± 0.1; HVA, 1.7 ± 0.1; 5-HT, 10.1 ± 0.4; 5-HIAA, 5.8 ± 0.3; NE, 15.6 ± 0.5; and brainstem—5-HT, 7.2 ± 0.2; 5-HIAA, 6.2 ± 0.4; NE, 6.8 ± 0.2 ng/mg of protein. ND, not detectable. One-way ANOVA were followed by Bonferroni comparisons (
significantly different from wildtype littermates).
NE, norepinephrine; DA, dopamine.
Behavior of Combined DAT and SERT Knockout Mice.
(i) Baseline behavioral assessments.
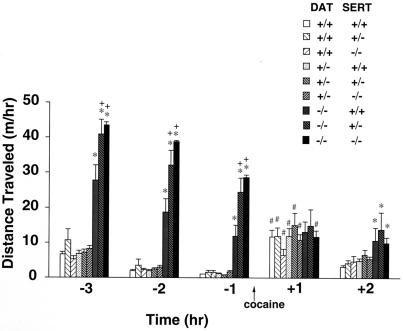
Combined DAT/SERT knockout mice lack gross behavioral deficits not already described for single knockouts. Locomotor activity in a novel environment is elevated in DAT−/− SERT+/+ mice, as previously described (9, 42). DAT−/− SERT−/− mice are even more active (Fig. 2). Both single and combined knockout mice habituate to the novel environment over at least the first 4 of the 5 hours of habituation testing (Fig. 2; see also refs. 9 and 42). Mice of each of these genotypes do not differ significantly from wild-type animals in tests of muscle tone, screen hang time, or ability to stay on a rotating rod. Although SERT−/− mice display enhanced anxiety responses as previously reported, SERT−/− DAT−/− animals display no further enhancements. Double knockout mice can also learn a passive avoidance task as readily as wild-type littermates.
Figure 2.
Locomotion in DAT/SERT double knockout mice during a habituation period and after 10 mg/kg of cocaine. Locomotor activities were recorded for 3-h periods in mice of several genotypes (n, 11–33). Mice were placed into an activity monitor cage, to which they had not been previously exposed, 3 h before injection of cocaine (10 mg/kg s.c.) (time 0, arrow). DAT homozygous knockout mice of any SERT genotype show greater locomotor activity when placed in a novel environment, but no incremental locomotor response to cocaine. *, P < 0.05 vs. wild-type littermate. +, P < 0.05 vs. DAT−/− SERT+/+ littermate; #, P < 0.05 vs. predrug value.
(ii) Cocaine: Locomotor and rewarding effects.
Because screening tests failed to identify double knockout features that would obviously invalidate results of further behavioral tests, mice of each genotype were tested for cocaine influences on locomotion and reward/reinforcement. Cocaine did not enhance locomotor activity in DAT−/− knockout mice habituated to a novel environment for 3 h, as previously reported (Fig. 2) (9, 42). Although evaluation of drug-induced locomotor increases is likely to be less sensitive in mice that exhibit altered baseline locomotion, deletions of either one or two copies of the SERT gene in mice that lacked DAT expression failed to complement or reverse this DAT knockout effect. DAT−/− mice that received cocaine continued to display habituation to the test apparatus and displayed no enhanced locomotor activity whether two, one, or no SERT gene copies were present.
To assess reward/reinforcement, we tested cocaine-conditioned place preference (9). This test provides a technically tractable and robust measure of drug reward in mice (49). It has been validated by its ability to detect the rewarding properties of virtually every class of substance abused by humans. It allows mice to express their drug preference 24 h after the last drug administration, when acute cocaine effects on motor performance are less likely to confound results (7, 8, 50). The failures of DAT−/− or SERT−/− single gene knockouts to decrease this measure of cocaine reward/reinforcement have been previously documented (9).
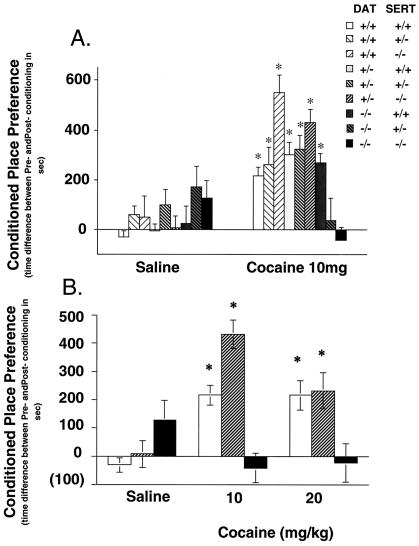
Pairing 10 mg/kg of cocaine with one side of a test apparatus reliably increased the time spent in this drug-paired environment in DAT−/− and in SERT−/− single gene knockout mice. Both of these strains thus show strong cocaine place preferences, as previously reported (9) (Fig. 3A). Even DAT+/− SERT−/− mice that express no SERT and only half of wild-type levels of DAT retain near-wild-type levels preference for cocaine-paired environments. However, neither DAT−/− SERT+/− nor DAT−/− SERT−/− mice exhibited any significant preference for the cocaine-paired environments. Removing all of one cocaine target, DAT, and either 50% or all of another cocaine target, SERT, eliminates cocaine reward. Dose-effect studies provide no clearcut evidence for leftward shifts in cocaine dose–response relationships in double knockouts compared with the DAT−/− mice (Fig. 3B).
Figure 3.
Lack of cocaine-conditioned place preference in DAT knockout mice with no or one copy of the SERT gene. (Upper) Conditioned place preference induced by 10 mg/kg of cocaine in mice of several genotypes (n, 8–56). Time scores shown represent differences between post- and preconditioning time spent in the cocaine-paired environment. DAT knockout mice with no or one copy of SERT displayed no preference for the places associated with 10 mg/kg of cocaine. *, P < 0.05 vs. saline. Average initial preference for the nonpreferred side for all groups was between 37 and 45%. (Lower) Conditioned place preference induced by 10- and 20-mg/kg of cocaine doses in mice of several genotypes (n, 8–56). Time scores shown represent differences between post- and preconditioning time spent in the cocaine-paired environment. Wild-type mice and SERT knockout mice with one copy of DAT displayed significant preferences for the place associated with either 10 or 20 mg/kg of cocaine, but double knockout mice did not have a significant preference for places associated with either dose. *, P < 0.05 vs. saline.
Discussion
The simplest hypotheses that explain the current data are that: (i) cocaine may normally work to provide rewarding actions at both DAT and SERT, and/or (ii) either DAT or SERT can mediate cocaine reward in the lifelong absence of the other transporter. Neurochemical rearrangements in single and double knockout mice demonstrate compensations for lifelong transporter deletion and might even follow long-term DAT or SERT blockade by drugs. However, double knockout mice that lack cocaine reward show few knockout influences on brain neurochemistry not found in the single knockout mice that retain cocaine reward. Retained cocaine reward in mice with as few as one DAT copy in the absence of SERT contrasts with the requirement for two SERT copies in the absence of DAT appears to indicate that fewer intact dopaminergic than serotonergic mechanisms are required for cocaine place preference.
Could knockout mouse data apply to cocaine reinforcement and reward in wild-type mice? The failure of single transporter gene knockouts to eliminate cocaine reinforcement and reward left open several possible roles for DAT and SERT in cocaine reward/reinforcement in wild-type mice (9), including the small possibility that previously known or novel nontransporter effects might mediate cocaine reward/reinforcement. The present results weigh strongly, to our knowledge for the first time, against this idea, and renew focus on transporter-based cocaine mechanisms. The most parsimonious explanation for the current data is that normal redundancy of transporter-related brain reward/reinforcement systems and/or adaptations to chronic deletions of single transporters render chronic ablation of both DAT and SERT necessary to completely block the cocaine reward/reinforcement that can be assessed by conditioned place preferences.
Adaptations to chronic DAT loss could alter cocaine's impact on serotonin systems important for rewarding or aversive properties. We have recently found that the SERT-specific blocker fluoxetine gains rewarding/reinforcing properties in DAT−/− SERT+/+ mice that are never found in wild-type animals (30). Previously available evidence has also fit the idea that removing both DAT and SERT might be required to block cocaine reward/reinforcement. Pharmacological studies support the idea that serotonin could normally interact with dopamine systems in ways important for psychostimulant reward/reinforcement. Agonists and antagonists selective for the serotonin 5-HT1B receptor modulate the reward/reinforcement induced by acute administration of DAT blockers (23, 51–54). Cocaine reinforcement differs between 5-HT1B knockout and wild-type mice (22). Serotonin can potently modulate dopamine levels in brain circuits associated with reward/reinforcement, such as the mesocortical/mesolimbic circuits projecting from the ventral tegmental area to the prefrontal cortex and nucleus accumbens (55–57). Serotonin systems could thus interact with dopamine systems in contributing to cocaine reward/reinforcement.
Cocaine has been reported to lose its ability to elevate dopamine efflux in DAT knockout mice (10). However, analyses of previous data and preliminary microdialysis studies in our mice demonstrate attenuated rather than absent effects (I.S., F.S.H., and G.R.U., unpublished observations). Our mouse strains that display cocaine reward/reinforcement, and those that do not, fail to display clearcut evidence that cocaine actions at SERT sustain cocaine reward/reinforcement in DAT knockouts simply by mimicking the increased dopamine overflow normally found after cocaine administration to wild-type mice.
How could deletion of SERT alone produce trends toward greater cocaine place preference, whereas double deletions of SERT and DAT ablate cocaine reward/reinforcement? These observations could fit with hypotheses that cocaine-elevated serotonin levels stimulate serotonin receptor subtypes in circuits that produce both reinforcing and aversive affects. Differential adaptations in these serotonin circuits could alter the overall balance of cocaine reward and aversive features in different knockout combinations. If blockade of NET and/or SERT in wild-type animals contributes to aversive cocaine effects, the enhanced cocaine reinforcement reported in NET knockout or SERT knockout mice, and even greater enhancements in NET/SERT double knockouts, makes sense (F.S.H., I.S., and G.R.U., unpublished work). Rearrangements in these systems in DAT knockout mice, clearly supported by their dramatically different responses to fluoxetine (30), could help preserve cocaine reward/reinforcement by altering the net balance of aversive and reinforcing effects of cocaine on serotonin circuits.
The effects of transporter gene copy numbers on cocaine place preference appear to indicate a greater role for DAT than SERT in cocaine reward/reinforcement in wild-type mice, consistent with previous pharmacological suggestions. Deleting two DAT copies and one SERT gene copy ablates cocaine preference just as effectively as deletion of both copies of both genes. By contrast, DAT+/− SERT−/− knockout mice that have a single wild-type DAT gene copy retain near-maximal cocaine reward/reinforcement and display no evidence for different cocaine dose-effect relationships. We are currently examining the drug-dose relationships in DAT−/− SERT+/− mice. If similar gene-dose relationships obtain in pharmacological interventions for human cocaine therapy, near-complete actions at DAT, accompanied by even subtotal actions at SERT, might provide an efficacious transporter-based strategy for reducing cocaine reward/reinforcement.
The present results suggest that medication strategies using drugs acting at both dopamine and serotonin brain systems might provide one minimal set of activities necessary to effectively combat cocaine addiction. Such drugs might be found. The sequence homologies between DAT and SERT (16) and work from structure/function studies that identify DAT domains selectively involved in cocaine recognition (58, 59) both support the idea that a single compound might provide uptake-sparing cocaine blockade at both transporters and a transporter-based anticocaine therapeutic. A second strategy could be based on involvement of selected dopamine and serotonin receptor subtypes. Drugs acting at both serotonin and dopamine receptor subtypes might represent a receptor-based medications development strategy targeting cocaine reward/reinforcement.
Acknowledgments
We are grateful to Kaori Itokawa, Nobue Kitanaka, Naraja Karmacharya, Nancy Goodman, Ira Baum, Qing-Rong Liu, David Huso, and the Charles River/Triad animal care staff. We also thank the National Institute on Drug Abuse, the National Institute of Mental Health, the Bundesministerium für Bildung, Wissenschaft, Forschung und Technologie, the Ministry of Education, Science, Sports, and Culture (Japan), and the Ministry of Health and Welfare (Japan). G.R.U. is grateful for his affiliation with the Departments of Neurology and Neuroscience, Johns Hopkins School of Medicine.
Abbreviations
- DAT
dopamine transporter
- SERT
serotonin transporter
- NET
norepinephrine transporter
- HVA
homovanillic acid
- DOPAC
3,4-dihydroxyphenylacetic acid
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Substance Abuse and Mental Health Services Administration. National Household Survey on Drug Abuse: Main Findings 1999. Rockville, MD: U.S. Department of Health and Human Services; 1999. [Google Scholar]
- 2.Caroll F I, Lewis A H. Pharmaceutical News. 1994;1:11–17. [Google Scholar]
- 3.Kuhar M J, Ritz M C, Boja J W. Trends Neurosci. 1991;14:299–302. doi: 10.1016/0166-2236(91)90141-g. [DOI] [PubMed] [Google Scholar]
- 4.Woolverton W L, Johnson K M. Trends Pharmacol Sci. 1992;13:193–200. doi: 10.1016/0165-6147(92)90063-c. [DOI] [PubMed] [Google Scholar]
- 5.Spealman R D, Madras B K, Bergman J. J Pharmacol Exp Ther. 1989;251:142–149. [PubMed] [Google Scholar]
- 6.Di Chiara G, Imperato A. Proc Natl Acad Sci USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pettit H O, Ettenberg A, Bloom F E, Koob G F. Psychopharmacology. 1984;84:167–173. doi: 10.1007/BF00427441. [DOI] [PubMed] [Google Scholar]
- 8.Roberts D C, Corcoran M E, Fibiger H C. Pharmacol Biochem Behav. 1977;6:615–620. doi: 10.1016/0091-3057(77)90084-3. [DOI] [PubMed] [Google Scholar]
- 9.Sora I, Wichems C, Takahashi N, Li X F, Zeng Z, Revay R, Lesch K P, Murphy D L, Uhl G R. Proc Natl Acad Sci USA. 1998;95:7699–7704. doi: 10.1073/pnas.95.13.7699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rocha B A, Fumagalli F, Gainetdinov R R, Jones S R, Ator R, Giros B, Miller G W, Caron M G. Nat Neurosci. 1998;1:132–137. doi: 10.1038/381. [DOI] [PubMed] [Google Scholar]
- 11.Chait L D, Uhlenhuth E H, Johanson C E. J Pharmacol Exp Ther. 1987;242:777–783. [PubMed] [Google Scholar]
- 12.Wilson A W, Neill J C, Costall B. Alcohol. 1998;16:249–270. doi: 10.1016/s0741-8329(98)00013-5. [DOI] [PubMed] [Google Scholar]
- 13.Yanagita T, Katoh S, Wakasa Y, Oinuma N. National Institute on Drug Abuse Res Monogr. 1982;41:208–214. [PubMed] [Google Scholar]
- 14.Javitch J A, Blaustein R O, Snyder S H. Mol Pharmacol. 1984;26:35–44. [PubMed] [Google Scholar]
- 15.Cooper J R, Bloom F E, Roth R H. The Biochemical Basis of Neuropharmacology. New York: Oxford Univ. Press; 1991. [Google Scholar]
- 16.Uhl G R, Hartig P R. Trends Pharmacol Sci. 1992;13:421–425. doi: 10.1016/0165-6147(92)90133-q. [DOI] [PubMed] [Google Scholar]
- 17.Miliaressis E. Pharmacol Biochem Behav. 1977;7:177–180. doi: 10.1016/0091-3057(77)90204-0. [DOI] [PubMed] [Google Scholar]
- 18.Poschel B P. Psychopharmacol Bull. 1974;10:46–47. [PubMed] [Google Scholar]
- 19.Redgrave P. Brain Res. 1978;155:277–295. doi: 10.1016/0006-8993(78)91023-5. [DOI] [PubMed] [Google Scholar]
- 20.Harrison A A, Parsons L H, Koob G F, Markou A. Psychopharmacology (Berlin) 1999;141:242–250. doi: 10.1007/s002130050831. [DOI] [PubMed] [Google Scholar]
- 21.Fletcher P J, Tampakeras M, Yeomans J S. Pharmacol Biochem Behav. 1995;52:65–71. doi: 10.1016/0091-3057(94)00441-k. [DOI] [PubMed] [Google Scholar]
- 22.Rocha B A, Scearce-Levie K, Lucas J J, Hiroi N, Castanon N, Crabbe J C, Nestler E J, Hen R. Nature (London) 1998;393:175–178. doi: 10.1038/30259. [DOI] [PubMed] [Google Scholar]
- 23.Fletcher P J, Korth K M. Psychopharmacology (Berlin) 1999;142:165–174. doi: 10.1007/s002130050876. [DOI] [PubMed] [Google Scholar]
- 24.Tran-Nguyen L T, Baker D A, Grote K A, Solano J, Neisewander J L. Psychopharmacology (Berlin) 1999;146:60–66. doi: 10.1007/s002130051088. [DOI] [PubMed] [Google Scholar]
- 25.Nomikos G G, Spyraki C. Pharmacol Biochem Behav. 1988;30:853–858. doi: 10.1016/0091-3057(88)90110-4. [DOI] [PubMed] [Google Scholar]
- 26.Papp M, Willner P. Psychopharmacology. 1991;103:99–102. doi: 10.1007/BF02244082. [DOI] [PubMed] [Google Scholar]
- 27.Frank R A, Zubrycki E. Pharmacol Biochem Behav. 1989;33:725–727. doi: 10.1016/0091-3057(89)90416-4. [DOI] [PubMed] [Google Scholar]
- 28.Tella S R. Pharmacol Biochem Behav. 1995;51:687–692. doi: 10.1016/0091-3057(94)00438-o. [DOI] [PubMed] [Google Scholar]
- 29.Xu F, Gainetdinov R R, Wetsel W C, Jones S R, Bohn L M, Miller G W, Wang Y M, Caron M G. Nat Neurosci. 2000;3:465–471. doi: 10.1038/74839. [DOI] [PubMed] [Google Scholar]
- 30.Hall F S, Sora I, Li X F, Uhl G. Soc. Neurosci. Abstr. 2000. [Google Scholar]
- 31.Uhl G R, Vandenbergh D J, Miner L L. Curr Biol. 1996;6:935–936. doi: 10.1016/s0960-9822(02)00630-9. [DOI] [PubMed] [Google Scholar]
- 32.Reith M E A. In: Cocaine: Pharmacology, Physiology, and Clinical Strategies. Lakoski J M, Golloway M P, White F J, editors. Boca Raton: CRC Press; 1991. pp. 203–227. [Google Scholar]
- 33.Ritz M C, Cone E J, Kuhar M J. Life Sci. 1990;46:635–645. doi: 10.1016/0024-3205(90)90132-b. [DOI] [PubMed] [Google Scholar]
- 34.Roberts D C, Phelan R, Hodges L M, Hodges M M, Bennett B, Childers S, Davies H. Psychopharmacology (Berlin) 1999;144:389–397. doi: 10.1007/s002130051022. [DOI] [PubMed] [Google Scholar]
- 35.Andrews A M, Murphy D L. J Neurochem. 1993;60:1167–1170. doi: 10.1111/j.1471-4159.1993.tb03271.x. [DOI] [PubMed] [Google Scholar]
- 36.Boja J W, Carroll F I, Rahman M A, Philip A, Lewin A H, Kuhar M J. Eur J Pharmacol. 1990;184:329–332. doi: 10.1016/0014-2999(90)90627-i. [DOI] [PubMed] [Google Scholar]
- 37.Marcusson J O, Bergstrom M, Eriksson K, Ross S B. J Neurochem. 1988;50:1783–1790. doi: 10.1111/j.1471-4159.1988.tb02479.x. [DOI] [PubMed] [Google Scholar]
- 38.Tejani-Butt S M, Brunswick D J, Frazer A. Eur J Pharmacol. 1990;191:239–243. doi: 10.1016/0014-2999(90)94155-q. [DOI] [PubMed] [Google Scholar]
- 39.Terai M, Hidaka K, Nakamura Y. Eur J Pharmacol. 1989;173:177–182. doi: 10.1016/0014-2999(89)90516-5. [DOI] [PubMed] [Google Scholar]
- 40.Boujrad F, Dauphin F, de Beaurepaire R. Brain Res. 1998;812:279–282. doi: 10.1016/s0006-8993(98)00970-6. [DOI] [PubMed] [Google Scholar]
- 41.Bolanos-Jimenez F, Manhaes de Castro R M, Seguin L, Cloez-Tayarani I, Monneret V, Drieu K, Fillion G. Eur J Pharmacol. 1995;294:531–540. doi: 10.1016/0014-2999(95)00590-0. [DOI] [PubMed] [Google Scholar]
- 42.Giros B, Jaber M, Jones S R, Wightman R M, Caron M G. Nature (London) 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- 43.Bengel D, Murphy D L, Andrews A M, Wichems C H, Feltner D, Heils A, Mossner R, Westphal H, Lesch K P. Mol Pharmacol. 1998;53:649–655. doi: 10.1124/mol.53.4.649. [DOI] [PubMed] [Google Scholar]
- 44.Bosse R, Fumagalli F, Jaber M, Giros B, Gainetdinov R R, Wetsel W C, Missale C, Caron M G. Neuron. 1997;19:127–138. doi: 10.1016/s0896-6273(00)80353-0. [DOI] [PubMed] [Google Scholar]
- 45.Maldonado R, Saiardi A, Valverde O, Samad T A, Roques B P, Borrelli E. Nature (London) 1997;388:586–589. doi: 10.1038/41567. [DOI] [PubMed] [Google Scholar]
- 46.Pert A. Adv Pharmacol. 1998;42:991–995. doi: 10.1016/s1054-3589(08)60913-8. [DOI] [PubMed] [Google Scholar]
- 47.Phillips T J, Brown K J, Burkhart-Kasch S, Wenger C D, Kelly M A, Rubinstein M, Grandy D K, Low M J. Nat Neurosci. 1998;1:610–615. doi: 10.1038/2843. [DOI] [PubMed] [Google Scholar]
- 48.Noble E P. Eur J Psychiatry. 2000;15:79–89. doi: 10.1016/s0924-9338(00)00208-x. [DOI] [PubMed] [Google Scholar]
- 49.Tzschentke T M. Prog Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- 50.Roberts D C, Koob G F. Pharmacol Biochem Behav. 1982;17:901–904. doi: 10.1016/0091-3057(82)90469-5. [DOI] [PubMed] [Google Scholar]
- 51.Parsons L H, Koob G F, Weiss F. Synapse. 1999;32:132–135. doi: 10.1002/(SICI)1098-2396(199905)32:2<132::AID-SYN6>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 52.Parsons L H, Weiss F, Koob G F. Psychopharmacology (Berlin) 1996;128:150–160. doi: 10.1007/s002130050120. [DOI] [PubMed] [Google Scholar]
- 53.Parsons L H, Weiss F, Koob G F. J Neurosci. 1998;18:10078–10089. doi: 10.1523/JNEUROSCI.18-23-10078.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Calcagnetti D J, Keck B J, Quatrella L A, Schechter M D. Life Sci. 1995;56:475–483. doi: 10.1016/0024-3205(94)00414-n. [DOI] [PubMed] [Google Scholar]
- 55.Ichikawa J, Meltzer H Y. Brain Res. 1999;842:445–451. doi: 10.1016/s0006-8993(99)01876-4. [DOI] [PubMed] [Google Scholar]
- 56.De Deurwaerdere P, Spampinato U. J Neurochem. 1999;73:1033–1042. doi: 10.1046/j.1471-4159.1999.0731033.x. [DOI] [PubMed] [Google Scholar]
- 57.Lorrain D S, Riolo J V, Matuszewich L, Hull E M. J Neurosci. 1999;19:7648–7652. doi: 10.1523/JNEUROSCI.19-17-07648.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kitayama S, Wang J B, Uhl G R. Synapse. 1993;15:58–62. doi: 10.1002/syn.890150107. [DOI] [PubMed] [Google Scholar]
- 59.Lin Z, Wang W, Kopajtic T, Revay R S, Uhl G R. Mol Pharmacol. 1999;56:434–447. doi: 10.1124/mol.56.2.434. [DOI] [PubMed] [Google Scholar]